Abstract
Background and objective: Acute liver failure (ALF) is a type of disease with high mortality and rapid progression with no specific treatment methods currently available. Glucocorticoids exert beneficial clinical effects on therapy for ALF. However, the mechanism of this effect remains unclear and when to use glucocorticoids in patients with ALF is difficult to determine. The purpose of this study was to investigate the specific immunological mechanism of dexamethasone (Dex) on treatment of ALF induced by lipopolysaccharide (LPS)/D-galactosamine (D-GaIN) in mice. Methods: Male C57BL/6 mice were given LPS and D-GaIN by intraperitoneal injection to establish an animal model of ALF. Dex was administrated to these mice and its therapeutic effect was observed. Hematoxylin and eosin (H&E) staining was used to determine liver pathology. Multicolor flow cytometry, cytometric bead array (CBA) method, and next-generation sequencing were performed to detect changes of messenger RNA (mRNA) in immune cells, cytokines, and Kupffer cells, respectively. Results: A mouse model of ALF can be constructed successfully using LPS/D-GaIN, which causes a cytokine storm in early disease progression. Innate immune cells change markedly with progression of liver failure. Earlier use of Dex, at 0 h rather than 1 h, could significantly improve the progression of ALF induced by LPS/D-GaIN in mice. Numbers of innate immune cells, especially Kupffer cells and neutrophils, increased significantly in the Dex-treated group. In vivo experiments indicated that the therapeutic effect of Dex is exerted mainly via the glucocorticoid receptor (Gr). Sequencing of Kupffer cells revealed that Dex could increase mRNA transcription level of nuclear receptor subfamily 4 group A member 1 (Nr4a1), and that this effect disappeared after Gr inhibition. Conclusions: In LPS/D-GaIN-induced ALF mice, early administration of Dex improved ALF by increasing the numbers of innate immune cells, especially Kupffer cells and neutrophils. Gr-dependent Nr4a1 upregulation in Kupffer cells may be an important ALF effect regulated by Dex in this process.
Keywords: Glucocorticoid, Dexamethasone, Kupffer cells, Acute liver failure, Nuclear receptor subfamily 4 group A member 1 (Nr4a1)
1. Introduction
Acute liver failure (ALF) is a type of clinical symptom that progresses rapidly. With its potential for rapid progression of acute hepatic dysfunction to multiorgan failure, prompt diagnosis and expeditious management are required. Liver transplantation is the ultimate cure for severe unresolved ALF. However, the lack of donors strongly limits the broad use of liver transplantation for treatment of ALF. Therefore, finding alternative treatments for ALF is an urgent medical need. Glucocorticoids have been used in the treatment of ALF for the prevention of hepatic encephalopathy with favorable clinical results, especially in Japan (Kakisaka et al., 2017). However, the questions regarding the optimal dosage and treatment duration of glucocorticoids in ALF therapy, and the best time for beginning treatment are still unresolved.
Immune damage, such as monocyte and macrophage dysfunction, is an important component in the onset and progression of ALF (Stravitz and Lee, 2019). Macrophages are innate immune cells with dual pro-inflammatory and anti-inflammatory effects, and they play a vital role in pathogenesis, progression, and remission of ALF (Possamai et al., 2014). The cytokine storm caused by macrophage is also a major factor in pathogenesis of ALF. The inherent plasticity of macrophages determines the diverse roles of these cells in different tissues, even at different stages of identical diseases. Determining the changes of macrophages in the progression of ALF will improve the efficacy of immunomodulatory therapy.
Glucocorticoids have strong immunosuppressive effects and their specific effect on regulating innate immune cells such as macrophages is a major focus of recent research (Robert et al., 2016). Previous studies have shown that glucocorticoids can affect apoptosis (Achuthan et al., 2018), phagocytosis (Garabuczi et al., 2015), cytokine secretion (van de Garde et al., 2014), and phenotypic differentiation (Heideveld et al., 2018) of macrophages to mitigate progression of inflammatory diseases. However, glucocorticoids are now known to have other functions as well, and their immunoregulatory mechanism is no longer considered to be limited to immunosuppressive effects (Cain and Cidlowski, 2017; Yu et al., 2019). Because of its strong anti-inflammatory effect, the corticosteroid dexamethasone (Dex) is recommended for the treatment of ALF. However, neither the immunological mechanisms by which glucocorticoids affect ALF progression nor the roles macrophages play in this process have thus far been clarified. Resolving these questions could provide scientific support for the use of glucocorticoids in the treatment of ALF, and facilitate determination of the optimal starting point for glucocorticoid administration.
In China, ALF is caused mainly by viral hepatitis. The pathogenesis of the lipopolysaccharide (LPS)/D-galactosamine (D-GaIN)-induced ALF mice model is considered similar to that of human liver failure caused by viral hepatitis (Zhao et al., 2018). Research has shown that D-GaIN is a liver-specific toxin that can selectively deplete uridine nucleotides, inhibit the syntheses of protein and messenger RNA (mRNA) in the liver, cause irreversible damage to liver cells, and increase liver sensitivity to LPS (Lyu et al., 2019). We have investigated the changes of immune cells and cytokines in the progression of LPS/D-GaIN-induced liver failure in mouse models. We further explored the specific immunological effects of glucocorticoids in ALF therapy to provide theoretical support for immunotherapy of ALF.
2. Materials and methods
2.1. Experiments on mice
Mouse studies were carried out in accordance with the National Institutes of Health guidelines and approved by the Animal Care and Use Committee of Zhejiang University, Hangzhou, China. Seven-eight week-old C57BL/6 male mice (weight 18–20 g) were purchased from Shanghai Slack Company, China and group-housed (4–5 mice per cage) in standard cages. All mice were housed in a temperature-controlled room on a 12 h:12 h light/dark cycle and had free access to water and standard mouse chow. The mice were fasted for 12 h before each experiment. Doses of 5 μg/kg LPS (Sigma-Aldrich, St. Louis, MO, USA) and 500 mg/kg D-GaIN (Sigma-Aldrich) dissolved with warm phosphate-buffered saline (PBS), were injected intraperitoneally to mice. The mice were sacrificed after LPS/D-GaIN treatment for 1–5 h. The liver samples were weighed and photographed, and blood was separated to collect the serum and immune cells. Dex (1 mg/kg; Sigma-Aldrich) in olive oil (Sangon Biotech, Shanghai, China) was injected intraperitoneally and mice were sacrificed 4 h after Dex stimulation. Glucocorticoid receptor (Gr) expression was suppressed with 100 μg/kg of the inhibitor RU486 (Sigma-Aldrich) in olive oil 1 h before Dex and LPS injection.
2.2. Measurement of liver necrosis
Paraffin-embedded liver samples were sectioned at 4-µm thickness and stained with hematoxylin and eosin (H&E) as described previously (Joshi et al., 2016). Serum alanine aminotransferase (ALT) and aspartate transaminase (AST) levels were determined using commercial reagents (Fujifilm, Tokyo, Japan).
2.3. Flow cytometry and data analysis
Digestive enzymes (type I collagenase 0.05% (0.5 g/L), type IV collagenase 0.05% (0.5 g/L), type I DNase 0.002% (0.02 g/L), and fetal bovine serum (FBS) 10% (0.1 g/mL)) were prepared with Hanks solution and placed in 37 °C water. After sacrificing experimental animals, mouse livers were taken out, rinsed with PBS, homogenized and placed in 10 mL of a solution with digestive enzymes, and digested on a 37 °C shaker for 15 min. The digested livers were filtered through a 70-μm cell strainer. Next, 50 g of hepatocytes and 500 g of non-parenchymal cells were centrifuged and pelleted at 4 °C for 10 min. Hepatic immune cells were isolated using 33% Percoll single density gradient centrifugation at 2000 r/min for 20 min and split using 500 µL red lysate. Finally, the cells were washed with PBS and filtered through a 40-µm sieve, and prepared for flow cytometry.
Anti-mouse antibodies CD45-FITC (30-F11), B220-APC (RA3-6B2), CD3-CY5.5 (145-2C11), CD49b-BV605 (HMa2), CD4-V500 (RM4-5), CD8-PE (53-6.7), CD11b-APC (M1/70), F4/80-BV421 (T45-2342), and Ly6G-AF700 (1A8) were purchased from BD Biosciences (NJ, USA), eBioscience (San Diego, CA, USA), and BioLegend (San Diego). FVS780 (BD Biosciences) was used to exclude dead cells. Stained cells were tested by 18-color flow cytometry BD™ LSRFortessa (BD Biosciences). Equal numbers of CD45+ cells were down-sampled from each sample and concatenated to a file (.fcs) for analysis using FlowJo v10.0 (BD Biosciences).
2.4. Serum cytokines
Serum cytokines interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), chemokine (C-C motif) ligand 2 (CCL-2), and IL-10 levels were quantitatively measured with a cytometric bead array (CBA) mouse inflammation kit (BD Biosciences) following the manufacturer’s protocol. Statistical analysis was performed with FCAP Array software v3.0 (BD Biosciences).
2.5. Immunohistochemistry
Liver sections were blocked in 3% normal goat serum in PBS for 45 min at room temperature and incubated with the primary antibody F4/80 (Abcam, Cambridge, UK; 1:400 (v/v)) overnight at 4 °C. Primary antibodies were detected using horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (CST, Boston, USA; 1:400 (v/v)) for 50 min, and 3,3'-diaminobenzidine (DAB) solution (Proteintech, Chicago, USA). All operations followed manufacturers’ instructions. Liver sections were scanned with panoramic MIDI (3DHISTECH, Budapest, Hungary).
2.6. Real-time polymerase chain reaction
RNA was extracted from liver samples by the TRIzol method. Kupffer cells were isolated using the methods of Leroux et al. (2012), and RNA was extracted from the cells using the RNeasy Plus Mini Kit (Qiagen, Bremen, Germany). Relative mRNA expression was determined using SYBR Green Master Mix (TaKaRa Bio, Kyoto, Japan). The primers used were the following: Cd86 forward 5'-ATGGACCCCAGA TGCACCAT-3', reverse 5'-CAACTTTTGCTGGTC CTGCC-3'; Cd163 forward 5'-TGCTGTCACTAA CGCTCCTG-3', reverse 5'-CATTGCATGCCAGGT CATCG 3'; Gr forward 5'-GAAAGTTGGGGGAGT GTGCT-3', reverse 5'-GGGTCTCATCTAATGGGC CG-3'; Nr4a1 forward 5'-TATCAAGCCCCAGCAG TGTG-3', reverse 5'-GCTGTCCTTCCACTGCTCTT-3'. Mix was run on a LightCycler 480 System. Running programs were as follows: pre-heating, 30 s at 95 °C; 40 cycles of 3 s at 95 °C and 30 s at 60 °C; 15 s at 95 °C, 1 min at 60 °C, 15 s at 95 °C. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene.
2.7. RNA-seq data process
RNA libraries of LPS/D-GaIN and LPS/D-GaIN+Dex were constructed by Microwell-seq and then sequenced by Illumina HiSeq (Illumina, California, USA). Because sequencing errors and low quality may produce false barcodes, we discarded read pairs with a base quality below 10. Cells with high proportions of transcript counts derived from mitochondria-encoded genes were also excluded. We used the SEURAT package in R for clustering and digital gene expression (DGE) data were used as inputs. DoHeatmap function was used to determine differential gene expression in LPS/D-GaIN and LPS/D-GaIN+Dex samples.
2.8. Statistical analysis
Data analysis was performed with GraphPad Prism 7.0 (San Diego). Continuous variables are shown as mean±standard error of the mean (SEM). Two-group comparisons were carried out using Student’s t-test. Comparison of three or more groups was performed using one-or two-way analysis of variance (ANOVA). Mouse survival was computed by the Kaplan-Meier estimator and statistical significance was calculated using log-rank test. Two-sided tests were used and only P values of <0.05 were considered significant.
3. Results
3.1. LPS/D-GaIN-induced mouse model of ALF
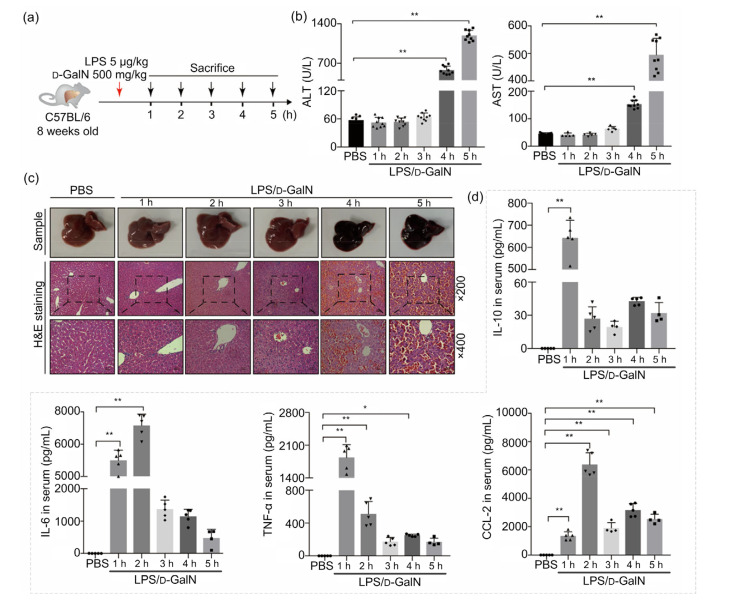
To construct a stable animal model of ALF, we injected LPS/D-GalN into eight-week-old mice for 5 h through intraperitoneal administration. To quantitatively assess the ALF model, liver enzymes and pathological tests were performed on mouse blood and liver at different time points (Fig. 1a). It was found that levels of ALT and AST started to rise after 4 h of administration (Fig. 1b). A macroscopic view of the treated ALF model livers showed obvious congestion (Fig. 1c). Liver H&E staining showed that interstitial congestion began to appear in mice at 4 h after the administration of the ALF model drugs, and became more severe at 5 h. The hepatic sinus was blocked and liver cells had inflammatory necrosis, accompanied by infiltration of liver non-parenchymal cells (Fig. 1c).
Fig. 1.
LPS/D-GaIN-induced mouse ALF model
(a) Schematic illustration of ALF mouse sample processing. (b) Changes of serum ALT and AST levels during mice ALF progression. All groups are compared with a PBS control group (n=5 or 9). (c) Representative images of mouse liver samples and hepatic hematoxylin and eosin (H&E) staining (n=5; scan bar=50 μm (×200) or 25 μm (×400)). (d) Serum levels of IL-10, IL-6, TNF-α, and CCL-2 after different time durations of LPS/D-GaIN stimulation (n=5 or 4). All groups are compared to a PBS control group. Data are expressed as mean±standard error of the mean (SEM). The number and value of each sample are shown by various marks. * P<0.05; ** P<0.01. LPS, lipopolysaccharide; D-GaIN, D-galactosamine; ALF, acute liver failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IL-10, interleukin-10; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; CCL-2, chemokine (C-C motif) ligand 2
It has been well established that cytokine damage related to immune damage plays an important role in the progression of liver failure. In this study, serum cytokine levels were measured at different stages of ALF progression in ALF mice induced by LPS/D-GalN. We found that IL-6 level significantly increased at 1 h, reached a peak at 2 h and then gradually decreased. Compared with the control group, serum TNF-α level initially increased, and then decreased rapidly after reaching a maximum at 1 h. CCL-2 level began to increase at 1 h and reached a maximum after 2 h. However, changes in the anti-inflammatory factor IL-10 was mostly consistent with that of TNF-α, decreasing after a brief rise at the initial stage (Fig. 1d).
3.2. Changes of immune cell numbers in the progression of LPS/D-GaIN-induced ALF in mice
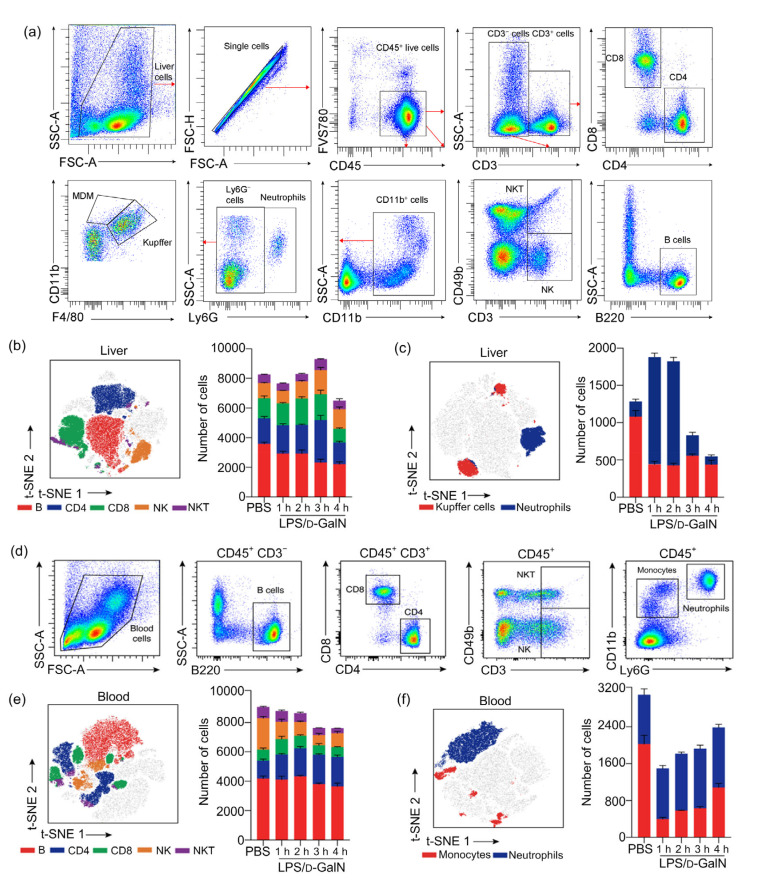
In order to clarify the changes in liver and blood immune cells in ALF induced by LPS/D-GalN, blood and liver samples at different time points were processed into single cell suspension, which was assayed using multicolor flow cytometry. Because the ALF mice were in the late stage of liver failure at 5 h and the number of immune cells decreased significantly, this experiment only monitored changes in liver immune cells at 1–4 h.
As shown in Figs. 2a and 2b, there were no significant changes in quantities of liver B cells, CD4+ T cells, CD8+ T cells, natural killer (NK) cells, or natural killer T (NKT) cells at 1–3 h. However, there was apparent evidence of liver failure as the inflammatory response progressed at 4 h when all of the above-mentioned cells decreased in number (Figs. 2a and 2b). The number of neutrophils among liver innate immune cells increased during 1 to 2 h, and began to decrease at 3 h. Kupffer cells of liver colonized macrophages began to decrease at 1 h (Figs. 2a and 2c). To clarify the changes of blood immune cells during ALF progression, the peripheral blood of ALF mice modeled with LPS/D-GalN was subjected to multicolor flow cytometry at 1–4 h. Among adaptive immune cells, in the blood, we found that B cells, CD4+ T cells, or CD8+ T cells did not change significantly as a whole; however, NK cells and NKT cells were reduced in number compared to the control group (Figs. 2d and 2e). There was no significant change in neutrophils belonging to blood innate immune cells. The number of monocytes decreased significantly at 1 h, and then increased gradually (Figs. 2d and 2f).
Fig. 2.
Changes of liver and blood immune cell numbers during LPS/D-GaIN-induced ALF in mice
(a) Gating strategy for surface maker analysis within the total liver nonparenchymal cells. (b, c) t-SNE map of CD45+ cells analyzed by expression of CD45, B220, CD3, CD4, CD8, CD49b, CD11b, Ly6G, and F4/80 in liver based on 15 000 CD45+ cells pooled from 15 mice and stacked bar plots of cell numbers within different subsets. (d) Gating strategy for makers within the blood immune cells. (e, f) t-SNE map distribution of CD45+ blood cells analyzed by intensities of CD45, B220, CD3, CD4, CD8, CD49b, CD11b, and Ly6G markers from 15 000 CD45+ cells and stacked bar plots of cell numbers within different subsets. The results are expressed as mean±standard error of the mean (SEM; n=3 per group per time point). LPS, lipopolysaccharide; D-GaIN, D-galactosamine; t-SNE, t-distributed stochastic neighbor embedding; SSC-A, side scatter area; FSC-A, forward scatter area; FSC-H, forward scatter height; MDM, monocyte derived macrophage; PBS, phosphate-buffered saline
3.3. Effects of early use of Dex on LPS/D-GaIN-induced ALF mice survival and liver damage
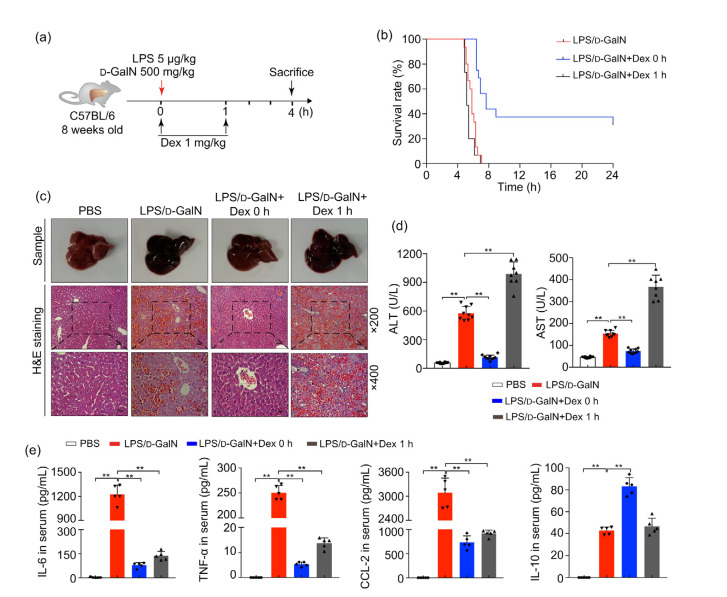
Early use of glucocorticoids is recommended for treatment of ALF (Fujiwara et al., 2014). To investigate the effect of glucocorticoid intervention on ALF, ALF mice induced by LPS/D-GaIN were treated with Dex at 0 and 1 h (Fig. 3a). Our results showed that the survival rates of ALF mice given Dex at 0 h were significantly improved compared with the group modeled by LPS/D-GaIN alone (Fig. 3b). However, there was no difference in survival rates of ALF mice given Dex at 1 h compared with the ALF mice (Fig. 3b). Applying Dex at 0 h can significantly improve the liver congestion and liver tissue structure. Hepatic sinus congestion was significantly improved, and liver ALT and AST levels were markedly reduced (Figs. 3c and 3d). Nevertheless, aggravated liver damage could still be seen after Dex treatment at 1 h (Figs. 3c and 3d). Detection of serum cytokines by the CBA method demonstrated that blood proinflammatory cytokines such as IL-6, TNF-α, and CCL-2 were significantly reduced after Dex 0 h therapy compared with the untreated LPS/D-GaIN mice (Fig. 3e). Nevertheless, application of Dex at 1 h could still play a role in inhibiting the secretion of inflammatory factors. At the same time, the serum anti-inflammatory cytokine IL-10 increased significantly in mice treated with Dex at 0 h; but IL-10 level in the late Dex administration group did not change significantly compared with the LPS/D-GaIN control group (Fig. 3e).
Fig. 3.
Effects of early administration of Dex on ALF mice survival and liver injury
Male C57 mice were treated with LPS/D-GaIN and Dex at early (0 h) and later (1 h) time. (a) Schematic illustration of mice processing. (b) Kaplan-Meier survival of ALF mice receiving Dex 1 mg/kg at 0 and 1 h (n=15). Log-rank test P<0.001 between LPS/D-GaIN group and LPS/D-GaIN+Dex 0 h group. Log-rank test P>0.05 between LPS/D-GaIN group and LPS/D-GaIN+Dex 1 h group. (c) Representative sample images and hematoxylin and eosin (H&E) staining of livers harvested at 4 h after the administrations of LPS/D-GaIN (n=5; scan bar=50 μm (×200) or 25 μm (×400)). (d) Changes in serum ALT and AST levels (n=8). (e) CBA methods detect serum levels of IL-6, TNF-α, CCL-2, and IL-10 (n=5). All groups are compared with the LPS/D-GaIN control group. The data are shown as mean±standard error of the mean (SEM). The number and value of each sample are shown by various marks. ** P<0.01. LPS, lipopolysaccharide; D-GaIN, D-galactosamine; Dex, dexamethasone; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CBA, cytometric bead array; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; CCL-2, chemokine (C-C motif) ligand 2; IL-10, interleukin-10
3.4. Effect of Dex on the number of Kupffer cells in mice with LPS/D-GaIN-induced ALF
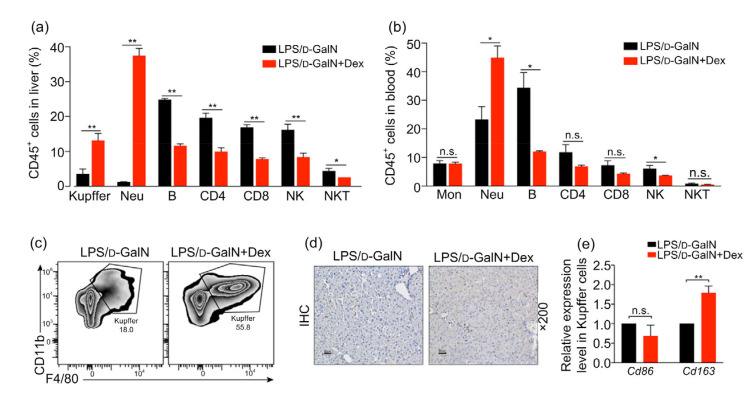
In order to clarify the immunological mechanism in the early Dex administration in improving ALF, multicolor flow cytometry was used to detect changes in liver and blood immune cells. Our study found that the use of Dex at 0 h in LPS/D-GaIN mouse models significantly increased the number of Kupffer cells and neutrophils in liver, while the numbers of B cells, CD4+ T cells, CD8+ T cells, NKT cells, and NK cells, which belong to adaptive immune cells, were significantly reduced (Fig. 4a). Similarly, the numbers of blood neutrophils increased, and numbers of B cells and NK cells decreased in the early Dex intervention group (Fig. 4b). Previous work has verified that glucocorticoids mainly exerted their effects as anti-allergic effects on macrophages and neutrophils, which form part of the innate immune system (Tuckermann et al., 2007). Considering the effects of glucocorticoids on multiple phenotypes of macrophages and the importance of macrophages in the progression of ALF (Possamai et al., 2014), we sorted Kupffer cells and examined their phenotypes (Figs. 4c and 4d). In ALF with early intervention of Dex, we found that the elevated numbers of liver macrophages were mainly M2 anti-inflammatory immune cells (Fig. 4e).
Fig. 4.
Effects of early use of Dex on immune cells in LPS/D-GaIN-induced ALF mice
Samples of LPS/D-GaIN-induced WT mice treated with or without Dex were collected at 4 h. (a, b) The proportion of different immune cells in CD45+ cells in liver (a) and blood (b) with or without Dex treatment. (c) Distribution of Ly6G− CD11b+F4/80+ Kupffer cells between ALF mice without and with Dex treatment by flow cytometry. (d) F4/80 protein expression in ALF mice without or with Dex treatment by immunohistochemical (IHC) techniques. Scan bar=50 μm. (e) Differential mRNA expression levels of Cd86 (left) and Cd163 (right) in Kupffer cells of ALF mice without or with Dex treatment. The data are shown as mean±standard error of the mean (SEM), n=5 per group. * P<0.05; ** P<0.01. n.s., not significant; LPS, lipopolysaccharide; D-GaIN, D-galactosamine; Dex, dexamethasone; WT, wild-type; Mon, monocyte; Neu, neutrophil
3.5. Effect of Dex on Nr4a1 expression in Kupffer cells in LPS/D-GaIN-induced ALF mice
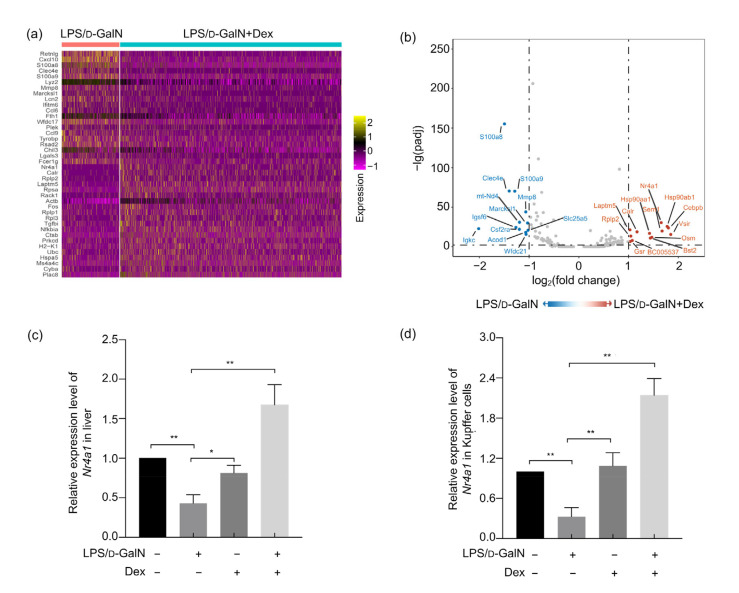
To further clarify the specific mechanism by which early Dex treatment mitigated, we sequenced RNA sequencing to detect Kupffer cells after flow sorting and found that the hepatocyte damage-related molecules, calcium-binding proteins S100A8, S100A9, and the chemokine CXCL-10 were significantly increased in Kupffer cells of LPS/D-GaIN-induced ALF mice. The Dex treatment group showed increased nuclear receptor Nr4a1 expression in Kupffer cells as well as changes in the heat shock protein HSP subfamily genes (Figs. 5a and 5b). Nr4a1 can participate in the systemic immune response by affecting cell proliferation. Therefore, we hypothesized that the effect of early use of Dex to increase liver Kupffer cells may be related to the increase of Nr4a1 expression. Quantitative polymerase chain reaction (qPCR) assays confirmed that expression of Nr4a1 in Kupffer cells and liver in the early Dex treatment group was significantly increased (Figs. 5c and 5d).
Fig. 5.
Effects of Dex on Nr4a1 expression in the liver and Kupffer cells in LPS/D-GaIN-induced ALF mice
LPS/D-GaIN-induced WT mice were treated with or without Dex and liver samples were collected 4 h later. (a) RNA sequencing of Kupffer cells sorted from the liver in two groups (mentioned above). Gene expression heatmap showed the top differentially expressed genes between two samples. Yellow indicates high expression; purple and black indicate low expression. (b) Volcano plot of the Kupffer cells sequencing data. (c, d) mRNA expression levels of Nr4a1 in mice liver samples (c) and Kupffer cells (d) between four groups (n=5 per group). All groups are compared with the LPS/D-GaIN group. The data are shown as mean±standard error of the mean (SEM). * P<0.05; ** P<0.01. LPS, lipopolysaccharide; D-GaIN, D-galactosamine; Dex, dexamethasone; WT, wild-type
3.6. Effects of Gr inhibition on LPS/D-GaIN-induced ALF in mice and Nr4a1 level in Kupffer cells
Glucocorticoid can bind to intracellular Gr (Panettieri et al., 2019), which translocates to the cell nucleus to interact with glucocorticoid response elements (GREs), thereby exerting genomic effects that alter protein expression. Interestingly, glucocorticoids also manifest almost immediate non-genomic actions by non-specific interactions with the cell membrane, or specific interactions with cytosolic Gr (cGR) or membrane-bound Gr (mGR) non-genomic mechanisms, which could not be eliminated by Gr inhibitors (Panettieri et al., 2019).
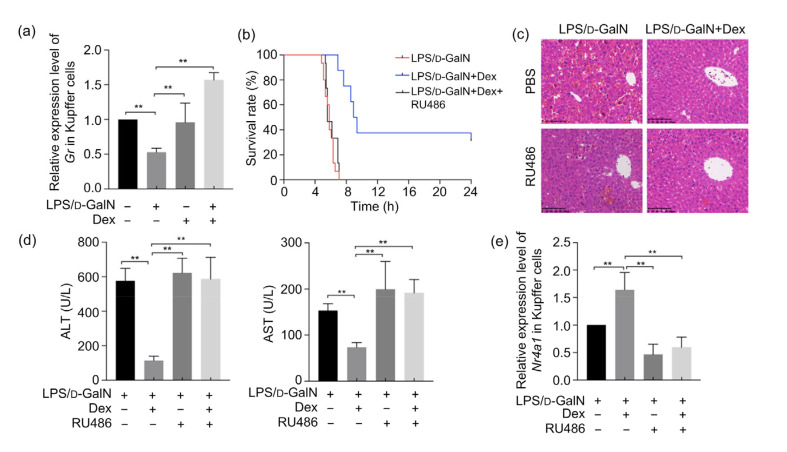
The results of this study show that Gr mRNA expression in Kupffer cells increases significantly in ALF mice treated early with Dex. Gr inhibitors completely inhibited the beneficial effect of Dex on survival of ALF mice (Figs. 6a and 6b). The results of H&E staining showed that liver and hepatic cord structures of ALF mice were disordered and that congestion occurred in the liver sinus after inhibiting the action of Gr (Fig. 6c). Serum ALT and AST levels also increased significantly after administration of Gr inhibitors (Fig. 6d), suggesting that the early use of Dex to improve the progress of mice ALF works mainly through the genomic effects of Gr. The qPCR results demonstrate that the Nr4a1 mRNA in Kupffer cells increased after Gr inhibition by RU486 (Fig. 6e).
Fig. 6.
Effects of Gr inhibition on LPS/D-GaIN-induced ALF in mice and Nr4a1 levels in Kupffer cells
(a) Gr mRNA expression of the Kupffer cells with or without LPS/D-GaIN and Dex administration (n=5). (b) Kaplan-Meier survival estimation of LPS/D-GaIN-challenged WT mice receiving no treatment, Dex treatment, or Dex and Gr antagonist RU486 (n=15). Log-rank test P<0.01 between Dex group and Dex-RU486 group. (c) Liver hematoxylin and eosin (H&E) staining after Dex or RU486 stimulation in LPS/D-GaIN-challenged WT mice (n=5; scan bar=100 μm). (d) Serum ALT and AST level changes among all groups (n=8). (e) Gr inhibition downregulates Nr4a1 mRNA levels in Kupffer cells in LPS/D-GaIN-induced WT mice (n=5). The results are shown as mean±standard error of the mean (SEM). ** P<0.01. LPS, lipopolysaccharide; D-GaIN, D-galactosamine; Dex, dexamethasone; Gr, glucocorticoid receptor; WT, wild-type; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PBS, phosphate-buffered saline; Nr4a1, nuclear receptor subfamily 4 group A member 1
4. Discussion
The etiology of liver failure is complex, and there are still inadequate numbers of specific animal models for different causes of liver failure (Stravitz and Lee, 2019). In 2018, the Guidelines for the Diagnosis and Treatment of Liver Failure of China proposed that the main cause of liver failure in China is viral hepatitis, especially hepatitis B virus (HBV) (CMA, 2019). Therefore, the application range of APAP (Acetaminophen)-induced ALF mice is limited. Our team previously found that LPS/D-GaIN could induce a model of ALF mice, but there still have some problems such as non-uniform dosage standards and poor repeatability. This study found that 18–20 g C57BL/6 male mice, which received LPS 5 μg/kg and D-GaIN 500 mg/kg intraperitoneally after fasting for 12 h, could show significant manifestations of ALF with good reproducibility.
Hepatocyte death, immune cell infiltration, and microcirculation disorders are typical histopathological findings of liver failure. Hepatocyte damage and necrosis can induce immune responses, which can further trigger microcirculation disorders and systemic inflammatory reactions because of the abundant hepatic blood flow. In this study, we tested the changes of liver and blood immune cells at different time points in the progression of ALF in our mouse model and verified the specific changes of immune cells and inflammatory factors. Consistent with the previous research indicating that ALF was mainly driven by the innate immune response (Possamai et al., 2014), our study demonstrated that cells involved in the innate immune response, especially monocytes and neutrophils, show relatively large changes during progression of LPS/D-GaIN-induced ALF.
ALF is accompanied by a severe systemic inflammatory response in many cases (Stravitz and Lee, 2019), and cytokine damage is thought to play a vital role in the progression of ALF. Researchers have found that increases in serum cytokines such as IL-6 and TNF-α can damage liver cells at the initial stage but promote hepatocyte regeneration in advanced stages (Scheving et al., 2007). Some recent studies used cytokines such as IL-6, TNF-α, and IL-10 as important indicators to determine liver failure progression (Wang et al., 2014; Bao et al., 2017). The results of this study indicate that LPS/D-GaIN-induced ALF mice had a significant cytokine storm at the onset of ALF. IL-6 and TNF-α increased sharply within 1 h of drug administration, accompanied by a significant increase in the anti-inflammatory cytokine IL-10, indicating that immune disorders appear in the initial stage of ALF (Bernal et al., 2010). Without early treatment, irreversible damage to hepatocytes would occur. Inflammatory cells recruited with elevated chemokine CCL-2 level in advanced stages would further aggravate ALF progression.
Many clinical studies have shown that early glucocorticoid therapy for ALF could improve patient survival (Fujiwara et al., 2014; Karkhanis et al., 2014), but there is a lack of supporting research to suggest the specific immunological mechanism for this effect. Dex and methylprednisolone are the recommended drugs for treating ALF (Cain and Cidlowski, 2017). In our mouse models, we chose the 4th hour of LPS/D-GaIN treatment as the end point of the study instead of the 5th hour because by the 5th hour of treatment, liver cells exhibited severe necrosis and disintegration, including a marked reduction in the numbers of non-parenchymal cells. We found that administration of Dex at 0 h in LPS/D-GaIN-induced ALF mice can achieve better results than treatment at later stages of disease progression. However, wild-type (WT) mice treated with LPS/D-GaIN for 1 h already showed evidence of a severe cytokine storm. Although Dex still could inhibit the secretion of pro-inflammatory cytokines such as IL-6, TNF-α, and CCL-2 at this stage, hepatocyte damage was already irreversible. Thus, administration of immunoregulators with suppressive functions such as Dex, at this stage will worsen the progression of ALF disease.
Studies have demonstrated that macrophage intervention is a promising treatment for ALF (Possamai et al., 2014; Lewis et al., 2020). We found that early use of Dex in LPS/D-GaIN-induced ALF mice could significantly increase the number of Kupffer cells, which were mainly M2 type macrophages that promoted tissue repair cells. Dex treatment combined with Gr that activates expression of nuclear genes, plays this protective function. Nr4a1 is an important nuclear receptor that affects cell proliferation and differentiation, promotes angiogenesis, and participates in hormone synthesis. It can participate in many physiological and pathological responses of the body by regulating the transcription of target genes (Carpenter et al., 2020; Gagliano et al., 2020; Wang et al., 2020). Studies have indicated that overexpression of Nr4a1 in macrophages can reduce the expression of pro-inflammatory cytokines IL-6 and CCL-2 (Bonta et al., 2006). It also can mediate differentiation of Ly6Chigh monocytes to non-classical Ly6Clow monocytes and eliminate damaged endothelial cells, thereby maintaining vascular integrity (Hanna et al., 2011; Honda et al., 2020). Our study found that the transcription level of Nr4a1 in Kupffer cells increased in ALF mice treated early with Dex, indicating that Nr4a1 plays a significant role in the process of phenotypic differentiation of macrophages, especially in transformation to an anti-inflammatory phenotype and cytokine secretion. In vivo experiments showed that Dex regulation of Nr4a1 in Kupffer cells was Gr-dependent. Contrary to the results of this study, another study found that Dex could downregulate mRNA level of Nr4a1 in the testis (Valdez et al., 2019), and Nr4a1 has diverse effects in different cells and diseases (Gagliano et al., 2020; Wang et al., 2020). There is a need for deeper investigation of the direct mechanism by which Dex affects Nr4a1 in Kupffer cells.
5. Conclusions
In summary, this study clarified the changes of immune cells and cytokines in the progression of LPS/D-GaIN-induced ALF in mice, and found that early use of Dex mitigated ALF by increasing the numbers of innate immune cells, especially Kupffer cells and neutrophils. Gr-dependent Nr4a1 upregulation in Kupffer cells may be an important effect of Dex treatment in ALF mice.
Acknowledgments
We thank Ying YANG (the First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China) for her financial support to this research, and Yan-ning LIU (the First Affiliated Hospital, School of Medicine, Zhejiang University) for guidance in research design.
Footnotes
Project supported by the Zhejiang Provincial Natural Science Foundation of China (No. LEZ20H260001) and the National Natural Science Foundation of China (No. 81700552)
Contributors: Jing-wen DENG and Qin YANG conceived and designed the experiments. Jing-wen DENG, Qin YANG, Jun GUAN, Jia-ming ZHOU, Qiu-xian ZHENG, and Xiao-peng CAI performed the experiments. Meng HONG, Yan-li REN, and Gang WANG collected the data. Jing-wen DENG, Wei-gao E, Yan-dong AN, and Shu-jing LAI analyzed the data. Jing-wen DENG and Xiao-peng CAI wrote the paper and edited the manuscript. Zhi CHEN supervised the study. All authors have read and approved the final version of the manuscript, and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Jing-wen DENG, Qin YANG, Xiao-peng CAI, Jia-ming ZHOU, Wei-gao E, Yan-dong AN, Qiu-xian ZHENG, Meng HONG, Yan-li REN, Jun GUAN, Gang WANG, Shu-jing LAI, and Zhi CHEN declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
- 1.Achuthan A, Aslam ASM, Nguyen Q, et al. Glucocorticoids promote apoptosis of proinflammatory monocytes by inhibiting ERK activity. Cell Death Dis. 2018;9(3):267. doi: 10.1038/s41419-018-0332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao SX, Zheng JM, Li N, et al. Role of interleukin-23 in monocyte-derived dendritic cells of HBV-related acute-on-chronic liver failure and its correlation with the severity of liver damage. Clin Res Hepatol Gastroenterol. 2017;41(2):147–155. doi: 10.1016/j.clinre.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Bernal W, Auzinger G, Dhawan A, et al. Acute liver failure. Lancet. 2010;376(9736):190–201. doi: 10.1016/s0140-6736(10)60274-7. [DOI] [PubMed] [Google Scholar]
- 4.Bonta PI, van Tiel CM, Vos M, et al. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006;26(10):2288. doi: 10.1161/01.ATV.0000238346.84458.5d. [DOI] [PubMed] [Google Scholar]
- 5.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter MD, Hu QW, Bond AM, et al. Nr4a1 suppresses cocaine-induced behavior via epigenetic regulation of homeostatic target genes. Nat Commun. 2020;11(1):504. doi: 10.1038/s41467-020-14331-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.CM A. Guideline for diagnosis and treatment of liver failure. Chin J Hepatol. 2019;27(1):18–26. doi: 10.3760/cma.j.issn.1007-3418.2019.01.006. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 8.Fujiwara K, Yasui S, Yonemitsu Y, et al. Efficacy of high-dose corticosteroid in the early stage of viral acute liver failure. Hepatol Res. 2014;44(5):491–501. doi: 10.1111/hepr.12148. [DOI] [PubMed] [Google Scholar]
- 9.Gagliano T, Shah K, Gargani S, et al. PIK3Cδ expression by fibroblasts promotes triple-negative breast cancer progression. J Clin Invest. 2020;130(6):3188–3204. doi: 10.1172/jci128313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garabuczi E, Sarang Z, Szondy Z. Glucocorticoids enhance prolonged clearance of apoptotic cells by upregulating liver X receptor, peroxisome proliferator-activated receptor-δ and UCP2. Biochim Biophys Acta. 2015;1853(3):573–582. doi: 10.1016/j.bbamcr.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Hanna RN, Carlin LM, Hubbeling HG, et al. The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C-monocytes. Nat Immunol. 2011;12(8):778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heideveld E, Hampton-O'Neil LA, Cross SJ, et al. Glucocorticoids induce differentiation of monocytes towards macrophages that share functional and phenotypical aspects with erythroblastic island macrophages. Haematologica. 2018;103(3):395–405. doi: 10.3324/haematol.2017.179341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda M, Surewaard BGJ, Watanabe M, et al. Perivascular localization of macrophages in the intestinal mucosa is regulated by Nr4a1 and the microbiome. Nat Commun. 2020;11(1):1329. doi: 10.1038/s41467-020-15068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi N, Kopec AK, Ray JL, et al. Fibrin deposition following bile duct injury limits fibrosis through an αMβ2-dependent mechanism. Blood. 2016;127(22):2751–2762. doi: 10.1182/blood-2015-09-670703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakisaka K, Kataoka K, Suzuki Y, et al. Appropriate timing to start and optimal response evaluation of high-dose corticosteroid therapy for patients with acute liver failure. J Gastroenterol. 2017;52(8):977–985. doi: 10.1007/s00535-017-1306-5. [DOI] [PubMed] [Google Scholar]
- 16.Karkhanis J, Verna EC, Chang MS, et al. Steroid use in acute liver failure. Hepatology. 2014;59(2):612–621. doi: 10.1002/hep.26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leroux A, Ferrere G, Godie V, et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57(1):141–149. doi: 10.1016/j.jhep.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 18.Lewis PS, Campana L, Aleksieva N, et al. Alternatively activated macrophages promote resolution of necrosis following acute liver injury. J Hepatol. 2020;73(2):349–360. doi: 10.1016/j.jhep.2020.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu C, Shi QL, Qin Q, et al. A review of experimental liver injury models in mice. Chin J Comp Med. 2019;29(1):107–113. doi: 10.3969/j.issn.1671-7856.2019.01.019. (in Chinese) [DOI] [Google Scholar]
- 20.Panettieri RA, Schaafsma D, Amrani Y, et al. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci. 2019;40(1):38–49. doi: 10.1016/j.tips.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Possamai LA, Thursz MR, Wendon JA, et al. Modulation of monocyte/macrophage function: a therapeutic strategy in the treatment of acute liver failure. J Hepatol. 2014;61(2):439–445. doi: 10.1016/j.jhep.2014.03.031. [DOI] [PubMed] [Google Scholar]
- 22.Robert O, Boujedidi H, Bigorgne A, et al. Decreased expression of the glucocorticoid receptor-GILZ pathway in Kupffer cells promotes liver inflammation in obese mice. J Hepatol. 2016;64(4):916–924. doi: 10.1016/j.jhep.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Scheving LA, Buchanan R, Krause MA, et al. Dexamethasone modulates ErbB tyrosine kinase expression and signaling through multiple and redundant mechanisms in cultured rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2007;293(3):G552–G559. doi: 10.1152/ajpgi.00140.2007. [DOI] [PubMed] [Google Scholar]
- 24.Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394(10201):869–881. doi: 10.1016/s0140-6736(19)31894-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuckermann JP, Kleiman A, Moriggl R, et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest. 2007;117(5):1381–1390. doi: 10.1172/jci28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdez R, Cavinder CA, Varner DD, et al. Dexamethasone downregulates expression of several genes encoding orphan nuclear receptors that are important to steroidogenesis in stallion testes. J Biochem Mol Toxicol. 2019;33(6):e22309. doi: 10.1002/jbt.22309. [DOI] [PubMed] [Google Scholar]
- 27.van de Garde MDB, Martinez FO, Melgert BN, et al. Chronic exposure to glucocorticoids shapes gene expression and modulates innate and adaptive activation pathways in macrophages with distinct changes in leukocyte attraction. J Immunol. 2014;192(3):1196–1208. doi: 10.4049/jimmunol.1302138. [DOI] [PubMed] [Google Scholar]
- 28.Wang HJ, Xu X, Guan X, et al. Liposomal 9-aminoacridine for treatment of ischemic stroke: from drug discovery to drug delivery. Nano Lett. 2020;20(3):1542–1551. doi: 10.1021/acs.nanolett.9b04018. [DOI] [PubMed] [Google Scholar]
- 29.Wang K, Wu ZB, Ye YN, et al. Plasma interleukin-10: a likely predictive marker for hepatitis B virus-related acute-on-chronic liver failure. Hepat Mon. 2014;14(7):e19370. doi: 10.5812/hepatmon.19370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu WQ, Zhang SY, Fu SQ, et al. Dexamethasone protects the glycocalyx on the kidney microvascular endothelium during severe acute pancreatitis. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2019;20(4):355–362. doi: 10.1631/jzus.B1900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao HL, Han QJ, Lu N, et al. HMBOX1 in hepatocytes attenuates LPS/D-GalN-induced liver injury by inhibiting macrophage infiltration and activation. Mol Immunol. 2018;101:303–311. doi: 10.1016/j.molimm.2018.07.021. [DOI] [PubMed] [Google Scholar]