Abstract
Yellow Fever (YF) is a severe disease caused by Yellow Fever Virus (YFV), endemic in some parts of Africa and America. In Brazil, YFV is maintained by a sylvatic transmission cycle involving non-human primates (NHP) and forest canopy-dwelling mosquitoes, mainly Haemagogus-spp and Sabethes-spp. Beginning in 2016, Brazil faced one of the largest Yellow Fever (YF) outbreaks in recent decades, mainly in the southeastern region. In São Paulo city, YFV was detected in October 2017 in Aloutta monkeys in an Atlantic Forest area. From 542 NHP, a total of 162 NHP were YFV positive by RT-qPCR and/or immunohistochemistry, being 22 Callithrix-spp. most from urban areas. Entomological collections executed did not detect the presence of strictly sylvatic mosquitoes. Three mosquito pools were positive for YFV, 2 Haemagogus leucocelaenus, and 1 Aedes scapularis. In summary, YFV in the São Paulo urban area was detected mainly in resident marmosets, and synanthropic mosquitoes were likely involved in viral transmission.
Subject terms: Virology, Viral epidemiology
Introduction
Yellow Fever virus (YFV) is an arbovirus member of the Flavivirus genus, family Flaviviridae and the causative agent of yellow fever (YF)1. The disease is considered endemic in parts of South America and Africa, with 200,000 cases and 30,000 deaths annually2. In Africa there are three transmission cycles: the sylvatic one, involving NHP and sylvatic Aedes africanus, the intermediate cycle, involving peridomestic Aedes spp, and the urban cycle, which involves mainly Aedes aegypti3,4. In Brazil, only the sylvatic YFV cycle is now described, subsequent to the eradication of Aedes aegypti and urban cycle transmission in 1942. Occasional spillover to non-vaccinated humans occur when they encroach into forested areas4. The current transmission cycle involves the mosquito vectors Haemagogus sp. and Sabethes sp., and several species of non-human primates (NHP), including the genera Alouatta, Sapajus, Callicebus and Callithrix5 . Most New World NHPs are susceptible to YFV, especially individuals of the Alouatta genus. The Brazilian surveillance system is based upon YFV detection in dead NHP6. Its identification triggers mass vaccination in local populations4.
In Brazil, during the late twentieth century and extending to the first decade of the twenty first century, intense YFV circulation extended from the Amazon region to the contiguous states of Central Brazil7. Subsequently, starting in 2016, several YFV outbreaks were reported in states in the most populous southeastern region, caused by a new YFV belonging to South American lineage I that was introduced from the Midwest region and disseminated to areas that were considered free of yellow fever8. This resulted in the most severe outbreak in past decades in the southeastern region, comprising the states of São Paulo, Rio de Janeiro, Minas Gerais and Espírito Santo. During the 2017–2018 outbreak, São Paulo State had 3,352 human cases with 333 fatalities, and a total of 534 YF epizootic events9,10. However, despite the magnitude of this outbreak, little is known about which mosquito species were involved in some of these YFV transmission areas.
We now present the results of YF confirmed epizootic events and entomological investigations conducted in Sao Paulo city during the intense circulation of YFV between 2017 and 2018. Particular attention is paid to urban and urban green areas, where no vectors classically involved in the sylvatic YFV transmission were found. Our objective was to describe YFV-positive epizootic events that were probably associated with Aedes spp. mosquitoes instead of the Haemagogus spp or Sabethes spp primary and secondary vectors.
Material and methods
Study area
This is a descriptive study regarding positive YFV NHP and mosquitoes collected in the city of Sao Paulo, which is the capital of Sao Paulo State, located in the Southeast Region of Brazil. It is the largest city in the country, with about 12 million inhabitants in an area of 1,521.11 km2 divided into 96 districts. São Paulo has a subtropical humid climate, with a rainy season during the summer (December-March) and a dry season during the autumn/winter (April-August).
Non-human primates sampling and diagnosis
From October 2017 to December, 2018, carcasses of NHP were collected by local authorities and later identified and autopsied by the veterinary staff from Divisão Técnica de Medicina Veterinária e Manejo da Fauna Silvestre (DEPAVE-3), and results reported to the surveillance system (Centro de Vigilância Epidemiológica—CVE). Samples of liver, brain and spleen were sent to Adolfo Lutz Institute, the reference laboratory for YFV, according to Ministry of Health guidelines6. Liver and brain samples were lysed using Magna Lyser green beads (Roche Life Sciences, Penzberg, Germany) and extracted using RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. YFV diagnosis was made by RT-qPCR11, (cut-off value = 38 Cycle threshold) (CT), and immunohistochemistry (IHC). For IHC analysis, liver tissue sections were tested with an in house primary polyclonal anti-YF antibody (1:40.000), signal amplification was achieved with Super HighDef (Enzo Life Sciences, Farmingdale) and visualization was done with diaminobenzidine (D-5637; Sigma, St. Louis, MO). Positive results at IHC were considered when there was immunolabeling in the cytoplasm of hepatocytes, with brown or red precipitates. NHP localization was based on the address where they were collected. Information regarding NHP was filled in the Sistema de Informação de Agravos de Notificação (SINAN) form, according to the Ministry of Health. When not available, coordinates were obtained using Google Earth Pro.
Mosquitoes sampling and YFV detection
Mosquitoes were collected by Sucen (Superintendence for Control of Endemic Diseases, State of São Paulo) at sites where epizootic events were recorded. They were captured at ground level between 9 am and 3 pm, by using an entomologic net and bottle-type manual vacuums in woods and green areas, and Nasci’s Aspirator in urban dwellings. After collection, they were frozen, transferred to cryogenic tubes, placed in liquid nitrogen containers for transportation to the laboratory and stored at -70 °C until processing. They were identified and separated according to species, place and date of collection into pools containing between 1–10 mosquitoes. Pools were triturated using FastPrep-24 5G Instrument (MP Biomedicals, OH) and magnetic beads in a 1 mL phosphate-buffered saline solution with 0.75% bovine albumin, penicillin (100 units/mL) and streptomycin (100 µg/mL). The resultant suspension was centrifuged at 1800 × g for 15 min, and the supernatant was withdrawn and frozen at −70 °C. Samples were extracted using the QIAamp RNA Viral Mini Kit (QIAGEN, Hilden, Germany), according to manufacturer’s instructions followed by RT-qPCR11. In addition, attempts for viral isolation were performed as follows: after medium removal, 20µL of positive RT-qPCR mosquito pools were inoculated into cell tubes containing monolayer cultures of C6/36 (Aedes albopictus clone C6/36 ATCC CRL-1660) and Vero (Vero ATCC CCL-81 Cercopithecus aethiops kidney normal) cells. C6/36 culture tubes were incubated for nine days at 28 °C with L-15 medium containing 2% FBS, penicillin (100units/mL) and streptomycin (100 µg/mL), while Vero tubes were incubated for nine days at 37 °C and 5% CO2 with 199 medium containing 2% FBS, penicillin (100units/mL) and streptomycin (100 µg/mL), and checked daily for a cytopathic effect. IFA tests were performed using YFV monoclonal antibodies provided by the U.S. Centers for Disease Control and Prevention, according to a published procedure12. A total of three passages were performed. In order to determine whether a positive pool of mosquito had recently consumed human or non-human primate blood an RNase P protocol13, used as an internal control for RT-qPCR, was performed. Mosquito density by entomological surveillance site was calculated as the arithmetic mean of collections performed, analyzed by collection method, effort and hours in each site14.
YFV from São Paulo phylogenetic analysis
Whole genome sequences from NHP and humans from São Paulo previously generated15,16 were aligned with YFV sequences from the 2016–2018 Brazilian outbreak using MAFFT (available at https://mafft.cbrc.jp/alignment/server/) and maximum likelihood (ML) phylogenetic analyses were conducted with a TN93 model with gamma variation17 (available at https://www.atgc-montpellier.fr/). Tree was edited using FigTree v.14.3 with a mid-point root. A YFV South American lineage II was used as an outer group (accession number MF004382).
Ethical statement
All animal research was approved by the Institutional Animal Care and Use Committee (IACUC) of the Adolfo Lutz Institute, São Paulo. The Adolfo Lutz Central Institute (The Central Public Health Laboratory from the State of São Paulo), an organ linked to the Health Department of the state of São Paulo, is the official laboratory for the diagnosis of YFV in humans and primates. NHP samples were sent by local authorities in accordance with Brazilian Ministry of Health guidelines (https://vigilancia.saude.mg.gov.br/index.php/download/guia-de-epizootias-febre-amarela-2a-edicao-2017/#). The use of NHP samples for research was also approved by ICMBIO protocol number 65181 (https://www.icmbio.gov.br/sisbio/).
Results
YFV in Non-human primates from urbanized areas
After almost a one hundred year absence, YFV was detected in Sao Paulo city in October 2017, in Alouatta guariba clamitans monkeys from Horto Florestal, an Atlantic Forest protection area located in the North region of the city (Tremembé district). During the 2017–2018 outbreak, a total of 542 NHP were sent for YFV diagnosis (Table 1), of which 162 (30%) were positive (n = 140 Alouatta guariba clamitans, median CT = 11, min = 7, max = 38); and 22 Callithrix sp. (median CT = 36, min = 33, max = 38). All positive Callithrix monkeys were classified as hybrid individuals based on their morphology. Most positive NHP, mainly of the Alouatta genera, were found near forest areas located in the North and South zones (Green Belt of São Paulo City, GBSPC) (Fig. 1). From the 22 Callithrix sp., 4 (18,1%) were collected in wooden areas (WO), 5 (22,8%) in urban green areas (UGA) and 13 (59,1%) in urban areas (UR). One Callithrix was a captive animal from the Mandaqui district (North Zone-animal ID 14), with no outdoor access according to its owner. A total of 18 animals were positive during the epidemic period (December-May), while 4 animals were positive during the non-epidemic period (June-November). The last confirmed epizootic event was in September 2018, and cases were detected throughout this period. Information regarding positive Callithrix is depicted in Table 2. Also, we found one positive free living Alouatta guariba clamitans monkey in the city Zoo on January 10th, 2018, which is located in a remnant Atlantic Forest area of the Cursino district. However, this location had no connection with sylvatic areas, and no epizootic events were confirmed nearby. The distribution of positive Callithrix and Alouatta NHP provides evidence for the restriction of the last monkey genera to woods and the dispersion of Callithrix (marmosets) throughout urbanized areas.
Table 1.
Total of notified and confirmed epizootic events in São Paulo, SP, Brazil, 2017–2018.
| NHP | 2017 | 2018 | Total | P(%) | ||
|---|---|---|---|---|---|---|
| N (%) | P (%) | N (%) | P (%) | |||
| Aloutta g. clamitans | 141 (53.6) | 95 (94) | 68 (24.4) | 45 (72.5) | 209 (38.5) | 140 (85.9) |
| Callithrix sp. | 119 (45.2) | 5 (6) | 206 (73.8) | 17 (27.4) | 324 (60) | 22 (14.1) |
| Sapajus nigritus | 2 (0.8) | 0 | 5 (1.8) | 0 | 7 (1.3) | 0 |
|
Callicebus nigrifus |
1 (0.4) | 0 | 0 | 0 | 1 (0.2) | 0 |
| Total | 263 | 100 | 279 | 62 | 541 | 162 |
Legend: NHP: Non-human primate. N: number. P: positive.
Figure 1.
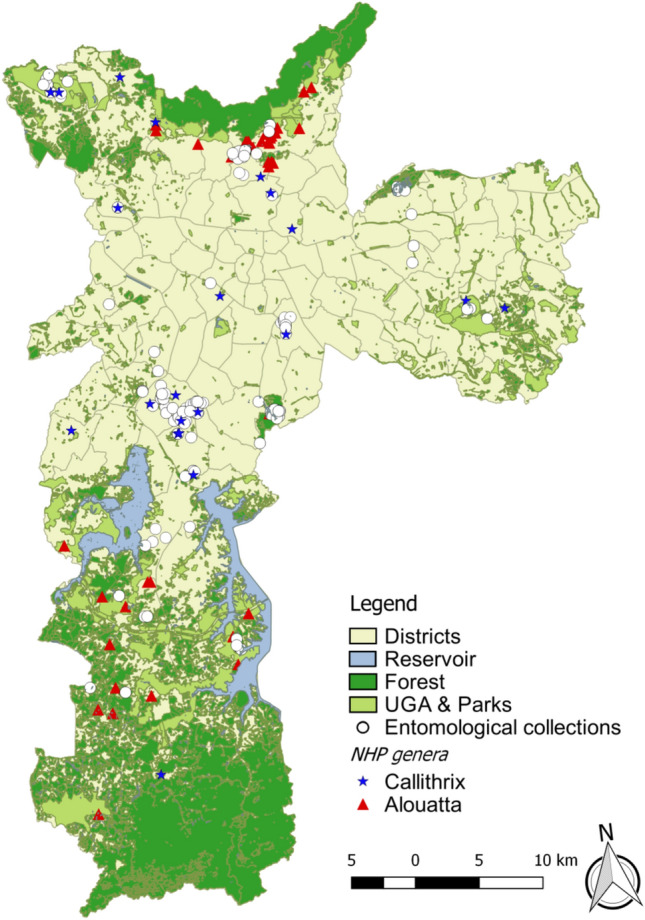
Map of São Paulo city with sites of Culicidae captures after confirmed epizootic events, 2017–2018. Districts represent the administrative divisions of the city. Reservoir represents the city water dams. Forest represents the Atlantic Forest protections area. Urban Green Areas (UGA) consists of urban parks and squares. Map was generated using QGIS version 2.14.9 (
available at https://www.qgis.org/pt_BR/site/).
Table 2.
Positive YFV Callithrix sp in São Paulo city and their respective distances from green areas or woods, 2017–2018.
| Callithrix ID | District (Region) | Area | Notification | Distance from green areas or woods |
|---|---|---|---|---|
| 1 | Anhanguera (N) | WO | Oct 16 2017 | 0 m |
| 2 | Anhanguera (N) | WO | Oct 16 2017 | 0 m |
| 3 | Vila Guilherme (N) | UR | Oct 25 2017 | 610 m |
| 4 | Santo Amaro (S) | UR | Dec 19 2017 | 540 m |
| 5 | Santana (N) | UR | Dec 19 2017 | 450 m |
| 6 | Santo Amaro (S) | UR | Jan 4 2018 | 634 m |
| 7 | Capão Redondo (S) | UGA | Jan 18 2018 | 0 m |
| 8 | Campo Grande (S) | UR | Jan 19 2018 | 290 m |
| 9 | José Bonifácio (E) | UGA | Jan 24 2018 | 0 m |
| 10 | Santo Amaro (S) | UR | Jan 25 2018 | 50 m |
| 11 | Brasilândia (N) | UR | Jan 27 2018 | 320 m |
| 12 | Pq do Carmo (E) | UGA | Jan 28 2018 | 0 m |
| 13 | Campo Grande (S) | UR | Feb 19 2018 | 220 m |
| 14 | Mandaqui (N) | UR (pet) | Feb 28 2018 | 210 m |
| 15 | Marsilac (S) | WO | Feb 28 2018 | 0 m |
| 16 | Campo Grande (S) | UGA | Mar 12 2018 | 0 m |
| 17 | Ipiranga (S) | UR | Mar 14 2018 | 520 m |
| 18 | Santo Amaro (S) | UGA | Apr 16 2018 | 0 m |
| 19 | Perus (N) | UR | Apr 22 2018 | 80 m |
| 20 | Cangaíba (E) | UR | May 25 2018 | 550 m |
| 21 | São Domingos (N) | UR | May 25 2018 | 40 m |
| 22 | Perus (N) | WO | Sep 12 2018 | 0 m |
Legend: S = South, N = North, E = East, WO = wood, UR = urban, UGA = urban green area.
YFV in mosquitoes
A total of 5591 female mosquito were collected, 2691 (48.1%) from Aedini Tribe or Sabethes genus. Results of entomological collections are shown in Table 3. Three mosquito species were more abundant and occurred frequently: Aedes scapularis (N = 1674, 29.9%) occurred in 78 collection sites (76.5%), followed by Aedes albopictus (N = 798, 14.3%) in 53 (52%) and Aedes aegypti (N = 83, 1.5%) in 24 sites(23.5%). These species were also collected in urban dwellings as well as in urban green areas. We also recorded Psorophora ferox in urban green areas and Haemagogus leucocelaenus in the woods (Table 3).
Table 3.
Distribution of potential mosquito species vectors of Yellow Fever in the habitats of entomological surveillance (woods, urban green areas and urban dwellings) during epizootics in São Paulo from October 2017 through December 2018.
| Species | Wood | Urban Green Areas | Urban Dwellings | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | (Std) | n coll | n | (Std) | n coll | n | (Std) | n coll | n | n coll | |
| Aedes aegypti | 5 | 0.2 (0.7) | 2 | 46 | 1.1 (2.2) | 14 | 32 | 3.8 (5.7) | 8 | 83 | 24 |
| Aedes albopictus | 149 | 5.0 (7.0) | 17 | 623 | 14.2 (29.8) | 33 | 26 | 0.8 (1.5) | 3 | 798 | 53 |
| Aedes argyrothorax | 1 | – | 1 | – | – | – | – | – | – | 1 | 1 |
| Aedes scapularis | 746 | 33.5 (94.3) | 26 | 894 | 24.2 (50.3) | 47 | 34 | 1.7 (3.9) | 5 | 1674 | 78 |
| Aedes terrens | 11 | 0 .4 (1 .7) | 4 | – | – | – | – | – | – | 11 | 4 |
| Haemagogus janthinomys/capricornii | 1 | – | 1 | – | – | – | – | – | – | 1 | 1 |
| Haemagogus leucocelaenus | 72 | 2 .7 (10 .7) | 11 | – | – | – | – | – | – | 72 | 11 |
| Psorophora ferox | 28 | 1 .3 (4 .7) | 7 | 3 | 0.1 (0.4) | 3 | - | - | - | 31 | 10 |
| Sabethes albiprivus | 10 | 0 .3 (1 .1) | 4 | – | – | – | – | – | – | 10 | 4 |
| Sabethes chloropterus | 1 | – | 1 | – | – | – | – | – | – | 1 | 1 |
| Sabethes purpureus | 7 | 0 .3 (0 .8) | 5 | – | – | – | – | – | – | 7 | 5 |
| Sabethes soperi | 2 | – | 1 | – | – | – | – | – | – | 2 | 1 |
| Total | 1033 | – | 33ª | 1566 | – | 56ª | 92 | - | 13ª | 2691 | 102b |
Legend: n: number of females, X ®(Std): arithmetic mean of collections by effort and hours in each site (standard deviation), n coll.: number of collections with presence of the species, a: total entomological collections according habitat type, b: total entomological collections.
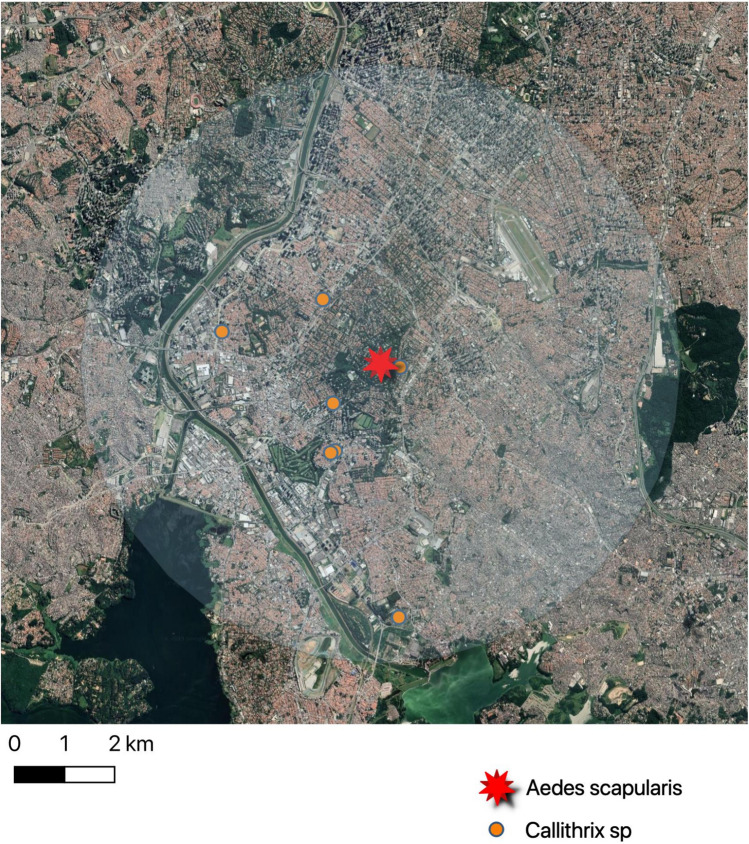
From a total of 437 mosquito pools, 2 from Haemagogus leucocelaenus in Horto Florestal (CT values of 19 and 18) collected on December 20th, 2017 and 1 from of Aedes scapularis, from the Santo Amaro district collected on February 19th, 2018 (Chácara Flora neighborhood, latitude −23,647,240; longitude −46,683,910), (CT value = 37), were YFV positive. Figure 2 shows the landscape of the Santo Amaro district, where the positive Aedes scapularis pool was collected following confirmed epizootic events in the area. YFV was not detected in Aedes aegypti or Aedes scapularis pools by RT-qPCR. YFV from each of Haemagogus leucocelaenus pools was isolated in Vero and C6/36 cells. However, even after 3 passages, YFV was not isolated from the Ae. scapularis pool, possible due to low viral load. All positive mosquito pools were tested negative for human RNAse P gene.
Figure 2.
Positive Callithrix monkeys and Aedes scapularis from Santo Amaro district. The buffer indicates a medium flight range of most Culicidae species (radius = 6Km).
The ML phylogenetic tree generated (S1) revealed that sequences belonging to Alouatta genus from São Paulo are basal to those of humans, revealing the sylvatic YFV circulation previous to human cases detected in the city.
Discussion
In 2016, a sylvatic YF outbreak occurred in Minas Gerais State, which spread to other Brazilian states, including São Paulo18. Continuous surveillance detected positive cases in humans and NHPs during subsequent years, indicating viral persistence in the Southeast region19,20. We now detected positive epizootic events in São Paulo city from October 2017 until September 2018. We describe 18 positive RT-qPCR YFV NHP belonging to the Callithrix genus found in urbanized areas within the metropolitan areas of São Paulo city, as well as pools of Hg. leucocelaenus from Horto Florestal and one Ae. scapularis pool from Santo Amaro. This last district has an area of 15.7 Km2 with a density of 38,70 inhabitants/ha, where thousands of people live and circulate daily. Our results show that apparently a unique YFV pattern may have possibly occurred during the 2017–2018 outbreak. Entomological collections executed in these areas did not detect the presence of strictly sylvatic mosquitoes, the main YFV vectors in the southeastern region21,22. This situation deserves attention since it may represent a new YF epidemiological pattern, since the circulation of YFV occurred in wooded neighborhoods within metropolitan São Paulo and close to highly populated areas. In addition, one positive Callithrix was an indoor pet with presumably no outside exposure.
In Brazil, several species of Callithrix monkeys (marmosets) are found, such as Callithrix jaccus, Cx. penicillata, Cx. geoffroyi and Cx. aurita. However, some species were introduced into new areas, and hybrid individuals are now present in the South and Southeastern regions; their susceptibility to infectious disease is poorly understood. In São Paulo, marmosets are found in city centers close to humans, and sometimes are even used as pets. However, unlike Alouatta monkeys, which are highly susceptible to YFV23,24, marmosets may not be efficient YFV amplifier hosts due to low viral loads, as previously reported25,26. As our results demonstrate, Alouatta monkeys were restricted to forest areas, mainly in Atlantic Forest preserved areas, while most marmosets were found in squares and other green areas, in close proximity to humans.
Interestingly, entomological collections performed in the city at sites where epizootic events were recorded showed a high frequency of the synanthropic mosquitoes Aedes scapularis and Aedes albopictus. Although we did not find Ae. albopictus and Ae. aegypti naturally infected with YFV in our collections, Brazilian populations of these species showed to be competent to transmit this virus 27.
According to a previous study that collected Culicidae in the parks of São Paulo during 2010–2011, Culex quinquefasciatus was the most common species collected28. The genus Aedes was represented mainly by Ae. (Och) fluviatilis and Ae. (Ste) albopictus, while Aedes scapularis were frequent in some parks24. However, information regarding mosquitoes diversity are scarce in São Paulo city, and more studies must be done to clarify the real frequency of Culicidae. In the present study, we hypothesize that Ae. scapularis mosquitoes could have played a role for YFV transmission in some green areas of São Paulo. This was the most frequent species identified in urbanized areas where YF positive NHP were found. Moreover, we detected one RT-qPCR positive pool in the Santo Amaro district, where 7 marmosets were positive from December 2017 until April 2018, while no Haemagogus or Sabethes were collected in this district. This pool was also negative for the endogenous primate control (RNAse P), showing the absence of recent human or NHP blood. Ae. scapularis is widely distributed in the Americas, and bites preferably in twilight at ground level in fragmented forests, open fields and forest edges29. This species shows a synanthropic tendency, occurring in modified environments and breeding in artificial containers30,31. It is an anthropophilic opportunistic mosquito that feeds on birds, humans, and NHP32. Other entomological surveys have demonstrated YFV-positive pools in Rio de Janeiro and Bahia15. More recently, one YFV-positive pool of Ae. scapularis was found in Urupês city, São Paulo State, at a site where epizootic cases were absent33.
The presence of YFV within an urban area is intriguing. According to recent reports, the movement rate of the current YFV lineage is on average 0.84 (0.50–2.19) km/day16 to 4.25 km/day8 within the sylvatic cycle, reflecting NHP movements. However, as our results show, during the epidemic period (i.e. January 2018), 7 marmosets and 1 free-living Alouatta from the city zoo were YFV positive in distant areas of São Paulo (East, North and South zones), with no forest areas connecting them. According to34, NHPs are not responsible for the rapid spread of YFV30. Conversely, infected mosquitoes and humans can disperse the virus over great distances7,35. Hypotheses concerning YFV dissemination during the 2016–2018 Southeast outbreak were (1) augmentation of conservation areas, (2) high population density of non-immune NHP in the coastal zone, and (3) increased occupation of modified environments by adapted marmosets in Haemagogus infested woods36. Arboviruses may also be dispersed by windblown infected mosquitoes, as previously demonstrated for Japanese Encephalitis virus in Australia37. Nevertheless, this dissemination was likely caused by intense monsoon winds, which is not observed in Brazil. Therefore, considering both the distance and sequence of epizootic events, we may consider the possibility of viral introduction by asymptomatic viremic people, as previously hypothesized5, which is not consistent with the classical sylvatic cycle. The spread of the virus with a pattern distinct from the classical sylvatic cycle in an ecological transition zone has also been described in Rio de Janeiro36 . It is also important to note that São Paulo city was not a vaccine-recommended area until recently, and most of the 12 million inhabitants were YF naïve before the outbreak. The occurrence of positive NHP and mosquitoes in areas with a susceptible population poses a serious threat for re-urbanization of the disease, and vaccination campaigns must be continued.
Limitations of our study must be acknowledged. We did not conduct experiments about vector competence, since a biosafety level 3 facility was not available, and we did not perform comparative viral sequencing in the mosquitoes and marmosets due to high Ct values. Experiments regarding vector competence of Ae. scapularis mosquitoes using this modern YFV lineage are needed, since the detection of the YFV genome in a mosquito does not necessarily mean that the insect can play a role as vector.
In conclusion, YFV was detected in the São Paulo urban area, affecting mainly resident marmosets, with synanthropic Aedes mosquitoes probably implicated in local viral transmission. This YF pattern is not consistent with the classic Brazilian cycles, urban or sylvatic, and it suggests a new scenario, probably due to anthropic action and modifications in natural landscapes and callitrichid behavior. Moreover, YFV surveillance based on analysis of deceased NHP has triggered rapid mass vaccination in São Paulo city to prevent human infection, and no human urban case was detected. This epidemiological pattern in a transition area between green and urban areas, with high population density, indicates the need for further studies to subsidize surveillance in order to prevent the re-urbanization of the disease in Brazil.
Supplementary information
Acknowledgements
We thank the SUCEN team for collecting mosquitoes and Thaís Caroline Sanches, Alice Soares de Oliveira, Marcelo Schiavo Nardi and Eric Thal Brambilla Cordeiro da Silva, from DEPAVE.
Author contributions
Manuscript preparation: M.S.C., J.T.D., N.C.C.F., J.M.G., L.S., S.S.W. Obtained funding and study supervision: M.S.C., M.C.S.T.T., E.C.S; Identification and necropsy: T.Z., J.L.S., A.A.C.C.; Mosquitoes collection and identification: R.M.T., R.M.T.M., M.P., J.T.D., E.C. Experiments of viral detection: G.S.C., M.S.C.; Performed the analyses: M.S.C., J.S.N., J.L.S., L.S., M.P., A.A.C.C., T.Z., L.F.M., S.S.W.. All authors reviewed, contributed to, and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72794-x.
References
- 1.Simmonds P, et al. ICTV virus taxonomy profile: flaviviridae. J. Gen. Virol. 2017;98:2–3. doi: 10.1099/jgv.0.000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasconcelos PFC, Monath TP. Yellow fever remains a potential threat to public health. Vector Borne Zoonotic Dis. 2016;16:566–567. doi: 10.1089/vbz.2016.2031. [DOI] [PubMed] [Google Scholar]
- 3.Douam F, Ploss A. Yellow fever virus: knowledge gaps impeding the fight against an old foe. Trends Microbiol. 2018;26:913–928. doi: 10.1016/j.tim.2018.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monath TP, Vasconcelos PFC. Yellow fever. J. Clin. Virol. 2015;64:160–173. doi: 10.1016/j.jcv.2014.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Vasconcelos, P. F. da C. Febre amarela. Revista da Sociedade Brasileira de Medicina Tropical36, 275–293 (2003). [DOI] [PubMed]
- 6.Da Saúde M. Guia De Vigilância De Epizootias Em Primatas Não Humanos E Entomologia Aplicada À Vigilância Da Febre Amarela. Segunda edición actualizada. 2017 doi: 10.1016/j.imlet.2011.02.006. [DOI] [Google Scholar]
- 7.da Vasconcelos PF, C. Yellow fever in Brazil: thoughts and hypotheses on the emergence in previously free areas. Revista de Saúde Pública. 2010;44:1144–1149. doi: 10.1590/S0034-89102010005000046. [DOI] [PubMed] [Google Scholar]
- 8.Faria NR, et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science. 2018;361:894–899. doi: 10.1126/science.aat7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MS. Emergência epidemiológica de febre amarela no Brasil, no período de dezembro de 2016 a julho de 2017. Bol. Epidemiológico SVS Ministério da Saúde48, 1–22 (2017).
- 10.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Monitoramento Dos Casos E Óbitos De Febre Amarela No Brasil - Informe – No 43/2017. 1–7 (2017).
- 11.Domingo C, et al. First international external quality assessment study on molecular and serological methods for yellow fever diagnosis. PLoS ONE. 2012;7:e36291. doi: 10.1371/journal.pone.0036291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gubler DJ, Kuno G, Sather GE, Velez M, Oliver A. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am. J. Trop. Med. Hyg. 1984;33:158–165. doi: 10.4269/ajtmh.1984.33.158. [DOI] [PubMed] [Google Scholar]
- 13.Who, T., Centre, C., Atlanta, C. D. C. & States, U. CDC protocol of realtime RTPCR for influenza A ( H1N1 ) Characterization of Swine Influenza ( version 2009 ) General Comments. Control1, (2009).
- 14.de Gomes A, C. Medidas dos níveis de infestação urbana para aedes (stegomyia) aegypti e aedes (stegomyia) albopictus em Programa de Vigilância Entomológica. Inf. Epidemiológico do Sus. 1998;7:49–57. doi: 10.5123/S0104-16731998000300006. [DOI] [Google Scholar]
- 15.Cunha MDP, et al. Origin of the São Paulo Yellow Fever epidemic of 2017–2018 revealed through molecular epidemiological analysis of fatal cases. Sci. Rep. 2019;9:20418. doi: 10.1038/s41598-019-56650-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Souza, R. P. et al. Genomic Surveillance of Yellow Fever Virus Epidemic Waves in São Paulo, Brazil, 2017–2018. bioRxiv 645341 (2019). 10.1101/645341
- 17.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 18.Silva NIO, et al. Recent sylvatic yellow fever virus transmission in Brazil: the news from an old disease. Virol. J. 2020;17:9. doi: 10.1186/s12985-019-1277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Abreu FVS, et al. Combination of surveillance tools reveals that Yellow Fever virus can remain in the same Atlantic Forest area at least for three transmission seasons. Mem. Inst. Oswaldo Cruz. 2019;114:e190076. doi: 10.1590/0074-02760190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Rezende IM, et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl. Trop. Dis. 2018;12:e0006538. doi: 10.1371/journal.pntd.0006538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Abreu FVS, et al. Haemagogus leucocelaenus and Haemagogus janthinomys are the primary vectors in the major yellow fever outbreak in Brazil, 2016–2018. Emerg. Microbes Infect. 2019;8:218–231. doi: 10.1080/22221751.2019.1568180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza RP, et al. Isolation of yellow fever virus (YFV) from naturally infected Haemagogus (Conopostegus) leucocelaenus (diptera, cukicudae) in Sao Paulo State, Brazil, 2009. Rev. Inst. Med. Trop. Sao Paulo. 2011;53:133–139. doi: 10.1590/S0036-46652011000300004. [DOI] [PubMed] [Google Scholar]
- 23.de Almeida MAB, et al. Yellow fever outbreak affecting Alouatta populations in southern Brazil (Rio Grande do Sul State), 2008–2009. Am. J. Primatol. 2012;74:68–76. doi: 10.1002/ajp.21010. [DOI] [PubMed] [Google Scholar]
- 24.Leal SG, et al. Frequency of histopathological changes in Howler monkeys ( Alouatta sp.) naturally infected with yellow fever virus in Brazil. Rev. Soc. Bras. Med. Trop. 2016;49:29–33. doi: 10.1590/0037-8682-0363-2015. [DOI] [PubMed] [Google Scholar]
- 25.de Mares-Guia MAM, M. , et al. Yellow fever epizootics in non-human primates, Southeast and Northeast Brazil (2017 and 2018) Parasit. Vectors. 2020;13:90. doi: 10.1186/s13071-020-3966-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunha MS, et al. Epizootics due to Yellow Fever Virus in Sao Paulo State, Brazil: viral dissemination to new areas (2016–2017) Sci. Rep. 2019;9:5474. doi: 10.1038/s41598-019-41950-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Couto-Lima D, et al. Potential risk of re-emergence of urban transmission of Yellow Fever virus in Brazil facilitated by competent Aedes populations. Sci. Rep. 2017;7:4848. doi: 10.1038/s41598-017-05186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros-Sousa AR, et al. Biodiversidade de mosquitos (Diptera: Culicidae) nos parques da cidade de São Paulo I. Biota Neotrop. 2013;13:317–321. doi: 10.1590/S1676-06032013000100030. [DOI] [Google Scholar]
- 29.Taipe-Lagos CB, Natal D. Culicidae mosquito abundance in a preserved metropolitan area and its epidemiological implications. Rev. Saude Publica. 2003;37:275–279. doi: 10.1590/S0034-89102003000300002. [DOI] [PubMed] [Google Scholar]
- 30.Forattini OP, de Gomes A, C., Natal, D., Kakitani, I. & Marucci, D. Preferências alimentares e domiciliação de mosquitos Culicidae no Vale do Ribeira, São Paulo, Brasil, com especial referência a Aedes scapularis e a Culex (Melanoconion) Revista de Saúde Pública. 1989;23:9–19. doi: 10.1590/S0034-89101989000100003. [DOI] [PubMed] [Google Scholar]
- 31.Forattini OP, Kakitani I, Sallum MAM. Encontro de criadouros de Aedes scapularis (Diptera: Culicidae) em recipientes artificiais. Revista de Saúde Pública. 1997;31:519–522. doi: 10.1590/S0034-89101997000600013. [DOI] [PubMed] [Google Scholar]
- 32.Mucci LF, et al. Feeding habits of mosquitoes (Diptera: Culicidae) in an area of sylvatic transmission of yellow fever in the state of Sao Paulo, Brazil. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21:6. doi: 10.1186/s40409-015-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunha MS, et al. Genomic evidence of yellow fever virus in Aedes scapularis, southeastern Brazil, 2016. Acta Trop. 2020;205:105390. doi: 10.1016/j.actatropica.2020.105390. [DOI] [PubMed] [Google Scholar]
- 34.Jung L, Mourthe I, Grelle CEV, Strier KB, Boubli JP. Effects of Local Habitat Variation on the Behavioral Ecology of Two Sympatric Groups of Brown Howler Monkey (Alouatta clamitans) PLoS ONE. 2015;10:e0129789. doi: 10.1371/journal.pone.0129789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mir D, et al. Phylodynamics of Yellow Fever Virus in the Americas: new insights into the origin of the 2017 Brazilian outbreak. Sci. Rep. 2017;7:7385. doi: 10.1038/s41598-017-07873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Possas C, et al. Yellow fever outbreak in Brazil: the puzzle of rapid viral spread and challenges for immunisation. Mem. Inst. Oswaldo Cruz. 2018;113:e180278. doi: 10.1590/0074-02760180278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna JN, et al. Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 1999;170:533–536. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- 38.Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for Bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.