Abstract
Health differences among the elderly and the role of medical treatments are topical issues in aging societies. We demonstrate the use of modern statistical learning methods to develop a data-driven health measure based on 21 years of pharmacy purchase and mortality data of 12,047 aging individuals. The resulting score was validated with 33,616 individuals from two fully independent datasets and it is strongly associated with all-cause mortality (HR 1.18 per point increase in score; 95% CI 1.14–1.22; p = 2.25e−16). When combined with Charlson comorbidity index, individuals with elevated medication score and comorbidity index had over six times higher risk (HR 6.30; 95% CI 3.84–10.3; AUC = 0.802) compared to individuals with a protective score profile. Alone, the medication score performs similarly to the Charlson comorbidity index and is associated with polygenic risk for coronary heart disease and type 2 diabetes.
Subject terms: Genomics, Public health, Statistics, Risk factors
Introduction
Health differences among the elderly and the role of medical treatments are topical issues in many aging societies. Older people suffer from multimorbidity1, presence of multiple chronic conditions and are susceptible to polypharmacy2, use of numerous potentially interacting medications. Both may lead to a severe medication cascade which, in effect, can cause severe adverse drug reactions, decrease quality of life, and even lead to premature death.
Considerable imbalance exists in the number of hospital visits and general medication use among the elderly which manifests in an uneven distribution of health care costs3. Understanding these differences better would help target resources and interventions more effectively to those at risk. Increasingly abundant digital health data and modern statistical tools have the potential to facilitate the development of novel ways to measure health differences in the aging population.
Several instruments have been developed to summarize disease diagnoses and exposure to medications into numeric scores using information from hospital databases and medication administration records, e.g. Charlson Comorbidity Index (CI)4, Elixhauser Index (EI)5, Rx-Risk-V6, Medication-Based Disease Burden Index7, and Chronic Disease Score (CDS)8. These instruments customarily consider a limited number of predefined severe health conditions and consider typically short time windows of a few years, depicting primarily acute changes in health. Given many of these measures are originally derived in hospitalized or institutionalized patient populations and they focus mainly on a priori defined set of severe diseases9,10, they could be strengthened by involving long-term prescription medication usage that can capture numerous chronic but less-acute health conditions in the general non-institutionalized population.
The objective of this study is to demonstrate novel data-driven ways to construct health measure from large-scale longitudinal health data and investigate how such score performs and could complement existing classic measures in predicting long-term mortality. In this paper, we derive a new score to measure chronic health differences in an aging population empirically with modern statistical learning methods using 21 years of Finnish pharmacy purchase and death data obtained from high-quality nationwide registries. We demonstrate the applicability of the resulting score in two fully independent prospective cohorts and investigate its relationship to known genetic predictors of late-onset diseases and diagnosis-based comorbidity index.
Results
We trained 28 medication score candidates (Supplementary Table S1) in the National FINRISK study11 where we included a subset of 20,078 participants who were alive and at least 46 years old at the beginning of 2006 (median age 60, IQR 53–67). We followed their mortality 10 years (2006–2015). To train the models with medication data, this mortality was contrasted against purchases 10 years prior to follow-up (1995–2004), with 1 year wash-out in-between (2005). Out of this sample, 15,995 (79.7%) had imputed whole genome genotypes available at the time of the study resulting in 15 million genetic variants per individual.
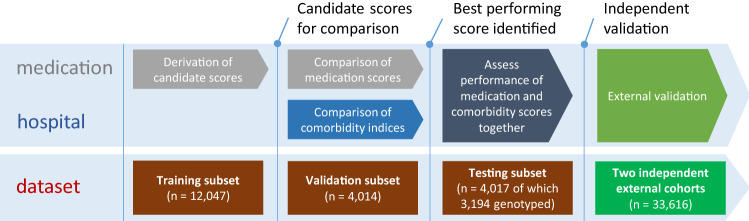
The study was conducted in four consecutive parts as described in Fig. 1. The included participants of the primary cohort (FINRISK) were randomly assigned into three non-overlapping groups: First, we separated a training set to derive candidate medication scores statistically in relation to all-cause mortality (12,047 individuals, 60%). Second, we extracted an independent validation set to compare the predictive performance of alternative medication scores (4,014 individuals, 20%) and two commonly used diagnosis-based comorbidity indices. Third, we evaluated the complement performance of best medication and comorbidity indices together in the testing hold-out set (4,017 individuals, 20%).
Figure 1.
Analysis workflow and FINRISK sample split.
Finally, we carried out external validation of the medication scores in two independent cohorts. For Finnish external validation, we used the Health 2000 Survey (H2000)12 where we included 5,410 participants who fulfilled the same inclusion criteria as the FINRISK sample. We only had access to the medication data for the H2000, and the cohort was therefore used solely for the validation and comparison of medication scores. Second external validation was conducted in the Estonian Biobank cohort13, using data on 28,206 aging individuals.
All included three cohorts were nationwide population studies with no substantial selection or sampling considerations. Cohorts are summarized in the Table 1.
Table 1.
Description of study cohorts, only aging population.
| Name | Data source/owner | Original collection criteria (nationality) | Total included sample size (% women) | Death events in the follow-up period | Age at the beginning of 10-year mortality follow-up | Cohort description | Purpose |
|---|---|---|---|---|---|---|---|
| National FINRISK study | THL Biobank | Random population sample (FIN) | 20,078 (52%) | 2,389 (12%) | 60 (53–67) | Borodulin et al.11 | Primary derivation and evaluation set |
| Health 2000 study | THL Biobank | Random population sample (FIN) | 5,410 (55%) | 1,082 (20%) | 61 (54–72) | Heistaro et al.12 | External validation (different subset of medications) |
| Estonian biobank | University of Tartu | Random population sample (EST) | 28,206 (68%) | 2,517 (8.9%) | 61 (53–71) | Leitsalu et al.13 | External validation (different timeframe) |
Data are n (%) or median (IQR).
Performance of medication scores
A score derived with elastic net regularization and dichotomous medication use indicator (i.e. purchased medicine ever) provided the best overall prediction performance. The result was consistent across all derivation methods: using continuous usage duration or dichotomous “used over 1 year” never improved the performance.
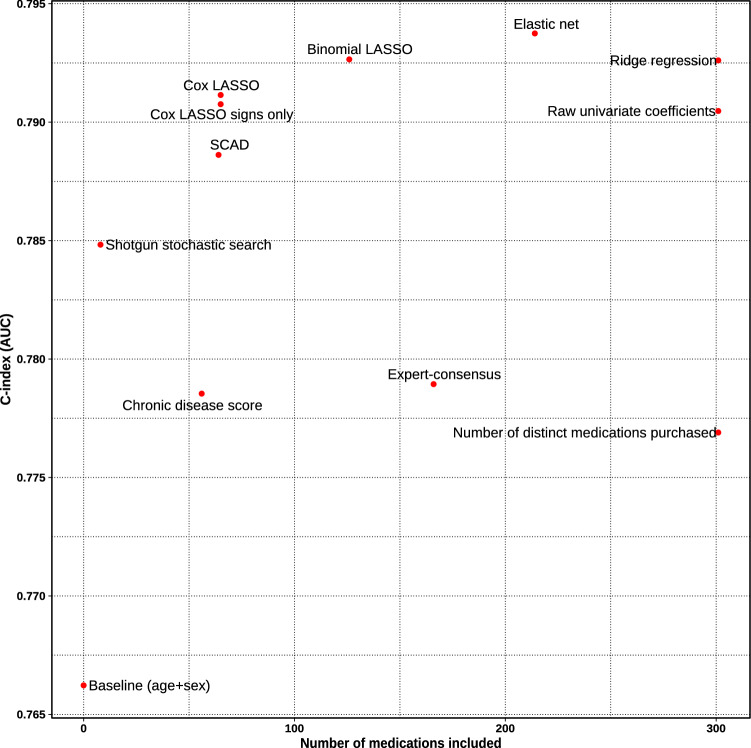
A score consisting of 166 medications hand-picked by an expert consensus panel of three medical doctors expectedly predicted mortality better than the simple baseline model (age and gender) alone (C-index 0.766 vs. 0.779, p = 1.83e−7). However, the expert score was inferior to all data-driven statistical approaches including the shotgun stochastic search that included only eight medications (C-index 0.785, p = 0.04) and it performed only marginally better than the classic polypharmacy measure of simply counting the number of distinct medications administered (C-index 0.777, p = 0.06). In our study setup, all statistical learning approaches seemed to outperform the Chronic Disease Score. Scores were built and tested by adjusting for age and sex. Performance of all scores is elaborated in the Supplementary Table S1 and the most interesting scores are illustrated in Fig. 2.
Figure 2.
Discriminative performance of the score versus parsimony in the FINRISK validation subset. All based on the “purchased ever” indicator.
Interestingly, a score involving only non-zero coefficient signs (− 1 and 1) of the Cox LASSO performed nearly as well as the actual Cox LASSO coefficient values (C-index 0.791 for both, p = 0.41, 65 medications). Performance was comparable to binomial LASSO model that included twice as many medications (C-index 0.793, p = 0.14). When comparing it to the best performing elastic net method that employs over 200 medications, the difference in performance seems to be marginal (C-index 0.794, p = 0.05).
After qualitatively considering trade-off between prediction performance, number of medications included, and general parsimony, the Cox LASSO signs-only score was chosen for further analyses. With comparable performance, it considers only a fraction of medications of marginally better performing alternatives. Furthermore, involving coefficient signs only makes the score easily deployable and robust to variance in different data sources and application domains. The resulting integer score is easily interpretable and intuitive without any transformations. The score distribution across the aging population of the whole FINRISK cohort is illustrated in Supplementary Fig. S1 and included medications are listed in Supplementary Table S2. We should note that the shotgun stochastic search score with eight medications could be also an attractive alternative if we preferred extreme parsimony, and it seems to be a direct subset of the 65 medications in the main score.
In order to confirm the result, we took some of the interesting score weights for evaluation to the two external validation cohorts. The results were highly concordant with the FINRISK findings, as summarized in Table 2. Only notable exception is the decreased performance of the elastic net score in the Estonian cohort. It may have an overfitting tendency to Finnish patterns given the large number of medications included. The systematically better absolute performance of all scores in the H2000 cohort can likely be explained with a higher number of mortality events and older age distribution.
Table 2.
Best performing medication scores evaluated in three separate validation datasets by C-index and Negelkerke R2.
| Score derivation method | Number of medications included | Main cohort FINRISK validation subset |
Replication cohorts | ||||
|---|---|---|---|---|---|---|---|
| R2 | C-index ∆C baseline |
||||||
| R2 | C-index (95% CI) ∆C baseline (95% CI) |
H2000 | Estonia | H2000 | Estonia | ||
| Baseline model (age and sex) | – | 0.134 | 0.766 (0.745–0.788) | 0.309 | 0.098 | 0.823 | 0.775 |
| Expert consensus panel | 166 | 0.149 | 0.779 (0.758–0.800) | 0.316 | 0.107 | 0.827 | 0.786 |
| + 0.013 (0.006–0.019) | + 0.004 | + 0.011 | |||||
| Number of medications | 301 | 0.148 | 0.777 (0.756–0.798) | 0.317 | 0.104 | 0.828 | 0.785 |
| + 0.011 (0.005–0.017) | + 0.005 | + 0.010 | |||||
| Shotgun stochastic search | 8 | 0.158 | 0.785 (0.764–0.806) | 0.317 | 0.102 | 0.827 | 0.781 |
| + 0.019 (0.01–0.027) | + 0.004 | + 0.006 | |||||
| Cox LASSO | 65 | 0.164 | 0.791 (0.771–0.812) | 0.324 | 0.109 | 0.830 | 0.793 |
| + 0.025 (0.017–0.033) | + 0.007 | + 0.018 | |||||
| Cox LASSO signs only | 65 | 0.162 | 0.791 (0.770–0.811) | 0.320 | 0.110 | 0.829 | 0.791 |
| + 0.025 (0.017–0.032) | + 0.006 | + 0.016 | |||||
| Ridge regression | 301 | 0.164 | 0.793 (0.772–0.813) | 0.322 | 0.113 | 0.830 | 0.795 |
| + 0.026 (0.018–0.034) | + 0.007 | + 0.020 | |||||
| Elastic net | 214 | 0.166 | 0.794 (0.773–0.814) | 0.324 | 0.108 | 0.830 | 0.784 |
| + 0.028 (0.019–0.036) | + 0.007 | + 0.009 | |||||
H2000 does not include all the ATC codes of the derivation set.
ATC hierarchies seem to perform comparably when we considered higher level categories, apart from the highest investigated level, therapeutic subgroup, that fared systematically worse (Supplementary Fig. S2). In this study we decided to concentrate on the full ATC code (i.e. chemical substance) as it gives most transparency to the score, is the easiest to apply based on e.g. interview, and the final number of codes included is not substantially higher.
Performance of comorbidity indices
In addition to medication scores, we compared two commonly used comorbidity indices using inpatient hospital admissions in the validation subset of the FINRISK and all diagnoses in the Estonian Biobank cohort. Although the more complex Elixhauser index has been argued to be superior specifically in hospital settings14, Charlson comorbidity index seems to be a better predictor of 10-year mortality in both cohorts (Supplementary Table S3).
Genetic risk factors
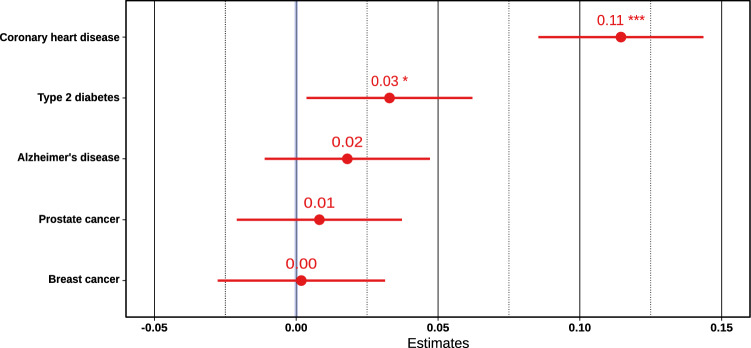
To investigate the genetic component of the score, we took polygenic risk scores of five major conditions that together account for over 60% of causes of death among the elderly15,16. Since genetic risk factors do not cause death directly but through increased disease risk, these polygenic disease risks were combined into a multivariable model to account for any shared genetic etiology and to adjust for age and sex. Together these scores seem to account for only 0.4% of the variance in the score, driven by coronary heart disease and type 2 diabetes (Fig. 3). This is expected given cardiometabolic conditions account for majority of early deaths among the aging and have a strong heritable component.
Figure 3.
Linear associations of polygenic risk scores with the Cox LASSO signs-only medication score, adjusted with age and sex, within the whole aging population of the FINRISK study (***p < 0.001; **p < 0.01; *p < 0.05).
Combining medication score, comorbidity index, and genetic risks
When the best medication score and comorbidity index (Cox LASSO signs and CI) were combined additively into one model in the FINRISK hold-out testing subset, measures seemed to complement each other well. Medication score appears to be rather independent of the comorbidity index and adds orthogonal information in relation to the risk of all-cause mortality (Table 3). The CI is only weakly correlated with the medication score (Spearman ρ = 0.233; p < 2.2e−16; Supplementary Fig. S3). Inclusion of five polygenic risks to this model did not seem to increase model performance but neither weakened the estimated effect of the two measures.
Table 3.
Cox proportional hazards model estimates in the genotyped FINRISK testing subset and Estonian validation cohort in relation to all-cause mortality.
| Model | FINRISK (testing subset with genotypes) | Estonian Biobank | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Components | Medications | Comorbidities | Polygenic risk scores | HRMed | HRComorb | C-index | p-value (removed variables) | HRMed | HRComorb | C-index | p-value (removed variables) |
| BL | 0.779 (0.757–0.801) | 0.775 (0.767–0.784) | |||||||||
| BL + CI | ✓ | 1.76 (1.56–1.98) | 0.794 (0.773–0.815) | 2.80e−16 (CI) | 1.15 (1.13–1.17) | 0.790 (0.782–0.798) | 2.16e−49 (CI) | ||||
| BL + M | ✓ | 1.18 (1.14–1.22) | 0.794 (0.773–0.815) | 2.25e−16 (M) | 1.19 (1.17–1.22) | 0.791 (0.783–0.799) | 3.82e−69 (M) | ||||
| BL + M + CI | ✓ | ✓ | 1.13 (1.08–1.17) | 1.50 (1.31–1.70) | 0.802 (0.781–0.822) |
2.49e−8 (M) 3.15e−8 (CI) |
1.15 (1.12–1.17) | 1.09 (1.06–1.11) | 0.796 (0.788–0.804) |
1.13e−46 (M) 8.13e−17 (CI) |
|
| BL + M + CI + PRS | ✓ | ✓ | ✓ | 1.13 (1.08–1.17) | 1.50 (1.31–1.70) | 0.803 (0.782–0.823) | 0.69 (PRS) | ||||
B baseline (age and sex covariates), M medication score, CI Charlson Comorbidity Index, PRS polygenic risk scores. Confidence intervals (95%) for estimates provided where applicable.
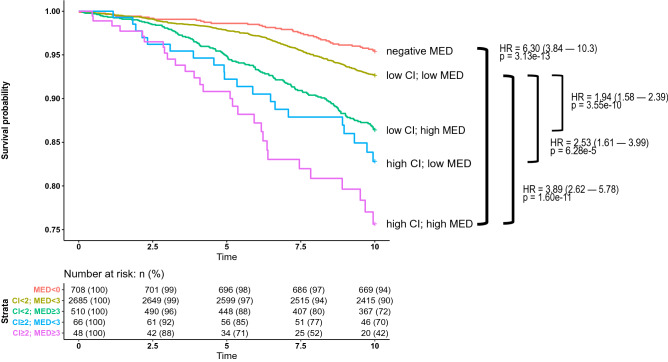
We can see a clear difference between mortality rates when we stratify both measures into low and high bins (CI ≥ 2 and MED ≥ 3). Notably, a negative medication score associates with lower risk of mortality compared to individuals with low or zero score (Fig. 4) suggesting a real protective correlation of the negatively weighted medications.
Figure 4.
Survival rates in the FINRISK testing dataset and a 10 year follow-up window, stratified by the medication index and Charlson Comorbidity Index. Both based on preceding ten years of medication and hospital data.
Discussion
We demonstrated how modern data-driven approaches and longitudinal secondary health data can be used to construct a novel measure of health differences from pharmacy purchases. We tested its association to mortality and inspected relationship to existing comorbidity indices and known genetic risk factors for late-onset diseases. We show that the resulting score is strongly associated with mortality in three datasets independent of the derivation set. When combined with an established comorbidity index, individuals with both elevated medication score and comorbidity index were at over six times higher risk compared to individuals with protective medication profile. The medication score was also associated with genetic risk scores for coronary heart disease and type 2 diabetes.
These findings allow us to draw several conclusions. First, classic indices can be effectively complemented with modern methods by mining large-scale secondary health data such as medication purchase histories. When such data-driven medication score is used alone, it performs similarly as a classic comorbidity index in predicting 10-year mortality in the general aging population. In contrast to most existing comorbidity and polypharmacy measures, our approach poses no presumptions of relevant medication indications and is based purely on an empirical analysis of the register data. Second, now derived medication score could be used as a tool for future population health and genetic research. It can indicate persons among large aging populations in need of a more detailed attention due to their potentially reduced health status and polypharmacy risk. As the score requires no new measurements from the individual and can be applied without complex algorithms on top of existing drug purchase or use databases, it scales effectively to larger populations.
Third, our medication score is a readout of the medication history over the last 10 years. Therefore, the method could be implemented in various health care environments having medication usage records. The resulting score is extremely simple to apply:
Identify prescription drugs one has used during the last 10 years (by medical substance, e.g. ibuprofen)
Count how many of these drugs are on the left-hand side of Supplementary Table S2 (associated with increased risk). From this number, subtract the count of drugs on the right-hand side of the same table (associated with decreased risk). This gives the medication score where each additional point has a HR 1.18 (95% CI 1.14–1.22).
Based on our replication results, the approach seems to generalize to another health care system and behaves robustly in a cohort where information of some of the included medications is missing.
Built from a predictive perspective, the score associations should not be considered as causal relationships between individual medications and mortality. They rather reflect complex correlations that can be harnessed into a practical measure to indicate aging individuals with an increased risk of long-term mortality and potentially diminished health status. The score associates specifically with life-years gained and does not value the subjective life-quality of prolonged survival in different chronic states. The score is primarily a surrogate for population level health differences, not a substitute for clinical assessment of geriatric frailty or functional impairment.
The methodology was based on fixed-time intervals and did not consider temporal aspects such as changes in medication prescription guidelines or introduction of new medications. The learning method itself could be implemented continuously in any health informatics setting that involves ATC coding and large enough training sample sizes to increase the adaptiveness of the score. In addition, the time windows and wash-out period durations were fixed in our study but could be considered as tunable parameters that could be similarly optimized from the data to maximize prediction power. This could be interesting future research question especially due to potential reverse-causation effect where some medications may end up being prescribed at the point when the lethal disease has already progressed for some time. The learning method itself could be implemented continuously in any health informatics setting that involves ATC coding and large enough training sample sizes to increase the adaptiveness of the score.
We also acknowledge the role of left-truncation and subsequent possible immortal time bias in model estimates. This, however, should not be a major concern when aiming to prediction in similar aging populations and ruling out any profound inferential conclusions about individual medications. Apart from potential left-truncation, the data is derived from nationwide registries that record all deaths, with loss of follow-up occurring only due to moving abroad which we cannot account for. The medication register includes all reimbursed prescription pharmacy purchases and thus excludes medications administered in institutions and over-the-counter medications. Such medications are out of scope of this study whose specific aim was to investigate predictive power of prescription pharmacy purchases in the general non-institutionalized population related to chronic diseases. The Charlson Comorbidity Index was based on standard Quan adaptations of Deyo–Charlson Comorbidity Index and could get marginal benefit from mapping to national Finnish ICD-10 adaptation.
As the aim of the study was to explore empirically the power of modern statistical methods to derive easily applicable measures from a secondary health data, we did not aim to model non-linear medication interactions or individual medication use patterns to the highest detail.
Moreover, our study did not address the role of sociodemographic factors in medication and healthcare usage which could confound some of the results. The Finnish healthcare is strongly based on public funding and medications are publicly subsidized which should mitigate considerably the effect of demographic differences.
Conclusion
Our study suggests that long-term and large-scale health data can be distilled into a composite measure to infer health differences in the general aging population. Together with Charlson comorbidity index, our novel polypharmacy score identifies 1.2% of elderly population with over six times higher risk of mortality compared to the individuals with a protective medication profile.
Given increasing availability of large-scale health data, statistical learning methods, and abundant computational power, scores aiming for health prediction could be more directly, yet transparently, mined from empirical health data to complement classic measures that are commonly founded on a priori expert opinion. The clinical utility of the newly developed score and relationship to the subjective life quality warrants further studies.
Materials and methods
To inspect medication usage, morbidity history, and death information in two Finnish cohorts, we used the Register for prescribed medication purchases, the Finnish Hospital Discharge Register, and the National Causes of Death Register17. In absence of longitudinal explicit health-related quality of life indicators, we measured health effects as gained life-years by modeling survival. To inspect our endpoint of interest, all-cause mortality, we derived data from the national causes of death register that includes all deaths in the study cohort, timestamps, and relevant diagnosis codes for major, acute, and contributory causes. Validity of the register has been demonstrated by e.g. Rapola18.
The Finnish medicine expenses register covers all pharmacy prescription drug purchases since 1995, purchase timestamps, and respective Anatomical Therapeutic Chemical Classification System codes (ATC). The full FINRISK study medication database constituted of 3.4 million medication purchase events in total. Hospital discharge register covers virtually all inpatient hospital visits in Finland since 1969 and has a demonstrated validity for discharges19. The register includes a timestamp of hospital stay and relevant diagnoses under the Finnish variant of the International Statistical Classification of Diseases and Related Health Problems scheme (ICD). The full FINRISK hospital discharge register covers nearly 300,000 hospital visits. In this study, we included only hospital visits registered with 10th revision of ICD between years 1996–2015 as ICD-10 was formally taken into use at the beginning of 1996 in Finland.
The Estonian cohort was comprised of participants of the Estonian biobank13, and the analyzed data combined time of death from the Estonian Causes of Death Registry and medication prescription information from the Estonian Health Insurance Fund. Diagnosis codes for comorbidity indices were derived from the databases of the Estonian National Health Information System, the Estonian Health Insurance Fund, Tartu University Hospital, North Estonia Medical Centre, and the Estonian Cancer Registry20.
In FINRISK, 11.9% of the included individuals deceased within the follow-up period, whereas the number was 20.0% in the H2000 and 8.9% in the Estonian Biobank cohort. The difference can be largely explained by the varying age distributions among the studies.
Analysis of the time-series register data
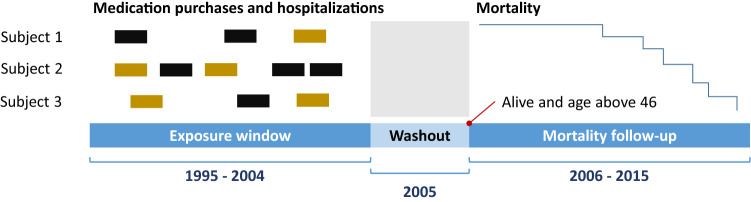
In both Finnish cohorts, we split our study time frame identically into three parts (Fig. 5): (i) a 10 year medication purchase and hospital visit exposure window (1995–2004), (ii) a 1-year washout period (2005) to mitigate the effect of palliative care and medications prescribed to terminally ill patients, and (iii) a 10 year follow-up window for all-cause mortality (2006–2015). In the Estonian Biobank cohort, we considered shorter windows: 7 years for exposure (2004–2010) and six years for mortality follow-up (2012–2017) with 1-year washout period in-between (2011).
Figure 5.
Exposure window and follow-up window illustrated in Finnish cohorts.
Longitudinal medication purchases were converted into cross-sectional data points by first generating three alternative indicators for each ATC code: (i) a dichotomous indicator for at least one purchase event within the exposure window, (ii) a dichotomous indicator for at least two purchases 1 year apart, and (iii) a continuous indicator for years between the last and first purchase, a rough proxy for treatment duration. If subject had at least one purchase event for a medication, a generic three-month constant was added to the continuous indicator to account for the duration of the last purchase and to cover single purchase scenarios. Given the ATC classification is a hierarchical coding system, we additionally explored how second, third and fourth levels of codes (i.e. therapeutic, pharmacological, and chemical subgroups) would work with used methods. We did not involve exhaustive register analysis frameworks, as we prioritized parsimony and application simplicity over marginal increases in accuracy.
Derivation of candidate medication scores and comorbidity indices
Candidate medication scores were derived with high-dimensional multivariable regression methods in the FINRISK training subset as described below. We aimed for a linear additive score that could be calculated effortlessly by taking a weighted sum over different medications. All models were adjusted for age and sex during the training.
First, univariate logistic regressions were run for all individual medications, imposing Firth’s penalty for bias reduction to address separation in rare cases21. We used resulting raw univariate regression coefficients as weights for the first score candidate. To find sparse combinations, univariate regression coefficients and medication correlations were also subject to shotgun stochastic search to identify the sparse linear configuration of associated medications with the highest posterior probability22.
As a direct multivariable approach, we examined the performance of classical regularized statistical learning methods (L1- and L2-regularization and combination of thereof, i.e. LASSO, ridge regression, and elastic net) and smoothly clipped absolute deviation (SCAD) that has an oracle property of asymptotically finding the true subset of variables under certain assumptions23,24. In addition to the above-mentioned methods that all were extensions of a binomial logistic regression, a L1-penalized (LASSO) Cox proportional hazards model was also tested.
As a reference point, we inspected the performance of a conventional score building scheme by including 166 aging-related drugs a priori selected by a consensus panel of three medical doctors from a list of the most commonly used medications in the FINRISK cohort. We further included a common polypharmacy surrogate that simply counts the number of distinct medications used. These two scores imposed equal weights for all included medications.
Two alternative diagnosis-based comorbidity indices evaluated were based on Quan adaptations of Deyo-Charlson Comorbidity Index and Elixhauser-van Walraven Comorbidity Index25,26. Comorbidity indices were constructed using the R-3.5.2 package icd and medication scores with glmnet and ncvreg27–30. Chronic disease score was calculated based on methodology of Lix et al.31.
Comparing medication score and comorbidity index performance
Each score candidate was evaluated in the non-overlapping FINRISK validation subset as a continuous variable in a Cox model using death as an endpoint and follow-up time as the time scale. Models were adjusted with age at the beginning of the follow-up and sex. Model fit was compared using Nagelkerke R2 and discriminative performance with C-index, a generalized area under the ROC curve measure32,33. We also included a reference baseline model that consisted of age and sex only.
Based on numerical validation results and qualitative assessment of score parsimony, we selected the best candidates amidst medication scores and comorbidity indices. These two were then further combined additively into a single Cox model to compare their complementary performance and to evaluate effect estimates in the FINRISK hold-out subsample (testing dataset) together with five genetic risk factors. The proportionality assumption was tested using Schoenfeld residuals and linearity assumption using penalized smoothing splines.
External validation
Medication score performance was evaluated within the H2000 and Estonian Biobank Cohort to test the robustness and generalizability of our medication score to different follow-up lengths, diverse medication category coverages, and sensitivity to differences in data sources. FINRISK and H2000 cohorts derive their data from the same national register resources and have the same temporal coverage, but medication categories are only partly intersecting (Supplementary Table S4). Estonian cohort, on the other hand, covers virtually all medications sold in Estonia but uses fundamentally different sources for medication information and has a shorter time window. We should also assume that medication prescription patterns differ between these two countries in general.
Genetic risk factors
To evaluate the relationship of medication score to genetic risk factors, we investigated the association of the best medication score with polygenic risk scores (PRS) based on six million genetic variants. The whole aging genotyped subset of the FINRISK cohort data was used to infer the correlation between best performing medication score and PRS scores. All were pre-adjusted for 10 first principal components and genotyping batch prior to analyses.
Ethical approval
The study was conducted in accordance with the principles of the Helsinki declaration. Written informed consent was obtained from all the study participants. FINRISK and Health 2000 cohorts were based on study specific consents and later transferred to the THL Biobank after approval by the National Supervisory Authority for Welfare and Health (Valvira). Recruitment protocols followed the biobank protocols approved by Valvira. All participants of the Estonian biobank have signed a broad informed consent that allows follow-up linkage of their electronic health records (EHR), thereby providing a longitudinal collection of phenotypic information for research.
The transfer of the FINRISK and Health 2000 sample collections to the THL biobank has been approved by the Coordinating Ethics Committee of Helsinki University Hospital on 10 October 2014 and by the Ministry of Social Affairs and Health on 9 March 2015. This study was conducted under the THL biobank permissions BB2015_31.1 (FINRISK), BB2017_100 (Health 2000), and the approval of the Research Ethics Committee of the University of Tartu 234/T-12 (Estonian biobank). All DNA samples and data in this study were pseudonymized.
Supplementary information
Acknowledgements
S.R. was supported by the Academy of Finland Center of Excellence in Complex Disease Genetics (Grant No 312062), Academy of Finland (Grant No 285380), the Finnish Foundation for Cardiovascular Research, the Sigrid Juselius Foundation and University of Helsinki HiLIFE Fellow grant. V.S. was supported by the Finnish Foundation for Cardiovascular Research. T.T. was supported with Estonian Research Council grant IUT34-4 and Estonian Centre of Excellence in IT (TK148). L.M. was supported by the Estonian Research Council Grant PRG184. A.S.H. was supported by the Academy of Finland (Grant No 321356). We thank the sample donors of the THL biobank and Estonian biobank for their contribution to this study.
Author contributions
P.H., S.R., M.D., A.P. and B.N. conceived and designed the study with clinical input from M.P., M.K. and A.P. P.H., A.H. and T.T. did the data analysis. N.M. and P.H. conducted polygenic risk score analyses. L.M., S.K. and V.S. were cohort custodians. P.H. and S.R. wrote the first draft of the article, which was critically revised and approved by all authors.
Data availability
The National FINRISK and Health 2000 studies can be applied from the THL Biobank. Access to the Estonian Genome Center Biobank Cohort can be requested from the Institute of Genomics, University of Tartu (https://genomics.ut.ee/en/biobank.ee/data-access). A detailed description of the analytical workflow can be requested from the authors.
Competing interests
A.P. is a member of Astra Zeneca Genomics Advisory Board. V.S. has participated in a conference trip sponsored by Novo Nordisk and received an honorarium for participating in an advisory board meeting. He also has ongoing research collaboration with Bayer Ltd. (All unrelated to the present study). T.T. is a shareholder, member of the board of directors and management team of Antegenes OÜ. The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72045-z.
References
- 1.Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. The Lancet. 2012;351:37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- 2.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am. J. Geriatr. Pharmacother. 2007;5:345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Linna M, Mikkola T, Malmström T, Tyni T. Iäkkäiden sosiaali-ja terveyspalveluiden kustannuserot: Palveluiden käyttö ja tuottavuus selittävinä tekijöinä. Focus Localis. 2017;45(3):7–16. [Google Scholar]
- 4.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 5.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med. Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Sloan KL, Sales AE, Liu CF, Fishman P, Nichol P, Suzuki NT, Sharp ND. Construction and characteristics of the RxRisk-V: A VA-adapted pharmacy-based case-mix instrument. Med. Care. 2003;41:761–774. doi: 10.1097/01.MLR.0000064641.84967.B7. [DOI] [PubMed] [Google Scholar]
- 7.George J, Vuong T, Bailey MJ, Kong DC, Marriott JL, Stewart K. Development and validation of the medication-based disease burden index. Ann. Pharmacother. 2006;40:645–650. doi: 10.1345/aph.1G204. [DOI] [PubMed] [Google Scholar]
- 8.Von Korff M, Wagner HE, Saunders K. A chronic disease score from automated pharmacy data. J. Clin. Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-G. [DOI] [PubMed] [Google Scholar]
- 9.Yourman LC, Lee SJ, Schonberg MA, Widera EW, Smith AK. Prognostic indices for older adults: A systematic review. JAMA. 2012;307:182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yurkovich M, Avina-Zubieta JA, Thomas J, Gorenchtein M, Lacaille D. A systematic review identifies valid comorbidity indices derived from administrative health data. J. Clin. Epidemiol. 2015;68:3–14. doi: 10.1016/j.jclinepi.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Borodulin K, et al. Cohort profile: The national FINRISK study. Int. J. Epidemiol. 2017;47:696–696i. doi: 10.1093/ije/dyx239. [DOI] [PubMed] [Google Scholar]
- 12.Heistaro, S. (ed.). Methodology Report, Health 2000 Survey. in Sampling Design no. B 26, National public health institute, Helsinki (2008)
- 13.Leitsalu L, et al. Cohort profile: Estonian biobank of the Estonian genome center, University of Tartu. Int. J. Epidemiol. 2014;44:1137–1147. doi: 10.1093/ije/dyt268. [DOI] [PubMed] [Google Scholar]
- 14.Ladha KS, et al. The Deyo-Charlson and Elixhauser-van Walraven Comorbidity Indices as predictors of mortality in critically ill patients. BMJ Open. 2015;5:e008990. doi: 10.1136/bmjopen-2015-008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Official Statistics of Finland. Causes of death. https://www.stat.fi/til/ksyyt/2017/ksyyt_2017_2018-12-17_tie_001_en.html (2017).
- 16.Mars, N.J. et al. Polygenic and clinical risk scores and their impact on age at onset of cardiometabolic diseases and common cancers. https://www.biorxiv.org/content/10.1101/727057v2.abstract (2019). [DOI] [PubMed]
- 17.Haukka J. Finnish health and social welfare registers in epidemiological research. Norsk Epidemiol. 2004 doi: 10.5324/NJE.V14I1.284. [DOI] [Google Scholar]
- 18.Rapola JM, et al. Validity of diagnoses of major coronary events in national registers of hospital diagnoses and deaths in Finland. Eur. J. Epidemiol. 1997;13:133–138. doi: 10.1023/A:1007380408729. [DOI] [PubMed] [Google Scholar]
- 19.Sund R. Quality of the Finnish Hospital Discharge Register: A systematic review. Scand. J. Public Health. 2012;40:505–515. doi: 10.1177/1403494812456637. [DOI] [PubMed] [Google Scholar]
- 20.Leitsalu L, Alavere H, Tammesoo ML, Leego E, Metspalu A. Linking a population biobank with national health registries—The estonian experience. J. Person. Med. 2015;5:96–106. doi: 10.3390/jpm5020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosmidis I, Pagui ECK, Sartori N. Mean and median bias reduction in generalized linear models. Stat. Comput. 2018;30:1–17. [Google Scholar]
- 22.Benner C, Spencer CC, Havulinna AS, Salomaa V, Ripatti S, Pirinen M. FINEMAP: Efficient variable selection using summary data from genome-wide association studies. Bioinformatics. 2016;32:1493–1501. doi: 10.1093/bioinformatics/btw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastie T, Tibshirani R, Wainwright M. Statistical Learning with Sparsity: The Lasso and Generalizations. Boca Raton: Chapman and Hall/CRC; 2015. [Google Scholar]
- 24.Fan J, Li R. Variable selection via nonconcave penalized likelihood and its oracle properties. J. Am. Stat. Assoc. 2001;96:1348–1360. doi: 10.1198/016214501753382273. [DOI] [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 26.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med. Care. 2009;47:626–633. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 27.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org (2018).
- 28.Wasey, J. O. icd: Comorbidity Calculations and Tools for ICD-9 and ICD-10 Codes. R package version 3.3. https://CRAN.R-project.org/package=icd (2018).
- 29.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breheny P, Huang J. Coordinate descent algorithms for nonconvex penalized regression, with applications to biological feature selection. Ann. Appl. Stat. 2011;5:232–253. doi: 10.1214/10-AOAS388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lix L, et al. Cancer Data Linkage in Manitoba: Expanding the Infrastructure for Research. Winnipeg: Manitoba Centre for Health Policy; 2016. [Google Scholar]
- 32.Nagelkerke NJ. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. doi: 10.1093/biomet/78.3.691. [DOI] [Google Scholar]
- 33.Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. doi: 10.1001/jama.1982.03320430047030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The National FINRISK and Health 2000 studies can be applied from the THL Biobank. Access to the Estonian Genome Center Biobank Cohort can be requested from the Institute of Genomics, University of Tartu (https://genomics.ut.ee/en/biobank.ee/data-access). A detailed description of the analytical workflow can be requested from the authors.