Abstract
A1874 is a novel BRD4-degrading proteolysis targeting chimera (PROTAC). In primary colon cancer cells and established HCT116 cells, A1874 potently inhibited cell viability, proliferation, cell cycle progression, as well as cell migration and invasion. The BRD4-degrading PROTAC was able to induce caspase and apoptosis activation in colon cancer cells. Furthermore, A1874-induced degradation of BRD4 protein and downregulated BRD-dependent genes (c-Myc, Bcl-2, and cyclin D1) in colon cancer cells. Significantly, A1874-induced anti-colon cancer cell activity was more potent than the known BRD4 inhibitors (JQ1, CPI203, and I-BET151). In BRD4-knockout colon cancer cells A1874 remained cytotoxic, indicating the existence of BRD4-independent mechanisms. In addition to BRD4 degradation, A1874 cytotoxicity in colon cancer cells was also associated with p53 protein stabilization and reactive oxygen species production. Importantly, the antioxidant N-acetyl-cysteine and the p53 inhibitor pifithrin-α attenuated A1874-induced cell death and apoptosis in colon cancer cells. In vivo, A1874 oral administration potently inhibited colon cancer xenograft growth in severe combined immuno-deficient mice. BRD4 degradation and p53 protein elevation, as well as apoptosis induction and oxidative stress were detected in A1874-treated colon cancer tissues. Together, A1874 inhibits colon cancer cell growth through both BRD4-dependent and -independent mechanisms.
Subject terms: Apoptosis, Oncogenes
Introduction
Colon cancer has become a common malignancy and a global health issue1,2, causing significant cancer-related human mortalities each year3,4. Current clinical treatments, including chemotherapy, surgery, radiation, and/or molecularly targeted therapies1,5,6, along with the advanced early screen and diagnosis techniques, have significantly improved the prognosis and five-year overall survival of colon cancer patients1,5,6. Yet the prognosis for the advanced, metastatic and recurrent colon cancer patients remains poor1. There is an urgent need to further explore the underlying pathological mechanisms of colon cancer development and progression1,5,6.
Bromodomain-containing protein 4 (BRD4) is an extensively studied BET (bromodomain and extra-terminal domain) family protein, that provides a promising therapeutic target for colon cancer7. BRD4 directly binds to acetylated histones and plays an essential role in regulating epigenetic processes8–10. In the process of mitosis, BRD4 is required for chromatin structure formation in the daughter cells9,11. Furthermore, BRD4 is important for transcription elongation and expression of key oncogenic genes including Bcl-2 and c-Myc. BRD4 associates with positive transcription elongation factor b (P-TEFb) to phosphorylate RNA polymerase II in proliferating cells9,11. Recent studies have proposed BRD4 as a novel oncogene as it is overexpressed in colon cancer12 and many other malignancies8,11.
Multiple small-molecule inhibitors of BRD4 have been developed, showing promising anticancer results in experimental and clinical cancer studies8,11,13. However, BRD4 inhibition results in feedback elevation of BRD4 protein in human cancer cells, leading to weak anti-proliferative activity and less apoptosis induction8,11,13. A recent study identified A1874 as a BRD4-targeting mouse double minute 2 homolog (MDM2)-based proteolysis targeting chimera (PROTAC)14.
Unlike the BRD4 inhibitors, A1874 can lead to a robust and sustained BRD4 protein degradation through ubiquitin system14, causing profound inhibition of BRD4-dependent cancers14. In this study, we tested the potential anticancer activity and underlying signaling mechanisms of A1874 against human colon cancer cells.
Materials and methods
Chemicals, reagents, and antibodies
A1874 was obtained from Hanxiang BioTech (Shanghai, China). Puromycin, JQ1, CPI203, I-BET151, N-acetyl-cysteine (NAC) and pifithrin-α, polybrene and CCK-8 were purchased from Sigma-Aldrich (St. Louis, MO). All cell culture reagents were provided by Hyclone Co. (Logan, UT). Antibodies for c-Myc (#9402), Cyclin D1 (#2922), BRD4 (#13440), Bcl-2 (#15707), Erk1/2 (#9102), p53 (#9282), cleaved-caspase-3 (#9664), cleaved-caspase-9 (#20750), cleaved-poly (ADP-ribose) polymerase (PARP) (#5625), Bcl-2 (#15707) and β-tubulin (#15115) were purchased from Cell Signaling Tech (Beverly, MA). Caspase inhibitors, z-VAD-fmk and z-DEVD-fmk, were provided by Thermo-Fisher (Shanghai, China). Lipofectamine 2000, TUNEL (terminal deoxynucleotidyl transferase dUTP nick end labeling), Annexin V and propidium iodide (PI) were purchased from Thermo-Fisher Invitrogen (Carlsbad, CA). The BrdU ELISA kit was provided by Roche Diagnostics (Basel, Switzerland).
Cell culture
The primary human colon cancer cells from four written-informed consent primary colon cancer patients, pCan1/2/3/4, and primary human colon epithelial cells from two healthy donors, pEpi1/2, were provided by Dr. Lu at Nanjing Medical University15–17. The primary human cells were cultured in the described medium15,18. The established HCT116 colon cancer cells were also provided by Dr. Lu15–17. All the established and primary cells were subjected to routine mycoplasma and microbial contamination examination every 2–3 months. To confirm the genotype of the cells, STR (short tandem repeat) profiling, population doubling time, and cell morphology were regularly checked. The protocols of this study were approved by Ethic Committee of Nanjing Medical University, according to Declaration of Helsinki.
Cell viability assay
The viable colon cancer cells or colon epithelial cells were seeded into 96-well plates at 5 × 103 cells per well. CCK-8 kit was utilized to determine cell viability, with CCK-8 optical densities (ODs) recorded at 550 nm.
Colony formation
Colon cancer cells with the applied A1874 treatment were re-suspended in 1 mL of DMEM with 0.5% agar (Sigma). Cells were then added onto the pre-solidified 10-cm cell culture dish. Medium was renewed every 2 days for a total of five rounds. Afterwards, the remaining cell colonies were stained and manually counted.
BrdU ELISA
Cells were seeded into 96-well plates at 5 × 103 cells per well. With the applied A1874 treatment, BrdU incorporation was tested by a BrdU ELISA kit according to the attached protocol. BrdU ELISA absorbance was recorded at 405 nm.
EdU staining
The viable colon cancer cells were seeded into 12-well plates (8 × 104 cells per well). With the applied A1874 treatment, EdU (5-ethynyl-20-deoxyuridine) staining assay was performed as described elsewhere19,20. EdU percentages (EdU vs. DAPI, %) of 1000 cells per treatment in five random views (under a fluorescence microscope at 1× 100 magnification) were recorded.
Cell migration and invasion assays
Colon cancer cells (3 × 104 cells per chamber, in serum-free medium) were seeded onto the “Transwell” chambers: (8-μm pore size, Corning Costar, Shanghai, China). Complete medium containing 10% FBS was added to the lower chambers21. After incubation for 24 h, the migrated cells were fixed and stained. For cell invasion assays, chambers were always coated with Matrigel (Sigma)22. Average number of migrated/invaded cells in five random views per treatment were recorded.
Cell cycle FACS
The primary human colon cancer cells were seeded into six-well plate at 2 × 105 cells per well. With the applied A1874 treatment, cells were washed, fixed, and incubated with DNase-free RNase and PI. Cell cycle distribution was recorded by using a FACSCalilur machine (BD Biosciences, Shanghai, China).
Caspase activity
The viable colon cancer cells were seeded into six-well plates (at 2 × 105 cells per well). Following treatment, 30 μg of cytosolic extract lysates (per treatment) were added to the caspase assay buffer23 together with the applied 7-amido-4-(trifluoromethyl)-coumarin (AFC)-conjugated caspase-3/-8/-9 substrate23. The AFC fluorescence intensity was tested under a Fluoroskan machine23.
Mitochondrial depolarization
JC-1 dye can aggregate into mitochondria and form green monomers in the apoptotic cells with mitochondrial depolarization24. The viable colon cancer cells were seeded into six-well plates (at 2 × 105 cells per well). Following treatment, cells were incubated with JC-1 (5.0 μg/mL). JC-1 intensity was tested immediately under a fluorescence spectrofluorometer at 488 nm. The representative merged JC-1 images were presented as well.
Annexin V-PI-FACS
The primary human colon cancer cells were seeded into six-well plate at 2 × 105cells per well. Forty-eight hours after the applied A1874 treatment, cells were harvested, washed, and incubated with Annexin V and PI (10 μg/mL each). Afterwards, cells were gated under a FACSCalibur machine (BD Biosciences). The percentage of Annexin V-positive cells was always recorded.
TUNEL staining
The viable colon cancer cells or colon epithelial cells were seeded into 12-well plates (8 × 104 cells per well). Forty-eight hours after the applied A1874 treatment, cells were incubated with both TUNEL and DAPI dyes. The nuclear TUNEL percentage (TUNEL vs. DAPI, %) of 1000 cells per treatment in five random views (under a fluorescence microscope at 1× 100 magnification) was recorded.
Trypan blue assay
Colon cancer cells were seeded into 12-well plates (8 × 104 cells per well). Seventy-two hours after the applied A1874 treatment, Trypan blue dye was added. Its ratio was recorded by using an automatic cell counter.
Western blotting
Colon cancer cells were seeded into six-well plate at 2 × 105cells per well. After the applied A1874 treatment, cells were incubated with the described lysis buffer25. Total protein lysates (30 μg per treatment in each lane) were analyzed. Western blotting protocols were described previously26. Data quantification was done through the ImageJ software (NIH).
Quantitative real-time PCR (qPCR)
Colon cancer cells were seeded into six-well plate at 2 × 105 cells per well. Following the applied treatment, TRIzol reagents were utilized to extract total RNA, and the latter was converted into complementary DNA (cDNA). qPCR was carried out using a SYBR Premix Ex Taq™ kit (TaKaRa, Tokyo, Japan) under the ABI Prism 7500 Fast Real-Time PCR system. GAPDH was always used as the reference gene and the internal control. Quantification was performed with the 2−ΔΔCt method. The RNA primer sequences employed in this study were from Dr. Zhu at Soochow University27.
Reactive oxygen species (ROS) assay
Colon cancer cells were seeded into six-well plate at 2 × 105 cells per well. After the applied A1874 treatment, cells were stained with CellROX dye (Beyotime, Wuxi, China) and thereafter tested via a fluorescence microscopy.
GSH/GSSG ratio
Reduced glutathione (GSH) is a vital ROS scavenger in human cells. Its ratio with the oxidized disulfide form glutathione (GSSG) was tested as a quantitative indicator of oxidative stress intensity28. Colon cancer cells were seeded into six-well plate at 2 × 105 cells per well. With the applied A1874 treatment, cells were lysed. The GSH/GSSG ratio was measured using a GSH/GSSG assay kit (Beyotime). GSH/GSSG ratio in human tissues was tested similarly.
Assaying DNA breaks
The viable colon cancer cells were seeded into 96-well plates at 5 × 103 cells per well. Following the applied A1874 treatment, a single strand DNA (ssDNA) ELISA kit (Roche, Shanghai, China) was utilized to test DNA breaks. The ssDNA ELISA absorbance was tested by 405 nm.
Exogenous BRD4 overexpression
The pSUPER-puro-GFP expression vector, containing the mutant BRD4 at the MDM2 binding sites, was provided by Dr. Zhao at Soochow University29. It was transfected to HEK-293 cells together with viral packaging proteins (VSVG and Hit-60) (provided by Dr. Zhao29) to generate BRD4-expressinglentivirus. Virus was then enriched, filtered and added to cultured colon cancer cells (in polybrene-containing complete medium), and stable cells selected by puromycin. Exogenous BRD4 overexpression was verified by Western blotting.
BRD4 knockout
A CRISPR/Cas9-BRD4-knockout (KO) plasmid (with puromycin selection gene, from Dr. Zhao at Soochow University29) was transfected into primary colon cancer cells via a Lipofectamine 2000 (Thermo-Fisher Invitrogen) protocol. Cells were distributed to 96-well plates to establish single cells and were subjected to BRD4-KO screening (qPCR). Stable cells were further selected by puromycin for 4–5 passages. BRD4 KO in the stable cells was always verified by Western blotting.
Tumor xenografts
The severe combined immuno-deficient (SCID) mice (5–6 week old, 18–19 g weight, all female) were purchased from the Animal Facility of Soochow University (Suzhou, China). The primary pCan1 colon cancer cells (8 × 106 cells per mouse) were subcutaneously (s.c.) injected to the flanks of SCID mice. Within three weeks the tumors reached the average volume of 100 mm3. Tumor-bearing mice were then randomly assigned into two groups (nine mice per group/n = 9). Mice were then treated with A1874 or the vehicle control. Tumor volumes were recorded using the described formula30. Estimated daily tumor growth (in mm3 per day) was calculated as described16. All animal studies were in accordance with regulations of the Institutional Animal Care and Use Committee and Ethics Committee of Nanjing Medical University (Nanjing, China).
Statistical analysis
The investigators were blinded to the group allocation during all experiments. In vitro experiments were repeated at least three times. Data were presented as mean ± standard deviation (SD). Statistics analyses were carried out through one-way ANOVA with the Scheffe’ and Tukey Test (SPSS 23.0, SPSS, Chicago, IL). To determine significance between two treatment groups, the unpaired t test was used (Excel 2007). Significance was determined as P < 0.05. All the protocols of this study were approved by Ethics Committee of Nanjing Medical University.
Results
A1874 inhibits colon cancer cell growth, proliferation, cell cycle progression, migration, and invasion
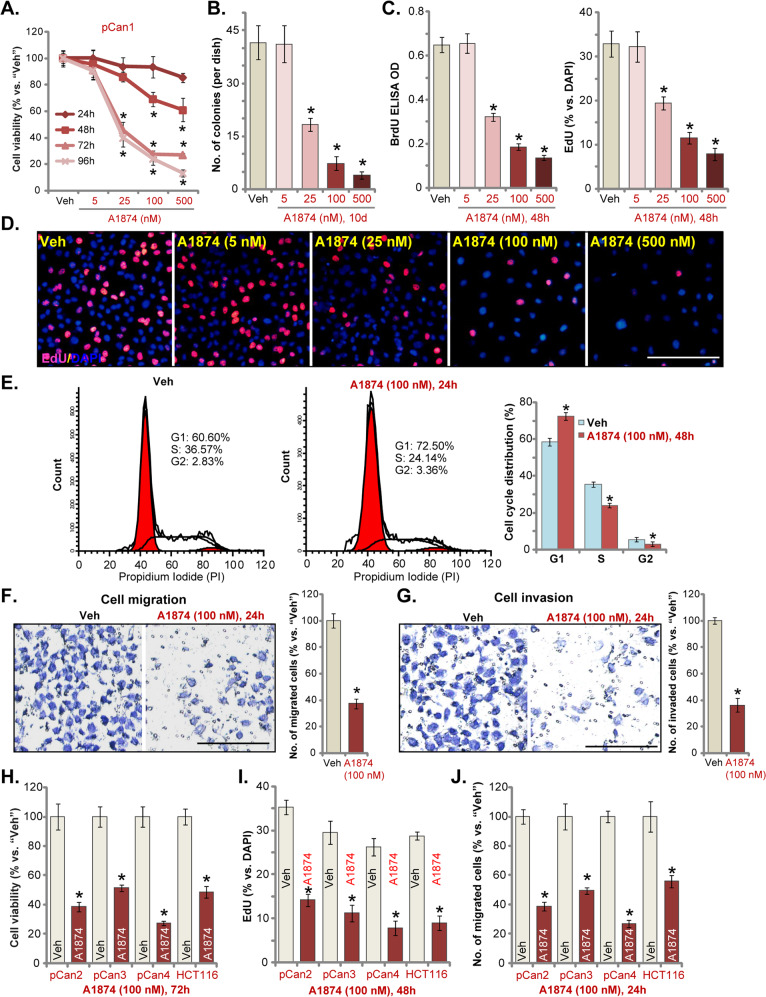
To examine the anti-proliferative activity of A1874, primary human colon cancer cells, pCan1, were cultured in FBS-containing complete medium and treated with increasing concentrations (5–500 nM) of A1874. A CCK-8 assay was carried out to test cell viability. As shown (Fig. 1a), A1874 decreased cell viability in pCan1 cells in a concentration-dependent manner. There was a significant reduction in viability following treatment with 25–500 nM of A1874 (Fig. 1a), whereas the lower concentration (5 nM) was ineffective (Fig. 1a). The BRD4-degrading PROTAC displayed a time-dependent response in inhibiting pCan1 cell viability (Fig. 1b). A1874 (25–500 nM) required at least 48 h to exert significant anti-survival activity (Fig. 1a). Furthermore, a colony formation assay (Fig. 1c) demonstrated that A1874 (25–500 nM) potently decreased the number of viable pCan1 cell colonies.
Fig. 1. A1874 inhibits colon cancer cell growth, proliferation, cell cycle progression, migration and invasion.
The primary human colon cancer cells, pCan1/2/3/4 (derived from different colon cancer patients) (a–j) or established HCT116 cells (h–j) were treated with applied concentration of A1874 (5–500 nM) or the vehicle control (“Veh,” 0.2% of DMSO). Cells were further cultured in complete medium for applied time periods, then cell viability (CCK-8 OD, a and h), colony formation (b) and cell proliferation (BrdU ELISA OD and nuclear EdU incorporation ratio, c, d and i) as well as cell cycle progression (PI-FACS, e), cell migration (f, j) and invasion (g) were tested by the listed assays. Data were presented as mean ± standard deviation (SD, n = 5). * P < 0.05 vs. “Veh” cells. Experiments in this figure were repeated three times, and similar results were obtained. Bar = 100 μm (d, f, g).
Additional studies showed that A1874 concentration-dependently suppressed BrdU incorporation in pCan1 cells (Fig. 1c). The percentage of cell nuclei with positive EdU staining was robustly decreased with A1874 (25–500 nM, 48 h) treatment (Fig. 1d). These results confirm the anti-proliferative activity of the BRD4-degrading PROTAC in pCan1 primary colon cancer cells. As the titration experimental results in Fig. 1a–d showed that 100 nM of A1874 efficiently inhibited pCan1 cell viability and proliferation, this concentration was selected for the additional studies.
By applying the PI-FACS assay, we found that A1874 (100 nM) resulted in cell cycle arrest by increasing the accumulation of pCan1 cells in G1-phase while reducing S- and G2/M-phase cells. Therefore, A1874-induced G1-S arrest (Fig. 1e). Examining the potential effect of A1874 on cancer cell migration, “Transwell” assay results, Fig. 1f, demonstrated that A1874 (100 nM) inhibited pCan1 cell migration in vitro. Furthermore, pCan1 cell invasion, tested by “Matrigel Transwell” assay, was suppressed by A1874 (Fig. 1g).
We also examined the potential activity of A1874 in other colon cancer cells. Primary colon cancer cells derived from three patients, pCan2/3/4, as well as established HCT116 cells, were tested. In these colon cancer cells, we found that A1874 (100 nM) potently inhibited cell viability, proliferation and migration, tested by CCK-8 (Fig. 1h), nuclear EdU incorporation (Fig. 1i) and “Transwell” (Fig. 1j) assays, respectively. Together, these studies demonstrate that A1874 potently inhibits colon cancer cell growth, proliferation, cell cycle progression, migration, and invasion.
A1874 induces apoptosis activation in colon cancer cells
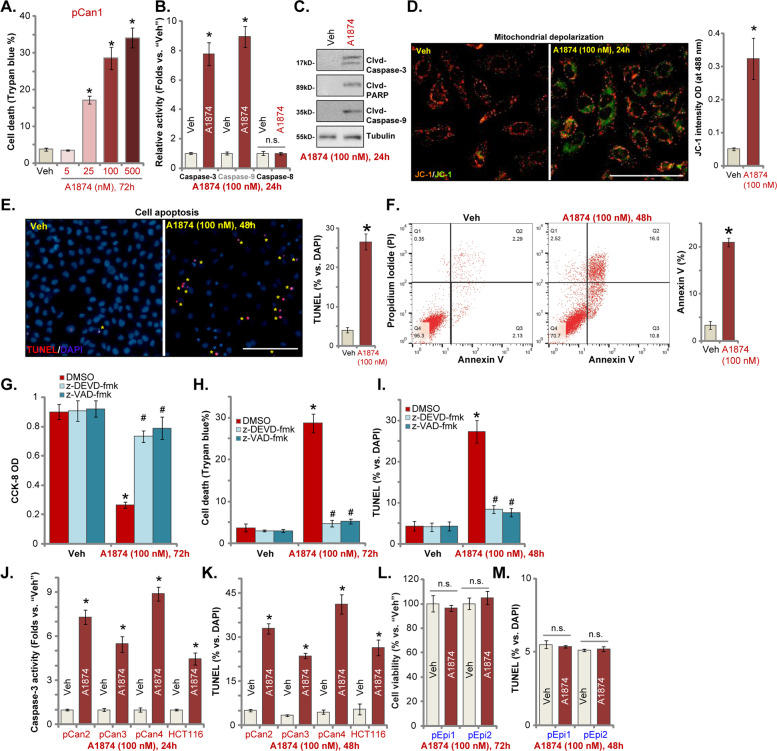
As proliferation inhibition and cell cycle arrest can induce cell apoptosis in colon cancer cells15,31,32, we tested whether A1874 can provoke cell death and apoptosis. Trypan blue staining assay results, Fig. 2a, confirmed that A1874 dose-dependently induced pCan1 colon cancer cell death (increased Trypan blue staining). A1874 (100 nM, 24 h) significantly increased caspase-3 and caspase-9 activity in pCan1 cells (Fig. 2b), leaving caspase-8 activity unaffected (Fig. 2b). Furthermore, cleavage of caspase-3, caspase-9, and poly ADP-ribose polymerase (PARP) was detected in A1874-treated pCan1 cells (Fig. 2c). A1874 also induced mitochondrial depolarization and caused JC-1 green monomers accumulation in the mitochondria of pCan1 cells (Fig. 2d). These results confirm the activation of mitochondrial apoptosis cascade33–36 following A1874 treatment (Fig. 2b–d). Further, A1874 (100 nM) induced apoptosis activation in pCan1 cells, causing increased ratios of TUNEL-positive nuclei (Fig. 2e) and Annexin V-positive cells (Fig. 2f).
Fig. 2. A1874 induces apoptosis activation in colon cancer cells.
The primary human colon cancer cells, pCan1/2/3/4 (derived from different patients) (a–f, j, k), HCT116 cells (j, k), or primary colon epithelial cells (“pEpi1/2”) (l, m) were treated with applied concentration of A1874 (5–500 nM) or the vehicle control (“Veh”, 0.2% of DMSO). Cells were further cultured in complete medium for applied time periods. Then cell death (Trypan blue-positive cell ratio, a), caspase activation (b, c, j), mitochondrial depolarization (JC-1 green monomers intensity, d), and cell apoptosis (nuclear TUNEL staining and Annexin V FACS assays, e, f, k, m) were tested, with cell viability in epithelial cells tested by CCK-8 assay (l). The pCan1 primary colon cancer cells were pretreated with 50 μM of the caspase-3 inhibitor z-DEVD-fmk or the pan caspase inhibitor z-VAD-fmk for 30 min, followed by A1874 (100 nM) stimulation and cultured for 72 h; Then cell viability, death and apoptosis were tested by CCK-8 (g), Trypan blue staining (h) and nuclear TUNEL staining (i) assays, respectively. TUNEL-positive nuclei were marked by the yellow stars (e). Data were presented as mean ± standard deviation (SD, n = 5). *P < 0.05 vs. “Veh” cells. #P < 0.05 vs. A1874 (100 nM) only treatment (g–i). “n.s.” stands for no statistic difference (l, m). Experiments in this figure were repeated three times, and similar results were obtained. Bar = 100 μm (d, e).
Significantly, the caspase-3 inhibitor z-DEVD-fmk and the pan caspase inhibitor z-VAD-fmk largely inhibited A1874 (100 nM)-induced decreased viability (CCK-8 OD) (Fig. 2g), cell death (Trypan blue staining assay, Fig. 2h), and apoptosis activation (nuclear TUNEL staining assay, Fig. 2i). Therefore, apoptosis activation appears to be the primary mechanism of A1874-induced cytotoxicity against pCan1 cells.
In other primary colon cancer cells (pCan2/3/4) and established HCT116 cells, A1874 (100 nM) treatment robustly increased caspase-3 activity (Fig. 2j) and TUNEL-positive nuclear ratio (Fig. 2k), indicating apoptosis activation. However, in the primary colon epithelial cells (“pEpi1/2,” from two donors18), A1874 (100 nM) treatment failed to decrease cell viability (Fig. 2l) and induce apoptosis activation (Fig. 2m), indicating a cancer cell-specific effect by the BRD4-degrading PROTAC.
A1874-induced anti-colon cancer cell activity is not solely dependent on BRD4 protein degradation
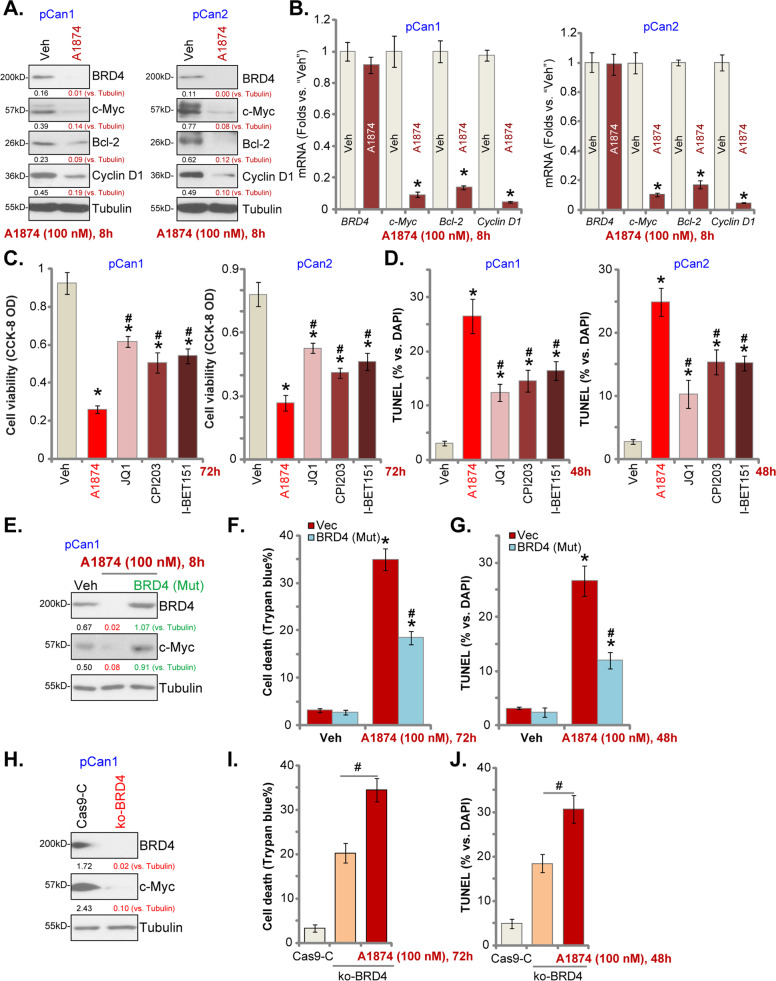
Because A1874 is a novel BRD4-degrading PROTAC14, we tested its effect on BRD4 signaling in colon cancer cells. In primary human colon cancer cells, pCan1 and pCan2, A1874 (100 nM) treatment led to robust degradation of BRD4 protein (Fig. 3a) without affecting BRD4 mRNA expression (Fig. 3b). Furthermore, mRNA and protein expression of BRD4-dependent genes, including c-Myc, Bcl-2, and cyclin D1, was significantly decreased in A1874-treated colon cancer cells (Fig. 3a, b). We then compared the anti-colon cancer cell activity of A1874 with other known BRD4-BET inhibitors, including JQ137,38, CPI20329,39, and I-BET72640–42. In pCan1 cells and pCan2 cells, A1874-induced viability (CCK-8 OD) reduction (Fig. 3c), and cell apoptosis (nuclear TUNEL staining assay, Fig. 3d) were significantly more potent than JQ1, CPI203, and I-BET726. These known inhibitors were utilized at even higher concentrations than A1874. These results suggest that A1874-induced anti-colon cancer cell activity might not be solely dependent on BRD4 protein degradation.
Fig. 3. A1874-induced anti-colon cancer cell activity is not solely dependent on BRD4 protein degradation.
The primary human colon cancer cells, pCan1 and pCan2, were treated with A1874 (100 nM) or the vehicle control (“Veh”, 0.2% of DMSO).Cells were further cultured in complete medium for applied time periods, and then expression of listed proteins (a) and mRNAs (b) were shown. The primary human colon cancer cells, pCan1 and pCan2, were treated with A1874 (100 nM), JQ1 (500 nM), I-BET726 (200 nM), CPI203 (500 nM) or the vehicle control (“Veh,” 0.2% of DMSO) and further cultured in complete medium for applied time periods, and then cell viability and apoptosis were tested by CCK-8 (c) and nuclear TUNEL staining (d) assays, respectively. Stable pCan1 cells with the mutant BRD4 expression construct [“BRD4 (Mut)”] or the empty vector (“Vec”) were treated with or without A1874 (100 nM). Cells were then further cultured in complete medium for applied time periods, and expression of listed proteins was shown (e); Cell death and apoptosis were tested by Trypan blue staining (f) and nuclear TUNEL staining (g) assays, respectively. The stable pCan1 cells with CRISPR/Cas9-BRD4-KO-GFP construct (“ko-BRD4” cells) were treated with or without A1874 (100 nM). The control cells with CRISPR/Cas9 empty vector (“Cas9-C”) were left untreated; Cells were further cultured in complete medium for applied time periods, and then expression of listed proteins was shown (h); Cell death and apoptosis were tested by Trypan blue staining (i) and nuclear TUNEL staining (j) assays, respectively. Expression of listed proteins was quantified and normalized to the loading control (a, e, h). Data were presented as mean ± standard deviation (SD, n = 5). *P < 0.05 vs. “Veh” cells. #P < 0.05 vs. A1874 treatment (c, d). #P < 0.05 vs. “Vec” cells (f, g). #P < 0.05 (I and J). Experiments in this figure were repeated three times, and similar results were obtained.
To explore whether A1874 acts solely to promote BRD4 protein degradation, a mutant BRD4 expression construct [“BRD4 (Mut)”], with a mutation at the MDM2’s binding site14, was stably transduced into pCan1 cells. Western blotting assay results, Fig. 3e, demonstrated that BRD4 and c-Myc protein expression was restored by the BRD4 (Mut) construct even after A1874 treatment. However, the BRD4 (Mut) only partially inhibited A1874 (100 nM)-induced cell death (Trypan blue ratio increase, Fig. 3f) and apoptosis (nuclear TUNEL staining assay, Fig. 3g). These studies support the existence of BRD4-independent mechanisms responsible for A1874-induced cytotoxicity in colon cancer cells.
To further support our hypothesis, we found that CRISPR/Cas9-induced BRD4 KO resulted in c-Myc downregulation (Fig. 3h), causing pCan1 cell death (Fig. 3i) and apoptosis (Fig. 3j). In BRD4-KO cells, A1874 (100 nM) was able to induce further cell death (Fig. 3i) and apoptosis (Fig. 3j). Thus, in addition to BRD4 protein degradation, parallel cell death mechanisms are also responsible for A1874-induced anti-colon cancer cell activity.
A1874 induces p53 protein stabilization and oxidative injury in colon cancer cells
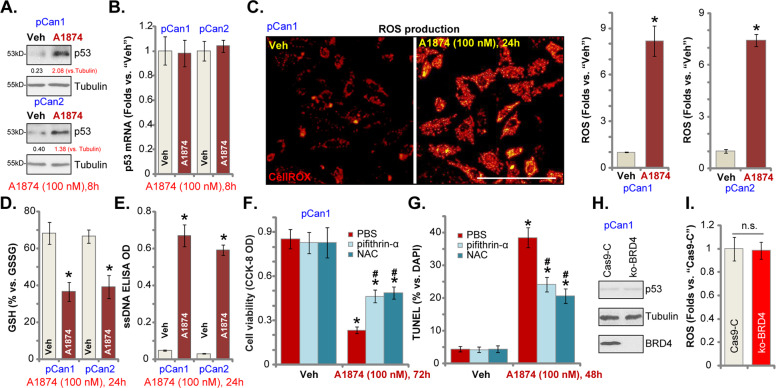
Possible A1874-induced parallel cell death mechanisms include p53 protein stabilization and oxidative injury. A1874 is a novel BRD4-targeting MDM2-based PROTAC, reported to stabilize p53 protein14. In the primary colon cancer cells, pCan1 and pCan2, treatment with A1874 (100 nM, 8 h) induced robust p53 protein elevation (Fig. 4a), whereas expression of p53 mRNA was unchanged (Fig. 4b). Interestingly, A1874-induced significant oxidative injury in colon cancer cells, increasing CellROX fluorescence intensity43 in pCan1 and pCan2 cells (Fig. 4c). A1874-induced oxidative stress in colon cancer cells was also indicated by the GSH/GSSG ratio reduction (Fig. 4d) and ssDNA accumulation (Fig. 4e, DNA breaks).
Fig. 4. A1874 induces p53 protein stabilization and oxidative injury in colon cancer cells.
The primary human colon cancer cells, pCan1 and pCan2, were treated with A1874 (100 nM) or the vehicle control (“Veh”, 0.2% of DMSO). Cells were further cultured in complete medium for applied time periods, and then expressions of p53 protein (a) and mRNA (b) were shown; The CellROX intensity (c), the GSH/GSSG ratio (d) and the single strand DNA (ssDNA) contents (e) were tested as well. The pCan1 cells were pretreated for 1 h with the antioxidant N-acetyl-cysteine (NAC, 400 μM) or the p53 inhibitor pifithrin-α (10 μM), followed by A1874 (100 nM) stimulation for another 48–72 h.Then cell viability was tested by CCK-8 assay (f), with cell apoptosis examined by nuclear TUNEL staining assay (g). Stable pCan1 cells with CRISPR/Cas9-BRD4-KO-GFP construct (“ko-BRD4” cells) or control cells with CRISPR/Cas9 empty vector (“Cas9-C”) were cultured for 24 h, and then expression of listed proteins (h) and ROS contents (CellROX intensity, i) were tested. Expression of listed proteins was quantified and normalized to the loading control (a). Data were presented as mean ± standard deviation (SD, n = 5). *P < 0.05 vs. “Veh” cells. #P < 0.05 vs. A1874 treatment (f, g). Experiments in this figure were repeated three times, and similar results were obtained. Bar = 100 μm (c). “n.s.” stands for no statistic difference (i).
To study whether p53 protein stabilization and oxidative injury participate in A1874-induced anti-colon cancer cell activity, the antioxidant NAC and the p53 inhibitor pifithrin-α44,45 were applied. As shown, A1874 (100 nM)-induced viability (CCK-8 OD) reduction was inhibited by NAC and pifithrin-α in pCan1 cells (Fig. 4f). Furthermore, NAC and pifithrin-α mitigated A1874-induced pCan1 cell apoptosis (nuclear TUNEL staining assay, Fig. 4g). Significantly, CRISPR/Cas9-induced BRD4 KO (see Fig. 3) failed to promote p53 protein upregulation (Fig. 4h) and ROS production (CellROX intensity, Fig. 4i) in pCan1 cells. These results demonstrate that p53 stabilization and oxidative injury act independently of BRD4 protein degradation to participate in A1874-induced anti-colon cancer cell activity.
A1874 oral administration inhibits colon cancer xenograft growth in SCID mice
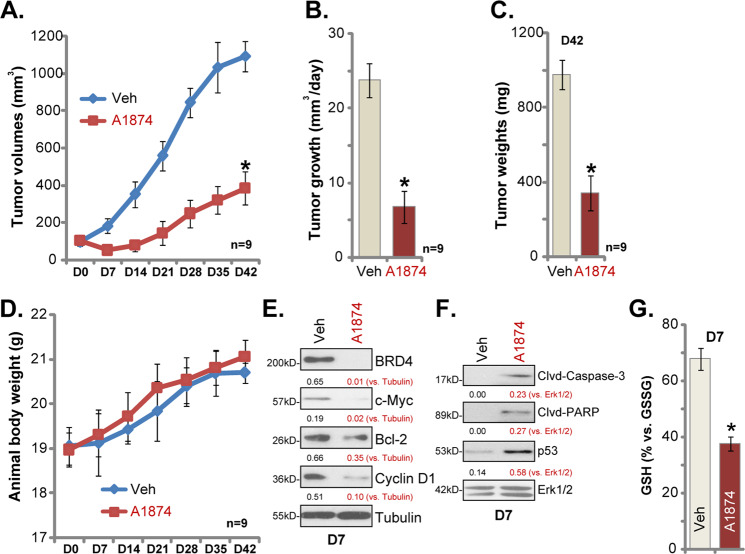
In order to study the potential anticancer activity of A1874 in vivo, pCan1 colon cancer cells were s.c. injected into the flanks of SCID mice. Within three weeks, colon cancer xenografts were established with tumor volumes close to 100 mm3 (Day-0/D0). By recording tumor growth curve, we demonstrated that A1874 oral administration (20 mg/kg, daily, 21 days) potently inhibited colon cancer xenograft growth in SCID mice (Fig. 5a). Calculating the estimated daily tumor growth, using the formula: (Tumor volume at D42—Tumor volume at D0)/42 (days), we found that colon cancer xenograft growth was largely inhibited in A1874-treated mice (Fig. 5b). Tumors from the two groups were isolated and weighted individually at experimental Day-42 (D42). Xenograft tumors with A1874 administration were significantly lighter than those of vehicle control mice (Fig. 5c). Mouse body weights were not significantly different between A1874-treated and vehicle control mice (Fig. 5d), and no noticeable toxicity was observed in the mice. These results show that oral administration of A1874 is able to inhibit colon cancer xenograft growth in SCID mice.
Fig. 5. A1874 oral administration inhibits colon cancer xenograft growth in SCID mice.
The SCID mice bearing pCan1 colon cancer xenografts were treated with A1874 (20 mg/kg body weight, oral administration, daily for 21 days) or the vehicle control (“Veh”); Tumor volumes (a) and mice body weights (d) were recorded weekly. The estimated daily tumor growth was calculated by using the described formula (b). At Day-42/D42, tumors of the two groups were isolated and weighted (c). At treatment Day7 (D7), one tumor of each group was isolated, and tumors were homogenized. Expression of the listed proteins in tumor tissue lysates was tested (e, f). The GSH/GSSG ratio in tumor tissue lysates was examined (g). Expression of listed proteins was quantified and normalized to the loading control (e, f). Data were presented as mean ± standard deviation (SD). *P < 0.05 vs. “Veh” group.
At treatment D7, one tumor from each group was isolated, and tumors were homogenized. Western blotting analyses showed that protein expression of BRD4, c-Myc, Bcl-2 and cyclin D1, were significantly decreased in A1874-treated tumor tissues (Fig. 5e), where caspase-3 and PARP cleavage was detected (Fig. 5f). Furthermore, p53 protein elevation was observed in xenograft tissues with A1874 administration (Fig. 5f). Additionally, the GSH/GSSG ratio was decreased in A1874-treated xenograft tissue lysates, indicating oxidative stress (Fig. 5g). Therefore, in line with in vitro findings, A1874 was able to induce BRD4 protein degradation, apoptosis activation, p53 elevation and oxidative stress in colon cancer xenografts.
Discussion
BRD4, a gene that is overexpressed in different human cancers, is associated with carcinogenesis, tumorigenesis, and progression of human malignancies. It is emerging as a promising therapeutic target8,46,47. The development and optimization of BRD4 small-molecule inhibitors as novel cancer therapeutics are currently a major focus of cancer research8,46,47. BRD4 binds directly to the acetylated histones to promote transcription and expression of multiple oncogenic genes, including c-Myc and several others8,46,47. BRD4 also acts as an associated factor of P-TEFb, stimulating RNA polymerase II-dependent transcription and cell cycle progression10,47,48.
BRD4 overexpression is detected in colorectal cancer (CRC)12. Hu et al., showed that MS417, a BRD4 blocker, potently inhibited CRC growth, epithelial-to-mesenchymal transition progression and metastasis12. Togel et al. demonstrated that BRD4 blockage downregulated c-Myc and inhibited CRC cell proliferation, which could be further augmented by targeting WNT or MAPK signaling49.
To date more than ten BET inhibitors have advanced to early stage clinical trials for patients with different types of cancer11,13. However, BRD4 inhibitors were found to only exert limited antitumor activity in patients11,13. One possibility is that BRD4 inhibition induces feedback upregulation of the BRD4 protein, leading to modest anti-proliferative ability and minor apoptotic induction8,11,13. Therefore, a new therapeutic approach is urgently needed to target BRD4 and other BET proteins8,11,13.
The BRD4 PROTACs have two covalently linked protein-binding domains: one capable of engaging an E3 ubiquitin ligase, and the other binding to BRD4 protein for ubiquitination-mediated degradation50. These compounds differ significantly from small-molecular BRD4 inhibitors in their cellular potency, phenotypic effects, pharmacokinetic kinetics and potential toxicity profiles50. A1874 is a first-in-class BRD4-targeting MDM2-based PROTAC14. Studies have shown that it results in robust and sustained BRD4 degradation14. Furthermore, A1874 increases p53 stabilization and protein levels in a dose-dependent manner14.
Here, in primary colon cancer cells and established HCT116 cells, A1874 potently inhibited cell viability, growth, proliferation and cell cycle progression, as well as cell migration and invasion. Furthermore, the BRD4-degrading PROTAC induced significant apoptosis activation in primary and established colon cancer cells. At the molecular level, A1874 is able to induce BRD4 protein degradation and the downregulation of BRD-dependent genes (c-Myc, Bcl-2 and cyclin D1) in colon cancer cells.
Although A1874-induced robust and potent BRD4 protein degradation, A1874-induced anti-colon cancer cell activity was not solely dependent on BRD4 degradation. First, A1874 was significantly more potent than other known BRD4/BET inhibitors (JQ1, CPI203, I-BET151) at inducing colon cancer cell apoptosis. Second, restoring BRD4 expression by the BRD4 (Mut) construct only partially inhibited A1874-induced anti-colon cancer cell activity. Third, the novel MDM2-recruiting PROTAC remained cytotoxic in the BRD4-KO colon cancer cells. A1874-induced p53 protein stabilization and oxidative stress in colon cancer cells, two actions that are independent of BRD4 depletion. Conversely, the antioxidant NAC and the p53 inhibitor pifithrin-α attenuated A1874-induced colon cancer cell apoptosis. Therefore A1874 acts via both BRD4-dependent and BRD4-independent (p53 stabilization and ROS production) mechanisms, providing an explanation for its superior anticancer activity against colon cancer cells.
Colon cancer and other CRC are among the third most common type of cancer, accounting for around 10% of all malignancies3,51. In 2018, there are 1.09 million new cases and 551,000 CRC deaths (mainly colon cancer). The 5-year survival rate of CRC in the United States is close to 65%3,51. Molecularly targeted therapies are the current focus of research for colon cancer52,53. Here we report that oral administration of a single dose of A1874 potently inhibits colon cancer xenograft growth in SCID mice. These results demonstrate that this novel compound is a promising therapeutic to treat colon cancer.
Acknowledgements
The present study was supported by the grant for Key Young Talents of Medicine in Jiangsu (QNRC2016250), Gusu medical talent project (GSWS2019026 and GSWS2019025), and Foundation of tumor clinical and basic research team of Affiliated Kunshan Hospital of Jiangsu University (KYC005).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by S. Tait
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: An-sheng Qin, Hua Jin, Yu Song and Yun Gao
Contributor Information
Li-na Zhou, Email: zhoulinaks@163.com.
Shu-sheng Wang, Email: drwangsszjg@163.com.
Xing-sheng Lu, Email: drluxsslyy@163.com.
References
- 1.Palma S, Zwenger AO, Croce MV, Abba MC, Lacunza E. From molecular biology to clinical trials: toward personalized colorectal cancer therapy. Clin. Colorectal Cancer. 2016;15:104–115. doi: 10.1016/j.clcc.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Hubbard JM, Grothey A. Colorectal cancer in 2014: progress in defining first-line and maintenance therapies. Nat. Rev. Clin. Oncol. 2015;12:73–74. doi: 10.1038/nrclinonc.2014.233. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker PH. Colorectal cancer: prevention and management of metastatic disease. Biomed. Res. Int. 2014;2014:782890. doi: 10.1155/2014/782890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmoll HJ, Stein A. Colorectal cancer in 2013: towards improved drugs, combinations and patient selection. Nat. Rev. Clin. Oncol. 2014;11:79–80. doi: 10.1038/nrclinonc.2013.254. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, et al. Inhibition of BRD4 suppresses cell proliferation and induces apoptosis in renal cell carcinoma. Cell Physiol. Biochem. 2017;41:1947–1956. doi: 10.1159/000472407. [DOI] [PubMed] [Google Scholar]
- 8.White ME, Fenger JM, Carson WE., III Emerging roles of and therapeutic strategies targeting BRD4 in cancer. Cell Immunol. 2019;337:48–53. doi: 10.1016/j.cellimm.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devaiah BN, Singer DS. Two faces of brd4: mitotic bookmark and transcriptional lynchpin. Transcription. 2013;4:13–17. doi: 10.4161/trns.22542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem. 2007;282:13141–13145. doi: 10.1074/jbc.R700001200. [DOI] [PubMed] [Google Scholar]
- 11.Hajmirza, A. et al. BET family protein BRD4: an emerging actor in NFkappaB signaling in inflammation and cancer. Biomedicines. 6 (2018). [DOI] [PMC free article] [PubMed]
- 12.Hu Y, et al. BRD4 inhibitor inhibits colorectal cancer growth and metastasis. Int J. Mol. Sci. 2015;16:1928–1948. doi: 10.3390/ijms16011928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu LL, et al. Inhibition of BET bromodomains as a therapeutic strategy for cancer drug discovery. Oncotarget. 2015;6:5501–5516. doi: 10.18632/oncotarget.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hines J, Lartigue S, Dong H, Qian Y, Crews CM. MDM2-recruiting PROTAC offers superior, synergistic antiproliferative activity via simultaneous degradation of BRD4 and stabilization of p53. Cancer Res. 2019;79:251–262. doi: 10.1158/0008-5472.CAN-18-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen MB, et al. Activation of AMP-activated protein kinase (AMPK) mediates plumbagin-induced apoptosis and growth inhibition in cultured human colon cancer cells. Cell Signal. 2013;25:1993–2002. doi: 10.1016/j.cellsig.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 16.Lu PH, et al. Aqueous Oldenlandia diffusa extracts inhibits colorectal cancer cells via activating AMP-activated protein kinase signalings. Oncotarget. 2016;7:45889–45900. doi: 10.18632/oncotarget.9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, et al. The preclinical evaluation of the dual mTORC1/2 inhibitor INK-128 as a potential anti-colorectal cancer agent. Cancer Biol. Ther. 2015;16:34–42. doi: 10.4161/15384047.2014.972274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li G, et al. Ninjurin 2 overexpression promotes human colorectal cancer cell growth in vitro and in vivo. Aging. 2019;11:8526–8541. doi: 10.18632/aging.102336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SS, et al. Triptonide inhibits human nasopharyngeal carcinoma cell growth via disrupting Lnc-RNA THOR-IGF2BP1 signaling. Cancer Lett. 2019;443:13–24. doi: 10.1016/j.canlet.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, et al. C6 ceramide dramatically enhances docetaxel-induced growth inhibition and apoptosis in cultured breast cancer cells: a mechanism study. Exp. Cell Res. 2015;332:47–59. doi: 10.1016/j.yexcr.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Zhou LN, Li P, Cai S, Li G, Liu F. Ninjurin2 overexpression promotes glioma cell growth. Aging. 2019;11:11136–11147. doi: 10.18632/aging.102515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv Y, et al. Overexpression of lymphocyte antigen 6 complex, locus E in gastric cancer promotes cancer cell growth and metastasis. Cell Physiol. Biochem. 2018;45:1219–1229. doi: 10.1159/000487453. [DOI] [PubMed] [Google Scholar]
- 23.Zheng B, et al. Pre-clinical evaluation of AZD-2014, a novel mTORC1/2 dual inhibitor, against renal cell carcinoma. Cancer Lett. 2015;357:468–475. doi: 10.1016/j.canlet.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Brooks MM, Neelam S, Fudala R, Gryczynski I, Cammarata PR. Lenticular mitoprotection. Part A: monitoring mitochondrial depolarization with JC-1 and artifactual fluorescence by the glycogen synthase kinase-3beta inhibitor, SB216763. Mol. Vis. 2013;19:1406–1412. [PMC free article] [PubMed] [Google Scholar]
- 25.Cao C, et al. Impairment of TrkB-PSD-95 signaling in Angelman syndrome. PLoS Biol. 2013;11:e1001478. doi: 10.1371/journal.pbio.1001478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye X, Xie J, Huang H, Deng Z. Knockdown of MAGEA6 activates AMP-activated protein kinase (AMPK) signaling to inhibit human renal cell carcinoma cells. Cell Physiol. Biochem. 2018;45:1205–1218. doi: 10.1159/000487452. [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, et al. MicroRNA-4651 targets bromodomain-containing protein 4 to inhibit non-small cell lung cancer cell progression. Cancer Lett. 2020;476:129–139. doi: 10.1016/j.canlet.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Zitka O, et al. Redox status expressed as GSH:GSSG ratio as a marker for oxidative stress in paediatric tumour patients. Oncol. Lett. 2012;4:1247–1253. doi: 10.3892/ol.2012.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang T, et al. Bromodomain protein BRD4 promotes cell proliferation in skin squamous cell carcinoma. Cell Signal. 2018;42:106–113. doi: 10.1016/j.cellsig.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Chen MB, et al. Itraconazole-induced inhibition on human esophageal cancer cell growth requires AMPK activation. Mol. Cancer Ther. 2018;17:1229–1239. doi: 10.1158/1535-7163.MCT-17-1094. [DOI] [PubMed] [Google Scholar]
- 31.Su C, et al. Targeting p38gamma to inhibit human colorectal cancer cell progression. Biochem. Biophys. Res. Commun. 2019;517:172–179. doi: 10.1016/j.bbrc.2019.07.038. [DOI] [PubMed] [Google Scholar]
- 32.Xu W, Xu B, Yao Y, Yu X, Shen J. The novel HDAC inhibitor AR-42-induced anti-colon cancer cell activity is associated with ceramide production. Biochem. Biophys. Res. Commun. 2015;463:545–550. doi: 10.1016/j.bbrc.2015.05.078. [DOI] [PubMed] [Google Scholar]
- 33.Fu M, Wan F, Li Z, Zhang F. 4SC-202 activates ASK1-dependent mitochondrial apoptosis pathway to inhibit hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2016;471:267–273. doi: 10.1016/j.bbrc.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 34.Fu M, Shi W, Li Z, Liu H. Activation of mPTP-dependent mitochondrial apoptosis pathway by a novel pan HDAC inhibitor resminostat in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2016;477:527–533. doi: 10.1016/j.bbrc.2016.04.147. [DOI] [PubMed] [Google Scholar]
- 35.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- 36.Chen M, Wang J. Initiator caspases in apoptosis signaling pathways. Apoptosis. 2002;7:313–319. doi: 10.1023/A:1016167228059. [DOI] [PubMed] [Google Scholar]
- 37.Lee DH, et al. Synergistic effect of JQ1 and rapamycin for treatment of human osteosarcoma. Int. J. Cancer. 2015;136:2055–2064. doi: 10.1002/ijc.29269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korb E, Herre M, Zucker-Scharff I, Darnell RB, Allis CD. BET protein Brd4 activates transcription in neurons and BET inhibitor Jq1 blocks memory in mice. Nat. Neurosci. 2015;18:1464–1473. doi: 10.1038/nn.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong C, et al. The bromodomain and extra-terminal inhibitor CPI203 enhances the antiproliferative effects of rapamycin on human neuroendocrine tumors. Cell Death Dis. 2014;5:e1450. doi: 10.1038/cddis.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, et al. I-BET726 suppresses human skin squamous cell carcinoma cell growth in vitro and in vivo. Cell Death Dis. 2020;11:318. doi: 10.1038/s41419-020-2515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gosmini R, et al. The discovery of I-BET726 (GSK1324726A), a potent tetrahydroquinoline ApoA1 up-regulator and selective BET bromodomain inhibitor. J. Med. Chem. 2014;57:8111–8131. doi: 10.1021/jm5010539. [DOI] [PubMed] [Google Scholar]
- 42.Wyce A, et al. BET inhibition silences expression of MYCN and BCL2 and induces cytotoxicity in neuroblastoma tumor models. PLoS ONE. 2013;8:e72967. doi: 10.1371/journal.pone.0072967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Celeghini, E. C. C., et al. Efficiency of CellROX deep red((R)) and CellROX orange((R)) fluorescent probes in identifying reactive oxygen species in sperm samples from high and low fertility bulls. Anim. Biotechnol. 1–7 (2019). [DOI] [PubMed]
- 44.Bu HQ, et al. Oridonin induces apoptosis in SW1990 pancreatic cancer cells via p53- and caspase-dependent induction of p38 MAPK. Oncol. Rep. 2014;31:975–982. doi: 10.3892/or.2013.2888. [DOI] [PubMed] [Google Scholar]
- 45.Komarov PG, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 46.Iftner T, Haedicke-Jarboui J, Wu SY, Chiang CM. Involvement of Brd4 in different steps of the papillomavirus life cycle. Virus Res. 2017;231:76–82. doi: 10.1016/j.virusres.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen R, Yik JH, Lew QJ, Chao SH. Brd4 and HEXIM1: multiple roles in P-TEFb regulation and cancer. Biomed. Res. Int. 2014;2014:232870. doi: 10.1155/2014/232870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jang MK, et al. The bromodomain protein Brd4 is a positive regulatory component of P-TEFb and stimulates RNA polymerase II-dependent transcription. Mol. Cell. 2005;19:523–534. doi: 10.1016/j.molcel.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Togel L, et al. Dual targeting of bromodomain and extraterminal domain proteins, and WNT or MAPK signaling, inhibits c-MYC expression and proliferation of colorectal cancer cells. Mol. Cancer Ther. 2016;15:1217–1226. doi: 10.1158/1535-7163.MCT-15-0724. [DOI] [PubMed] [Google Scholar]
- 50.Yang CY, Qin C, Bai L, Wang S. Small-molecule PROTAC degraders of the bromodomain and extra terminal (BET) proteins—a review. Drug Discov. Today Technol. 2019;31:43–51. doi: 10.1016/j.ddtec.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 52.Chu E. An update on the current and emerging targeted agents in metastatic colorectal cancer. Clin. Colorectal Cancer. 2012;11:1–13. doi: 10.1016/j.clcc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Lea MA. Recently identified and potential targets for colon cancer treatment. Future Oncol. 2010;6:993–1002. doi: 10.2217/fon.10.53. [DOI] [PubMed] [Google Scholar]