Abstract
Ongoing neuroinflammation may contribute to symptoms of autism spectrum disorder (ASD) in at least a portion of affected individuals. Mesenchymal stromal cells (MSCs) have demonstrated the capacity to modulate neuroinflammation, but safety and feasibility of MSC administration in children with ASD have not been well established. In this open‐label, phase I study, 12 children with ASD between 4 and 9 years of age were treated with intravenous (IV) infusions of human cord tissue mesenchymal stromal cells (hCT‐MSCs), a third‐party MSC product manufactured from unrelated donor umbilical cord tissue. Children received one, two, or three doses of 2 × 106 cells per kilogram at 2‐month intervals. Clinical and laboratory evaluations were performed in person at baseline and 6 months and remotely at 12 months after the final infusion. Aside from agitation during the IV placement and infusion in some participants, hCT‐MSCs were well tolerated. Five participants developed new class I anti‐human leukocyte antigen (HLA) antibodies, associated with a specific lot of hCT‐MSCs or with a partial HLA match between donor and recipient. These antibodies were clinically silent and not associated with any clinical manifestations to date. Six of 12 participants demonstrated improvement in at least two ASD‐specific measures. Manufacturing and administration of hCT‐MSCs appear to be safe and feasible in young children with ASD. Efficacy will be evaluated in a subsequent phase II randomized, placebo‐controlled clinical trial.
Keywords: autism, cellular therapy, clinical trials, cord tissue, mesenchymal stromal cells
In this phase I study, intravenous infusion of human cord tissue mesenchymal stromal cell was safe and feasible in young children with autism. Autism symptoms improved in half of children, and 40% developed clinically silent but detectable anti‐human leukocyte antigen antibodies post‐infusion.
Lessons learned.
Unrelated donor human umbilical cord tissue‐derived mesenchymal stromal cells can be successfully manufactured under GMP conditions for use in clinical trials.
Unrelated donor human umbilical cord tissue‐derived mesenchymal stromal cells can be successfully manufactured under GMP conditions for use in clinical trials.
Rigorously conducted placebo‐controlled clinical trials are necessary to assess the efficacy of cord tissue mesenchymal stromal cell therapies for the treatment of autism spectrum disorder.
Significance statement.
In this phase I study, intravenous infusion of mesenchymal stromal cells derived from donated human umbilical cord tissue (hCT‐MSCs) was safe and feasible in young children with autism spectrum disorder. Half of treated children showed improvements in autism symptoms, although it is uncertain whether these improvements are related to the treatment. The ability of hCT‐MSC to improve core symptoms of autism is being studied in a subsequent randomized, placebo‐controlled clinical trial.
1. INTRODUCTION
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental disorder that presents in early childhood with symptoms including repetitive behaviors, restricted range of activities, and impairments in social communication. 1 Treatment of ASD includes intensive behavioral, occupational, and speech therapies as well as educational support and psychotropic medications. Notably, available U.S. Food and Drug Administration (FDA)‐approved medicines for ASD do not address core symptoms. Moreover, despite available therapies, ASD often causes significant functional impairments that require lifelong support, dependence on caretakers, and accommodations.
The etiology of ASD involves a complex interaction between multiple genetic and environmental risk factors. 2 Given the close interplay between the nervous and immune systems, particularly in early development, a causative role of neuroinflammation, microglial activation, and/or immune dysregulation has been hypothesized in a significant portion of individuals with ASD. 3 , 4 , 5 As such, immunomodulatory therapies are currently being investigated in the treatment of selected populations of children with ASD.
Mesenchymal stromal cells (MSCs) are a heterogeneous group of undifferentiated, multipotent cells that can be isolated from several different tissues in the body. They do not express MHC class II or other costimulatory molecules, resulting in low immunogenicity and eliminating the need to HLA‐match donors and recipients. MSCs do not engraft in the recipient but rather exert their effects via paracrine and trophic signaling. They can modify a wide range of cellular activities including angiogenesis, (anti)apoptosis, chemoattraction, growth and differentiation of progenitor cells, and immune functions 6 and, as such, are under investigation for the treatment of a variety of conditions. MSCs have also been shown to modulate neuroinflammation, provide neuroprotection, and enhance synaptic function, making them attractive candidates for therapy in certain neurologic conditions including ASD. 7 , 8
Human cord tissue MSC (hCT‐MSC) is a third‐party, allogeneic, human MSC product manufactured from donor umbilical cord tissue that is digested and expanded in culture, harvested, cryopreserved, and stored in the vapor phase of liquid nitrogen until use. In in vitro preclinical models, hCT‐MSCs suppress T‐cell responses and decrease microglial activation. We hypothesized that hCT‐MSCs might improve core symptoms of ASD in affected children through these mechanisms and performed a phase I study testing intravenous infusions of one, two, or three doses of hCT‐MSCs in children with ASD.
2. MATERIALS AND METHODS
2.1. Study design
This study was designed as a phase I, open‐label, dose escalation trial of one, two, or three intravenous infusions of hCT‐MSCs in young children with ASD (figure 1). The study was approved by the Duke University Institutional Review Board (Pro00079421), conducted under IND 17313 from the FDA, and registered at Clinicaltrials.gov (NCT03099239).
2.2. Participant eligibility
Participants ages 2‐11 years with a confirmed diagnosis of ASD per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria, informed by the Autism Diagnostic Observation Schedule, Second Edition (ADOS‐2), were eligible. Children were ineligible if they had a coexisting psychiatric condition (except attention deficit hyperactivity disorder), a genetic syndrome, or a history of autoimmune disease or immunosuppressive therapy, had an available qualified autologous cord blood unit, or had previously received any type of cellular therapy. They also had to have whole‐exome sequencing or chromosome microarray that did not indicate a genetic cause of ASD as well as normal Fragile X testing, organ function, and immune screen via medical history and absolute lymphocyte count on a complete blood count with differential prior to study entry.
2.3. Human cord tissue mesenchymal stromal cell
hCT‐MSC is a product of allogeneic cells manufactured from umbilical cord tissue donated to the Carolinas Cord Blood Bank, an FDA‐licensed, Foundation for the Accreditation of Cellular Therapy‐accredited, public cord blood bank at Duke University Medical Center. Maternal donors provided written informed consent for donation and completed donor screening questionnaires, and maternal blood was tested for communicable diseases by a Clinical Laboratory Improvement Amendments‐certified donor screening laboratory per FDA regulations (21 CFR 1271.75, 1271.80, and 1271.85). After delivery of the placenta and cord and aseptic drainage of the cord blood, a minimum of 20 cm of contiguous cord tissue was sterilely harvested from the placentas of male babies delivered by elective cesarean section, placed in a sterile bottle containing Plasmalyte‐A (Hospira, Lake Forest, Illinois), and transported at room temperature to the Good Manufacturing Practice (GMP) laboratory.
In the clean room manufacturing suite of the Robertson GMP Laboratory, in a biologic safety cabinet, the cord tissue was placed in sterile dishes, cut into small pieces, and then minced and digested in the Miltenyi Biotec GentleMacs Octo Dissociator (Bergisch Gladbach, Germany) with four GMP‐grade enzymes (α‐hyaluronidase, DNAase, collagenase, papain [source proprietary]). The resultant cell suspension was placed in culture in Prime XV MSC Expansion XSFM (Irvine Scientific, Santa Ana, California) media with 1% platelet lysate (Compass Biomedical, Hopkinton, Massachusetts) and grown to confluence (~7‐14 days) to establish the P0 culture. To establish the master cell bank, P0 was harvested and cryopreserved in cryovials with Cryostor 10 media (BioLife, Bothell, Washington) and stored in the vapor phase of liquid nitrogen. P1 and P2 cultures were grown under similar conditions, in hyperflasks without platelet lysate, as needed to create the working cell bank and product for administration, respectively. The final product was derived from the P2 cultures, which were harvested with 10× TripLE Select (Life Technologies Corporation, Grand Island, New York) into plasmalyte‐A with 5% human serum albumin, washed, and cryopreserved in 5 compartment cryobags (Syngen, Sacramento, California) in 5 mL containing 50‐100 million cells in a final concentration of 10% dimethyl sulfoxide (DMSO) with Dextran40 (Akron Scientific, Boca Raton, Florida). At each passage, the cell product was characterized by assessing cell surface phenotype by flow cytometry and functional assays via T‐cell proliferation and organotypic models of microglial activation. A representative P2 compartment of each lot was thawed and tested for quality control (QC) release and shown to be sterile, endotoxin‐free, and negative for mycoplasma and adventitial viruses. The P2 cells also had to inhibit third‐party lymphocyte proliferation and microglial activation in laboratory assays developed in the Robertson Research laboratory of the Marcus Center for Cellular Cures. For dosing, release testing after thaw and dilution included total nucleated cell (TNC) count and viability, which was tested on a Nexcelom Cellometer 2000 (Lawrence, Massachusetts) that enumerates viable and dead nucleated cells through A0/P1 staining. Sterility cultures (14 day) were also initiated, but results were not available at the time of infusion.
2.4. hCT‐MSC Infusion
On the day of infusion, the hCT‐MSC product was thawed and tested for viability and count on the Cellometer. Post‐thaw viability had to be ≥70%. The amount of product required to deliver 2 × 106 cells per kilogram was removed and placed in 20 mL Plasmalyte A + 5% human serum albumin for administration. Participants were premedicated with intravenous diphenhydramine 0.5 mg/kg and methylprednisolone 0.5 mg/kg 15‐30 minutes prior to administration of the hCT‐MSCs. Some children also received premedication with oral versed to facilitate IV placement. The hCT‐MSC product was administered intravenously through a peripheral IV over 30‐60 minutes in an outpatient infusion center (the Valvano Day Hospital, Duke Children's Hospital) with monitoring of vital signs and oximetry under direct physician supervision. Participants received maintenance IV fluids as tolerated and were monitored for 1‐2 hours after the infusion.
2.5. Safety and ASD Evaluations
Participants were evaluated with physical examinations, laboratory studies, and clinical psychological measures at baseline and 6 months after their initial hCT‐MSC infusion. Laboratory studies included standard blood counts and chemistries, C‐reactive protein (CRP), erythrocyte sedementation rate (ESR), HLA typing, type and screen with Coombs test, humoral immune profile, immune reconstitution panel, and anti‐HLA antibodies. Descriptive efficacy assessments were performed at baseline and 6 months. These included (a) the Vineland Adaptive Behavior Scales, third edition, Survey Interview Form (VABS‐3), a standardized caregiver interview that measures adaptive functioning, socialization, communication, daily living skills, and motor skills; (b) the Pervasive Developmental Disorder‐Behavior Inventory (PDDBI), a caregiver‐completed questionnaire that assesses social, language, and learning/memory skills and problem behaviors; and (c) the Clinical Global Impression (CGI)‐Severity, a clinician assessment rated at baseline and 6 months, with separate scores for overall severity of social communication symptoms, restricted and repetitive behaviors, and overall functioning, with 1 indicating “normal” and 7 “extreme impairment.” At 6 months, CGI‐Improvement (CGI‐I) ratings were made to indicate degree of improvement or worsening in overall severity ranging from 1 (“very much improved”) to 7 (“very much worse”). Additional safety assessments were conducted in person 1 day after infusion and remotely via questionnaires at 2 weeks and 2, 4, and 6 months after the initial infusion and 6 and 12 months after the final dose.
2.6. Statistical analysis
Adverse events (AEs) were classified based on Common Terminology Criteria for Adverse Events version 5.0. The frequency of AEs was analyzed descriptively by participant, severity, dose cohort, lot number, and relatedness to study product as determined by the investigator. The number of events reported per participant was compared between dose cohorts using the Jonckheere‐Terpstra test (a nonparametric test for trend) and between lot numbers using the Kruskal‐Wallis test. Efficacy outcome measures were analyzed descriptively.
3. RESULTS
3.1. Participant characteristics
Twelve children (nine boys, three girls) with ASD, with a median age of 6.4 years (range 4‐9 years), were enrolled between June and October 2017 (Table 1). Eleven participants were white, one was Asian; two were Hispanic or Latino. Median nonverbal IQ at baseline was 38.5 (range 22‐91), and median ADOS‐2 comparison score was 9 (range 6‐10, with higher scores indicating more symptoms). One participant did not have complete data on the baseline PDDBI; all other assessments were completed as planned. Participants were not exposed to any other cellular products throughout the study period.
TABLE 1.
Participant characteristics (n = 12)
| Participant characteristics | Median | Range or % |
|---|---|---|
| Age, years | 6.4 | 4–9 |
| Sex | ||
| Male | 9 | 75% |
| Female | 3 | 25% |
| Race | ||
| White | 11 | 92% |
| Nonwhite | 1 | 8% |
| Ethnicity | ||
| Hispanic | 2 | 17% |
| Non‐Hispanic | 10 | 83% |
| ADOS Severity Score | 9 | 6–10 |
| Nonverbal Intelligence Quotient | 38.5 | 22–91 |
Abbreviation: ADOS, Autism Diagnostic Observation Schedule.
3.2. hCT‐MSC characteristics
hCT‐MSCs all expressed the full complement of MSC surface markers (CD73, CD90, CD105, CD166, and HLA class I) and were negative for CD3, CD31, CD45, and HLA class II (Table 2). A total of 27 hCT‐MSC doses were administered from three different manufacturing lots from three different donor cord tissue samples (Table 3). Three participants received one and two infusions each, and six participants received three infusions over a 6‐month time period. In each dosing cohort, participants were exposed to manufacturing lots from each of the three donors, with each participant receiving MSCs from a single lot. The mean infused dose at each administration was 2.0 × 106 (TNC) per kilogram (SD 0.4 × 106 TNC per kilogram; Table 3) based on the post‐thaw count, with a median viability of 76.6% (range 60‐82%). One participant received a lower dose of 1.79 × 106 TNC per kilogram owing to a laboratory miscalculation. All hCT‐MSC products had negative post‐thaw sterility cultures.
TABLE 2.
Cell yields and flow cytometric analysis of human cord tissue mesenchymal stromal cell lots
| Lot | Umbilical cord weight, g | Manufacturing TNCCs | Release testing cell count and flow cytometry | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P0 Seeded a | P0 Harvest | P1 Seeded | P1 Harvest | P2 Seeded | P2 Harvest | TNCC | CD90 | CD73 | CD166 | CD3 | CD45 | CD31 | CD105 | ||
| A (GMP‐075) | 34.42 | — | 6.47 × 107 | 6.8 × 106 | 2.64 × 108 | 3.4 × 107 | 1.06 × 109 | 5.60 × 107 | 99.89% | 99.88% | 96.48% | 0.03% | 0.05% | 3.05% | 79.60% |
| B (GMP‐087) | 40.33 | — | 6.17 × 107 | 6.8 × 106 | 3.59 × 108 | 3.4 × 107 | 1.38 × 109 | 6.54 × 107 | 99.96% | 92.99% | 85.72% | 0.04% | 0.03% | 0.44% | 88.96% |
| C (GMP‐088) | 25.87 | — | 1.82 × 107 | 6.8 × 106 | 2.25 × 108 | 3.4 × 107 | 1.02 × 109 | 6.40 × 107 | 99.95% | 99.65% | 94.45% | 0.05% | 0.05% | 4.00% | 88.88% |
Abbreviations: —, no data; hCT‐MSC, human cord tissue mesenchymal stromal cell; TNCC, total nucleated cell count.
Cell counts not performed at P0 seeding.
TABLE 3.
Dosing cohorts
| Participant no. | No. of doses | hCT‐MSC lot no. | Mean weight at infusion, kg | Mean infused hCT‐MSC dose (×106) | |
|---|---|---|---|---|---|
| Cohort 1 | 1 | 1 | A | 19.0 | 38.5 |
| 2 | 1 | B | 17.3 | 35.2 | |
| 3 | 1 | C | 19.7 | 39.8 | |
| Cohort 2 | 4 | 2 | A | 34.4 | 68.9 |
| 5 | 2 | B | 22.3 | 45.2 | |
| 6 | 2 | C | 23.8 | 47.4 | |
| Cohort 3 | 7 | 3 | A | 46.5 | 93.0 |
| 8 | 3 | B | 19.8 | 39.6 | |
| 9 | 3 | C | 15.1 | 29.9 | |
| 10 | 3 | A | 30.0 | 60.5 | |
| 11 | 3 | B | 21.1 | 42.3 | |
| 12 | 3 | C | 20.4 | 39.3 |
Abbreviation: hCT‐MSC, human cord tissue mesenchymal stromal cell.
3.3. Safety of hCT‐MSC Infusions
All participants received their planned doses as intended. Two participants experienced product‐related adverse events during or immediately following an hCT‐MSC infusion. One participant in Cohort 2 experienced a hypersensitivity reaction and mild hypotension during their second infusion associated with a parental request for a deviation (no diphenhydramine) in the premedication schedule for the infusion. The infusion was immediately stopped at the onset of symptoms, after which an IV fluid bolus and an additional dose of methylprednisolone were administered, and the participant recovered completely. The remainder of the hCT‐MSC infusion was successfully completed after a subsequent dose of IV diphenhydramine, which had been omitted initially. One participant in Cohort 3 experienced moderate hypotension (low of 78/30, 10th percentile and fourth percentile for age, respectively) after the completion of their third infusion, which resolved with additional IV fluids.
FIGURE 1.
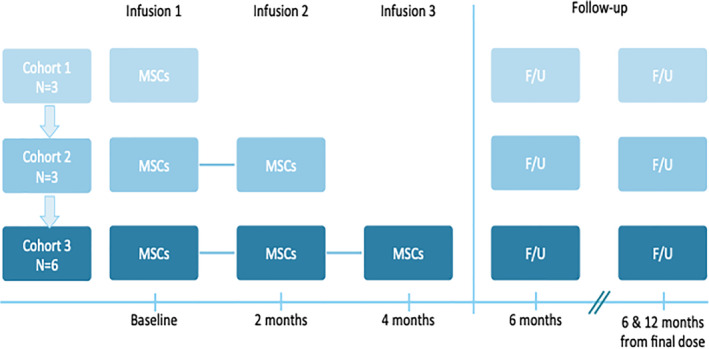
Study design. MSCs, mesenchymal stromal cells
A total of 66 nonserious AEs that were not specifically attributed to the study product were reported among 11 of the 12 enrolled participants (Figure 2). All of these reported events were mild. The most frequently occurring event, agitation during the infusion procedure, was associated with the requirements of placing and maintaining an IV and being confined to a hospital room for the infusion, and all of these events resolved on the same day. A total of 22 other psychiatric or behavioral symptoms were reported in seven different participants: two participants/two events in Cohort 1, one participant/five events in Cohort 2, and four participants/15 events in Cohort 3. These symptoms included aggression (n = 2), agitation (n = 5), anxiety (n = 3), defiant behavior (n = 2), depression (n = 1), emotional lability (n = 1), insomnia (n = 3), intentional self‐injury (n = 1), and stereotypies (n = 4). Of note, three participants accounted for 17/22 of the psychiatric or behavioral adverse events. Aside from agitation during the infusion procedure, there was a trend of increasing frequency of nonserious AEs with increasing number of doses administered, but this was not statistically significant (Cohort 1: median = 2 events per patient [range: 1‐3]; Cohort 2: median = 5 [range: 0‐10]; Cohort 3: median = 7.5 [range: 3‐13]; p Jonckheere‐Terpstra = .05). There were no differences observed in the frequency of AEs according to lot number (GMP‐075: median = 9 events per participant, range: 1‐12; GMP‐087: median = 4.5, range: 0‐13; GMP‐088: median = 3.5, range: 3‐50; p Kruskal‐Wallis = .58).
FIGURE 2.
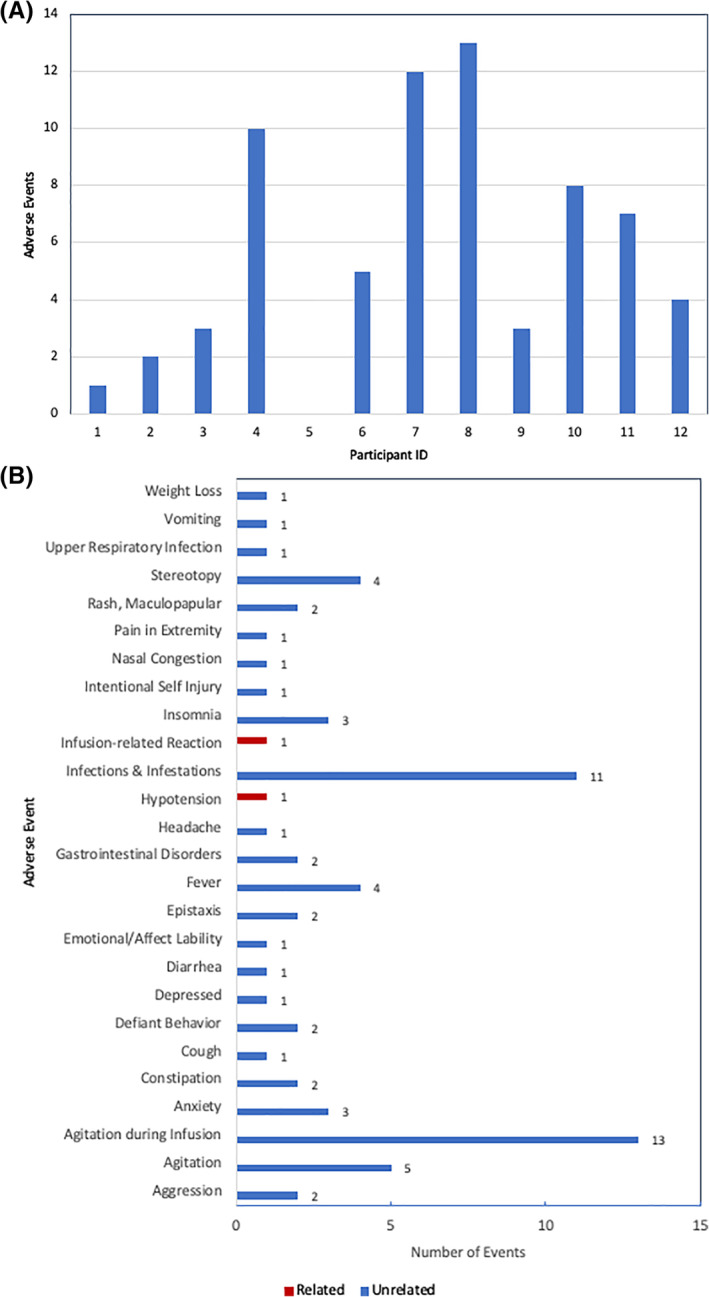
Adverse events (AEs) observed up to 20 months after initial human cord tissue mesenchymal stromal cell infusion. A, Frequency of AEs by participant. B, Frequency of AEs by type of AE
There were no concerning changes in blood counts, chemistries, basic inflammatory markers (CRP, ESR), or humoral and cellular immune profiles throughout the study, and there was no evidence of graft‐vs‐host disease in any participant. All participants remained Coombs negative. Of note, anti‐HLA antibody data were collected in all 12 participants at baseline and 6 months, and 11/12 participants at ≥12 months after their final hCT‐MSC dose. Three participants had detectable anti‐HLA class I antibodies at baseline prior to hCT‐MSC treatment. Of the nine participants who did not have detectable anti‐HLA antibodies before treatment, development of low titer anti‐HLA class I antibodies was observed in five participants 6 months after the initial hCT‐MSC dose and persisted at ≥12 months (Figure 3). The anti‐HLA antibodies were directed against HLA alleles/antigens expressed on the MSCs and not by the participant. One donor appeared to elicit formation of anti‐HLA antibodies more than the other two.
FIGURE 3.
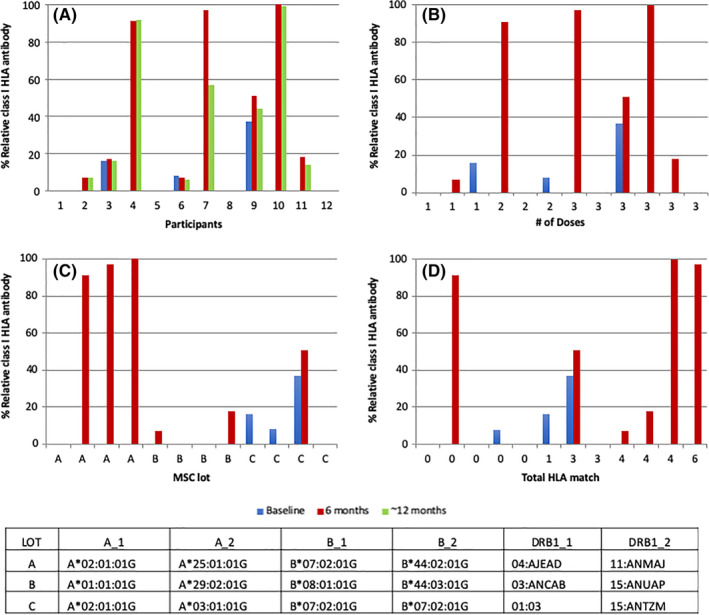
Class I anti‐HLA antibodies. A, Presence of class I HLA antibodies at baseline, 6 months, and >12 months by participant (≥12‐month data not available for participants 3, 4, 11, and 12). B, Class I HLA antibodies and baseline and 6 months by number of hCT‐MSC doses. C, Class I HLA antibodies at baseline and 6 months by lot of hCT‐MSC. D, Class I HLA antibodies by HLA match (at HLA‐A, B, C, DRB1) between hCT‐MSC donor and recipient. HLA, human leukocyte antigen; MSC, mesenchymal stromal cell
Class I anti‐HLA antibodies are shown in Figure 3 according to the number of doses, lot of hCT‐MSC, and degree of HLA match between hCT‐MSC donor and recipient. New class I anti‐HLA antibodies developed in 1/3 participants who received one dose of hCT‐MSCs, 1/3 participants who received two doses, and 3/6 participants who received three doses. All three participants who developed broad‐spectrum class I anti‐HLA antibodies were treated with the same lot of hCT‐MSCs. In addition, 4/4 participants who by chance were at least haploidentical to their hCT‐MSC donor developed new‐onset anti‐HLA antibodies (broad spectrum or donor‐specific), vs 1/8 participants who were 0/8, 1/8, 2/8, or 3/8 HLA‐matched at HLA‐A, B, C, and DRB1. None of the detected anti‐HLA antibodies have been clinically significant.
3.4. Clinical outcome assessments
Results of clinical psychological outcome assessments are shown in Table 4. Measures reported include assessments of social communication skills (VABS‐3 Socialization Subscale Scores) with increases of 3 points and above indicating improvement, 9 severity of autism symptoms (PDDBI Autism Composite) with decreases of at least 5 points indicating improvement, and expert clinical judgment (CGI‐I) ranging from no improvement to much improvement.
TABLE 4.
Clinical outcomes
| ID | No. of doses | Sex | Nonverbal DQ | VABS a | PDDBI | CGI | No. of assessments indicating improvement |
|---|---|---|---|---|---|---|---|
| 1 | 1 | M | 62 | −2 | — | Min | 1 |
| 2 | 1 | M | 68 | 4 | 6 | Min | 2 |
| 3 | 1 | M | 45 | 22 | −22 | Min | 3 |
| 4 | 2 | F | 59 | 0 | −6 | Much | 2 |
| 5 | 2 | M | 40 | −10 | −1 | No | 0 |
| 6 | 2 | M | 36 | 8 | −22 | Min | 3 |
| 7 | 3 | M | 42 | −2 | 0 | Min | 1 |
| 8 | 3 | M | 54 | −8 | −4 | No | 0 |
| 9 | 3 | M | 71 | −3 | 6 | Min | 1 |
| 10 | 3 | M | 82 | 19 | −20 | Min | 3 |
| 11 | 3 | F | 59 | 4 | −7 | Min | 3 |
| 12 | 3 | F | 95 | 7 | −2 | No | 1 |
Clinically significant improvement = 3 points.
Abbreviations: CGI, Clinical Global Impression‐Improvement scale; DQ, Developmental Quotient; F, female; M, male; Max, maximum; Min; minimum; PDDBI, Pervasive Developmental Disorder Behavior Inventory Autism Composite; VABS, Vineland Adaptive Behavior Scales, Third Edition, Socialization Subscale Standard Score.
Fifty percent (6/12) of participants showed an improvement on ≥2/3 measures; 4/12 on all three measures, and 2/12 on two measures. Of the six children who improved on ≥2/3 of the outcome measures, two received one dose of hCT‐MSCs, two had two doses, and two had three doses.
4. DISCUSSION
In this report, we describe the results of an open‐label, safety and feasibility, phase I dose escalation trial testing intravenous administration of hCT‐MSCs, an MSC product derived from donated, allogeneic, third‐party umbilical cord tissue, in 12 children with ASD. Overall, infusions were safe and well tolerated. Suggestions of improvement in core symptoms of ASD were described in 50% of participants, as evidenced by improvement on two out of three clinical outcome measures. Outcome was variable, however, with some participants showing substantial improvements and others showing little or no improvement. Because this was an open‐label trial and in light of the well‐known placebo effect in ASD clinical trials, it is uncertain whether these improvements are related to the treatment.
Behavioral challenges and comorbid psychiatric symptoms are common in children with ASD, so it would be expected that seven participants reported at least one behavioral or psychiatric AE during the 12‐month study period. In most of these participants, the AE was self‐limited and resolved. Ten out of 12 children in the study, including three of the participants whose caregiver reported multiple psychiatric/behavioral AEs, demonstrated improvement in at least one of the three measures of ASD symptoms performed. This highlights the challenges inherent in characterizing and reporting changes in ASD symptoms over time.
An interesting finding in this study was the observation that 1/3 of participants developed new anti‐HLA antibodies to class I HLA antigens that were either broad‐spectrum antibodies or specific to donor HLA‐antigens. Development of broad‐spectrum anti‐HLA antibodies was observed in participants who received a particular lot of hCT‐MSCs, indicating that some HLA haplotypes may be more antigenic than others. Anti‐HLA antibodies were also more prevalent in partially‐HLA‐matched donor/recipient pairs, suggesting that the degree of matching may play a role in sensitization. The number of doses received and improvement on ASD assessment measures were not associated with anti‐HLA antibodies, although the sample size in this study may be too small to detect any such relationships. Importantly, none of the anti‐HLA antibodies were directed against self‐antigens, and none had any clinical sequelae. Development of anti‐HLA antibodies has been described previously after treatment with bone marrow 10 , 11 , 12 , 13 and adipose‐derived MSCs. 14 Although no clinical consequences of these anti‐HLA antibodies have been described, even among solid organ transplant recipients, the implications of anti‐HLA antibody development with multiple MSC doses and in different patient populations deserves additional investigation.
There are only two other clinical reports testing allogeneic hCT‐MSCs in children with ASD in the medical literature. In one study, 15 children were treated with four doses of umbilical cord blood mononuclear cells (CBMNCs) given both intravenously and intrathecally (n = 14), CBMNCs + intrathecal cord tissue MSCs (n = 9), or standard therapy (n = 14). The only treatment‐related side effect was transient fever in five participants. At six months after treatment, both treated groups demonstrated greater improvement in ASD measures than the placebo group. A second recently published study 16 reported results of cord tissue MSC therapy in 20 children, treated in an open‐label fashion, receiving IV doses of ~36 million cells divided over four days every 12 weeks over a nine‐month time period. Cells were manufactured in media containing fetal bovine serum and expanded out to passage 5 before cryopreservation. Premedication for infusions were not administered, and during treatment, post‐thaw characteristics of the cells were not assessed, and children were exposed to multiple donors over time. Safety was assessed clinically, and mild to moderate AEs were reported to occur after 20% of doses administered. Follow‐up did not occur in all participants, but improvements in the Autism Treatment Evaluation Checklist (ATEC) were described.
Although hCT‐MSC infusions in our study were medically well tolerated, agitation during the infusion procedure was common. The process of coming to a new medical center, having an IV placed and labs drawn, being monitored with a pulse oximeter and blood pressure cuff, and being observed in a foreign environment among strangers is stressful for most children, and particularly for children with ASD. In some inflammatory conditions, repeated doses or prolonged courses of therapy are required for a sustained effect. In this small study, number of doses was not associated with measures of clinical improvement. Thus, given the stressful nature of the infusion experience, we have elected to pursue a strategy of increased dose, rather than increased number of doses, in our next clinical trial. We also used oral versed as a premed in some children to decrease anxiety and facilitate cooperation with IV placement. Two children in this series experienced infusion reactions during MSC administration requiring medical interventions. Although the definitive etiology of these reactions cannot be clear, it is likely that they were due to reactions to DMSO, which has been widely reported with administration of cryopreserved products. In one case, premeds were held owing to a parental request. In the other participant who received premedication per protocol, hypotension occurred and required treatment. Both children recovered, but the occurrence of these events emphasizes the need for children to be treated with MSC therapy in a controlled setting with a secure IV, physiological monitoring, and medical personnel in attendance to identify and treat reactions at their earliest presentation.
5. CONCLUSION
We report favorable safety outcomes of a small, phase I trial of hCT‐MSCs in children with ASD. Additional trials are needed to fully assess the safety and efficacy of this approach. Accordingly, our center recently activated a phase II randomized, double‐blind study to assess the safety and efficacy of hCT‐MSCs for improving social communication abilities in young children with ASD (NCT04089579).
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
J.M.S., G.D., J.K.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of the manuscript; L.F., C.M., B.K., B.W.P., N.M.: collection and/or assembly of data, final approval of the manuscript; J.H.: conception and design, collection and/or assembly of data, final approval of the manuscript; J.T.: data analysis and interpretation, manuscript writing, final approval of the manuscript.
ACKNOWLEDGMENTS
This study was supported by a grant from The Marcus Foundation, without which this work could not have been done. We thank the administrative staff and the clinical trials associates from the Marcus Center for Cellular Cures and Center for Autism and Brain Development at Duke for their work on behalf of this study. We are indebted to the participants and families for their dedication and commitment to participating in this trial.
Sun JM, Dawson G, Franz L, et al. Infusion of human umbilical cord tissue mesenchymal stromal cells in children with autism spectrum disorder. STEM CELLS Transl Med. 2020;9:1137–1146. 10.1002/sctm.19-0434
Authored by a member of CBA.
Funding information The Marcus Foundation
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. King BH, Navot N, Bernier R, Webb SJ. Update on diagnostic classification in autism. Curr. Opin. Psychiatry. 2014;27:105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abrahams BS, Geschwind DH. Connecting genes to brain in the autism spectrum disorders. Arch. Neurol. 2010;67:395‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Takano T. Role of microglia in autism: recent advances. Dev. Neurosci. 2015;37:195‐202. [DOI] [PubMed] [Google Scholar]
- 4. Zantomio D, Chana G, Laskaris L, et al. Convergent evidence for mGluR5 in synaptic and neuroinflammatory pathways implicated in ASD. Neurosci. Biobehav. Rev. 2015;52:172‐177. [DOI] [PubMed] [Google Scholar]
- 5. Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol. Teratol. 2013;36:67‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 2011;6:457‐478. [DOI] [PubMed] [Google Scholar]
- 7. Jaimes Y, Naaldijk Y, Wenk K, Leovsky C, Emmrich F. Mesenchymal stem cell‐derived microvesicles modulate LPS‐induced inflammatory responses to microglia cells. Stem Cells. 2017;35:812‐823. [DOI] [PubMed] [Google Scholar]
- 8. Ooi YY, Dheen ST, Tay SS. Paracrine effects of mesenchymal stem cells‐conditioned medium on microglial cytokines expression and nitric oxide production. Neuroimmunomodulation. 2015;22:233‐242. [DOI] [PubMed] [Google Scholar]
- 9. Chatham CH, Taylor KI, Charman T, et al. Adaptive behavior in autism: minimal clinically important differences on the Vineland‐II. Autism Res. 2018;11:270‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garcia‐Sancho J, Sanchez A, Vega A, Noriega DC, Nocito M. Influence of HLA matching on the efficacy of allogeneic mesenchymal stromal cell therapies for osteoarthritis and degenerative disc disease. Transplant. Direct. 2017;3:e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hare JM, DiFede DL, Rieger AC, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON‐DCM trial. J. Am. Coll. Cardiol. 2017;69:526‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic mesenchymal precursor cells (MPC) in diabetic nephropathy: a randomized, placebo‐controlled, dose escalation study. EBioMedicine. 2016;12:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skyler JS, Fonseca VA, Segal KR, MSB‐DM003 Investigators . Allogeneic mesenchymal precursor cells in type 2 diabetes: a randomized, placebo‐controlled, dose‐escalation safety and tolerability pilot study. Diabetes Care. 2015;38:1742‐1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panes J, Garcia‐Olmo D, Van Assche G, et al. Expanded allogeneic adipose‐derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double‐blind controlled trial. Lancet. 2016;388:1281‐1290. [DOI] [PubMed] [Google Scholar]
- 15. Lv YT, Zhang Y, Liu M, et al. Transplantation of human cord blood mononuclear cells and umbilical cord‐derived mesenchymal stem cells in autism. J. Transl. Med. 2013;11:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riordan NH, Hincapie ML, Morales I, et al. Allogeneic human umbilical cord mesenchymal stem cells for the treatment of autism spectrum disorder in children: safety profile and effect on cytokine levels. Stem Cells Translational Medicine. 2019;8:1008‐1016. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


