Abstract
Objective
The aim was to study the effect of non-mandatory transitioning from etanercept originator to etanercept biosimilar on retention rates in a setting promoting shared decision-making.
Methods
In 2016, all patients treated with etanercept originator and stable disease at the Rheumatology department in Bernhoven were offered transitioning to etanercept biosimilar by an opt-in approach. A historical cohort of patients treated with etanercept originator in 2015 was identified as the control group. Etanercept discontinuation was compared between the cohorts using Cox regression. To study the nocebo effect, reasons for discontinuation were categorized into objective reasons (e.g. laboratory abnormalities, increase in swollen joint count, allergic reaction) and subjective health complaints (symptoms perceptible only to the patient, e.g. tiredness, arthralgia). An adjusted Kaplan–Meier curve for retention of the etanercept biosimilar was made, censoring subjective health complaints as the reason for discontinuation.
Results
Seventy of the 79 patients eligible for transitioning agreed to transition (89%). The 1-year crude retention rate of etanercept in the transition cohort was 73% (95% CI: 0.62, 0.83), compared with a retention rate of 89% (95% CI: 0.81, 0.95) in the historical cohort (P = 0.013). This resulted in a higher risk of treatment discontinuation in the transition cohort (adjusted hazard ratio = 2.73; 95% CI: 1.23, 6.05, P = 0.01). After adjusting for the nocebo effect, the cohorts had comparable retention rates (86 vs 89%, P = 0.51).
Conclusion
Non-mandatory transition from etanercept originator to its biosimilar using an opt-in approach in a setting promoting shared decision-making resulted in a higher discontinuation of etanercept compared with the historical cohort. This could be attributed largely to the nocebo effect.
Keywords: etanercept, biologic therapy, longitudinal studies
Key messages
Transitioning from bio-originator to biosimilar has been associated with the nocebo effect.
Shared decision-making between doctor and patient is thought to be crucial in preventing the nocebo effect.
Remarkably, we did find a nocebo effect in a setting promoting shared decision-making.
Introduction
In January 2016, the first etanercept biosimilar (EB) was approved by the European Medicines Agency [1]. At that point, a large randomized clinical trial had shown that the efficiency of the EB was comparable to that of the etanercept originator (EO) in a blinded setting [2–4]. In many countries, substitution of a bio-originator with a biosimilar was assumed for treatment of bio-naïve patients [5]. However, non-medical transitioning from the bio-originator to its biosimilar was debatable, and this discussion is still ongoing. No consensus has been reached about how and when to transition [6–8].
A recent study looked at the effect of open-label transitioning from EO to EB on the retention rates of etanercept in a mandatory setting. That study showed a lower retention rate for etanercept after transitioning to EB compared with a historical cohort being treated with the EO. The reasons for EB withdrawal were mainly subjective and were hypothesized to be attributable to the nocebo effect [9]. The nocebo effect is the counterpart of the placebo effect. The placebo effect can occur when there is a positive perception of the treatment being administered, whereas the nocebo effect may occur when there is a negative perception. Contrary to the placebo effect, the nocebo effect leads to a more negative outcome [10]. The current hypothesis is that by improving the shared decision-making process, educating the medical professional in techniques of communication and improving their ability to interact with patients, in addition to providing patients with structured information, the nocebo effect can be reduced and retention rates improved [11–14]. However, this has not yet been demonstrated in the transitioning to biosimilars. The importance of shared decision-making and adequate patient information is stressed in the 2015 statement of the Dutch Medicines Evaluation Board declaring that ‘the exchange between biologic medicines is permitted, but only if adequate clinical monitoring is performed and the patient is properly informed’ [11]. This is in line with current guidelines promoting shared decision-making for the treatment of RA [12] and the findings of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases, who stated that ‘Treatment of rheumatic diseases is based on a shared decision-making process between patients and their rheumatologists’ [7]. Therefore, in line with current views regarding shared decision-making [7, 12] and in an attempt to counter the nocebo effect [13], a non-mandatory transition in a setting promoting shared decision-making might be preferred.
The aim of this observational study was to assess the 1-year retention of EB after open-label non-mandatory transitioning from EO in patients with stable inflammatory rheumatic disease in a setting promoting shared decision-making. Secondary analyses aimed to assess the acceptance rate of the non-mandatory transition [1] and the influence of the nocebo effect on the retention of the EB [2].
Methods
Study design and method of transition
This observational study assessed the open-label non-mandatory transition from EO to EB at the Rheumatology Department of Bernhoven, a general hospital in Uden, in the south of The Netherlands. Since 2015, Bernhoven has been actively promoting the shared decision-making strategy, in an attempt to improve shared decision-making in the hospital [14]. The transition was part of the usual care delivered at Bernhoven and, as such, shared decision-making was an important part of the transitioning to the EB. Firstly, all health professionals of the outpatient department were informed about the transition process and educated about the biosimilars. At the same time, all patients receiving EO were informed by a standardized letter containing information on both the biosimilar and the proposed transition process to EB. Secondly, the possibility of transitioning was discussed between the patient and the rheumatologist during the next outpatient visit. This gave patients the opportunity to ask questions regarding biosimilars and transitioning to a biosimilar. At the same time, it gave the rheumatologist time to assess whether the patient's disease was stable. In addition, it was once more stressed that patients could return to treatment with the originator if they encountered difficulties with biosimilar treatment. An opt-in approach was used, whereby patients had to agree actively to transition, before they were transitioned to the EB. If the patient still had questions regarding, for instance, the transition or administration of the biosimilar, a consultation with the nurse specialist was planned to address these and any other questions.
Patients
As part of usual care, patients at the Rheumatology Department of Bernhoven were proposed to transition to EB if they met the following disease-related criteria: they were diagnosed with RA, according to the 2010 ACR/EULAR criteria [15], or with either PsA or ankylosing spondylitis (AS), according to the 2009 Assessment of Spondyloarthritis International Society criteria [16]; they were being treated with EO (50 mg in a prefilled pen or syringe) between 1 June 2016 and 22 October 2017; and they had stable disease activity according to the physician's opinion.
All patients agreeing to transition to EB were included in the transition cohort. All patients being treated with the EO at the same department on 1 June 2015 were identified as the historical cohort. All procedures were performed in accordance with the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. According to Dutch regulation, this study did not require ethical approval because only data used for daily clinical practice were collected. All patients had provided written informed consent for the use of their data for scientific purposes at an earlier time point.
Data collection
Data regarding demographics, disease and treatment were recorded at the time of inclusion and during the follow-up visits performed in usual care in the year after transition. Disease activity was measured with the DAS 28 joints (DAS28) for RA and PsA. The BASDAI was measured for AS. Reasons for etanercept discontinuation were documented by the rheumatologists in the electronic patient records. Reasons for discontinuation were categorized into objective reasons (e.g. laboratory abnormalities, increase in swollen joint count, allergic reaction) and subjective health complaints (a descriptive term for symptoms perceptible only to the patient, e.g. tiredness, arthralgia).
Statistical analyses
All continuous variables were expressed as the mean with s.d. or median with range, depending on distribution, and tested with Student’s two-tailed t-test or Wilcoxon’s rank sum test, respectively. All categorical variables were expressed as proportions and analysed using a χ2 test.
Firstly, the acceptance of non-mandatory transitioning was studied. Differences in baseline characteristics between patients accepting and patients declining the transition to biosimilar were assessed.
Secondly, the 1-year retention rate of etanercept was explored in both the transition cohort and the historical cohort using a Kaplan–Meier curve, and the difference in retention rate distributions was tested using the log-rank test. Patients who discontinued treatment because they achieved clinical remission were not coded for an event but were censored at the time of discontinuation.
Thirdly, the hazard ratio (HR) of treatment discontinuation between the transition cohort and the historical cohort was calculated using Cox regression. An adjusted HR of treatment discontinuation was calculated to account for possible baseline differences [in age, sex, diagnosis, treatment duration categorized in two groups (>1 year and ≤1 year), dose interval, combination therapy and CRP level] between the transition cohort and the historical cohort using a multivariate Cox regression. A robust variance estimator was applied in the Cox regression to account for repeated subjects (i.e. patients included in both the transition cohort and the historical cohort). To address missing values, especially for CRP level, multiple imputation was used. The fully conditional specification method was used because this allows any missing data pattern, and the cumulative hazard instead of time to retention was used in the imputation model as advised in the literature [17].
Fourthly, to study the possible nocebo effect in the transition cohort, an adjusted Kaplan–Meier curve for the retention of the EB was made, censoring subjective health complaints as a reason for discontinuation. Subjective health complaints were defined as worsening of disease perceived by the patient, in the absence of clinical signs of arthritis according to the rheumatologist or change in the disease activity score. The reasons for discontinuation and the course of action after discontinuation were also described.
Additional (sub)analyses are presented in the Supplementary Material and Supplementary Table S1, available at Rheumatology Advances in Practice online.
The analysis for this paper was generated using SAS software, v.9.2 with v.9.4 of the SAS System for Windows (copyright 2011 SAS Institute Inc.). Values of P < 0.05 were considered as statistically significant.
Results
Patients
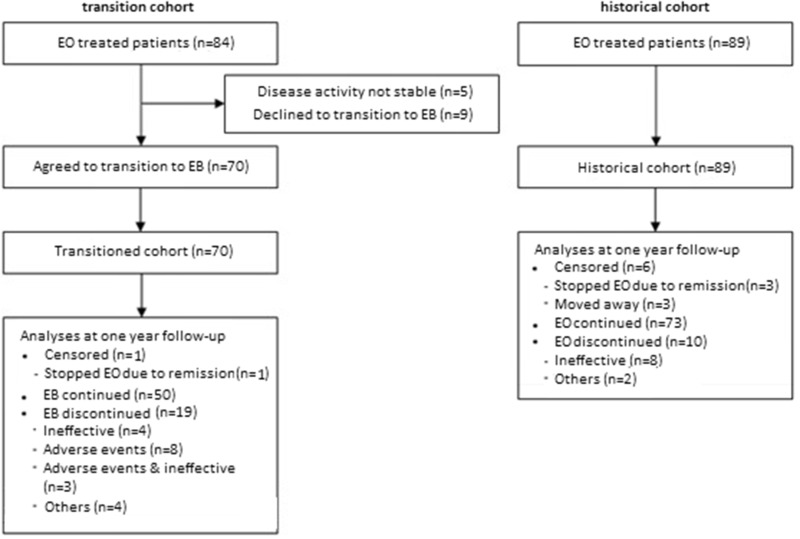
A total of 84 patients were being treated with EO (50 mg) between 1 June 2016 and 23 October 2017 (Fig. 1). Of these patients, five did not have a stable disease activity according to their rheumatologist and were therefore not eligible for transitioning. Of the 79 patients who were eligible, 70 (89%) accepted transitioning. As the historical cohort, 89 patients being treated with EO (50 mg) on 1 June 2015 were identified. A total of 56 patients were included in both the transition cohort and the historical cohort. Patient, disease and treatment characteristics of the transition cohort and the historical cohort are given in Table 1. Patients accepting and patients declining the transition showed similar baseline characteristics.
Fig. 1.
Flowchart of follow-up in the transition cohort and the historical cohort
EB: etanercept biosimilar; EO: etanercept originator.
Table 1.
Baseline characteristics of the transition cohort and the historical cohort
| Baseline characteristics | Transition cohort (n = 70) |
Historical cohort (n = 89) |
||
|---|---|---|---|---|
| At transitioning | n | 1 June 2015 | n | |
| Patient characteristics | ||||
| Age, mean (s.d.), years | 58 (14) | 70 | 56 (19) | 89 |
| Female sex, % | 51 | 36 | 55 | 49 |
| Diagnosis | ||||
| RA, % | 69 | 48 | 73 | 65 |
| PsA, % | 16 | 11 | 11 | 10 |
| AS, % | 16 | 11 | 16 | 14 |
| Disease characteristics | ||||
| Disease duration, median (IQR), years | 10 (6–14) | 67 | 9 (6–17) | 87 |
| CRP, median (IQR), mg/l | 2 (2–4) | 63 | 2 (2–4) | 63 |
| DAS28, median (IQR) | 2.7 (2.2–3.7) | 39 | 3.0 (2.4–3.8) | 47 |
| RF positive, % | 72 | 31 | 71 | 42 |
| Anti-CCP positive, % | 71 | 30 | 73 | 40 |
| BASDAI, median (IQR) | 1.4 (1.2–2.6) | 6 | 2.3 (1.3–3.9) | 7 |
| HLAB27 positive, % | 54 | 7 | 62 | 8 |
| Treatment characteristics | ||||
| Number of previous biologics, median (IQR) | 0 (0–0) | 70 | 0 (0–0) | 89 |
| Etanercept treatment duration, median (IQR), years | 5 (2–8) | 68 | 4 (2–7) | 88 |
| csDMARD combination therapy, % | 52 | 70 | 48 | 89 |
| Etanercept dose interval, median (IQR), days | 7 (7–7) | 70 | 7 (7–7) | 89 |
Anti-CCP: anti-CCP antibody; csDMARD: conventional synthetic DMARD; DAS28: DAS 28 joints; IQR: interquartile range.
Biosimilar discontinuation
The discontinuation of etanercept is shown in Fig. 2. The 1-year crude retention rate of Etanercept in the transition cohort was 73% (95% CI: 0.62, 0.83), compared with a retention rate of 89% (95% CI: 0.81, 0.95) in the historical cohort (P = 0.013). Therefore, patients in the transition cohort had a higher risk of treatment discontinuation (HR = 2.56; 95% CI: 1.19, 5.49, P = 0.016). Adjusting for baseline differences and taking repeated measures into account did not significantly alter the risk of treatment discontinuation (adjusted HR = 2.73; 95% CI: 1.23, 6.05, P = 0.01).
Fig. 2.
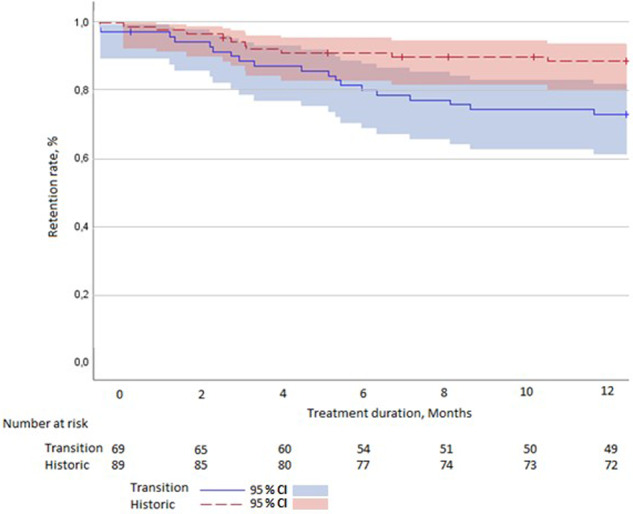
Kaplan–Meier curve showing the discontinuation of etanercept in the historical cohort and the transition cohort
The nocebo effect
To assess the influence of the nocebo effect, the reasons for stopping treatment were analysed.
Reasons for treatment discontinuation in the 19 patients who discontinued etanercept treatment in the transition cohort and the 10 patients who discontinued etanercept treatment in the historical cohort are specified in Table 2. In the historical cohort, all 10 patients had objective reasons for discontinuation. Eight patients (80%) had clinical worsening of the disease, assessed by a DAS28 of >4.0, one patient (10%) had to stop owing to scheduled surgery, and one patient (10%) had to stop owing to a terminal illness. In the transition cohort, seven patients (37%) reported clinical worsening of the disease. However, four of those did not have any clinical signs of worsening of disease activity. In total, nine patients (47%) discontinued because of subjective health complaints. This amounted to a nocebo response of 13% in the transition cohort. After adjusting for subjective reasons for discontinuation, the transition cohort and the historical cohort had comparable retention rates (86 vs 89%, P = 0.51; Fig. 3). Only one serious adverse event was reported in the transition cohort. The serious adverse event seemed to be a drug hypersensitivity reaction after transitioning. During follow-up, this reaction also occurred without any treatment, suggesting a cause other than the biosimilar. Of the patients who discontinued EB treatment in the transition cohort, 12 patients (63%) returned to treatment with the EO, two patients (11%) switched to another biologic, and five patients (26%) discontinued biologic treatment altogether.
Table 2.
Health complaints and reasons for discontinuation in the transition cohort and the historical cohort
| Health complaints and reasons for discontinuation | Transition cohort (n) | Historical cohort (n) |
|---|---|---|
| Objective health complaints | 10 | 10 |
| Clinical worsening | 1 | 8 |
| Clinical worsening and painful injection | 1 | – |
| Diarrhoea | 1 | – |
| Infections | 1 | – |
| Mucositis and clinical worsening | 1 | – |
| Hypersensitivity reaction | 1 | – |
| Stopping of medication owing to scheduled surgery | – | 1 |
| Stopping of medication owing to terminal illness | 2 | 1 |
| Switch to other medication owing to AS | 1 | – |
| General decline | 1 | – |
| Subjective health complaints | 9 | – |
| General discomfort/overall malaise | 2 | – |
| Increased tiredness | 1 | – |
| Arthralgia without clinical sign of arthritis | 3 | – |
| Muscle aches in arms | 1 | – |
| Tingling in hands and feet | 1 | – |
| Arthralgia without clinical sign of arthritis and general discomfort/overall malaise | 1 | – |
Fig. 3.
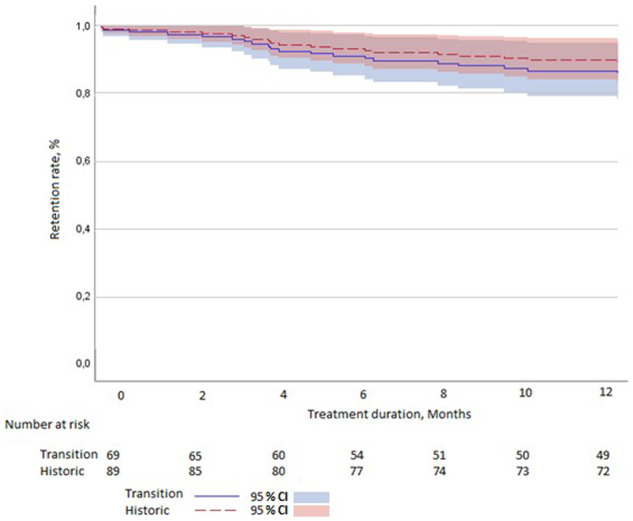
Adjusted Kaplan–Meier curve
(censoring for subjective reasons for discontinuation).
Discussion
This study focused on a non-mandatory open-label transition from EO to EB in a setting promoting shared decision-making. Acceptance of the transition using an opt-in method was high (89%). However, after transitioning there was higher discontinuation of etanercept in comparison to discontinuation in a historical cohort in the same setting. This difference in discontinuation was mainly driven by subjective health complaints. During 1-year follow-up, the effectiveness of the EO and the EB was similar.
One of the strengths of this design is that real-world data were collected and assessed. This offered the possibility for studying the retention rates in a setting promoting shared decision-making and comparing these with those of the bio-originator in the same setting. A weakness of the design is that the control group consisted of a historical cohort. Therefore, calendar time bias could occur, with stricter adherence to the treat-to-target principle in the later time period. This could lead to higher discontinuation of the biologic therapy in the transition cohort. Given that patients in the transition cohort were selected on the basis of stability of the disease, this could have led to selection bias, whereby the selected group was less likely to discontinue, because unstable patients are more likely to discontinue treatment [9, 18]. If this effect occurred, the observed difference in discontinuation of etanercept is an underestimate of the true difference in discontinuation.
There is a large heterogeneity in the methods used to transition patients and the way in which patients are informed about the transition [19]. There are differences regarding whether the approach is mandatory or non-mandatory and whether an opt-in or an opt-out method is used. These differences in approach are relevant, because they are hypothesized to influence acceptance rates and retention rates [15]. In our study, we tried to empower patients using a non-mandatory opt-in method, whereby patients were involved in the decision to transition and in the decision to (dis)continue biosimilar treatment. It is thought that such a method might lead to higher retention rates by countering the nocebo effect, and at the same time it fits with shared decision-making [7, 13, 19]. However, we observed an increased discontinuation of biologic therapy after transition to the biosimilar. This increased discontinuation appears to have been influenced by our transition method, which offered patients the option to return to the originator if they encountered difficulties with the biosimilar. This assumption is strengthened by the high number of patients with subjective health complaints who discontinued treatment. Of the patients who discontinued treatment, 63% returned to the originator, instead of switching to another biological, because no signs of increased disease activity were present. In these cases, complaints were possibly attributable to the nocebo effect, and restarting the originator therapy was likely to be successful.
After adjusting for the nocebo effect, the retention rate in the transition cohort increased from 73 to 86% and was comparable to the retention rate in the historical cohort. This observed incidence of discontinuation because of the nocebo effect of 13% in the transition cohort matches the 13% incidence of the nocebo effect observed in an earlier study that transitioned patients with immune-mediated inflammatory diseases from the infliximab bio-originator to its biosimilar on the basis of shared decision-making [20]. During the same time period as our study, a study was performed by Tweehuysen et al. [18], which was similar to ours in design but differed in the way in which patients were informed. They used a more directive approach, informing the patients that transition was necessary, while at the same time using a ‘wait and see’ approach, if patients experienced subjective health complaints. As can be expected, a lower discontinuation rate after transitioning was found. After 6 months, the retention rate in the transition cohort compared with the historical cohort was 90 vs 92% [18].We observed a comparable small difference in 1-year retention rates between our transition cohort and the historical cohort, after adjusting for the discontinuation attributable to the nocebo effect. These findings imply that our method of transitioning does not seem to counter the nocebo effect sufficiently. On the contrary, the information given by the health-care personnel and the informed consent procedure could, instead of reducing nocebo effects, introduce these negative feelings in the patient and facilitate the nocebo response.
The above-mentioned study by Tweehuysen et al. [18] found an acceptance rate of 99% using an opt-out approach, whereby patients were transitioned to the biosimilar unless they actively objected, in contrast to our acceptance rate of 89% using an opt-in approach. These results suggest that the method of transitioning and doctor–patient communication also influence the acceptance rate of transitioning.
These findings make the ongoing discussion about selective non-disclosure of information to patients to negate the nocebo effect relevant [21–23]. It has been hypothesized that a paternalistic non-disclosure of information might decrease nocebo-induced adverse events and lead to higher retention rates [19]. Current evidence, where a more directive approach results in higher acceptance rates and retention rates, supports these hypothesis [18]. Therefore, using a more directive approach seems a logical step when maximizing cost reduction, by maximizing biosimilar utilization, is the primary goal. However, this approach does not take the opinion of the patient seriously and is directly contrary to the latest guidelines for the treatment of RA and the findings of the Task Force on the Use of Biosimilars to Treat Rheumatological Diseases, both of which promote shared decision-making [12]. Therefore, it would be interesting to study the satisfaction of patients with these different approaches.
Our shared decision-making approach used for transitioning from EO to EB resulted in a lower retention rates of EB compared with a historical cohort. At the same time, effectiveness was comparable. A difference in retention rates was caused by an increase in subjective health complaints. The acceptance rate and retention rate observed using a shared decision-making approach were lower compared with those observed using a more directive approach. These findings contradict the hypothesis that more patient involvement in decision-making and patient empowerment reduce the nocebo effect and improve retention rates. Furthermore, it implies that there is a tension between maximal cost reduction and the promotion of shared decision-making in the case of transitioning to biosimilars.
Supplementary Material
Acknowledgements
W.D.M., S.A.A.R.-v.D., E.M.M.A. and P.L.C.M.v.R. were involved in the conception and design of the study. W.D.M. and S.A.A.R.-v.D. acquired the data. W.D.M. and S.T. analysed the data, and all authors were involved in the interpretation of the data. W.D.M. drafted the article. W.D.M., S.A.A.R.-v.D., S.T., E.M.M.A. and P.L.C.M.v.R. revised the article critically for important intellectual content. All authors read and approved the final manuscript. All authors had access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. W.D.M. attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. He is the guarantor.
Funding: This work was supported by a grant from Stichting RUN. The opinions, results and conclusions reported in this paper are those of the authors and are independent of the funding sources. No endorsement by Stichting RUN is intended or should be inferred.
Disclosure statement: P.L.C.M.v.R. has received grant funding from the Rheumatoid Arthritis Foundation to support this work and has received speaker fees or grants from the following companies: Abbvie, Eli Lilly, Pfizer and UCB in the past 3 years. The other authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1.Biosimilars approved in Europe ; 2011[updated 2nd of February 2018]. http://www.gabionline.net/Biosimilars/General/Biosimilars-approved-in-Europe (3 April 2018, date last accessed).
- 2. Emery P, Vencovský J, Sylwestrzak A. et al. A phase III randomised, double-blind, parallel-group study comparing SB4 with etanercept reference product in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis 2017;76:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Emery P, Vencovský J, Sylwestrzak A. et al. 52-week results of the phase 3 randomized study comparing SB4 with reference etanercept in patients with active rheumatoid arthritis. Rheumatology (Oxford) 2017;56:2093–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Emery P, Vencovský J, Sylwestrzak A. et al. Long-term efficacy and safety in patients with rheumatoid arthritis continuing on SB4 or switching from reference etanercept to SB4. Ann Rheum Dis 2017;76:1986–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. La Noce A, Ernst M. Switching from reference to biosimilar products: an overview of the European approach and real-world experience so far. EMJ 2018;3:74–81. [Google Scholar]
- 6. Wiland P, Batko B, Brzosko M. et al. Biosimilar switching – current state of knowledge. Reumatologia 2018;56:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kay J, Schoels MM, Dorner T, Emery P. et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis 2018;77:165–74. [DOI] [PubMed] [Google Scholar]
- 8. Inotai A, Prins CPJ, Csanádi M. et al. Is there a reason for concern or is it just hype? – A systematic literature review of the clinical consequences of switching from originator biologics to biosimilars. Expert Opin Biol Ther 2017;17:915–26. [DOI] [PubMed] [Google Scholar]
- 9. Glintborg B, Loft AG, Omerovic E. et al. To switch or not to switch: results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann Rheum Dis 2019;78:192–200. [DOI] [PubMed] [Google Scholar]
- 10. Glintborg B, Sørensen IJ, Loft AG. et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis 2017;76:1426–31. [DOI] [PubMed] [Google Scholar]
- 11. Medicines Evaluation Board. Biosimilar Medicines 2015. [updated 31 March 2015]. https://www.cbg-meb.nl/actueel/nieuws/2015/03/31/standpunt-cbg-over-voorschrijven-van-biosimilars (21 December 2018, date last accessed).
- 12. Smolen JS, Landewé R, Bijlsma J. et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. [DOI] [PubMed] [Google Scholar]
- 13. Colloca L, Miller FG. Harnessing the placebo effect: the need for translational research. Philos Trans R Soc Lond B Biol Sci 2011;366:1922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. van Leersum N, Bennemeer P, Otten M. et al. Cure for increasing health care costs: The Bernhoven case as driver of new standards of appropriate care. Health Policy 2019;123:306–11. [DOI] [PubMed] [Google Scholar]
- 15. Neogi T, Aletaha D, Silman AJ. et al. American College of Rheumatology/European League Against Rheumatism classification criteria for rheumatoid arthritis: phase 2 methodological report. Arthritis Rheum 2010;62:2582–91. The 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rudwaleit M, van der Heijde D, Landewé R. et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis 2009;68:777–83. [DOI] [PubMed] [Google Scholar]
- 17. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009;28:1982–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tweehuysen L, Huiskes VJB, van den Bemt BJF. et al. Open-label, non-mandatory transitioning from originator etanercept to biosimilar SB4: six-month results from a controlled cohort study. Arthritis Rheumatol 2018;70:1408–18. [DOI] [PubMed] [Google Scholar]
- 19. Odinet JS, Day CE, Cruz JL, Heindel GA. The biosimilar nocebo effect? A systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm 2018;24:952–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boone NW, Liu L, Romberg-Camps MJ. et al. The nocebo effect challenges the non-medical infliximab switch in practice. Eur J Clin Pharmacol 2018;74:655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fortunato JT, Wasserman JA, Menkes DL. Nonmaleficence, nondisclosure, and nocebo: response to open peer commentaries. Am J Bioeth 2017;17:W4–W5. [DOI] [PubMed] [Google Scholar]
- 22. Fortunato JT, Wasserman JA, Menkes DL. When respecting autonomy is harmful: a clinically useful approach to the nocebo effect. Am J Bioeth 2017;17:36–42. [DOI] [PubMed] [Google Scholar]
- 23. Schoene-Seifert B. Beware of nocebo-paternalism: pitfalls of tailored nondisclosure. Am J Bioeth 2017;17:56–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.