Abstract
Background
Staphylococcus aureus is the leading cause of prosthetic joint infection (PJI). Beyond the antibiogram, little attention has been paid to the influence of deep microbiological characteristics on patient prognosis. Our aim was to investigate whether microbiological genotypic and phenotypic features have a significant influence on infection pathogenesis and patient outcome.
Methods
A prospective multicenter study was performed, including all S. aureus PJIs (2016–2017). Clinical data and phenotypic (agr functionality, β-hemolysis, biofilm formation) and genotypic characteristics of the strains were collected. Biofilm susceptibility to antimicrobials was investigated (minimal biofilm eradication concentration [MBEC] assay).
Results
Eighty-eight patients (39.8% men, age 74.7 ± 14.1 years) were included. Forty-five had early postoperative infections (EPIs), 21 had chronic infections (CPIs), and 19 had hematogenous infections (HIs). Twenty (22.7%) were caused by methicillin-resistant S. aureus. High genotypic diversity was observed, including 16 clonal complexes (CCs), with CC5 being the most frequent (30.7%). agr activity was greater in EPI than CPI (55.6% vs 28.6%; P = .041). Strains causing EPI were phenotypically and genotypically similar, regardless of symptom duration. Treatment failure (36.5%) occurred less frequently among cases treated with implant removal. In cases treated with debridement and implant retention, there were fewer failures among those who received combination therapy with rifampin. No genotypic or phenotypic characteristics predicted failure, except vancomycin minimal inhibitory concentration ≥1.5 mg/L (23.1% failure vs 3.4%; P = .044). MBEC50 was >128 mg/L for all antibiotics tested and showed no association with prognosis.
Conclusions
S. aureus with different genotypic backgrounds is capable of causing PJI, showing slight differences in clinical presentation and pathogenesis. No major microbiological characteristics were observed to influence the outcome, including MBEC.
Keywords: biofilm, bone infections, pathogenesis, prosthetic joint infections, Staphylococcus aureus
Prosthetic joint infection (PJI) is a serious complication of arthroplasty, resulting in significant morbidity and costs. Although the outcome of PJI is largely dependent on specialized surgery and appropriate antimicrobial treatment, the etiology is also very important for defining the pathogenesis and prognosis.
The ability of bacteria to cause PJI depends on virulence factors that enable attachment, biofilm development, tissue damage, and intracellular invasion, among other complications. The specific bacteria responsible for the infection have a significant influence on clinical presentation [1]. Common classifications of PJI are based on timing and have proved to be useful for predicting the etiology of infection and taking management decisions. However, specific definitions and time limits are sometimes arbitrary and do not explain why specific bacterial species lead to particular types of PJI [2, 3].
Staphylococcus aureus, a leading cause of PJI, is a versatile microorganism, able to cause various types of PJI, mainly acute postoperative and hematogenous, but also chronic [2, 4]. This flexibility is probably due to the large number of virulence factors, which are mainly regulated by the accessory gene regulator (agr) [5]. There is little information about differences in genetic background or the molecular mechanisms of S. aureus strains according to the clinical presentation of PJI. Moreover, few studies have analyzed the impact of microbiological characteristics and virulence factors on the prognosis of the infection [6, 7].
It is well known that the in vitro activity of antimicrobials, commonly measured as the minimal inhibitory concentration (MIC), correlates poorly with clinical outcome in the setting of biofilm-associated infections. Other approaches based on measuring the minimal biofilm inhibitory concentration (MBIC) and/or the minimal biofilm eradication concentration (MBEC) have been proposed [8]. While these parameters have been studied in strains causing PJI, the correlation with outcome has not been addressed [9].
In this study, we characterized in detail a prospective multicenter cohort of patients with S. aureus PJI with the aim of investigating the influence of a wide range of phenotypic and genotypic characteristics of this microorganism on clinical presentation and also on the outcomes of patients.
METHODS
Setting and Patients
A prospective observational multicenter pilot study that included every S. aureus PJI between May 2016 and September 2017 was conducted at 11 teaching hospitals in Madrid (Spain).
Staphylococcal PJI was defined as ≥1 surgical, joint aspirate, or blood culture yielding S. aureus, along with a compatible clinical presentation [3]. Data collected included clinical data on baseline features, prosthesis characteristics, and clinical presentation, along with information about surgical and medical treatment. Patient management was decided by the treating medical team on an individualized basis and followed current recommendations [1, 3, 10]. Antibiotics were administered according to the antimicrobial susceptibility profile, with a preference for rifampin-based combinations.
Clinical Definitions: Types of PJIs and Outcomes
PJI was considered acute or chronic depending on whether it began within the first 90 days after prosthesis placement or later, respectively. Hematogenous infection was defined as acute onset after a clinically suspected or proven bacteremia. Cases that were considered hematogenous were excluded from being classed as early or chronic postoperative infections. Alternatively, PJI could also be caused by the spread of a contiguous suppurative focus and by “positive intraoperative cultures” for cases of prosthesis revision due to presumed aseptic loosening [2].
Failure of therapy was considered in cases of death from any cause within 90 days after surgery, persistent or relapsing signs of staphylococcal infection, and/or the need for salvage therapy due to S. aureus, including antimicrobial suppressive therapy and unplanned surgeries (except for extra debridements in the first 30 days after the initial therapeutic surgery). Patient follow-up was carried out until death, failure, or loss to follow-up for at least 1 year.
Microbiological and Molecular Characterization of S. aureus Isolates
Identification and Antimicrobial Susceptibility Testing
Isolation and identification of S. aureus were based on standard microbiological procedures at each laboratory. All isolates were sent to a central laboratory (Hospital 12 de Octubre). Antimicrobial susceptibility testing was performed with the MicroScan Walkway System (Siemens, West Sacramento, CA, USA), and MICs were interpreted according to EUCAST criteria. The E-test macromethod was also performed to screen for heteroresistant vancomycin-intermediate S. aureus (h-VISA) phenotypes in isolates with vancomycin MIC ≥1.5 mg/L (by E-test) [11].
Antimicrobial Susceptibility Testing in Biofilm: Calgary Biofilm Device
The Calgary Biofilm Device (CBD; Innovotech, Edmonton, AB, Canada) was used to study the MBEC and MBIC. Studies involving the CBD were conducted as previously described [8] with minor modifications (Supplementary Data, Supplementary Table 1) on isolates from patients treated with debridement, antibiotics, and implant retention (DAIR) and on antibiotics administered for a significant period of time (≥14 days in the first month after DAIR and/or ≥21 days during the whole treatment period). The antibiotics were oxacillin, daptomycin, levofloxacin, and rifampin, with a concentration range of 0.5–256.0 mg/L. The combination of levofloxacin plus rifampin was also tested: rifampin (in a concentration range of 0.5–256.0 mg/L) with a fixed concentration of levofloxacin (3.0 mg/L), then levofloxacin (in a concentration range of 0.5–256.0 mg/L) with a fixed concentration of rifampin (5.0 mg/L). Fixed concentrations of levofloxacin and rifampin were chosen to approximate those expected in bone (Supplementary Data).
Phenotypic Characterization
The activity of the agr operon was measured by δ-hemolysin production [12] and categorized as negative, weak, or strong. β-hemolysis production produced by α-hemolysin (Hla) activity was also analyzed [13]. Biofilm formation was assessed in triplicate with the 0.7% crystal violet method on microtiter plates using 33% glacial acetic acid as the discoloring solution [14]. Absorbance was measured at 595 nm, and the results were interpreted in accordance with Stepanovic [15]. S. aureus colony phenotype, including small colony variants (SCVs), was also observed.
Genotypic Characterization
Virulence and antibiotic resistance genes [16] were determined by DNA microarrays based on the ArrayTube platform, in accordance with the manufacturer’s instructions (S. aureus Genotyping Kit 2.0, Alere, Jena, Germany).
Statistical Analysis
As this was an exploratory study, no sample size calculations were made. Continuous variables were compared using the t test or Mann-Whitney U test, and categorical parameters were compared using the χ 2 or Fisher’ exact test, as appropriate. For the analysis of antimicrobial treatment among patients managed by DAIR, treatment with a specific drug was considered if it was administered for at least 14 days in the first 30 days after surgery and/or for at least 21 days during the entire antimicrobial treatment period [17]. All tests were 2-tailed, and a P value <.05 was considered statistically significant. Statistical analyses were carried out using SPSS, version 20.0, and figures were created using GraphPad Prism, version 6.
PATIENT CONSENT STATEMENT
The study was designed and performed in accordance with the ethical standards of the Helsinki Declaration. It was evaluated and approved by the Hospital Research Ethics Committee (Expte 16/188). Due to the observational nature of the study, no informed consent was deemed necessary.
RESULTS
Clinical and Microbiological Description of the Cohort
Eighty-eight patients were included in the study. Forty-five subjects had early postoperative infection (51.1%), 21 were chronic (23.9%), and 19 were hematogenous (21.6%); in addition, 2 patients had an infection as a result of contiguous spread from a suppurative focus, and 1 patient had a positive intraoperative culture.
Clinical features and microbiological characteristics (phenotypic and genotypic) are shown in detail in Table 1 and Supplementary Table 2. Twenty isolates (22.7%) were methicillin-resistant (MRSA), and all harbored the mecA gene. All strains were vancomycin-susceptible, 11 (12.5%) showed MICs ≥1.5 mg/L, and none had an h-VISA phenotype. Most isolates showed β-hemolysis (87.5%, n = 77) and biofilm formation (95.5%, n = 84). Four strains showed uncommon phenotypes: 3 were SCVs and 1 showed the mucous phenotype. Molecular epidemiology analysis showed that S. aureus isolates belonged to 16 different clonal complexes (CCs), with CC5 being the most frequent (30.7%, n = 27) (Table 1; Supplementary Table 2). Of interest, 6 cases (6.8%) with S. aureus infection belonged to CC398 (1 MRSA and 5 MSSA).
Table 1.
Case Series and Microbiological (Phenotypic and Genotypic) Description and Comparative Analysis According to Methicillin Susceptibility and Clinical Presentation of the Prosthetic Joint Infection
| All Cases (n = 88), No. (%) | MRSA (n = 20), No. (%) | MSSA (n = 68), No. (%) | P | Hematogenous Infection (n = 19),a No. (%) | Postsurgical <90 d (EPI) (n = 45),a No. (%) | Postsurgical >90 d (CPI) (n = 21),a No. (%) | P (HI vs EPI) | P (EPI vs CPI) | P (HI vs CPI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline features | ||||||||||
| Sex (men) | 35 (39.8) | 8 (40.0) | 27 (39.7) | .981 | 8 (42.1) | 15 (33.3) | 11 (52.4) | .504 | .140 | .516 |
| Age, yb | 74.7 ± 14.1 | 79.8 ± 8.8 | 73.2 ± 15.0 | .102 | 77.4 ± 13.9 | 74.2 ± 14.1 | 73.5 ± 15.3 | .293 | .962 | .307 |
| Diabetes mellitus | 16 (18.2) | 5 (25.0) | 11 (16.2) | .509 | 4 (21.1) | 8 (17.8) | 3 (14.3) | .739 | 1.000 | .689 |
| Chronic renal impairment | 14 (15.9) | 3 (15.0) | 11 (16.2) | 1.000 | 4 (21.1) | 6 (13.3) | 4 (19.0) | .466 | .714 | 1.000 |
| Rheumatoid arthritis | 7 (8.0) | 2 (10.0) | 5 (7.4) | .655 | 2 (10.5) | 3 (6.7) | 1 (4.8) | .629 | 1.000 | .596 |
| Prosthesis location (knee)c | 40 (45.5) | 8 (40.0) | 32 (47.1) | .619 | 16 (84.2) | 18 (40.0) | 4 (19.0) | .002* | .160 | <.001* |
| Revision prosthesis | 22 (25.0) | 4 (20.0) | 18 (26.5) | .770 | 5 (26.3) | 9 (20.0) | 7 (33.3) | .742 | .355 | .736 |
| Clinical presentation | ||||||||||
| Hematogenous infectiona | 19 (21.6) | 2 (10.0) | 17 (25.0) | .220 | - | - | - | - | - | - |
| Polymicrobial infection | 14 (15.9) | 3 (15.0) | 11 (16.2) | 1.000 | 0 (0.0) | 8 (17.8) | 6 (28.6) | .093 | .346 | .021* |
| Bacteremia | 18 (20.5) | 3 (15.0) | 15 (22.1) | .753 | 9 (47.4) | 6 (13.3) | 2 (9.5) | .008* | 1.000 | .007* |
| Temperature >37ºC | 31 (35.2) | 6 (30.0) | 25 (36.8) | .578 | 11 (57.9) | 16 (35.6) | 3 (14.3) | .098 | .075 | .004* |
| Sinus tract | 24 (27.3) | 8 (40.0) | 16 (23.5) | .146 | 0 (0.0) | 7 (15.6) | 16 (76.2) | .094 | <.001* | <.001* |
| Leukocytes, ×109/Lb,d | 10.3 ± 6.3 | 7.6 ± 6.8 | 11.1 ± 5.9 | .010* | 11.4 ± 7.6 | 10.4 ± 5.4 | 9.0 ± 6.5 | .397 | .247 | .124 |
| C-reactive protein, mg/Lb,d | 140.4 ± 128.9 | 121.4 ± 127.1 | 145.8 ± 129.9 | .367 | 244.8 ± 130.1 | 128.5 ± 122.3 | 75.0 ± 89.4 | .002* | .096 | <.001* |
| Surgical management | ||||||||||
| DAIRg | 58 (65.9) | 9 (45.0) | 49 (72.1) | .025* | 16 (84.2) | 33 (73.3) | 8 (38.1) | .521 | .006* | .003* |
| Antimicrobial resistance | ||||||||||
| Oxacillin | 20 (22.7) | - | - | - | 2 (10.5) | 9 (20.0) | 7 (33.3) | .483 | .239 | .133 |
| Levofloxacin | 21 (23.9) | 17 (85.0) | 4 (5.9) | <.001* | 3 (15.8) | 9 (20.0) | 8 (38.1) | 1.000 | .117 | .115 |
| Rifampin | 3 (3.4) | 1 (5.0) | 2 (2.9) | .543 | 0 (0.0) | 1 (2.2) | 2 (9.5) | 1.000 | .236 | .488 |
| Vancomycin MIC ≥1.5 mg/L | 11 (12.5) | 6 (30.0) | 5 (7.4) | .015* | 2 (10.5) | 1 (2.2) | 7 (33.3) | .208 | .001* | .133 |
| Phenotypic characteristics | ||||||||||
| agr functionality | ||||||||||
| Negative | 30 (34.1) | 6 (30.0) | 24 (35.3) | 6 (31.6) | 13 (28.9) | 9 (42.9) | ||||
| Weak | 24 (27.3) | 4 (20.0) | 20 (29.4) | .474 | 10 (52.6) | 7 (15.6) | 6 (28.6) | .003* | .117 | .335 |
| Strong | 34 (38.6) | 10 (50.0) | 24 (35.3) | 3 (15.8) | 25 (55.6) | 6 (28.6) | ||||
| β-hemolysis | 77 (87.5) | 20 (100) | 57 (83.8) | .063 | 15 (78.9) | 42 (93.3) | 17 (81.0) | .182 | .196 | 1.000 |
| Biofilm formation, OD 595 nmb | 0.12 ± 0.11 | 0.18 ± 0.17 | 0.11 ± 0.07 | .001* | 0.09 ± 0.04 | 0.13 ± 0.14 | 0.13 ± 0.07 | .150 | .117 | .008* |
| Molecular epidemiology | ||||||||||
| Clonal complex | ||||||||||
| CC5 | 27 (30.7) | 17 (85.0) | 10 (14.7) | 2 (10.5) | 15 (33.3) | 8 (38.1) | ||||
| CC15 | 7 (8.0) | 0 (0.0) | 7 (10.3) | 1 (5.3) | 4 (8.9) | 2 (9.5) | ||||
| CC30 | 13 (14.8) | 0 (0.0) | 13 (19.1) | <.001* | 3 (15.8) | 5 (11.1) | 5 (23.8) | .162 | .605 | .088 |
| CC45 | 12 (13.6) | 1 (5.0) | 11 (16.2) | 2 (10.5) | 8 (17.8) | 2 (9.5) | ||||
| Othere | 29 (33.0) | 2 (10.0) | 27 (39.7) | 11 (57.9) | 13 (28.9) | 4 (19.0) | ||||
| agr group | ||||||||||
| agr I | 26 (29.5) | 2 (10.0) | 24 (35.3) | 8 (42.1) | 13 (28.9) | 4 (19.0) | ||||
| agr II | 35 (39.8) | 17 (85.0) | 18 (26.5) | <.001* | 5 (26.3) | 18 (40.0) | 10 (47.6) | .495 | .685 | .225 |
| agr III | 27 (30.7) | 1 (5.0) | 26 (38.2) | 6 (31.6) | 14 (31.1) | 7 (33.3) |
Abbreviations: CC, clonal complex; CPI, chronic postoperative infection; DAIR, debridement, antibiotics, and implant retention; EPI, early postoperative infection; HI, hematogenous infection; MIC, minimum inhibitory concentration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; OD, optical density.
aThree cases (2 infections caused by a contiguous suppurative focus and 1 positive intraoperative culture) were excluded from this comparison.
bMean ± SD.
cThere were 40 knee prostheses and 46 hip prostheses (14 hemiarthroplasties and 32 total hip replacements).
dData obtained at diagnosis, before the performance of surgical treatment (either debridement or prosthesis removal).
eOther CCs in all cases (n = 88): 1 (1.1%) CC1, 2 (2.3%) CC6, 4 (4.5%) CC8, 2 (2.3%) CC9, 1 (1.1%) CC10, 5 (5.7%) CC22, 2 (2.3%) CC25, 1 (1.1%) CC188, 6 (6.8%) CC398, 3 (3.4%) CC509, 1 (1.1%) CC707, and 1 (1.1%) CC1021.
*These results are statistically significant.
Comparative Analysis According to Methicillin Susceptibility
The clinical characteristics of MRSA and methicillin-susceptible S. aureus (MSSA) cases were similar (Table 1; Supplementary Table 2) except for type of infection: MRSA more frequently presented as a chronic PJI. Consequently, PJIs caused by MSSA were more frequently acute and managed with DAIR than those caused by MRSA (72.1% vs 45.0%; P = .025).
From a microbiological perspective, MRSA isolates mostly belonged to CC5 (85%), while MSSA strains were distributed in 16 CCs, with CC30 being the most frequent (19.1%). MRSA strains formed more biofilm (OD 595 nm 0.18 ± 0.17 vs 0.11 ± 0.07; P = .001).
With respect to virulence genes, those with a significantly higher representation in MRSA isolates included the enterotoxins seg, sei, sem, sen, seo, and seu; the leukocidins lukD, lukE, and lukY; the serine proteases splA and splB; the staphylococcal exotoxin-like proteins setB2 and setB3; capsule type 5 (cap 5); the microbial surface component–recognizing adhesive matrix molecules (MSCRAMMs) fib, fnbB, and sasG; and immunodominant surface antigen B (isaB). By contrast, the serine protease splE, collagen-binding adhesin (cna), and chemotaxis-inhibiting protein (chp) genes were significantly more frequent among MSSA isolates.
Comparative Analysis According to Clinical Presentation
Hematogenous infection occurred more frequently in immunocompromised patients, on knee prostheses, and showed more fever, bacteremia, and higher C-reactive protein (Table 1; Supplementary Table 2). Clonal diversity was also higher in hematogenous infection (19 isolates belonged to 13 different CCs) than in early postoperative (12 CCs for 45 isolates) or chronic infection (8 different CCs for 21 isolates). CC5 was less frequent in HI than in EPI and CPI (10.5%, 33.3%, and 38.1%, respectively). Interestingly, S. aureus isolates causing HI were less resistant to penicillin (68.4% vs 95.6%; P = .007) and less frequently harbored the blaZ (beta-lactamase) gene (73.7% vs 93.3%; P = .044).
In the subset of HIs, blood cultures were negative or positive in 10 and 9 cases, respectively. Overall, bacteremic and nonbacteremic cases were similar from a clinical and microbiological perspective (Supplementary Table 3), although nonbacteremic cases occurred relatively sooner (interquartile range) after placement of the prosthesis (1.26 [0.66–7.65] years vs 7.05 [2.91–21.4] years; P = .043) and the number of revision prostheses was higher (40% vs 11%; P > .05). No significant differences in agr functionality were detected, although biofilm formation was observed to be slightly higher among nonbacteremic cases (OD 595 nm 0.10 ± 0.04 vs 0.07 ± 0.01; P = .133). In the subset of patients with EPI, we compared the characteristics of patients with onset of symptoms in the first month after prosthesis placement and those with infection appearing between days 30 and 90. As shown in Table 2 and Supplementary Table 3, no clinical or microbiological differences were observed between the 2 groups.
Table 2.
Comparative Analysis of Clinical and Microbiological (Phenotypic and Genotypic) Characteristics Between Prosthetic Joint Infections With Onset of Symptoms <30 Days After Surgery and Prosthetic Joint Infections With Onset of Symptoms in the 30–90 Days After Surgery
| Postsurgical <30 d (n = 30), No. (%) | Postsurgical 30–90 d (n = 15), No. (%) | P | |
|---|---|---|---|
| Baseline features | |||
| Sex (men) | 10 (33.3) | 5 (33.3) | 1.000 |
| Age, mean ± SD, y | 76.1 ± 12.8 | 70.4 ± 16.2 | .201 |
| Diabetes mellitus | 4 (13.3) | 4 (26.7) | .410 |
| Chronic renal impairment | 5 (16.7) | 1 (6.7) | .647 |
| Rheumatoid arthritis | 2 (6.7) | 1 (6.7) | 1.000 |
| Prosthesis location (knee) | 10 (33.3) | 8 (53.3) | .197 |
| Prosthesis revision | 6 (20.0) | 3 (20.0) | 1.000 |
| Clinical presentation | |||
| Polymicrobial infection | 5 (16.7) | 3 (20.0) | 1.000 |
| Bacteremia | 4 (13.3) | 2 (13.3) | 1.000 |
| Temperature >37ºC | 11 (36.7) | 5 (33.3) | .826 |
| Sinus tract | 4 (13.3) | 3 (20.0) | .670 |
| Leukocytes, mean ± SD,a ×109/L | 10.2 ± 5.4 | 10.7 ± 5.5 | .779 |
| C-reactive protein, mean ± SD,a mg/L | 121.7 ± 120.4 | 140.5 ± 128.9 | .864 |
| Surgical management | |||
| DAIR | 21 (70.0) | 12 (80.0) | .722 |
| Antimicrobial resistance | |||
| Oxacillin | 5 (16.7) | 4 (26.7) | .454 |
| Levofloxacin | 5 (16.7) | 4 (26.7) | .454 |
| Rifampin | 1 (3.3) | 0 (0.0) | 1.000 |
| Vancomycin MIC ≥1.5 mg/L | 1 (3.3) | 0 (0.0) | 1.000 |
| Phenotypic characteristics | |||
| agr functionality | |||
| Negative | 9 (30.0) | 4 (26.7) | |
| Weak | 5 (16.7) | 2 (13.3) | 1.000 |
| Strong | 16 (53.3) | 9 (60.0) | |
| β-hemolysis | 27 (90.0) | 15 (100.0) | .540 |
| Biofilm formation, mean ± SD, OD 595 nm | 0.13 ± 0.15 | 0.13 ± 0.09 | .682 |
| Molecular epidemiology | |||
| Clonal complex | |||
| CC5 | 9 (30.0) | 6 (40.0) | |
| CC15 | 2 (6.7) | 2 (13.3) | |
| CC30 | 5 (16.7) | 0 (0.0) | .462 |
| CC45 | 6 (20.0) | 2 (13.3) | |
| Other | 8 (26.7) | 5 (33.3) | |
| agr group | |||
| agr I | 9 (30.0) | 4 (26.7) | |
| agr II | 10 (33.3) | 8 (53.3) | .381 |
| agr III | 11 (36.7) | 3 (20.0) |
Abbreviations: CC, clonal complex; DAIR, debridement, antibiotics, and implant retention; MIC, minimum inhibitory concentration; OD, optical density.
aData obtained at diagnosis, before the performance of surgical treatment (either debridement or prosthesis removal).
Compared with acute infections (EPI and HI), CPI cases more frequently occurred on hip prostheses, were less inflammatory, and more frequently presented with a sinus tract. CPI isolates showed a higher vancomycin MIC than EPI (geometric mean: 1.01 vs 0.87 mg/L; P = .051) and less frequently harbored the blaZ gene (71.4% vs 93.3%; P = .024). There were fewer isolates in CPI with strong agr functionality than in EPI isolates (28.6% vs 55.6%; P = .041). In postoperative infections, biofilm formation was similar, but higher than in strains causing HI.
Comparative Analysis of Clinical and Microbiological Characteristics According to Outcome of Infection
Thirty-one failures (36.5%) were observed in the 85 patients (96.6%) whose outcome we were able to evaluate (follow-up 1.26 ± 0.6 years) (Table 3; Supplementary Table 4). Most cases with poor outcome were managed with DAIR compared with those with a good outcome (83.9 % vs 53.7%; P = .005). Among the former, failure was more frequent in patients with CPI and a longer delay to debridement. The use of rifampin and the exchange of removable components were associated with a good prognosis in patients treated with DAIR. A comprehensive comparison of phenotypic and genotypic microbiological characteristics showed no factors associated with failure, with the single exception of vancomycin MIC ≥1.5 mg/L, which was higher in cases in patients treated with DAIR with a poor outcome (23.1% vs 3.4%; P = .044). There was also a trend toward greater presence of S. aureus belonging to CC5 (30.8% vs 13.8%; P = .192) and agr type II (46.2% vs 24.1%; P = .099) among the failures.
Table 3.
Comparative Analysis of Clinical and Microbiological (Phenotypic and Genotypic) Characteristics According to the Outcome of the Prosthetic Joint Infection
| All (n = 85) | Managed With DAIR (n = 55) | Managed With Prosthesis Removal (n = 30) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Failure (n = 31), No. (%) | Cure (n = 54), No. (%) | P | Fail (n = 26), No. (%) | Cure (n = 29), No. (%) | P | Fail (n = 5), No. (%) | Cure (n = 25), No. (%) | P | |
| Baseline features | |||||||||
| Sex (men) | 15 (48.4) | 19 (35.2) | .232 | 10 (38.5) | 10 (34.5) | .759 | 5 (100.0) | 9 (36.0) | .014* |
| Age, mean ± SD, y | 76.3 ± 13.1 | 73.4 ± 14.9 | .298 | 75.6 ± 13.8 | 73.5 ± 14.3 | .320 | 80.2 ± 8.8 | 73.4 ± 15.9 | .627 |
| Diabetes mellitus | 5 (16.1) | 10 (18.5) | .781 | 5 (19.2) | 6 (20.7) | .893 | 0 (0.0) | 4 (16.0) | 1.000 |
| Chronic renal impairment | 5 (16.1) | 8 (14.8) | 1.000 | 3 (11.5) | 4 (13.8) | 1.000 | 2 (40.0) | 4 (16.0) | .254 |
| Rheumatoid arthritis | 2 (6.5) | 5 (9.3) | 1.000 | 1 (3.8) | 1 (3.4) | 1.000 | 1 (20.0) | 4 (16.0) | 1.000 |
| Prosthesis location (knee) | 14 (45.2) | 24 (44.4) | 1.000 | 13 (50.0) | 18 (62.1) | .422 | 1 (20.0) | 6 (24.0) | 1.000 |
| Prosthesis revision | 4 (12.9) | 18 (33.3) | .043* | 4 (15.4) | 8 (27.6) | .339 | 0 (0.0) | 10 (40.0) | .140 |
| Clinical presentation | |||||||||
| Hematogenous infection | 9 (29.0) | 9 (16.7) | .269 | 8 (30.8) | 7 (24.1) | .763 | 1 (20.0) | 2 (8.0) | .433 |
| Polymicrobial infection | 3 (9.7) | 10 (18.5) | .358 | 2 (7.7) | 6 (20.7) | .257 | 1 (20.0) | 4 (16.0) | 1.000 |
| Bacteremia | 8 (25.8) | 9 (16.7) | .311 | 7 (26.9) | 4 (13.8) | .224 | 1 (20.0) | 5 (20.0) | 1.000 |
| Temperature >37ºC | 13 (41.9) | 18 (33.3) | .428 | 12 (46.2) | 13 (44.8) | .921 | 1 (20.0) | 5 (20.0) | 1.000 |
| Sinus tract | 9 (29.0) | 15 (27.8) | .902 | 6 (23.1) | 5 (17.2) | .589 | 3 (60.0) | 10 (40.0) | .628 |
| Leukocytes, mean ± SD,a ×109/L | 10.4 ± 5.0 | 12.3 ± 5.6 | .106 | 10.8 ± 5.3 | 12.0 ± 5.0 | .348 | 8.8 ± 2.7 | 12.8 ± 6.2 | .162 |
| C-reactive protein, mean ± SD,a mg/L | 161 ± 139 | 122 ± 122 | .219 | 152 ± 132 | 168 ± 148 | .957 | 207 ± 181 | 71 ± 52 | .245 |
| Surgical management | |||||||||
| DAIR | 26 (83.9) | 29 (53.7) | .005* | - | - | - | - | - | - |
| Time to debridement, median (IQR), d | - | - | - | 10 (6–24) | 5 (2–10) | .001* | - | - | - |
| Polyethylene exchange | - | - | - | 15 (57.7) | 22 (75.9) | .152 | - | - | - |
| Need for ≥2 debridements | - | - | - | 2 (7.7) | 6 (20.7) | .257 | - | - | - |
| Rifampin ≥14 db | - | - | - | 12 (57.0) | 25 (86.0) | .021* | - | - | - |
| Prosthesis removal | 5 (16.1) | 25 (46.3) | .005* | - | - | - | - | - | - |
| Antibiotic-loaded spacer | - | - | - | - | - | - | 1/3 (33.3) | 11/14 (78.6) | .191 |
| Antimicrobial resistance | |||||||||
| Oxacillin | 7 (22.6) | 12 (22.2) | .970 | 4 (15.4) | 4 (13.8) | 1.000 | 3 (60.0) | 8 (32.0) | .327 |
| Levofloxacin | 9 (29.0) | 11 (20.4) | .365 | 5 (19.2) | 3 (10.3) | .455 | 4 (80.0) | 8 (32.0) | .128 |
| Rifampin | 1 (3.2) | 2 (3.7) | 1.000 | 1 (3.8) | 0 (0.0) | .473 | 0 (0.0) | 2 (8.0) | 1.000 |
| Vancomycin MIC ≥1.5 mg/L | 7 (22.6) | 4 (7.4) | .089 | 6 (23.1) | 1 (3.4) | .044* | 1 (20.0) | 3 (12.0) | .538 |
| Phenotypic characteristics | |||||||||
| agr functionality | |||||||||
| Negative | 9 (29.0) | 20 (37.0) | 8 (30.8) | 11 (37.9) | 1 (20.0) | 9 (36.0) | |||
| Weak | 8 (25.8) | 16 (29.6) | .548 | 7 (26.9) | 6 (20.7) | .806 | 1 (20.0) | 10 (40.0) | .392 |
| Strong | 14 (45.2) | 18 (33.3) | 11 (42.3) | 12 (41.4) | 3 (60.0) | 6 (24.0) | |||
| β-hemolysis | 28 (90.3) | 47 (87.0) | .740 | 23 (88.5) | 25 (86.2) | 1.000 | 5 (100.0) | 22 (88.0) | 1.000 |
| Biofilm formation, mean ± SD, OD 595 nm | 0.12 ± 0.07 | 0.12 ± 0.12 | .373 | 0.13 ± 0.07 | 0.13 ± 0.16 | .231 | 0.10 ± 0.03 | 0.12 ± 0.07 | .957 |
| Molecular epidemiology | |||||||||
| Clonal complex | |||||||||
| CC5 | 10 (32.3) | 16 (29.6) | 8 (30.8) | 4 (13.8) | 2 (40.0) | 12 (48.0) | |||
| CC15 | 4 (12.9) | 3 (5.6) | 3 (11.5) | 2 (6.9) | 1 (20.0) | 1 (4.0) | |||
| CC30 | 4 (12.9) | 8 (14.8) | .574 | 4 (15.4) | 5 (17.2) | .276 | 0 (0.0) | 3 (12.0) | .625 |
| CC45 | 2 (6.5) | 9 (16.7) | 1 (3.8) | 6 (20.7) | 1 (20.0) | 3 (12.0) | |||
| Other | 11 (35.5) | 18 (33.3) | 10 (38.5) | 12 (41.4) | 1 (20.0) | 6 (24.0) | |||
| agr group | |||||||||
| agr I | 7 (22.6) | 18 (33.3) | 5 (19.2) | 10 (34.5) | 2 (40.0) | 8 (32.0) | |||
| agr II | 15 (48.4) | 19 (35.2) | .434 | 12 (46.2) | 7 (24.1) | .196 | 3 (60.0) | 12 (48.0) | .675 |
| agr III | 9 (29.0) | 17 (31.5) | 9 (34.6) | 12 (41.4) | 0 (0.0) | 5 (20.0) | |||
Three patients had unknown outcomes.
Abbreviations: CC, clonal complex; DAIR, debridement, antibiotics, and implant retention; IQR, interquartile range; MIC, minimum inhibitory concentration; OD, optical density.
aData obtained at diagnosis, before the performance of surgical treatment (either debridement or prosthesis removal).
bCalculated for patients not failing in the first 30 days of treatment.
*These results are statistically significant.
Antimicrobial Susceptibility in Biofilm (CBD)
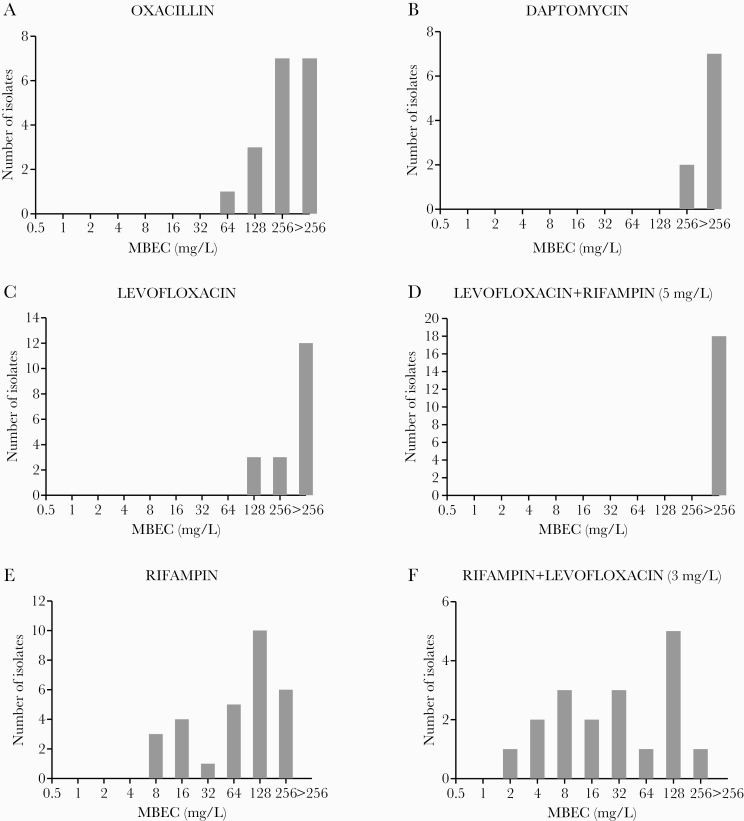
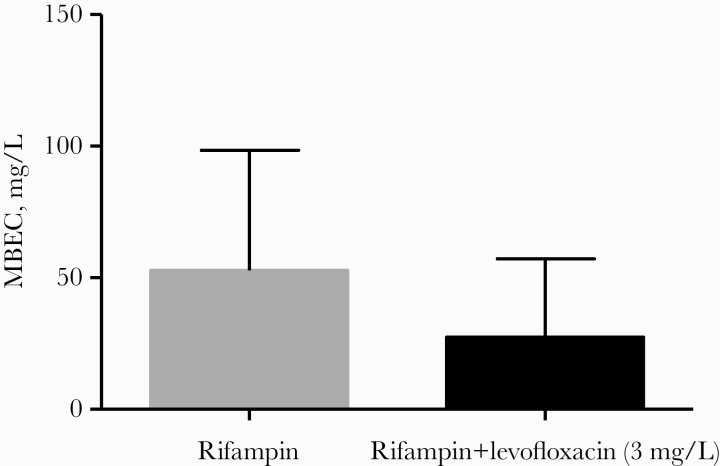
Biofilm susceptibility to antimicrobials was assessed in antibiotics administered for a significant period of time (≥14 days in the first 30 days or ≥21 days over the whole treatment) in cases managed with DAIR. All strains were susceptible to daptomycin oxacillin, levofloxacin, and rifampin by MIC criteria. The MBEC distribution for these antimicrobials is represented in Figure 1 and Supplementary Table 5. MBEC50/90 values for all antibiotics in monotherapy were >256.0 mg/L, except for oxacillin (MBEC50 256.0 mg/L) and rifampin (MBEC50/90 128.0 and 256.0 mg/L, respectively). The addition of a fixed concentration of levofloxacin showed a nonsignificant reduction in the MBEC of rifampin (Figures 1F and 2). Conversely, the addition of a fixed concentration of rifampin led to a nonsignificant increase in the MBEC of levofloxacin (Figure 1D). Overall, we found no association between MBEC values and clinical outcome (Supplementary Table 6).
Figure 1.
Distribution of minimal biofilm eradication concentration (MBEC) for oxacillin (A), daptomycin (B), levofloxacin (monotherapy) (C), levofloxacin in combination with a fixed concentration of 5 mg/L of rifampin (D), rifampin (monotherapy) (E), and rifampin in combination with a fixed concentration of 3 mg/L of levofloxacin (F).
Figure 2.
Geometric mean (95% CI) in all cases of rifampin minimal biofilm eradication concentration (MBEC) for rifampin monotherapy and in combination with a fixed concentration of 3 mg/L of levofloxacin.
DISCUSSION
In this prospective multicenter study, we explored in some detail the relationships between clinical and microbiological (phenotypic and genotypic) features of staphylococcal PJI beyond species and the antibiogram that could lead to a better understanding of pathogenesis and prognosis.
We observed a high clinical, microbiological, and genetic diversity of S. aureus causing PJI. Sixteen different staphylococcal CCs were observed in our cohort, with CC5 being the most frequent [6, 18–20]. The frequency of MRSA in our study was similar to other studies [17, 21, 22]. The genetic backgrounds of MSSA and MRSA are known to be different [23], as is reflected in the distribution of genes. Remarkably, MRSA exhibited a higher biofilm-producing ability. Nevertheless, we found few clinical differences between MRSA and MSSA infections, except for a lower frequency of hematogenous acquisition among the former [17]. Indeed, MRSA PJI is fundamentally a postoperative phenomenon, as is illustrated by its greater CC homogeneity (85% CC5), which is consistent with hospital-related source of acquisition [18].
As expected, the clinical presentation of staphylococcal PJI showed a predominance of acute-onset forms (EPI and HI) [1, 2]. Although we were unable to draw firm conclusions, the different types of infection may be partly explained by the different agr functionality, which is less functional in CPI cases. In acute presentations, agr generally enhances pathogenesis by increasing the expression of aggressive virulence determinants. In contrast, dysfunctional agr has a more complex role in chronic infections, leading to biofilm formation [5]. There is also a virulence tradeoff in favor of antibiotic resistance, which was most frequent in our cases of CPI [24].
A comparison of bacteremic and nonbacteremic HI could shed some light on the pathogenesis of this type of PJI. We observed more revision prostheses among nonbacteremic cases (which are at a higher risk of infection during surgery [25]), it took less time to develop infection, and strains had a higher biofilm-forming ability. Although the absence of bacteremia does not rule out a hematogenous route of infection, it may suggest that some of them were the result of reactivation of a long-term latent inoculum of staphylococci that most likely reached the prosthesis at the time of prosthesis placement. Staphylococcal reactivation in bone tissue is indeed a well-described phenomenon in osteomyelitis [26]. In this connection, some authors have suggested the term “late-acute infection” in order to include any type of PJI presenting as an acute infection at some point after prosthesis placement [27].
With respect to pathogenesis, we observed no significant differences in the EPI group between cases with onset of symptoms <30 days after the index surgery and those with symptoms beginning between days 30 and 90. This is worth highlighting because the time limits defining EPI and CPI are sometimes arbitrary and have changed over time [1–3, 28], and labeling patients with 1 type of PJI or another has direct implications for surgical management and the possibility of performing DAIR [1–3, 10]. Our results are consistent with the similar prognosis for cases managed by DAIR reported elsewhere [17].
The failure rate observed was notable (37% overall, 47% for cases managed with DAIR), but comparable to previous reports [17, 22]. In spite of a thorough analysis of multiple genes and phenotypic microbiological features, we did not find any factor that could be related to the patient’s clinical outcome. Although some reports have associated MRSA infection with an increased risk of failure [6, 29, 30], this has been contested by others [17], and we did not observe a worse prognosis among MRSA infections. Overall, PJI is a very complex infection, in which specific microbiological features may be diluted in the sophisticated interplay of host, surgical, foreign body, and therapeutic factors [31]. Nevertheless, we observed a slightly higher vancomycin MIC in cases with unfavorable outcomes. Although the performance of the vancomycin E-test can produce significant variability [32], a higher vancomycin MIC has been associated with agr dysfunction and biofilm-associated complications [33].
Our results agree with a recent study by Wildeman et al. [34], who observed an association between an antibiotic resistance phenotype, use of non-biofilm-active antimicrobial treatment, and failure, but did not find any association between genetic traits and outcomes of patients with PJIs caused by S. aureus.
As previously mentioned, the standard parameters of antibiotic susceptibility such as MIC show a poor correlation with outcome [1, 35]. There is a need for standardized susceptibility methods for biofilm-associated infections [36–38]. The CBD has been used previously in the setting of PJI [9], although, as far as we know, this is the first study to address the possible correlation between a specific antibiotic MBEC and the clinical results. Overall, the MBECs of oxacillin, daptomycin, and levofloxacin were higher than clinically achievable concentrations and did not show a correlation with patient outcome. As expected, rifampin showed the lowest MBECs, and hence the highest biofilm activity [39], which is consistent with the positive clinical results reported in the literature [17, 22, 28]. Still, the specific rifampin MBEC values were very high and did not show a correlation with outcome. We also explored the MBEC of combinations by adding fixed clinically relevant concentrations of levofloxacin to rifampin, and vice versa, although the results were no better than the monotherapies. There may be several reasons for the lack of correlation between MBEC and outcome: there may be PK/PD factors that influence bone and biofilm antimicrobial activity, such as the postantibiotic effect or the accumulation of antibiotics intracellularly and in biofilms [37, 40]. More research is needed to standardize the activity of antibiotics against biofilm in this clinical scenario.
Our study has some limitations. First, although the number of cases is not small compared with other series, the sample size and comparisons between different strata do not allow us to draw definitive conclusions. Nor can we rule out that some of the associations observed were caused by chance, as the number of comparisons was many. Second, although our study included a wide range of molecular markers of S. aureus, the presence of a given gene does not necessarily imply a specific protein product or cell function. Further phenotypic and transcriptomic studies are needed to achieve a better understanding of the influence of virulence factors of S. aureus on the evolution of PJI.
To conclude, despite a thorough clinical, microbiological, and molecular analysis of staphylococcal PJI, we have not found significant phenotypic or genotypic parameters that may account for the clinical presentation or prognosis of the infection, including the MBEC.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Mercedes Murcia (Servicio de Microbiología, Hospital Universitario 12 de Octubre) for her valuable technical assistance and Janet Dawson for revising the English language in the manuscript.
Collaborators. Raúl Parrón Cambero,1 Álvaro Auñón Rubio,1 Ricardo Fernández Roblas,2 Luis Alcalá Hernández,3 Javier Marco Martínez,4 Berta Laguna Fonseca,5 Juana Cacho,6 and Gloria Pérez Caballero7
Collaborators’ affiliations. 1Servicio de Traumatología y Cirugía Ortopédica, Hospital Universitario Fundación Jiménez Díaz, Madrid, Spain; 2Servicio de Microbiología, IIS-Fundación Jiménez Díaz, Madrid, Spain; 3Servicio de Microbiología y Enfermedades Infecciosas, Hospital General Universitario Gregorio Marañón, Madrid, Spain; 4Servicio de Medicina Interna, Hospital Clínico San Carlos, Madrid, Spain; 5Servicio de Microbiología, Hospital Clínico San Carlos, Madrid, Spain; 6Servicio de Microbiología, Hospital Universitario de Getafe, Getafe, Madrid, Spain; and 7Servicio de Medicina Interna, Hospital Universitario de Getafe, Getafe, Madrid, Spain
Financial support. This work was supported by Planes Nacionales de I+D+i 2008-2011/2013-2016/2019–2020 (Expte PI18/01623) and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía y Competitividad, Spanish Network for Research in Infectious Diseases (REIPI RD16/0016/0002 and PI15/02013), co-financed by the European Development Regional Fund “A Way to Achieve Europe” and operative program Intelligent Growth 2014-2020. The study was also supported by a grant from the Spanish Society of Infectious Diseases and Microbiology (SEIMC) and by a Juan Rodés fellowship grant (Instituto de Salud Carlos III; JR 18/00048 to E.V.) and a Río Hortega fellowship grant (Instituto de Salud Carlos III; CM19/00226 to M.M.L.).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Grupo de Infección Osteoarticular de la Comunidad de Madrid:
Raúl Parrón Cambero, Álvaro Auñón Rubio, Ricardo Fernández Roblas, Luis Alcalá Hernández, Javier Marco Martínez, Berta Laguna Fonseca, Juana Cacho, and Gloria Pérez Caballero
References
- 1. Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med 2004; 351:1645–54. [DOI] [PubMed] [Google Scholar]
- 2. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am 1996; 78:512–23. [DOI] [PubMed] [Google Scholar]
- 3. Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2013; 56:1–25. [DOI] [PubMed] [Google Scholar]
- 4. Zimmerli W, Moser C. Pathogenesis and treatment concepts of orthopaedic biofilm infections. FEMS Immunol Med Microbiol 2012; 65:158–68. [DOI] [PubMed] [Google Scholar]
- 5. Le KY, Otto M. Quorum-sensing regulation in staphylococci—an overview. Front Microbiol 2015; 6:1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ferry T, Uçkay I, Vaudaux P, et al. Risk factors for treatment failure in orthopedic device-related methicillin-resistant Staphylococcus aureus infection. Eur J Clin Microbiol Infect Dis 2010; 29:171–80. [DOI] [PubMed] [Google Scholar]
- 7. Kim CK, Karau MJ, Greenwood-Quaintance KE, et al. Superantigens in Staphylococcus aureus isolated from prosthetic joint infection. Diagn Microbiol Infect Dis 2015; 81:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ceri H, Olson ME, Stremick C, et al. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J Clin Microbiol 1999; 37:1771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Molina-Manso D, del Prado G, Ortiz-Pérez A, et al. In vitro susceptibility to antibiotics of staphylococci in biofilms isolated from orthopaedic infections. Int J Antimicrob Agents 2013; 41:521–3. [DOI] [PubMed] [Google Scholar]
- 10. Ariza J, Cobo J, Baraia-Etxaburu J, et al. ; Spanish Network for the Study of Infectious Diseases and the Sociedad Española de Enfermedades Infecciosas; Microbiología Clínica (SEIMC) Executive summary of management of prosthetic joint infections. Clinical practice guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm Infecc Microbiol Clin 2017; 35:189–95. [DOI] [PubMed] [Google Scholar]
- 11. Walsh TR, Bolmström A, Qwärnström A, et al. Evaluation of current methods for detection of staphylococci with reduced susceptibility to glycopeptides. J Clin Microbiol 2001; 39:2439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Traber KE, Lee E, Benson S, et al. agr function in clinical Staphylococcus aureus isolates. Microbiology 2008; 154:2265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stulik L, Malafa S, Hudcova J, et al. α-hemolysin activity of methicillin-susceptible Staphylococcus aureus predicts ventilator-associated pneumonia. Am J Respir Crit Care Med 2014; 190:1139–48. [DOI] [PubMed] [Google Scholar]
- 14. Lázaro-díez M, Remuzgo-martínez S, Rodríguez-Mirones C. Effects of subinhibitory concentrations of ceftaroline on methicillin-resistant Staphylococcus aureus (MRSA) biofilms. PLoS One 2016; 11:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stepanović S, Vuković D, Hola V, et al. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007; 115:891–9. [DOI] [PubMed] [Google Scholar]
- 16. Monecke S, Slickers P, Ellington MJ, et al. High diversity of Panton-Valentine leukocidin-positive, methicillin-susceptible isolates of Staphylococcus aureus and implications for the evolution of community-associated methicillin-resistant S. aureus. Clin Microbiol Infect 2007; 13:1157–64. [DOI] [PubMed] [Google Scholar]
- 17. Lora-Tamayo J, Murillo O, Iribarren JA, et al. ; REIPI Group for the Study of Prosthetic Infection A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin Infect Dis 2013; 56:182–94. [DOI] [PubMed] [Google Scholar]
- 18. Muñoz-Gallego I, Lora-Tamayo J, Pérez-Montarelo D, et al. Influence of molecular characteristics in the prognosis of methicillin-resistant Staphylococcus aureus prosthetic joint infections: beyond the species and the antibiogram. Infection 2017; 45:533–7. [DOI] [PubMed] [Google Scholar]
- 19. Pérez-Montarelo D, Viedma E, Larrosa N, et al. Molecular epidemiology of Staphylococcus aureus bacteremia: association of molecular factors with the source of infection. Front Microbiol 2018; 9:2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Post V, Wahl P, Uçkay I, et al. Phenotypic and genotypic characterisation of Staphylococcus aureus causing musculoskeletal infections. Int J Med Microbiol 2014; 304:565–76. [DOI] [PubMed] [Google Scholar]
- 21. Byren I, Bejon P, Atkins BL, et al. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother 2009; 63:1264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Senneville E, Joulie D, Legout L, et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin Infect Dis 2011; 53:334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Branger C, Gardye C, Galdbart JO, et al. Genetic relationship between methicillin-sensitive and methicillin-resistant Staphylococcus aureus strains from France and from international sources: delineation of genomic groups. J Clin Microbiol 2003; 41:2946–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Painter KL, Krishna A, Wigneshweraraj S, Edwards AM. What role does the quorum-sensing accessory gene regulator system play during Staphylococcus aureus bacteremia ? Trends Microbiol 2014; 22:676–85. [DOI] [PubMed] [Google Scholar]
- 25. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998; 27:1247–54. [DOI] [PubMed] [Google Scholar]
- 26. Libraty DH, Patkar C, Torres B. Staphylococcus aureus reactivation osteomyelitis after 75 years. N Engl J Med 2012; 366:481–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wouthuyzen-Bakker M, Sebillotte M, Lomas J, et al. ; ESCMID Study Group for Implant-Associated Infections (ESGIAI) Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention. J Infect 2019; 78:40–7. [DOI] [PubMed] [Google Scholar]
- 28. Zimmerli W, Widmer AF, Blatter M, et al. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA 1998; 279:1537–41. [DOI] [PubMed] [Google Scholar]
- 29. Bradbury T, Fehring TK, Taunton M, et al. The fate of acute methicillin-resistant Staphylococcus aureus periprosthetic knee infections treated by open debridement and retention of components. J Arthroplasty 2009; 24:101–4. [DOI] [PubMed] [Google Scholar]
- 30. Salgado CD, Dash S, Cantey JR, Marculescu CE. Higher risk of failure of methicillin-resistant Staphylococcus aureus prosthetic joint infections. Clin Orthop Relat Res 2007; 461:48–53. [DOI] [PubMed] [Google Scholar]
- 31. Trampuz A, Zimmerli W. Prosthetic joint infections: update in diagnosis and treatment. Swiss Med Wkly 2005; 135:243–51. [DOI] [PubMed] [Google Scholar]
- 32. Falcón R, Madrid S, Tormo N, et al. Intra- and interinstitutional evaluation of an etest for vancomycin minimum inhibitory concentration measurement in Staphylococcus aureus blood isolates. Clin Infect Dis 2015; 61:1490–2. [DOI] [PubMed] [Google Scholar]
- 33. Viedma E, Sanz F, Orellana MA, et al. Relationship between agr dysfunction and reduced vancomycin susceptibility in methicillin-susceptible Staphylococcus aureus causing bacteraemia. J Antimicrob Chemother 2014; 69:51–8. [DOI] [PubMed] [Google Scholar]
- 34. Wildeman P, Tevell S, Eriksson C, et al. Genomic characterization and outcome of prosthetic joint infections caused by Staphylococcus aureus. Sci Rep 2020; 10:5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science 1999; 284:1318–22. [DOI] [PubMed] [Google Scholar]
- 36. Macià MD, Rojo-Molinero E, Oliver A. Antimicrobial susceptibility testing in biofilm-growing bacteria. Clin Microbiol Infect 2014; 20:981–90. [DOI] [PubMed] [Google Scholar]
- 37. Sendi P, Zimmerli W. The use of rifampin in staphylococcal orthopaedic-device-related infections. Clin Microbiol Infect 2017; 23:349–50. [DOI] [PubMed] [Google Scholar]
- 38. Coenye T, Goeres D, Van Bambeke F, Bjarnsholt T. Should standardized susceptibility testing for microbial biofilms be introduced in clinical practice? Clin Microbiol Infect 2018; 24:570–2. [DOI] [PubMed] [Google Scholar]
- 39. Zimmerli W, Sendi P. Role of rifampin against staphylococcal biofilm infections in vitro, in animal models, and in orthopedic-device-related infections. Antimicrob Agents Chemother 2019; 63:e01746-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Coiffier G, Albert JD, Arvieux C, Guggenbuhl P. Optimizing combination rifampin therapy for staphylococcal osteoarticular infections. Joint Bone Spine 2013; 80:11–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.