Abstract
Background
Due to increasing multidrug-resistant (MDR) infections, there is an interest in assessing the use of bacteriophage therapy (BT) as an antibiotic alternative. After the first successful case of intravenous BT to treat a systemic MDR infection at our institution in 2017, the Center for Innovative Phage Applications and Therapeutics (IPATH) was created at the University of California, San Diego, in June 2018.
Methods
We reviewed IPATH consult requests from June 1, 2018, to April 30, 2020, and reviewed the regulatory process of initiating BT on a compassionate basis in the United States. We also reviewed outcomes of the first 10 cases at our center treated with intravenous BT (from April 1, 2017, onwards).
Results
Among 785 BT requests to IPATH, BT was administered to 17 of 119 patients in whom it was recommended. One-third of requests were for Pseudomonas aeruginosa, Staphylococcus aureus, and Mycobacterium abscessus. Intravenous BT was safe with a successful outcome in 7/10 antibiotic-recalcitrant infections at our center (6 were before IPATH). BT may be safely self-administered by outpatients, used for infection suppression/prophylaxis, and combined successfully with antibiotics despite antibiotic resistance, and phage resistance may be overcome with new phage(s). Failure occurred in 2 cases despite in vitro phage susceptibility.
Conclusions
We demonstrate the safety and feasibility of intravenous BT for a variety of infections and discuss practical considerations that will be critical for informing future clinical trials.
Keywords: bacteriophage therapy, phage therapy, multidrug-resistant infections
Bacteriophage therapy (BT) is an emerging therapeutic strategy against multidrug resistant infections. We demonstrate safety and successful outcome in 7/10 cases treated with intravenous BT and share lessons learned, BT referral pattern, and regulatory aspects in the US.
Bacteriophages (phages) are viruses that infect specific bacterial hosts to set up lytic and/or lysogenic replicative cycles; lytic phages lead to lysis of bacterial cells, with the newly released virions infecting other bacterial cells in an exponential manner. Phages were first described in the early 20th century and were used in the 1920s and 1930s for treatment of various infectious syndromes, including dysentery, furunculosis, and urinary and respiratory tract infections (though success rates are unclear). Advent of the antibiotic era led to discontinuation of bacteriophage therapy (BT) in Western medicine, though treatment centers have persisted in Eastern Europe and the former Soviet Union [1].
Increased rates of multidrug-resistant (MDR) and extensively drug-resistant bacterial infections have ushered us into the postantibiotic era. We described the first successful case of intravenous BT to treat a systemic MDR infection at our institution in 2017 and since then have pioneered the clinical use of BT in antibiotic-recalcitrant infections in the United States [2]. To assist in this endeavor, the Center for Innovative Phage Applications and Therapeutics (IPATH) was created at the University of California, San Diego, in June 2018. In this report, we describe the outcomes of consult requests made to IPATH and discuss the first 10 cases of BT treated at our center. We focus on incremental knowledge gains made from these early cases that will inform treatment decisions for future cases and phage-related clinical trials.
METHODS
We reviewed all BT consult requests made to IPATH from June 1, 2018, through April 30, 2020. This was done by retrospective review of a prospectively developed database that has de-identified data including reason for request, bacterial pathogen, and disease process. Per institutional review board (IRB) recommendations, review of the de-identified database did not require informed consent. We also reviewed outcomes of 10 cases of BT treated at our center under Food and Drug Administration (FDA) and IRB oversight with informed consent of the patient.
Testing of Susceptibility of Bacteria to Bacteriophages
There is currently no gold standard testing method for developing a “phagogram,” that is, assessing bacterial susceptibility to phage. Phagograms for patients treated at our center were conducted by 2 main methodologies by the phage solution manufacturer.
Biolog method: This consists of inoculation of standardized bacterial suspensions with bacteriophages individually and in combination in 96-well microtiter plates containing tryptic soy media in 1% (vol/vol) tetrazolium dye and incubated at 37°C in a Biolog machine for 24 hours. Bacterial respiration leads to reduction of the tetrazolium dye, leading to a color change, which is depicted as relative units of bacterial growth [3].
Double agar overlay plaque assay: A lawn of the bacterial isolate is grown on an agar plate, and isolates are classified as susceptible when individual plaques are observed in drop tests with serial dilutions of the phage solution [4].
Synergy testing between antibiotics and phages was not routinely performed.
RESULTS AND DISCUSSION
IPATH Consultation Experience
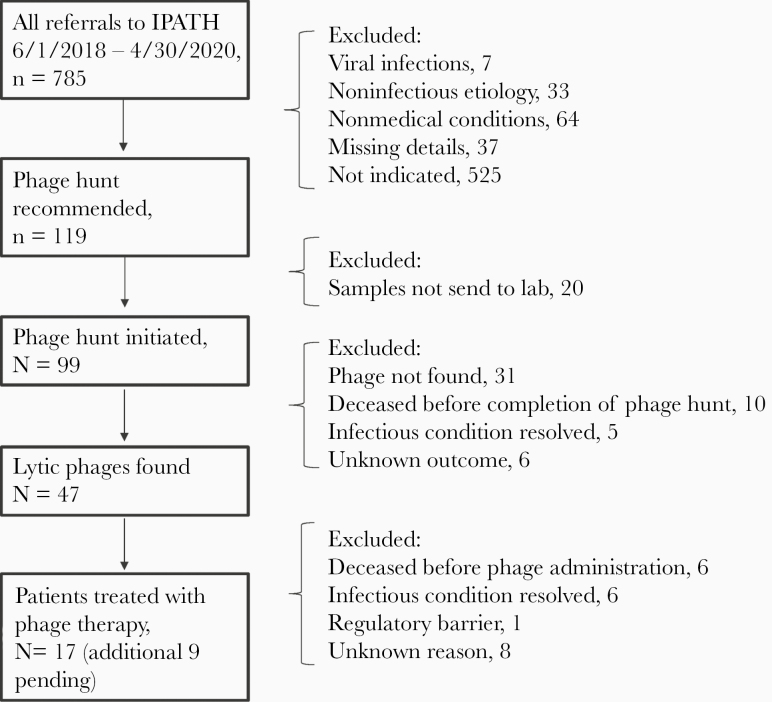
Between June 1, 2018, and April 30, 2020, IPATH fielded a total of 785 requests for BT from patients and physicians within the United States (n = 536, 68.2%) and internationally (n = 158, 20%; missing designation n = 91, 11.6%). Of all requests, the majority were for bacterial infections (n = 644, 82%), 7 (0.9%) were for viral infections, 33 (4.2%) were for noninfectious diseases, and 64 (8.2%) were for nonmedical conditions. IPATH’s role was to assess the validity of the consult requests in order to determine if BT was clinically indicated. We then matched consult requests with commercial/academic laboratories that had experience in developing phages active against a particular bacterial species. We facilitated single-use Investigational New Drug (IND) applications to the FDA and the local IRB and related applications by providing templated material, walking treating physicians through the process, and helping formulate treatment plans. We recommended a phage hunt for BT in 119/644 (18.5%) bacterial cases, though only a minority of patients received BT under compassionate use indications, 17/119 (14.3%), as noted in Figure 1. Reasons for attrition are also noted in Figure 1 and include lack of bacterial isolates for phage susceptibility testing, resolution of the infectious condition, or death of the patient before finding matching lytic phages or before phage administration if matching phages were found.
Figure 1.
Flowchart depicting outcome of all bacteriophage therapy requests at the Center for Innovative Phage Applications and Therapeutics. Abbreviation: IPATH, Center for Innovative Phage Applications and Therapeutics.
Table 1 describes the outcomes of BT requests by bacterial pathogen. As can be noted, the majority of requests among all bacterial infections were for infections due to Pseudomonas aeruginosa (92/644, 14.3%), Staphylococcus aureus (77/644, 12%), and Mycobacterium abscessus (47/644, 7.3%), together accounting for a third of all requests. Other common organisms included enteric gram-negative bacilli Escherichia coli (39/644, 10.7%), Klebsiella pneumoniae (27/644, 4.2%), Enterobacter species (9/644, 1.4%), Burkholderia species (20/644, 3.1%), and Mycobacterium avium (23/644, 3.6%). As noted in Table 1, there was a significant delay from time to request for phage therapy to actual administration to the patient, ranging from 28 to 386 days with a median of 170.5 days.
Table 1.
Outcomes of Request for Phage Therapy Received at IPATH From June 1, 2018, to April 30, 2020, Based on Infecting Bacterial Pathogen
| Number of Requests | Phage Hunt Initiated | Lytic Phage(s) Found | Phage Therapy Administered | Phage Therapy Pending | Median Time From Request to Administration (Range), d | |
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | 92 | 26 | 18 | 5 | 1 | 156.5 (58–374) |
| Escherichia coli | 39 | 6 | 4 | 2 | 2 | 260 (165–355) |
| Klebsiella pneumoniae | 27 | 2 | 0 | 0 | 0 | - |
| Acinetobacter baumannii | 14 | 1 | 0 | 0 | 0 | - |
| Achromobacter species | 8 | 5 | 2 | 0 | 1 | - |
| Enterobacter cloacae | 6 | 2 | 2 | 0 | 1 | - |
| Enterobacter aerogenes | 1 | 1 | 1 | 1 | 0 | 252 |
| Enterobacter species | 2 | 0 | 0 | 0 | 0 | - |
| Serratia marcescens | 4 | 1 | 1 | 0 | 0 | - |
| Citrobacter species | 1 | 1 | 1 | 1 | 0 | 53 |
| Burkholderia cenocepacia | 8 | 6 | 0 | 0 | 0 | - |
| Burkholderia cepacia | 10 | 6 | 1 | 0 | 0 | - |
| Burkholderia gladioli | 1 | 1 | 0 | 0 | 0 | - |
| Bacillus vietnamensis | 1 | 1 | 0 | 0 | 0 | - |
| Pandoraea pulmonicola | 1 | 1 | 0 | 0 | 0 | - |
| Ralstonia mannitolilytica | 1 | 1 | 0 | 0 | 0 | - |
| Staphylococcus aureus | 77 | 4 | 1 | 1 | 0 | 28 |
| Staphylococcus epidermidis | 2 | 0 | 0 | 0 | 0 | - |
| Staphylococcus lugdunensis | 1 | 1 | 0 | 0 | 0 | - |
| Enterococus faecalis | 10 | 0 | 0 | 0 | 0 | - |
| Enterococcus faecium | 2 | 2 | 2 | 2 | 0 | 127 (50–204) |
| Clostridiodes difficile | 8 | 0 | 0 | 0 | 0 | - |
| Mycobacterium abscessus | 47 | 18 | 9 | 4 | 3 | 176 (87–386) |
| Mycobacterium chimera | 7 | 5 | 4 | 1 | 0 | 168 |
| Mycobacterium avium | 23 | 1 | 0 | 0 | 0 | - |
| Mycobacterium smegatis | 1 | 0 | 0 | 0 | 0 | - |
| Mycobacterium chelonae | 2 | 0 | 0 | 0 | 0 | - |
| Mycobacterium xenopi | 1 | 0 | 0 | 0 | 0 | - |
| Mycobacterium bolletii | 1 | 0 | 0 | 0 | 0 | - |
| Mycobacterium genavense | 1 | 0 | 0 | 0 | 0 | - |
| Mycobacterium species | 7 | 0 | 0 | 0 | 0 | - |
| Borrelia burgdorferi | 37 | 0 | 0 | 0 | 0 | - |
| Other organisms | 45 | 0 | 0 | 0 | 0 | - |
| Polymicrobial | 86 | 7 | 1 | 0 | 1 | - |
| Not applicable | 141 | 0 | 0 | 0 | 0 | - |
| Missing organism name | 70 | 0 | 0 | 0 | 0 | - |
| Total | 785 | 99 | 47 | 17 | 9 | Overall median, 170.5 (28–386) |
Among 17 cases who received phage therapy during this time period, 4 were treated at IPATH. This included cases of infection due to Pseudomonas aeruginosa (n = 2), Staphylococcus aureus (n = 1), and Escherichia coli (n = 1).
Abbreviation: IPATH, Center for Innovative Phage Applications and Therapeutics.
Current Status of Compassionate Use Bacteriophage Therapy in the United States
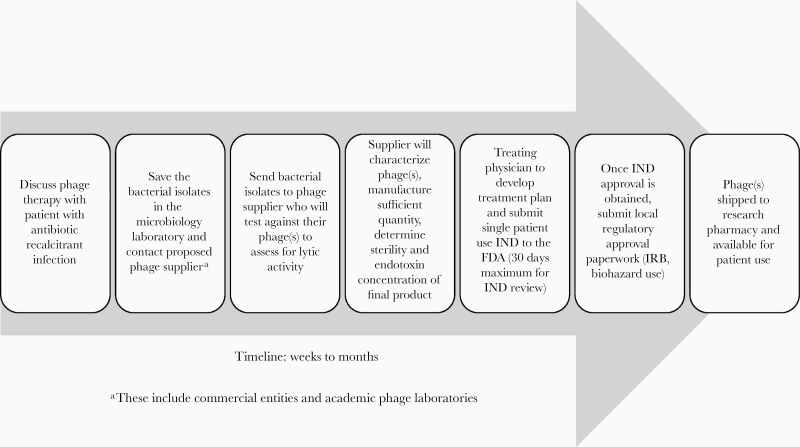
As phage therapy remains experimental, each case required approval from the FDA under a single-use IND. Requirements for a successful application include evidence of clinical need, proof of in vitro bacterial susceptibility to the phage(s), genetic characterization of the phage(s) with particular focus on plasmids encoding for resistance mechanisms, lack of lysogenic activity, sterility of the final product based on USP 71 testing requirements, and minimal endotoxin concentration. Drug resistance genes are often carried on plasmids, which may be transferred to other bacteria through several mechanisms, including horizonal gene transfer via bacteriophages. Temperate phages integrate into the host bacterial genome (then called prophage) and, when they switch to a lytic cycle based on certain environmental triggers, can activate antibiotic resistance genes [5]. The FDA currently requires genetic sequencing of the phages to be used for BT to demonstrate lack of known antimicrobial resistance plasmids in the genome and lack of lysogeny as a safety measure. As phage solutions are manufactured by growing the phage in the host bacterial broth, there is concern for endotoxin contamination. Per the FDA, the maximal allowable endotoxin limit for a single dose is 5-EU/kg body weight per hour of administration. The process from identification of a potential patient to actual clinical phage administration under compassionate use indications is detailed in Figure 2 and, as noted earlier, involves a significant time lag.
Figure 2.
Timeline of the process to initiate bacteriophage therapy for a patient on a compassionate use basis in the United States. Abbreviations: FDA, Food and Drug Administration; IND, Investigational New Drug; IRB, institutional review board.
Lessons Learned From Clinical Use of Bacteriophage Therapy
A brief overview of the first 10 cases of BT at our center is provided in Table 2. Five of these have been published in granular detail in prior reports, and others are pending [2, 6–9]. In general, we treated MDR and antibiotic-recalcitrant cases of abdominal abscesses, pulmonary infections in patients with cystic fibrosis and lung transplant, bone and prosthetic joint infections, ventricular assist device infections, recurrent urinary tract infection, and recurrent bacteremia from an infected aortic graft. All active infections were treated with a combination of phage and systemic antibiotics. Each patient was treated after informed consent and through a single-use IND application and under close oversight by the FDA and local IRB.
Table 2.
Bacteriophage Therapy Cases Treated at the University of California, San Diego; of Note, All Cases Had Previously Failed Multiple Attempts of Antibiotic Treatment and/or Were Due to Highly Drug-Resistant Organisms
| Age, Gender | Type of Infection | Organism | Bacteriophage | Phage Concentration, Dosing, and Duration | Key Clinical Points and Outcome | |
|---|---|---|---|---|---|---|
| 1 | 68-year-old male [2] | Infected pancreatic pseudocyst, abdominal abscesses, and septic shock | Acinetobacter baumannii | φIV (4 phages), φIVB (2 phages), φPC (4 phages)a | 5 × 109 PFU/mL q2h h (16 wk) followed by 5 × 109 PFU/mL IV q6h (2 wk); φPC was instilled percutaneously into abdominal drains q8–12h (18 wk) | Patient had an episode of necrotizing pancreatitis complicated by XDR A. baumannii infected pseudocyst, abdominal abscesses, and critically ill on 3 vasopressors (ongoing infection for 4 mo before BT). Received BT+ antibiotics. Patient recovered fully from the infection. |
| Duration from phage request to administration: 21 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Success | ||||||
| 2 | 67-year-old male [3] | Pneumonia in lung transplant recipient | Pseudomonas aeruginosa | Episode 1: AB-PA01 (4 phages)b | Episode 1: 4 × 109 PFU/mL IV q6h, nebulized q12h (4 wk) | Post-transplant course complicated by recurrent MDR P. aeruginosa pneumonia. Had 2 distinct pneumonia episodes resolved with BT + antibiotics followed by phage alone as suppressive therapy. No infection recurrence while on suppressive phage. |
| Episode 2: AB-PA 01 m1 (5 phages)b and Navy phage cocktail 1 (3 phages)a | Episode 2: 5 × 109 PFU/mL IV q6h, nebulized q12h and 1 × 109 PFU/ mL IV q2h, nebulized q4h (4 wk) | Duration from phage request to administration: 22 d | ||||
| Suppression: Navy phage cocktail 2 (2 phages)a | Suppression: 5 × 107 PFU/mL IV q4h (8 wk) | AE: No phage-related adverse events | ||||
| Outcome: Success | ||||||
| 3 | 77-year-old male [6] | Subdural and epidural empyema and cranial osteomyelitis | Acinetobacter baumannii | Single phagea | 2.14 × 107 PFU/mL IV q2h (8 d) | Patient underwent a craniectomy for trauma complicated by MDR A. baumanni subdural and epidural empyema and cranial osteomyelitis status post debridement and flap repair. Flap healed while on BT but patient was transitioned to hospice due to lack of neurologic improvement. |
| Duration from phage request to administration: 12 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Un-interpretable | ||||||
| 4 | 26-year-old female [5] | Pneumonia in cystic fibrosis | Pseudomonas aeruginosa | AB-PA 01 (4 phages)b | 4 × 109 PFU/mL IV q8h (8 wk) | Acute on chronic respiratory failure due to MDR P. aeruginosa pneumonia unresponsive to antibiotics. Resolved with BT + antibiotics and no further CF flare for the next 3 mo; underwent lung transplant 9 mo later. Colistin was stopped while on BT and acute kidney injury resolved. |
| Duration from phage request to administration: 23 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Success | ||||||
| 5. | 60-year-old male | Ventricular assist device infection | Pseudomonas aeruginosa | GD-1 (3 phages)a | 1.9 × 107 PFU/mL IV q8h (6 wk) | Patient developed MDR P. aeruginosa infection of the driveline complicated by sternal osteomyelitis and recurrent bacteremia for ~9 mo before BT with multiple surgical debridements and prolonged antibiotic courses. Developed bacteremia 1 wk after starting BT, resolved with change in antibiotics. Developed recurrent DL drainage following end of BT. |
| Duration from phage request to administration: 37 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Failure | ||||||
| 6 | 65-year-old male [4] | VAD infection | Staphylococcus aureus | AB-SA 01 (3 phages)b | 3 × 109 PFU/mL q12h (4 wk) | Patient had a VAD infection, sternal osteomyelitis, and recurrent bacteremia for >2 y before BT with multiple surgeries and prolonged courses of IV antibiotics and an open chest with visible device. Infection resolved with BT + antibiotics, and he underwent successful heart transplant. |
| Duration from phage request to administration: 28 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Success | ||||||
| 7 | 61-year-old female | Prosthetic joint infection | Staphylococcus aureus | Episode 1: AB-SA01 (3 phages)b | Episode 1: 3 × 109 PFU/mL q12h (2 wk) + 1-time intra-articular injection | Patient had persistently infected right total knee arthroplasty for >2 y before BT with several surgeries, prosthesis exchange, and prolonged IV antibiotics. |
| Episode 2: SaGR51øK (single phage)a | Episode 2: 2.89 × 1010 PFU/mL 1-time intra-operative dose + IV q12h (6 wk) | Patient’s pain, swelling, and purulent drainage significantly improved at the end of 2 wk of treatment in episode 1 but then recurred 5 d later. She was then re-treated almost 6 months later (episode 2) with surgical revision, systemic antibiotics, and BT with resolution of S. aureus infection. | ||||
| Duration from phage request to administration: 28 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Success | ||||||
| 8 | 82-year-old male | VAD infection | Pseudomonas aeruginosa | Episode 1: SDSU1 (2 phages: PAK_P, E217) and SDSU2 (2 phages: PAK_P1, PAK_P5)b | Episode 1: 2 × 105 PFU/mL IV q8h (6 wk) + 1-time intra-operative dose followed by PAK_P1 7.58 × 105 PFU/mL alone for 10 d followed by SDSU2 4 × 1010 IV q12h for 3 wk | Patient had a persistently infected VAD complicated by multiple hospitalizations, surgical debridements, and recurrent bacteremia for >2 y before BT. He underwent 2 rounds of BT, developed recurrent bacteremia within 1 wk of ending BT in episode 1. Retreated with BT 3.5 mo later but had recurrent bacteremia 4 wk into episode 2 while still on BT. |
| Episode 2: PPM3 (4 phages)b | Episode 2: 1 × 109 PFU/mL q12h (4 wk) | Duration from phage request to administration: 58 d | ||||
| AE: Developed fever, wheezing, and shortness of breath after 2 infusions of SDSU2 1 × 011 PFU/mL concentration; same phage was well tolerated at the 1010 PFU/mL concentration. Other phage formulations well tolerated. | ||||||
| Outcome: Failure | ||||||
| 9 | 56-year-old male | Recurrent urinary tract infection in liver transplant recipient | Escherichia coli | UCS1 (4 phages)b | 1.0 × 109 PFU/mL IV q12h (2 wk) | Recurrent extended-spectrum beta-lactamase-producing E. coli UTI and prostatitis in a patient with chronic kidney disease, kidney stones, and immunosuppression. Pre-BT, patient was intermittent on systemic antibiotics for 1 y with recurrence of symptoms 1–3 wk after antibiotics discontinuation. After end of BT + ertapenem, there was a lack of recurrent symptomatic UTI symptoms with 12 wk of follow-up though urine culture was positive. |
| Duration from phage request to administration: 355 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Success | ||||||
| 10 | 64-year-old male | Recurrent bacteremia and probable aortic graft infection | Pseudomonas aeruginosa | PPM2 (3 phages)b | 2.6 × 106 PFU/mL IV q12h (6 wk) | Recurrent bacteremia for the past 1.5 y with prolonged antibiotic courses and breakthrough infection. Negative blood cultures while on BT + ciprofloxacin. Weekly surveillance blood cultures remained negative ×4 with no recurrence for 12 wk after end of therapy (previous bacteremia recurrences occurred 7–10 d off antibiotics). |
| Duration from phage request to administration: 374 d | ||||||
| AE: No phage-related adverse event | ||||||
| Outcome: Success |
All patients received BT along with concomitant systemic antibiotics except for suppressive phage therapy in Patient 2. The first 6 patients received BT before the formation of IPATH. Phage manufacturers and bacterial susceptibility testing methods are noted in the footnote [3, 4].
Phage manufacturers: φIV, φIVB, φPC and single phage for Patient 3—Naval Medical Research Center, Fort Detrick, MD, USA; Navy Phage Cocktail 1, Navy Phage Cocktail 2, GD-1, and SaGR51øK—Adaptive Phage Therapeutics, Gaithersburg, MD, USA; ABPA-01, ABPA-01m1, and ABSA-01—Armata Pharmaceuticals (formerly Ampliphi Corp), Marina del Rey, CA, USA; SDSU1 and SDSU2—Roach Laboratory, San Diego State University, San Diego, CA, USA; USC1—Tailored Antimicrobials and Innovative Laboratories for Phage φ Research, Baylor College of Medicine, Houston, TX, USA; PPM2 and PPM3 manufactured by Walter Reed Army Institute of Research, Silver Spring, MD, USA.
Abbreviations: AE, adverse event; BT, bacteriophage therapy; CF, cystic fibrosis; DL, driveline; IV, intravenous; MDR, multidrug-resistant; PFU, phage-forming units; UTI, urinary tract infection; VAD, ventricular assist device; XDR, extensively drug resistant.
Bacterial susceptibility testing (phagogram) methods:
aBiolog method.
bDouble agar overlay plaque assay.
Intravenous and Nebulized Phage Administration Appears Safe
The first BT case made it clear that application of bacteriophages is a viable therapeutic option for the treatment of systemic MDR infections. This was the first time, to our knowledge, that BT was administered via the intravenous (IV) route and was well tolerated [2]. We have since treated all our patients through the IV route. Case 2 received nebulized phage in addition to IV phage, which was well tolerated, without bronchospasm. Nebulized phage administration was staggered, so there was a 1-week period of nebulized BT only and 1 week of IV BT only during episode 1, in addition to 2 weeks of combined IV and nebulized phage (with concomitant antibiotics). In this case, we determined that an equivalent amount of phage was obtained from the bronchoalveolar lavage specimens regardless of route of administration. However, as noted in Table 2, the nebulized version required 4 times the volume per dose compared with the IV route due to environmental loss, and thus we have focused on using the IV route for subsequent pulmonary infections [6].
One patient, Case 8, developed an adverse event while receiving BT. This occurred when a new phage cocktail at a concentration of 1×1011 phage-forming units (PFU)/mL was first administered. The patient developed fever, wheezing, and shortness of breath ~2 hours after each of 2 consecutive doses, which resolved with acetaminophen, solumedrol, albuterol nebulization, and diphenhydramine. The same phage cocktail was subsequently well tolerated when multiple titrations were administered as a dose escalation study; the patient subsequently received a 1010-PFU/mL dose without incident. Source of fever in this case was unclear; the endotoxin concentration of the original preparation was 4.3 EU per dose (well below the allowable limit of 5 mg/kg per hour per FDA requirements [298 EU in this patient]), the phage solution was sterile, and blood cultures remained negative. We hypothesize that there may have been additional pyrogens in the solution (perhaps related to solvents used during dilution or manufacture), which were then diluted with subsequent lower concentrations of the phage solution.
Outpatient Self-Administered BT Is Feasible
Most of the patients we treated with BT had persistent infections before BT and were well versed with outpatient self-administered parenteral antibiotic therapy. With this in mind and with the object of preventing a long hospitalization (and associated costs) in otherwise ambulatory patients, we have now treated 6 patients (Cases 5–10 in Table 2) with outpatient self-administered BT via an indwelling peripherally inserted central catheter. The first dose of BT was administered in a dedicated research clinic space by S.A. with frequent vital sign monitoring every 15 minutes for the first 2–3 hours following the first dose. An anaphylaxis kit containing antihistamine, epinephrine, and steroid was available at the bedside. Each patient underwent teaching and was given clear instructions on phage storage and administration at home. We generally provided enough phage doses for self-administration for a 1-week period (with some overage), the patient had weekly clinic visits for an in-person evaluation and blood draws and a pharmacy visit for an additional 1-week phage supply. Patients completed a daily symptom diary to assess for adverse events and documented date and timing of phage administration. Case 10 was treated during the COVID-19 pandemic, and S.A. used weekly telemedicine video visits to assess the patient after the initial in-person visit for the first dose of phage administration.
None of the patients treated with BT as an outpatient had a phage-related adverse event while administering phage at home, and all patients reported feeling comfortable with this process. Outpatient administration and avoidance of hospitalization are very important from the patient perspective, and all those treated were appreciative of the convenience while receiving experimental therapy. This was a cost-saving approach, reduced the risk of patients acquiring nosocomial infections, and allowed patients to continue with work or other regular routines. We believe that outpatient administration will be important as clinical trials are developed, in particular if prolonged therapy for a particular clinical indication is needed.
BT May Be Used Alone Safely as Suppressive Therapy
Case 2 received IV BT alone for almost 8 weeks as suppressive therapy in an attempt to prevent recurrence of MDR P. aeruginosa pneumonia [6]. During this time period, he was taken off systemic antibiotics and had no recurrence of P. aeruginosa infection while on BT and for the subsequent 3 months. We demonstrated the safety of phage alone as well as potential efficacy as a preventive approach.
Bacterial Resistance to Phage Can Develop During Therapy but May Be Overcome With New Phage(s)
We noted development of phage resistance during BT in 3 patients (Cases 1, 2, and 8); in each case, this was overcome clinically by matching resistant bacterial isolates to additional new phages in a personalized treatment strategy. Development of new phages occurred in real time, and we were able to treat patients effectively with this approach. Most patients were treated with a combination of several phages with different bacterial receptors to reduce the risk of developing bacterial resistance to the phage(s), though conversely development of resistance may come at a fitness cost to the bacteria as well. One patient, Case 7 with S. aureus prosthetic joint infection, was treated successfully with a single lytic phage without development of resistant organisms. Case 3 was also treated with a single phage, as only 1 was found, though treatment duration was too short to interpret any outcome. In Cases 1, 2, 5, and 8, new bacterial isolates emerged either during the course of treatment or thereafter with different antibiotic susceptibility profiles than the original infecting strain that were more amenable to antibiotic therapy.
Phage and Antibiotics Combination Can Lead to Successful Outcome Despite Presence of In Vitro Antibiotic Resistance
This was assessed in depth for Case 1, whose A. baumannii isolate demonstrated in vitro resistance to minocycline, but the combination of phage and minocycline showed synergistic activity against the organism [2]. We have not assessed phage–antibiotic synergy systematically to date, though there is great interest in developing phage–antibiotic synergy testing assays that will help determine optimal treatment choices for a patient [10]. Phage and antibiotic combinations were tested before BT in Case 9 only and did not show antagonism between the phages and ertapenem, though there was lack of synergy.
Treatment Failure Can Occur Despite In Vitro Phage Susceptibility
Both cases of P. aeruginosa VAD infection were associated with therapeutic failure of adjunctive BT. Both of these were chronic (>1-year) biofilm-based infections. We hypothesize that treatment failure may be related to poor biofilm penetration of the phages and/or presence of multiple biofilm-based pseudomonal strains at baseline that were not all susceptible to the phage(s) administered. Both also developed bacteremia within the first week of BT from pseudomonal strains with different antimicrobial susceptibility patterns that cleared with a change in systemic antibiotic administration. These new bacterial isolates remained susceptible to administered phages in vitro. Case 5 was noted to have phage-specific neutralizing serum at the time of recurrent bacteremia, but we did not have baseline samples for comparison. Additionally, filamentous lysogenized phages are known to occur in P. aeruginosa biofilms as a biofilm-promoting virulence factor [11]; these prophages may be associated with inhibition of adsorption of extraneous phage administered as therapy. Lastly, loss of potency of stored phage is possible, and thus stability studies will be helpful for future cases.
CONCLUSIONS
Our experience with BT highlights the burgeoning interest and need for alternative antimicrobial therapeutics for effective management of infections that are recalcitrant to traditional antibiotics mainly due to multidrug resistance and biofilm characteristics; in just 2 years, we received 785 requests. Based on our referral pattern, the top 3 organisms of interest for BT are P. aeruginosa, S. aureus, and M. abscessus, together comprising ~30% of all referrals, though we noted a wide spectrum of organisms. Identification of the main organisms of interest for BT is helpful for researchers in order to focus their efforts based on clinical need. The safety profile and favorable outcomes of our locally treated cases are highly encouraging for increasing use of BT both on a compassionate use basis and in particular for upcoming clinical trials. Since the creation of IPATH as the first BT center in the United States, other sites in the United States have developed clinical BT centers as well, including Baylor College of Medicine, Mayo Clinic, and Johns Hopkins University. There are also well-established international centers including the Eliava Institute (Tbilisi, Georgia), Queen Astrid Military Hospital (Brussels, Belgium), Centre Hospitalier Universitaire de Lyon (Lyon, France), Westmead Institute for Medical Research (Sydney, Australia), and HUJI-HMC Phage Therapy Institute (Jerusalem, Israel).
The National Institutes of Health and Cystic Fibrosis Foundation have funded phage-related clinical trials and development of bacteriophage libraries focusing on P. aeruginosa and Burkholderia species, and a number of commercial entities are in the process of initiating clinical trials; multiple other funding opportunities are available for basic science, translational, and clinical phage-related work. Factors we believe will be important for successful trials include development of a broadened host range for phage(s) used for BT using genetic engineering techniques, stability testing of the final phage product to ensure that the concentration delivered to the patient at bedside is at goal, pharmacokinetic/dynamic studies to evaluate for optimal dosing concentration, interval, and duration of BT, impact of immune response to the phage on clinical outcome, and rapid phage susceptibility platforms and assessment of phage–antibiotic synergy to identify optimal treatment [12, 13].
In conclusion, our experience with BT highlights the promise of BT for multiple clinical indications. Significant work is needed to identify predictors of success and for design of clinical trials that will lead to more widespread use.
Acknowledgments
We would like to acknowledge the efforts of the Investigational Drug Services Unit at UCSD, in particular the efforts of Mischelle LeFebvre and Jennifer Faccio.
Financial support. The work described in this paper was made by possible by a grant from the UC San Diego Chancellor’s Innovation Fund.
Potential conflicts of interest. Saima Aslam—grant funding from the Mallory Smith Legacy Fund and Cystic Fibrosis Foundation, consultant to Merck unrelated to the current manuscript. Elizabeth Lampley—none. Darcy Wooten—none. Maile Karris—none. Steffanie Strathdee—stock in Adaptive Phage Therapeutics, uncompensated
scientific advisor to NextBiotics. Constance Benson—grants from UCSD Board of Regents Chancellor’s Fund during the conduct of the study; grants from NIH/NIAID, personal fees from GlaxoSmithKline, grants from Gilead Pharmaceuticals, personal fees from IAS-USA, and personal fees from IDSA outside the submitted work. Robert T. Schooley—consultant to CytoDyn, stock options in CytoDyn and Antiva Biosciences, member of the Gilead Sciences Scientific Advisory Board, previously served as an uncompensated member of the AmpliPhi Scientific Advisory Board. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. Each patient in the case series was treated with written informed consent approved by the University of California San Diego Human Research Protections Program (UCSD HRPP) and a specific IND from the Food and Drug Administration. The retrospective review of the case series was approved by the UCSD HRPP protocol number 200163. The review of the de-identified consult request data set did not require institutional review board oversight as determined by the UCSD HRPP.
References
- 1. Green S, Ma L, Maresso A. Phage therapy. In: Schmidt T, ed. Encyclopedia of Microbiology. 4th ed. Amsterdam, Netherlands: Elsevier; 2019:485–95. [Google Scholar]
- 2. Schooley RT, Biswas B, Gill JJ, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2017; 61:e00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henry M, Biswas B, Vincent L, et al. Development of a high throughput assay for indirectly measuring phage growth using the OmniLog™ system. Bacteriophage 2012; 2:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kropinski AM, Mazzocco A, Waddell TE, Lingohr E, Johnson RP. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol Biol 2009; 501:69–76. [DOI] [PubMed] [Google Scholar]
- 5. Torres-Barceló C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg Microbes Infect 2018; 7:168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aslam S, Courtwright AM, Koval C, et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am J Transplant 2019; 19:2631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aslam S, Pretorius V, Lehman SM, Morales S, Schooley RT. Novel bacteriophage therapy for treatment of left ventricular assist device infection. J Heart Lung Transplant 2019; 38:475–6. [DOI] [PubMed] [Google Scholar]
- 8. Law N, Logan C, Yung G, et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 2019; 47:665–8. [DOI] [PubMed] [Google Scholar]
- 9. LaVergne S, Hamilton T, Biswas B, et al. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect Dis 2018; 5:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Segall AM, Roach DR, Strathdee SA. Stronger together? Perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Curr Opin Microbiol 2019; 51:46–50. [DOI] [PubMed] [Google Scholar]
- 11. Secor PR, Sweere JM, Michaels LA, et al. Filamentous bacteriophage promote biofilm assembly and function. Cell Host Microbe 2015; 18:549–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schooley RT, Strathdee S. Treat phage like living antibiotics. Nat Microbiol 2020; 5:391–2. [DOI] [PubMed] [Google Scholar]
- 13. Aslam S, Schooley RT. What’s old is new again: bacteriophage therapy in the 21st century. Antimicrob Agents Chemother 2019; 64:e01987-19. [DOI] [PMC free article] [PubMed] [Google Scholar]