Abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD) is one of the most common chronic liver diseases. The development of NAFLD is closely associated with hepatic lipotoxicity, inflammation, and oxidative stress. The new concept of NAFLD treatment is to seek molecular control of lipid metabolism and hepatic redox hemostasis. Phoenixin is a newly identified neuropeptide with pleiotropic effects. This study investigated the effects of phoenixin 14 against high-fat diet (HFD)-induced NAFLD in mice.
Materials and Methods
For this study, we used HFD-induced NAFLD mice models to analyze the effect of phonenixin14. The mice were fed on HFD and normal diet and also given phoenixin 14 (100 ng/g body weight) by gastrogavage for 10 weeks. The peripheral blood samples were collected for biochemical assays. The liver tissues were examined for HFD-induced tissue fibrosis, lipid deposition and oxidative activity including SOD, GSH, and MDA. The liver tissues were analyzed for the inflammatory cytokines and oxidative stress pathway genes.
Results
The results indicate that phoenixin 14 significantly ameliorated HFD-induced obesity and fatty liver. The biochemical analysis of blood samples revealed that phoenixin 14 ameliorated HFD-induced elevated circulating alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol, and triglyceride levels, suggesting that phoenixin 14 has a protective role in liver function and lipid metabolism. Hematoxylin-eosin (HE) and Oil Red O staining of the liver showed that phoenixin 14 alleviated HFD-induced tissue damage and lipid deposition in the liver. Furthermore, the mice administered with phoenixin 14 had increased hepatic SOD activity, increased production of GSH and reduced MDA activity, as well as reduced production of TNF-α and IL-6 suggesting that phoenixin 14 exerts beneficial effects against inflammation and ROS. The findings suggest an explanation of how mechanistically phoenixin 14 ameliorated HFD-induced reduced activation of the SIRT1/AMPK and NRF2/HO-1 pathways.
Conclusion
Collectively, this study revealed that phoenixin 14 exerts a protective effect in experimental NAFLD mice. Phoenixin could be of the interest in preventive modulation of NAFLD.
Keywords: phoenixin 14, nonalcoholic fatty liver disease, NAFLD, oxidative stress, inflammation, NRF2/HO-1, SIRT1/AMPK
Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined as a series of conditions consisting of the accumulation of excessive fat in the liver, that are not related to alcohol consumption.1 NAFLD is one of the most common chronic liver diseases worldwide, with an estimated prevalence reaching 25%.2 Current studies recognize that NAFLD is involved in the pathogenesis of type 2 diabetes and cardiovascular diseases.3,4 Pathologically, NAFLD is characterized as excessive hepatic lipotoxicity, inflammation, and oxidative stress. The damage caused by lipid deposition includes the production of oxidative products such as malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), or the reduced function of the antioxidant system, including the activity of superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase. As a result, most NAFLD patients have increased serum levels of MDA and 4-HNE, even with hepatic steatosis at an advanced stage.5 Lifestyle intervention is considered to be an option of treating NAFLD, and the antidiabetic drug pioglitazone is also being recommended to improve insulin sensitivity.6 Recent progress in therapeutic implications has been focused on targeting the key pathway of lipid metabolism and hepatic inflammation. AMP-activated protein kinase (AMPK) is the cellular energy sensor, thereby playing a critical role in the regulation of lipid metabolism and oxidative stress. Targeted AMPK activation has been proposed as a promising strategy for the intervention of NAFLD.7 Erythroid 2-related factor 2 (NRF2) is a master regulator of many anti-oxidative proteins. Positive regulation of NRF2 has been considered as another potential molecular target to treat NAFLD.8
Phoenixin is a newly discovered neuropeptide. The original study suggests that phoenixin is a regulator of the reproductive system.9 However, recent research has shown that phoenixin has a wide range of regulatory functions, including glucose metabolism, pain sensation, appetite, anxiety, and cardiovascular regulation.10–12 Phoenixin is highly expressed in different regions of the brain, including the hypothalamus,9 but is also detectable in the circulation and peripheral tissues.10 Phoenixin is known to be involved in gut-brain communication.11 The study of fish shows that both phoenixin 14 and phoenixin 20 target the liver by promoting the expression of the growth hormone receptor 1 (GHR1).13 Similarly, the immunoreactivity of phoenixin is detected in zebrafish liver cells.14 These facts suggest phoenixin 14 could have a direct regulatory role in the liver. So far, only two isoforms of phoenixins have been identified: the 20 amino acid peptide phoenixin-20 and the 14-amino acid peptide phoenixin14. Phoenixin14 is the truncated form of phoenixin 20; both of these isoforms have demonstrated a similar function in most studies. This study investigated the potential role of phoenixin 14 in NAFLD mice models with metabolic disturbance.
Materials and Methods
Mice and Drug Administration
We purchased 8-week old male C57BL/6 mice from Jackson Laboratories (Bar Harbor, U.S.A.). The bodyweights of the experimental mice were measured to be within the range of 20–25 g. The mice were allocated into four groups (10 mice in each group): normal chow (NC) control group; phoenixin 14 treatment group; high-fat diet (HFD) group; HFD + phoenixin 14 group. The mice were housed in a pathogen-free environment with a 12-h light/dark cycle and free access to food and water. Starting at the age of 10 weeks, the mice were fed with either NC (10% of kilocalories fat) or HFD (60% of kilocalories fat; Product D12492, Research Diets, New Brunswick, United States).13,14 At the same time, two groups of mice were given phoenixin 14 (100 ng/g body weight) daily by gastrogavage for a duration of ten weeks. At the end of the experiment, the mice were sacrificed and the body weights were recorded. To record the liver weight, the fat pad was removed from the liver. To harvest the organs, the bodies of mice were perfused with PBS, and the liver tissues were collected for the different experiments. All animal experiments were performed according to the guidelines approved by the Animal Care and Use Committee of Guilin People’s Hospital (GLH-AEA-20170023).
Blood Sample Assay
Peripheral blood samples were obtained after a fasting period of 5 h by orbital eye-bleeding, using a capillary blood collection tube precoated with heparin. The mouse serum was collected by centrifugation at 2500 ×g for 5 min at 4°C and stored at −20°C. To test the blood, the liver-function markers alanine aminotransferase (ALT) and aspartate aminotransferase (AST), and serum levels of triglyceride (TG), and total cholesterol (TC) were determined using a standard biochemical analyzer 7180 (Hitachi Ltd, Tokyo, Japan).
Oxidative Marker Assay
To measure the activity of SOD, GSH, and MDA, fresh liver tissues were homogenized in PBS at 4°C supplemented with protease inhibitor (Roche Diagnostics, Shanghai). The tissue mixtures were centrifuged at 2500 × g for 5 min at 4°C, and the supernatants were collected and aliquoted for different assays. The protein concentrations in the supernatants were measured using a BCA protein assay kit (Thermo Fisher Scientific, USA). The activity of SOD was detected using a commercial SOD colorimetric activity kit (Thermo Fisher Scientific, cat# EIASODC). The expression of glutathione was assessed using a glutathione fluorescent detection kit (Thermo Fisher Scientific, cat# EIAGSHF). The malondialdehyde expression was measured by an MDA assay kit (ABCAM, cat# ab118970). All these procedures were performed by following the corresponding manufacturer’s protocols.
Hematoxylin-Eosin (HE) Staining
The perfused liver tissues were fixed in 4% formalin overnight. The fixed liver tissues were processed in ethyl alcohol and xylene for 5 min each and then embedded in paraffin. Slices of liver sections were dewaxed in xylene three times for 5 min each time, then dehydrated with gradient alcohol (95%, 90%, and 70%), and washed with tap water for 5 min. The slices were then stained with standard HE for 3 min, differentiated with 1% hydrochloric acid for 30 seconds, and stained with 1% eosin-alcohol for 5 min. Next, the slices were hydrated with regular gradient alcohol (70%, 90%, and 95%) and mounted in oil. The histological images were captured by an inverse microscope equipped with an LCD camera (Olympus, Tokyo, Japan).
Oil Red O Staining and Masson Staining
Freshly harvested liver tissues were embedded in optimum cutting temperature (OCT) compound (Sigma; St. Louis, USA) on dry ice. The OCT sections were sliced at −20°C. Subsequent to drying for 5 min, the sliced tissues were stained with Oil Red O solution (Sigma; St. Louis, USA) for 10 min, followed by differentiation with propylene glycol solution for 5 min and hematoxylin counterstaining for 2 min. The slices were flushed with tap water for 5 min and then mounted in oil under a cover slide. The stained sections were imaged under a light microscope (Olympus, Tokyo, Japan). The Oil Red O-stained area was measured using Image J software (NIH, U.S.A.). The data are presented as fold-changes after normalization to control. For Masson staining, the standard protocol of the manufacturer, that is the use of Weigert’s iron haematoxylin solution Set and Trichrome Stain (Masson) Kit (Sigma-Aldrich, USA) was followed.15
Real-Time PCR Analysis
Freshly harvested liver tissues were processed using a glass homogenizer in saline on ice. The cell pellets were collected, and total tissue RNAs were extracted using a commercial High Pure RNA Isolation Kit (Roche Diagnostics, Shanghai). The concentration of RNAs was determined using a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, U.S.A.). Then, 1~2 µg RNAs were used to synthesize cDNA with a One Step PrimeScript RT-PCR Kit (Takara Ltd., Japan). For real-time PCR, the reaction was performed on an ABI 7500 system (Applied Biosystems Inc., Foster City, U.S.A.). The fast PCR conditions recommended by the product were followed: pre-denaturation at 94°C for 3 min, 40 cycles of denaturation at 94°C for 20 s, annealing at 60°C for 30 s, and extension at 68°C for 10 s. GAPDH was selected as a quality control gene, and a 2−ΔΔCT formula was adopted to profile the relative expression levels of the above genes.
Western Blot Analysis
Fresh liver tissue homogenates were collected as described above. The cell pellets were then washed in phosphate-buffered saline (PBS) solution and lysed in RIPA buffer supplemented with protease inhibitor (Roche Diagnostics, Shanghai) on ice. The total protein concentration of the dissolved lysates was quantified using the Bradford method. A sample of 20 μg protein was electrophoresed using polyacrylamide gel (PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane using a wet transfer apparatus (Bio-Rad, Hercules, USA). The blots were then blocked with 5% skimmed milk PBST for 1 h followed by overnight binding with specific antibodies in a cold room, incubation with the secondary antibody for 1 h, and extensive washing. Finally, the reaction was detected using enhanced horseradish peroxidase (HRP) substrate (Thermo Fisher Scientific, Waltham, U.S.A.). The specific antibodies were used to detect murine NRF2 (1:500, ABCAM), HO-1 (1:500, ABCAM), NQO-1 (1:500, ABCAM), p-AMPK (1:1000, Cell Signaling Technologies), total-AMPK (1:1000, Cell Signaling Technologies), SIRT1 (1:1000, Cell Signaling Technologies), β-actin (1:5000, Cell Signaling Technologies), and the secondary rabbit or mouse IgG HRP conjugated antibody (1:3000, Thermo Fisher Scientific, USA). The expression levels of the target proteins were normalized to β-actin.
ELISA
Fresh liver tissue homogenates were obtained as described above. The homogenates were then spun at 10,000 × g for 5 min to collect the supernatant. Two ELISA kits (R&D Systems, cat#. DY410 and DY406) were used to measure the levels of liver TNF-α and IL-6. The assay steps were performed by following the manufacturer’s instructions. The data were collected with a 96-plate reader spectrometry (BioTek, China). The absolute values were obtained from a standardized 4-PL curve.
Statistics
All the experimental data are expressed as mean ± S.D. We performed statistical testing using SPSS 20.0 software (IBM Corp., Armonk, U.S.A.). Differences in more than two groups were tested using the two-way ANOVA method. A value of p<0.05 was considered to be statistically significant.
Results
Phoenixin 14 Decreases Body and Liver Weight in NAFLD Mice
To examine the effect of phoenixin 14 on liver diseases, we administered 100 ng/g body weight of phoenixin 14 to high-fat diet (HFD)-induced NAFLD mice and normal chow (NC)-fed mice. Our results showed that the administration of phoenixin 14 did not change the bodyweights of the NC-fed mice but significantly decreased the bodyweights of the HFD-induced NAFLD mice (Figure 1A). Meanwhile, the administration of phoenixin 14 significantly decreased the liver weights of HFD-fed mice (Figure 1B).
Figure 1.
Administration of phoenixin 14 decreases body weight and liver weight in NAFLD experimental mice. Mice were divided into four groups: normal chow (NC) group; normal chow (NC) + phoenixin 14 group; a high-fat diet group (HFD) group; a high-fat diet + phoenixin 14 (100 ng/g body weight) group. (A) Body weights of mice; (B) Liver weights of mice (**P<0.01 vs vehicle group; ##P<0.01 vs HFD group, $P>0.05 vs vehicle group).
Phoenixin 14 Ameliorates HFD-Induced Damaged Liver Function and Plasma Lipid Levels in NAFLD Mice
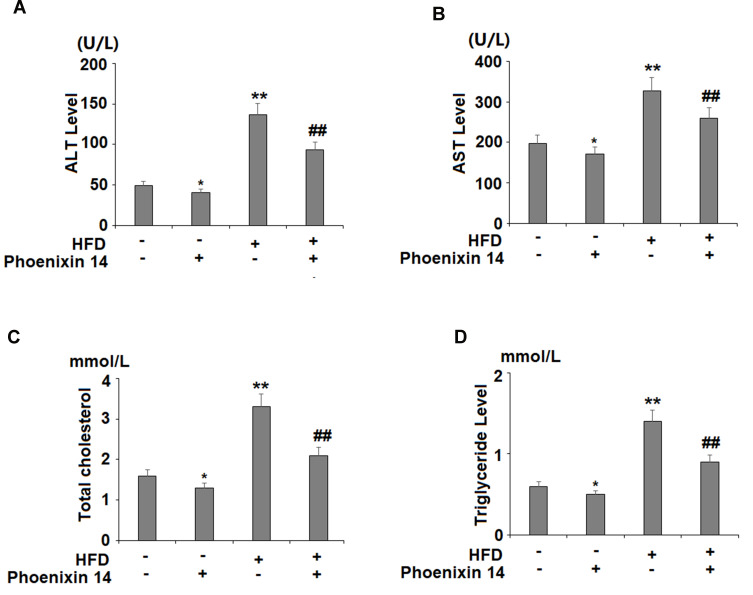
Consecutively, we evaluated the role of phoenixin 14 on liver function by measuring blood ALT and AST activities. Our results showed that HFD elevated both ALT (Figure 2A) and AST levels (Figure 2B). Phoenixin 14 had no effects on ALT and AST levels in NC-fed mice. However, the administration of phoenixin 14 in HFD-fed mice significantly reduced the elevations in ALT (Figure 2A) and AST (Figure 2B). We also measured the plasma total cholesterol and triglyceride levels. HFD feeding increased mice plasma cholesterol (Figure 2C) and triglyceride concentrations (Figure 2D). In NC-fed mice, phoenixin 14 had no effects on the total cholesterol and triglyceride levels. However, phoenixin 14 significantly reduced blood cholesterol (Figure 2C) and triglyceride (Figure 2D) levels in HFD-fed mice.
Figure 2.
Administration of phoenixin 14 improves liver function and increases lipid levels in NAFLD mice. (A) ALT; (B) AST; (C) Total cholesterol; (D) Triglyceride (*, **P<0.05, 0.01 vs vehicle group; ##P<0.01 vs HFD group).
Phoenixin 14 Alleviates HFD-Induced Liver Tissue Damage
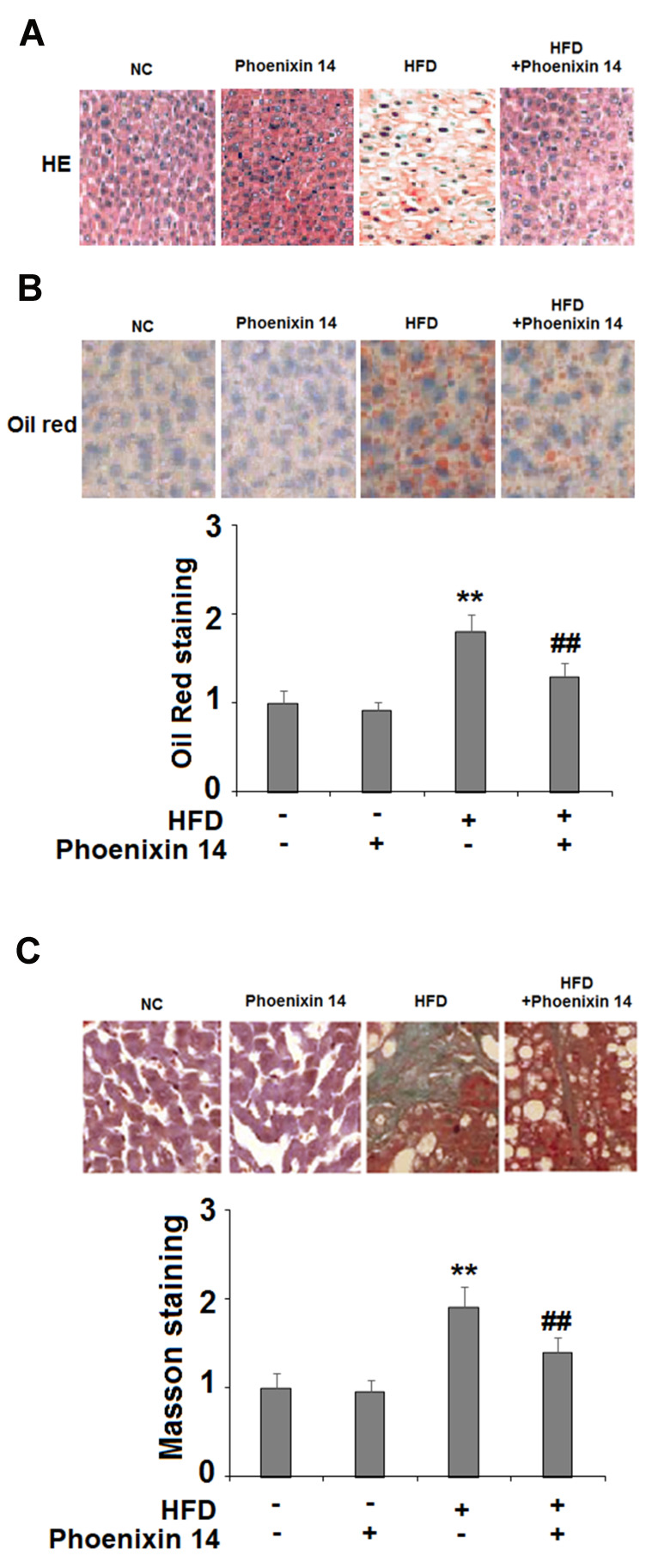
Subsequently, we examined the histopathological features of liver tissues by following the different treatments. NC-fed mice displayed histological features associated with a normal liver, whereas the HFC-fed group exhibited extensive fibrosis and collagen deposition. However, the phoenixin 14 treated group showed substantial alleviation of liver tissue injury, induced by HFD (Figure 3A). Meanwhile, Oil Red O staining showed that mice administered with phoenixin 14 had reduced lipid deposition in the liver tissue induced by HFD (Figure 3B).
Figure 3.
Administration of phoenixin 14 ameliorates hepatic tissue damage, lipid deposition, and fibrosis in HFD-fed mice. (A) Representative liver histological section images; (B) Representative images and quantitative graph of liver Oil Red staining for the four groups of mice; (C) Representative images and quantitative graph of Masson staining for the four groups of mice (**P<0.01 vs vehicle group; ##P<0.01 vs HFD group).
Phoenixin 14 Relieves Liver Oxidative Stress in NAFLD Mice
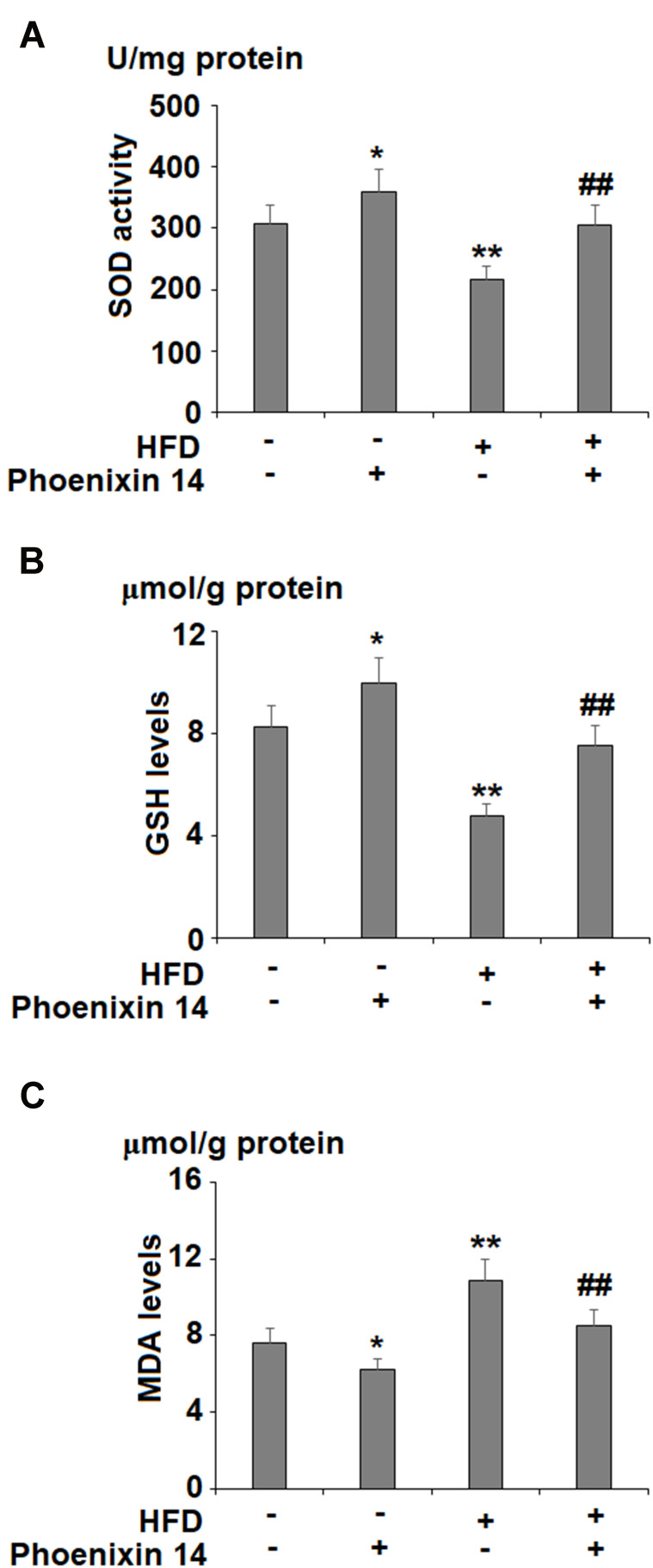
To evaluate the effect of phoenixin 14 on oxidative stress in the liver, we examined liver SOD activity, GSH, and MDA (malondialdehyde) levels. HFD-fed mice showed reduced SOD activity. However, phoenixin 14 treatment increased SOD activity in both the NC- and HFD-fed groups of mice (Figure 4A). Although HFD reduced the GSH level, phoenixin 14 administration exhibited an alleviative effect in both the NC- and HFD-fed mice (Figure 4B). Despite increased MDA expression in the HFD-fed group of mice, phoenixin 14 treatment showed an alleviative effect on MDA levels in both the NC- and HFD-fed groups of mice (Figure 4C).
Figure 4.
Administration of phoenixin 14 ameliorates hepatic oxidative stress in NAFLD mice. (A) SOD activity; (B) GSH levels; (C) MDA levels (*, **P<0.05, 0.01 vs vehicle group; ##P<0.01 vs HFD group).
Phoenixin 14 Reduces Pro-Inflammatory Cytokine Production in Liver
To assess the effect of phoenixin 14 on the inflammatory response, we profiled the production of two major pro-inflammatory cytokines: TNF-α and IL-6. Despite increased TNF-α and IL-6 production in the HFD-fed group, phoenixin 14 administration showed an alleviative effect on both TNF-α and IL-6 levels in NC- and HFD-fed mice, respectively (Figure 5A and B).
Figure 5.
Administration of phoenixin 14 reduces hepatic TNF-α and IL-6 production in NAFLD mice. (A) TNF-α; (B) IL-6 (*, **P<0.05, 0.01 vs vehicle group; ##P<0.01 vs HFD group).
Phoenixin 14 Increases Antioxidant Gene Expression
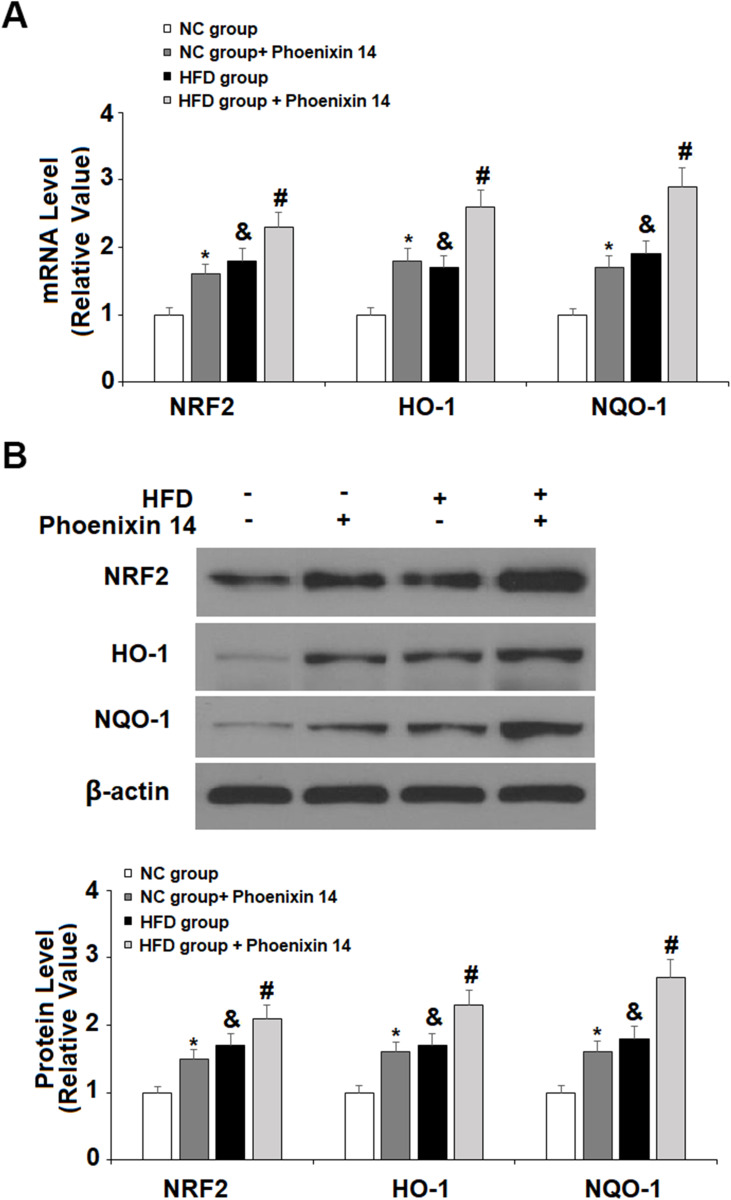
To find the potential molecular targets of phoenixin 14, we tested the expression of several antioxidant genes. At the mRNA level, phoenixin 14 treatment increased the expressions of NRF2, HO-1, and NQO-1 in the liver tissues of NC-fed mice. In HFD-fed mice, its presence promoted the expression of these antioxidant genes induced by HFD (Figure 6A). As for their protein expression, phoenixin 14 similarly increased the expression of these antioxidant proteins (Figure 6B).
Figure 6.
Administration of phoenixin 14 increases the hepatic expression of antioxidant regulators in NAFLD mice. (A) mRNA of NRF2, HO-1, and NQO-1; (B) Protein of NRF2, HO-1, and NQO-1 (*, &P<0.05, vs vehicle group; #P<0.01 vs HFD group).
Phoenixin 14 Ameliorates HFD-Induced Reduced AMPK Activation and SIRT1 Expression
Lastly, we examined the role of phoenixin 14 on the hepatic SIRT1/AMPK pathway. In NC-fed mice, phoenixin 14 induced basal SIRT-1 expression and phospho-AMPKα. In HFD-fed mice, phoenixin 14 treatment largely alleviated decreased SIRT1 expression and phospho-AMPKα induced by HFD (Figure 7).
Figure 7.
Administration of phoenixin 14 mitigates the hepatic reduction of SIRT1 expression and AMPK inactivation in NAFLD mice. Expression of SIRT1 and phosphorylated AMPKα (*, &P<0.05, vs vehicle group; #P<0.01 vs HFD group).
Discussion
Recent research has shown that phoenixins are a class of attractive pleiotropic neuropeptides. Phoenixin 14 is cleaved from the C-terminal of its precursor protein SMIM20. Currently, its availability and degradation in vivo remains unclear. The 14 amino acid phoenixin14 is considered unstable based on amino acid composition, length and an estimated half-life that is 1.1 hour. Emerging data suggest that circulating phoenixin levels are positively correlated with several sex hormones and body weight index (BMI) in women.16 In hypothalamic neurons, phoenixin expression is responsive to specific fatty acid stimulation.17 As active short peptides, phoenixins can be directly delivered in vivo. Through this approach, a recent study revealed that phoenixin 14 injections dose-dependently stimulate food intake in rats in the light phase of housing.18 These facts indicate that phoenixins could act as nutrient sensors to regulate energy homeostasis. Previous studies of fish demonstrated the expression of phoenixins and its possible role in their liver.13 As an important part of the digestive system, we hypothesized that the expression of phoenixin 14 in the liver could play a certain role in energy metabolism. To investigate its function in vivo, we used an HFD-induced NAFLD mouse model. Animal models are crucial to understanding the pathogenesis and treatment of NAFLD. HFD-induced NAFLD mimics several key risk factors of the human condition, such as obesity and metabolic syndrome, and this is a widely used approach in NAFLD rodent studies.19
This study demonstrated that the neuropeptide phoenixin 14 plays an active role in liver lipid metabolism by preventing HFD-induced obesity and fatty liver in NAFLD mice models. When NAFLD mice were administered phoenixin 14 at a dosage of 100 ng/g body weight, the treated mice showed significant loss of body weight and decreased liver mass, indicating that phoenixin 14 is an important regulator of metabolism in the liver or even perhaps in the whole body. The liver is the prime organ for glucose and lipid metabolism, and its physiological function involves the synthesis and storage of cholesterol, free fatty acids, and triglycerides.20 In light of its primary role in metabolism, we examined the liver function in phoenixin 14 treated NAFLD mice. We found that mice administered with phoenixin 14 had improved liver function as revealed by the alleviation of elevated plasma ALT and AST levels. Further, we found that phoenixin 14 significantly mitigated HFD-induced increased plasma cholesterol and triglyceride levels, which may be the result of the improvement in liver function. Thus, we concluded that phoenixin 14 plays a role in regulating lipid metabolism in the liver. Through histological examination, we confirmed that mice that were administered with phoenixin 14 had a better-preserved structure and a reduced lipid deposition in the liver. Biochemical assay of plasma samples revealed that phoenixin 14 treated mice had recovered levels of SOD and GSH, as well as reduced MDA activity, which had been impaired by HFD, thereby indicating an antioxidant capacity of phoenixin 14. Moreover, we found that administration of phoenixin 14 mitigated HFD-induced increased systematic inflammation as revealed by reduced hepatic TNF-α and IL-6. As reported before, the cytokine TNF-α is induced early in the process of liver injury, and the production of TNF-α could facilitate the release of IL-6 and other inflammatory mediators, thus contributing to the progression of NAFLD.21 Hepatic infiltration of neutrophils contributes to HFD-induced tissue injury and fibrosis.22 Macrophages play a central role in HFD-induced inflammation, steatosis and fibrosis.23 A recent publication shows that phoenixin 20 suppresses inflammation in microglial cells (macrophages in CNS),24 suggesting phoenixin may play a role in residential macrophages.
Phoenixin has been shown to stimulate food uptake behavior after fasting and refeeding.13 The mechanism of action of phoenixin 14 on feeding is primarily center controlled in the brain, as intraperitoneal injection of phoenixin 14 during the light phase had no effect on feeding behavior.18 Mice are active in the dark phase and inactive in the light phase. In this study, mice were fed with phoenixin 14 at the light phase and the mice were allowed to access food freely in the dark phase. Thus, we reasoned that it is less likely that the body weight loss could be due to food uptake behavior; instead, it is largely due to its influence on internal metabolism. Phoenixin 14 mediated weight loss could be one reason for the reduction in inflammation, which in turn contributes to alleviating liver injury in NAFLD. Further, phoenixin has been reported to stimulate the expression of genes responsible for glycolysis in cultured zebrafish liver cells.14 The liver plays a major role in the control of glucose metabolism, and it is likely that the administration of phoenixin 14 could have an influence on glucose metabolism in the liver. Its connection to the insulin pathway is another interesting topic to pursue.
This study revealed that phoenixin 14 treatment had an ameliorative effect on AMPK activation and the expression of SIRT1, NRF2, and HO-1 in the livers of mice. AMPK is a central regulator of energy expenditure and metabolism.7 Most recently, liver-specific genetic studies have confirmed the essential role of AMPK in liver damage and steatosis in HFD-induced NAFLD mice.25 SIRT1 is a NAD-dependent histone deacetylase involved in NAFLD, and its activation has been shown to play beneficial roles in regulating hepatic lipid metabolism, oxidative stress, and hepatic inflammation.26 AMPK and SIRT1 activate each other,27 and the activation of this pathway has been shown to play a beneficial role in several studies using NAFLD rodent models.28–30 The NRF2/HO-1 pathway is a key regulatory pathway involved in cellular defense, which controls a series of antioxidant and anti-inflammatory responses in the liver.8,31 NRF2 and its direct target HO-1 play dual roles by regulating both lipid metabolism and antioxidant defense in NAFLD experimental mice.32 Importantly, recent work has shown that AMPK is a direct regulator of NRF2. AMPK promotes nuclear accumulation of NRF2 by direct phosphorylation of NRF2,33 and their crosstalk has been verified both in vitro and in vivo.34 Thus, we hypothesize that phoenixin 14 could act on NRF2/HO-1 pathways mediated by SIRT1/AMPK. We summarized our mechanistic findings in a graphical scheme (Figure 8).
Figure 8.
Graphical summary of the mechanism described in this study.
A major limitation of our study is the inability to demonstrate the mechanistic link between phoenixin 14 and NAFLD. Although the study confirmed that phoenixin 14 ameliorated HFD-induced activation of AMPK and NRF2 pathways, the direct molecular targets of phoenixin 14 remain unclear. AMPK and NRF2 control a broad spectrum of genes for systematic metabolism and stress response, and their connection to phoenixin hormone remains elusive. Another critical question is whether the action of phoenixin 14 is dependent on cell surface receptor. Recent studies report that GPR173 could be a putative receptor of phoenixin,35 and it remains elusive if the action of phoenixin 14 is dependent on GPR173 expression in liver. Furthermore, our study was limited to a one-time point and all mice were exposed to HFD and phoenixin 14 feeding for the duration of 10 weeks. However, it is also possible that long-term administration of phoenixin 14 may change HFD-induced mice behavior that is linked to obesity. It would be informative to examine multiple time points during the process to fully evaluate other factors involved in the progression of NAFLD.
Conclusion
In conclusion, our study revealed a novel function of phoenixin 14 in experimental NAFLD mice. Phoenixin 14 administration in mice exhibited several beneficial effects, including weight loss, liver mass reduction, anti-inflammation, and anti-ROS. Phoenixin 14 alleviated liver injury through the activation of the AMPK/SIRT1 and NRF2/HO-1 pathways. This study provides preclinical evidence supporting the notion that phoenixin 14 could be an interesting agent in the prevention of NAFLD.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 81460160).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: definitions, risk factors, and workup. Clin Liver Dis (Hoboken). 2012;1(4):99–103. doi: 10.1002/cld.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–8276. doi: 10.3748/wjg.v23.i47.8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith BW, Adams LA. Nonalcoholic fatty liver disease and diabetes mellitus: pathogenesis and treatment. Nat Rev Endocrinol. 2011;7(8):456–465. doi: 10.1038/nrendo.2011.72 [DOI] [PubMed] [Google Scholar]
- 4.Patil R, Sood GK. Non-alcoholic fatty liver disease and cardiovascular risk. World J Gastrointest Pathophysiol. 2017;8(2):51–58. doi: 10.4291/wjgp.v8.i2.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fatma U, Sevilay S, Serpil E, Sumeyya A, Ferah A, Omer A. The relationship between oxidative stress and nonalcoholic fatty liver disease: its effects on the development of nonalcoholic steatohepatitis. Redox Rep. 2013;18(4):127–133. doi: 10.1179/1351000213Y.0000000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to treatment. Frontline Gastroenterol. 2014;5(4):277–286. doi: 10.1136/flgastro-2013-100404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730–E740. doi: 10.1152/ajpendo.00225.2016 [DOI] [PubMed] [Google Scholar]
- 8.Xu D, Xu M, Jeong S, et al. The role of Nrf2 in liver disease: novel molecular mechanisms and therapeutic approaches. Front Pharmacol. 2019;9:1428. doi: 10.3389/fphar.2018.01428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yosten GL, Lyu RM, Hsueh AJ, et al. A novel reproductive peptide, phoenixin. J Neuroendocrinol. 2013;2013(25):206–215. doi: 10.1111/j.1365-2826.2012.02381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stein LM, Haddock CJ, Samson WK, Kolar GR, Yosten GLC. The phoenixins: from discovery of the hormone to identification of the receptor and potential physiologic actions. Peptides. 2018;106:45–48. doi: 10.1016/j.peptides.2018.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schalla MA, Stengel A. Phoenixin-A pleiotropic gut-brain peptide. Int J Mol Sci. 2018;19:6. doi: 10.3390/ijms19061726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schalla MA, Stengel A. The role of phoenixin in behavior and food intake. Peptides. 2019;114:38–43. doi: 10.1016/j.peptides.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Deng SP, Chen HP, et al. Phoenixin participated in regulation of food intake and growth in spotted scat, Scatophagus argus. Comp Biochem Physiol B Biochem Mol Biol. 2018;226:36–44. doi: 10.1016/j.cbpb.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 14.Rajeswari JJ, Unniappan S. Phoenixin-20 stimulates mRNAs encoding hypothalamo-pituitary-gonadal hormones, is pro-vitellogenic, and promotes oocyte maturation in zebrafish. Sci Rep. 2020;10:6264. doi: 10.1038/s41598-020-63226-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szymańska-Dębińska T, Karkucińska-Więckowska A, Piekutowska-Abramczuk D, et al. Leigh disease due to SCO2 mutations revealed at extended autopsy. J Clin Pathol. 2015;68:397–399. doi: 10.1136/jclinpath-2014-202606 [DOI] [PubMed] [Google Scholar]
- 16.Ullah K, Ur Rahman T, Wu DD, et al. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin Chim Acta. 2017;471:243–247. doi: 10.1016/j.cca.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 17.McIlwraith EK, Loganathan N, Belsham DD. Phoenixin expression is regulated by the fatty acids palmitate, docosahexaenoic acid and oleate, and the endocrine disrupting chemical bisphenol a in immortalized hypothalamic neurons. Front Neurosci. 2018;15(12):838. doi: 10.3389/fnins.2018.00838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schalla M, Prinz P, Friedrich T, et al. Phoenixin-14 injected intracerebroventricularly but not intraperitoneally stimulates food intake in rats. Peptides. 2017;96:53–60. doi: 10.1016/j.peptides.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 19.Asai A, Chou PM, Bu HF, et al. Dissociation of hepatic insulin resistance from susceptibility of nonalcoholic fatty liver disease induced by a high-fat and high-carbohydrate diet in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G496–G504. doi: 10.1152/ajpgi.00291.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canbay A, Bechmann L, Gerken G. Lipid metabolism in the liver. Z Gastroenterol. 2007;45(1):35–41. doi: 10.1055/s-2006-927368 [DOI] [PubMed] [Google Scholar]
- 21.Tilg H, Moschen AR, Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;64:955–965. doi: 10.1002/hep.28456 [DOI] [PubMed] [Google Scholar]
- 22.Zhou Z, Xu MJ, Cai Y, et al. Neutrophil-hepatic stellate cell interactions promote fibrosis in experimental steatohepatitis. Cell Mol Gastroenterol Hepatol. 2018;5(3):399–413. doi: 10.1016/j.jcmgh.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alharthi J, Latchoumanin O, George J, Eslam M. Macrophages in metabolic associated fatty liver disease. World J Gastroenterol. 2020;26(16):1861–1878. doi: 10.3748/wjg.v26.i16.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng X, Li Y, Ma S, Tang Y, Li H. Phoenixin-20 ameliorates lipopolysaccharide-induced activation of microglial NLRP3 inflammasome. Neurotox Res. 2020;10:1007. [DOI] [PubMed] [Google Scholar]
- 25.Garcia D, Hellberg K, Chaix A, et al. Genetic liver-specific AMPK activation protects against diet-induced obesity and NAFLD. Cell Rep. 2019;26(1):192–208.e6. doi: 10.1016/j.celrep.2018.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017 [DOI] [PubMed] [Google Scholar]
- 27.Hou X, Xu S, Maitland-Toolan KA, et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen XY, Cai CZ, Yu ML, et al. LB100 ameliorates nonalcoholic fatty liver disease via the AMPK/Sirt1 pathway. World J Gastroenterol. 2019;25(45):6607–6618. doi: 10.3748/wjg.v25.i45.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salomone F, Barbagallo I, Godos J, et al. Silibinin restores NAD? Levels and induces the SIRT1/AMPK pathway in non-alcoholic fatty liver. Nutrients. 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liou CJ, Dai YW, Wang CL, Fang LW, Huang WC. Maslinic acid protects against obesity-induced nonalcoholic fatty liver disease in mice through regulation of the Sirt1/AMPK signaling pathway. FASEB J. 2019;33:11791–11803. doi: 10.1096/fj.201900413RRR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colak Y, Yesil A, Mutlu HH, et al. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J Gastrointest Liver Dis GLD. 2014;23:311–319. doi: 10.15403/jgld.2014.1121.233.yck [DOI] [PubMed] [Google Scholar]
- 32.Chambel SS, Santos-Gonçalves A, Duarte TL. The dual role of Nrf2 in nonalcoholic fatty liver disease: regulation of antioxidant defenses and hepatic lipid metabolism. Biomed Res Int. 2015;2015:597134. doi: 10.1155/2015/597134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo MS, Kim WD, Lee KY, et al. Accumulation of Nrf2 by Phosphorylating at Serine 550. Mol Cell Biol. 2016;36(14):1931–1942. doi: 10.1128/MCB.00118-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mo C, Wang L, Zhang J, et al. The crosstalk between Nrf2 and AMPK signal pathways is important for the anti-inflammatory effect of berberine in LPS-stimulated macrophages and endotoxin-shocked mice. Antioxid Redox Signal. 2014;20(4):574–588. doi: 10.1089/ars.2012.5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIlwraith EK, Loganathan N, Belsham DD. Regulation of Gpr173 expression, a putative phoenixin receptor, by saturated fatty acid palmitate and endocrine-disrupting chemical bisphenol A through a p38-mediated mechanism in immortalized hypothalamic neurons. Mol Cell Endocrinol. 2019;485:54–60. doi: 10.1016/j.mce.2019.01.026 [DOI] [PubMed] [Google Scholar]