For more than two decades, antagonism of the deleterious effects of neurohormonal system activation has been the prime focus of treatment for patients with heart failure with reduced ejection fraction (HFrEF).1 Indeed, until ivabradine and the subsequent demonstration of the impressive clinical effects of neprilysin inhibition,2 no new treatment beyond beta-blockade and renin–angiotensin–aldosterone system antagonism had proved effective. We are in an era of rapid growth in evidence for the use of drugs antagonising a much wider range of pathophysiological pathways, including inhibitors of sodium-glucose co-transport protein 2 (SGLT2).2 The VICTORIA (Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction) trial3 now broadens the therapeutic armamentarium even further by demonstrating meaningful benefits of soluble guanylate cyclase (sGC) agonism in patients with worsening or high-risk HFrEF.4
The 5050 patients enrolled in VICTORIA were randomized to placebo or treatment with the sGC stimulator, vericiguat, including a brief titration phase (based on blood pressure and symptoms) aiming to achieve a target 10 mg once daily oral dose. Importantly, patients were required to have evidence of high-risk features of heart failure including heart failure hospitalization in the preceding 6 months or the use of intravenous diuretic in the prior 3 months. Patients in VICTORIA were a higher-risk group than those in PARADIGM-HF [Prospective Comparison of Angiotensin Receptor-Neprilysin Inhibitor (ARNI) with Angiotensin-Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in Heart Failure]2 and DAPA-HF (Dapagliflozin And Prevention of Adverse outcomes in Heart Failure).3 Forty percent of patients had New York Heart Association (NYHA) Class 3 or 4 symptoms (vs. 25% and 32% of patients in PARADIGM and DAPA-HF, respectively) and median N-terminal brain natriuretic peptide (NT-proBNP) was 2816 pg/mL (vs. 1608 pg/mL and 1437 pg/mL in PARADIGM and DAPA-HF, respectively). Consequently, event rates in VICTORIA were high—more than two-fold higher than in PARADIGM and DAPA-HF—and were even greater than expected.4,5 After a median follow-up of only 10.8 months, there was a significantly lower incidence of the primary composite outcome of death from a cardiovascular cause or first heart failure hospitalization in those in the active treatment arm [16.4% with vericiguat vs. 17.5% with placebo (hazard ratio 0.93; 95% confidence interval 0.81–1.06)]. This was driven by a reduction in heart failure hospitalization but there was no reduction in cardiovascular mortality.4 Although the 10% relative risk reduction of the primary outcome may be less striking than that seen in PARADIGM6 or DAPA-HF,3 the absolute reduction in events in this much higher-risk population was similar.5 The number needed to treat with vericiguat for 1 year to avoid one primary outcome event was only 24. Of particular importance, in a population who might now be considered to be ‘at risk’ from polypharmacy, vericiguat was well tolerated with over 89% achievement of the 10 mg target dose and there was no difference in symptomatic hypotension or syncope between groups. While renal function and electrolytes were unaffected, there was a concerning and as yet unexplained greater incidence of anaemia found in those in the vericiguat arm.4
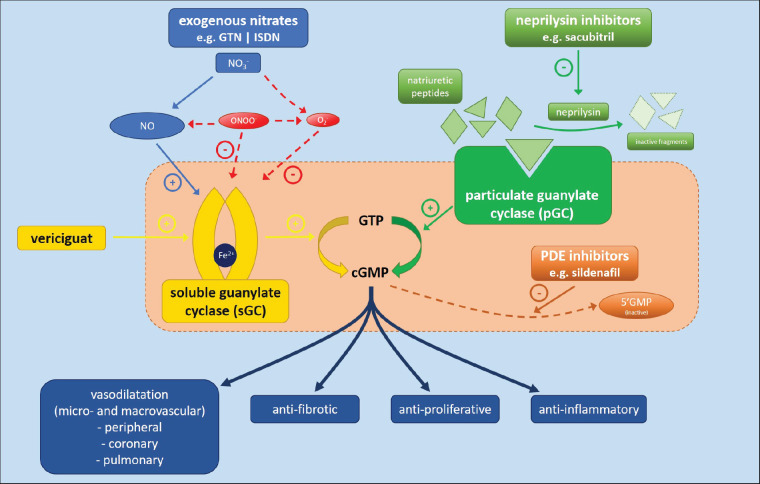
Vericiguat represents a major success in finally harnessing the therapeutic potential of the nitric oxide (NO) pathway (Figure 1). NO exerts its activity via binding to a heme group on sGC to catalyse the synthesis of its second messenger, guanosine 3′,5′-cyclic monophosphate (cGMP). By increasing cGMP, NO has many potentially beneficial effects including peripheral, coronary and pulmonary vasorelaxation and inhibition of smooth muscle proliferation as well as anti-inflammatory, anti-fibrotic and anti-platelet aggregatory properties.7,8 Pharmacological manipulation of the NO pathway is one of the oldest concepts in heart failure therapy and there has been huge effort in this area but the results have, until now, been disappointing.8 Nitrates are still used as an exogenous source of NO in chronic HFrEF1 but, except when used in combination with hydralazine in black patients,9 do not have robust contemporary evidence demonstrating any effect on outcomes and are badly affected by tachyphylaxis.7
Figure 1.
Mechanism of action of pharmacological agents targeting the NO/sGC and neprilysin/pGC pathways to increase cGMP generation. cGMP, 3′,5′-cyclic guanosine monophosphate; GTN, glyceryl trinitrate; GTP, guanosine triphosphate; ISDN, isosorbide dinitrate; NO, nitric oxide; NOS, nitric oxide synthase; PDE, phosphodiesterase; pGC, particulate guanylate cyclase; sGC, soluble guanylate cyclase. Adapted from Friebe et al.13
Oxidative stress associated with cardiovascular disease may change sGC-associated heme from a ferrous to ferric state, weakening its binding to sGC and consequently producing a relatively NO-resistant state.7 It has also been a challenge to capture the potentially beneficial effects of NO without also invoking potentially cytotoxic effects as a consequence of NO reacting with superoxide to produce the reactive oxygen species, peroxynitrite.7 Importantly, however, the beneficial effects of NO seem to be the consequence of sGC-dependent mechanisms while the deleterious effects are independent phenomena. This makes sGC itself a particularly attractive therapeutic target. Vericiguat acts as an sGC stimulator, maintaining the enzyme in its active configuration by stabilizing its nitrosyl-heme interaction. This sGC stimulation allows cGMP production that is independent of NO. It also acts synergistically with NO by sensitizing sGC to even low substrate bio-availability.10 In contrast with the neurohormonal blockers used in contemporary heart failure treatment, vericiguat stands alone in its mechanism of action but, in particular, because it is an agonist. In keeping with the premise of neprilysin inhibition,6 it serves to further highlight the importance of efforts to boost endogenous protective pathways and mechanisms as well as to counteract those that are harmful.
We now find ourselves in the enviable situation of having multiple evidence-based therapies for the treatment of patients with HFrEF. Vericiguat has not been shown to beneficial in all patients with HFrEF and personalized therapy has probably never been more important. VICTORIA enrolled patients with more advanced heart failure4 than other major trials in HFrEF have done in recent years and the findings cannot immediately be extrapolated to patients without recent heart failure hospitalization or the need for intravenous diuretic treatment. On the far end of the spectrum, sub-group analysis of VICTORIA suggested that those in the highest quartile of natriuretic peptide concentration might not have benefitted from vericiguat,4 with cautious interpretation suggesting that these patients may be too sick to benefit. Fewer than 5% of patients in VICTORIA were black.4 This group may be more sensitive to the benefits of NO augmentation and future trials should investigate these potential racial differences.9 Further consideration must also be made to the potential for an incremental effect of vericiguat in addition to treatment with an SGLT2 inhibitor or angiotensin-neprilysin inhibitor (ARNI). The numbers receiving an SGLT2 inhibitor at baseline were not disclosed, although are likely to be small, and only 15% of patients were treated with an ARNI. Neprilysin inhibition also augments guanylate cyclase although its actions are indirect and are upon particulate guanylate cyclase, the membrane-bound receptor for natriuretic peptides, rather than sGC.11 Because of these distinct effects, combined neprilysin inhibition and vericiguat may have synergistic benefits in patients with HFrEF but this remains to be demonstrated.11 Its effects in the presence of phosphodiesterase inhibition are also unknown. Vericiguat was well tolerated in VICTORIA4 and appears easy to titrate, particularly in terms of renal function and blood pressure, two of the frequent limiting factors in the titration of other heart failure medications. However, the association between vericiguat and the development of anaemia requires further evaluation. We also await the results of VICTORIA’s pharmacogenomic and biomarker sub-studies 12 with interest.
Vericiguat has finally delivered sGC and the NO pathway as an effective therapeutic target in HFrEF. Amidst the sudden broadening of treatment options for these patients, the challenge now lies in the appropriate personalization of therapy. This can be achieved by focusing scientific endeavour to understand increasingly complex interactions between multiple pathophysiologic mechanisms and pharmacotherapies. Integrating these basic science data with information derived from large, well-conducted clinical trials will be key to maximizing the huge potential of novel treatments for HFrEF while minimizing the potential risks of polypharmacy.
Conflict of interest: none declared.
Funding
This work was supported by a British Heart Foundation Centre of Research Excellence Award (BHF; RE/18/6/34217).
Authors

Biography: Ninian N. Lang is Clinical Senior Lecturer in Cardiology in the Institute of Cardiovascular and Medical Sciences at the University of Glasgow and also serves as Honorary Consultant Cardiologist at the Queen Elizabeth University Hospital, Glasgow. He is a graduate of the University of Edinburgh Medical School where he also undertook clinical and research training. His clinical focus is upon heart failure and cardiovascular oncology and this is integrated with his translational research in heart failure therapeutics as well as mechanistic studies of vascular and myocardial injury.

Biography: Stephen J.H. Dobbin is a Clinical Research Fellow in the Institute of Cardiovascular and Medical Sciences at the University of Glasgow. He also studied medicine at the University of Glasgow, and he is training as a Cardiology Specialty Registrar in the West of Scotland Deanery. His main research interests are in heart failure and cardiovascular oncology, focusing on the mechanisms of vascular and myocardial injury from cancer therapeutic agents.

Biography: Mark C. Petrie is Professor of Cardiology in the Institute of Cardiovascular and Medical Sciences at the University of Glasgow. He was Director of the Scottish National Advanced Heart Failure Service 2010–2014. He established and chaired the Scottish Heart Failure Hub 2014–2017. He has leadership roles in many clinical trials in heart failure and was an author of the 2011 European Society of Cardiology NSTEMI Guidelines and a reviewer of the 2016 ESC Heart Failure Guidelines. His research interests include coronary artery disease in heart failure, peripartum cardiomyopathy, trials of SGLT2is and GLP1RAs, intravenous iron in heart failure, and myocardial remodelling.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, P van der M, Members AF, Reviewers D; Authors/Task Force Members. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 2. McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR.. Angiotensin–neprilysin inhibition versus enalapril in heart failure. New Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 3. McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, Böhm M, Chiang C-E, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde A-M.. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 4. Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, Lam CSP, Ponikowski P, Voors AA, Jia G, McNulty SE, Patel MJ, Roessig L, Koglin J, O’Connor CM.. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 5. Butler J, Anstrom KJ, Armstrong PW.. Comparing the benefit of novel therapies across clinical trials: insights from the VICTORIA trial. Circulation 2020;382:1883–1893. [DOI] [PubMed] [Google Scholar]
- 6. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen H-D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP.. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 7. Stasch J-P, Pacher P, Evgenov OV.. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 2011;123:2263–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singh P, Vijayakumar S, Kalogeroupoulos A, Butler J.. Multiple avenues of modulating the nitric oxide pathway in heart failure clinical trials. Curr Heart Fail Rep 2018;15:44–52. [DOI] [PubMed] [Google Scholar]
- 9. Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M, Cohn JN; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049–2057. [DOI] [PubMed] [Google Scholar]
- 10. Follmann M, Ackerstaff J, Redlich G, Wunder F, Lang D, Kern A, Fey P, Griebenow N, Kroh W, Becker-Pelster E-M, Kretschmer A, Geiss V, Li V, Straub A, Mittendorf J, Jautelat R, Schirok H, Schlemmer K-H, Lustig K, Gerisch M, Knorr A, Tinel H, Mondritzki T, Trübel H, Sandner P, Stasch J-P.. Discovery of the soluble guanylate cyclase stimulator vericiguat (BAY 1021189) for the treatment of chronic heart failure. J Med Chem 2017;60:5146–5161. [DOI] [PubMed] [Google Scholar]
- 11. Mitrovic V, Hernandez AF, Meyer M, Gheorghiade M.. Role of guanylate cyclase modulators in decompensated heart failure. Heart Fail Rev 2009;14:309–319. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, Ezekowitz J, Hernandez AF, Koglin J, O'Connor CM.. A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail 2018;6:96–104. [DOI] [PubMed] [Google Scholar]
- 13. Friebe A, Sandner P, Schmidtko A.. cGMP: a unique 2nd messenger molecule—recent developments in cGMP research and development. Naunyn-Schmied Arch Pharmacol 2020;393:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]