Abstract
Progressive hand interphalangeal joint (IPJ) osteoarthritis is associated with pain, reduced function and impaired quality of life. However, the evidence surrounding risk factors for IPJ osteoarthritis progression is unclear. Identifying risk factors for IPJ osteoarthritis progression may inform preventative strategies and early interventions to improve long-term outcomes for individuals at risk of IPJ osteoarthritis progression. The objectives of the study were to describe methods used to measure the progression of IPJ osteoarthritis and identify risk factors for IPJ osteoarthritis progression. MEDLINE, EMBASE, Scopus, and The Cochrane Library were searched from inception to 19th February 2020 (PROSPERO CRD42019121034). Eligible studies assessed potential risk factor/s associated with IPJ osteoarthritis progression. Risk of bias was assessed using a modified QUIPS Tool, and a best evidence synthesis was performed. Of eight eligible studies, all measured osteoarthritis progression radiographically, and none considered symptoms. Eighteen potential risk factors were assessed. Diabetes (adjusted mean difference between 2.06 and 7.78), and larger finger epiphyseal index in males (regression coefficient β = 0.202) and females (β = 0.325) were identified as risk factors (limited evidence). Older age in men and women showed mixed results; 13 variables were not risk factors (all limited evidence). Patients with diabetes and larger finger epiphyseal index might be at higher risk of radiographic IPJ osteoarthritis progression, though evidence is limited and studies are biased. Studies assessing symptomatic IPJ osteoarthritis progression are lacking.
Electronic supplementary material
The online version of this article (10.1007/s00296-020-04687-1) contains supplementary material, which is available to authorized users.
Keywords: Hand interphalangeal joint, Osteoarthritis, Risk factors, Disease progression
Introduction
Osteoarthritis is one of the leading causes of worldwide disability [1], and, in the USA alone, carries a cost of $10 billion just from economic loss [2]. Hand osteoarthritis is one of the most common types of radiographic osteoarthritis [1]. Hand osteoarthritis also presents in a younger population than osteoarthritis at other joints, with a prevalence of 3% in men and 8% in women aged 45–64 years [3, 4]. It is considered a chronic disease, with some cases progressing and the prevalence increasing to 5% in men and 9% in women aged 65–74 years [5]. Symptomatic treatment for progressive hand osteoarthritis is limited, with patients often requiring surgical management, such as arthrodesis or arthroplasty [6].
Measuring progressive hand osteoarthritis is difficult, with no consensus for defining or quantifying worsening of disease [7]. The Osteoarthritis Research Society International (OARSI) 2006 Task Force described hand osteoarthritis progression as being joint specific, whereby osteoarthritis in one hand joint evolves independently from other hand joints [8]. However, analysis from a large cohort study suggests there are patterns of symmetry, osteoarthritis clustering by row (across distal interphalangeal joints (DIPJs) or across proximal interphalangeal joints (PIPJs)), and clustering by ray (within a finger) also exist [9]. There is also poor correlation between radiographic and symptomatic disease [10, 11].
The aetiology for the progression of hand osteoarthritis is also poorly understood, and therefore identifying patients at highest risk for needing surgical management is limited. When managing hip and knee osteoarthritis surgically, shared decision making between clinicians and patients has been shown to be beneficial [12]. In the hand, a better understanding of whether a patient is at increased risk of progressive disease would help to inform shared decision making. In particular, it would enable earlier investigations, more personalised treatment pathways, and targeted interventions for prevention and treatment. These priorities have been highlighted by the recent Commission on the Future of Surgery [13]. Similarly, being able to identify patients with osteoarthritis who will not progress will prevent the over-investigation and excessive medical treatment of these patients. A review has found that abnormal scintigraphy scans, higher Australian/Canadian Hand Osteoarthritis Index (AUSCAN) scores, number of osteoarthritis joints at baseline, more pain, and nodal osteoarthritis were risk factors for the progression of radiographic or clinical hand osteoarthritis [14]. However, this review combined interphalangeal joint (IPJ) and base of thumb [first carpometacarpal joint (CMCJ)] osteoarthritis under the umbrella of ‘hand osteoarthritis.’ Finger IPJ and first CMCJ osteoarthritis are now thought to be different subsets of the disease, with different risk factors, pathophysiology and patterns of progression [15].
Therefore, the primary aim of this systematic review is to identify risk factors for the progression of finger IPJ osteoarthritis. The secondary aim is to describe the measurements used to define the progression of IPJ osteoarthritis.
Methods
The reporting of this systematic review followed the Preferred Reporting Items for Systematic reviews and Meta-Analysis (PRISMA) Statement [16]. The protocol was prospectively registered on PROSPERO [17] (CRD42019121034).
Search strategy
The search strategy was constructed with the assistance of a specialist health-care librarian. The search was conducted in four electronic databases: (1) Medline by Ovid, (2) Embase by Ovid, (3) Scopus, (4) the Cochrane library. The search string included a range of search terms for (1) hands and fingers, (2) osteoarthritis, and (3) progression, and was amended for each database (Electronic Supplementary Material 1). The PICOS tool [18] was used to frame the search strategy as follows: population: adults with IPJ osteoarthritis, intervention/prognostic factor: potential risk factor(s) for IPJ osteoarthritis progression, comparison: no exposure to the risk factor(s), outcome: progression of IPJ osteoarthritis, study type: quantitative methodology. The search was conducted on 17th October 2018 and duplicates were removed. The search was updated on 19th February 2020. The reference lists of all eligible articles were manually assessed for additional studies. Rayyan QCRI Tool was used to import all papers [19].
Two groups of reviewers (Group 1: KS, Group 2: XY and JCEL) independently screened titles and abstracts for eligibility. Any articles with insufficient title or abstract information were referred for full text review. Articles for which the full text was not available were requested directly from the authors. Any disagreements in eligibility assessment was resolved at a consensus meeting by a third reviewer (SRF).
Study eligibility criteria
Studies were considered eligible if they (a) included participants with evidence of radiographic or clinical IPJ osteoarthritis at baseline; (b) the participants were followed up for at least 1 year (as it has been shown that progression of radiographic hand osteoarthritis can be detected over a 1 year time frame [20]); (c) IPJ osteoarthritis (separate from first CMCJ osteoarthritis) progression was measured at follow-up, using radiographic and/or symptomatic criteria (IPJ osteoarthritis progression was defined as an increase in radiographic or symptomatic criteria/score at follow-up compared to baseline); (d) the association between a potential risk factor and the progression of IPJ osteoarthritis was investigated at follow-up.
Case reports were excluded. Letters to editors might contain important information about studies, such as new information or discussions of further weaknesses of original studies [21]. Therefore, letters to editors which exist in the context of original studies, included in our review, were examined to inform the risk of bias assessment and as additional sources of information [22]. Conference abstracts are considered to have high variability in terms of data reliability, accuracy and detail, and therefore these were excluded [21, 23].
Studies of inflammatory arthritis, erosive arthritis, with participants under the age of 18 years (to avoid confounding by juvenile arthritis), and studies where IPJ osteoarthritis results could not be separated from other joints including the first CMCJ, and were not provided on request of the corresponding author within 2 months were excluded. Animal, cadaver, and cell studies were excluded. Articles not in English, and articles which lacked accessible full texts (online or in paper copy throughout the UK, or after requesting them from the corresponding author with no reply within 2 months) were excluded.
Data extraction
One reviewer (KS) independently extracted participant demographics (e.g. age and sex), study characteristics (e.g. study design), the potential risk factor/s assessed, effect measure and size/s and the definition/s used to measure osteoarthritis progression. A potential risk factor was defined as any factor investigated for an association with IPJ osteoarthritis progression.
If data was reported at multiple time points, results from all time points were extracted. If articles or supplementary material did not contain sufficient data, the corresponding author was contacted to request additional data, with a 2 month turnaround policy. For any articles which reported data from a study described in detail elsewhere, the source of the data was retrieved and data extracted as appropriate. Data extraction was input into a Microsoft Excel file and cross-checked by a second independent reviewer (XY).
Risk of bias assessment
Two independent reviewers (KS and XY) rated the risk of bias of included studies using a modified version of the Quality in Prognosis Studies (QUIPS) risk of bias tool [24] (Electronic Supplementary Material 2). The following five domains were assessed: (1) study participation, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) statistical analysis and reporting [24]. We excluded the domain assessing ‘Confounding factors’, as confounders can themselves be considered to be prognostic factors, and thus the term ‘confounders’ is a misnomer in prognostic factor studies [25]. As there is currently limited established literature in the field of IPJ osteoarthritis progression, any ‘confounder’ identified in the literature was treated as a potential risk factor for this review. Each domain was given an overall score of ‘low’, ‘moderate’ or ‘high’ risk of bias (Electronic Supplementary Material 2). The overall risk of bias of a study was classified by examining the risk of bias in each of the five domains. If one or more domains were classified as having high risk of bias, then this study was classified as having an overall high risk of bias [24, 26–28]. If three or more domains were classified as having a moderate risk of bias, then this study was classified as having an overall moderate risk of bias [24, 26, 27, 29]. If all domains were classified as having a low risk of bias, or less than three domains had a moderate risk of bias, then this study was classified as having an overall low risk of bias [28, 30]. Any disagreement between reviewers was discussed at a consensus meeting with a third reviewer (SRF).
Analysis and best evidence synthesis
Risk factors for all definitions of IPJ osteoarthritis progression were identified, followed by a subgroup analysis for DIPJ and PIPJ separately. If studies were homogenous with regard to study populations, potential risk factors assessed, effect measures used, and measurements of IPJ osteoarthritis progression, a pooled meta-analysis was considered using Review Manager software [31], and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence [32].
If studies were heterogeneous, we chose not to report effect measures of different types and instead used a qualitative narrative summary. The association between a potential risk factor and IPJ osteoarthritis progression was categorised as:
A risk factor: positive effect measure.
Not a risk factor: negative effect measure; or, no statistical association.
Conflicting evidence: effect measures not in the same direction.
A best evidence synthesis was used to summarise the data for each potential risk factor assessed [33–36]. The criteria were applied sequentially. If multiple analyses were performed within one study, the consistent findings approach described below was applied to the study to decide whether it showed consistent or mixed evidence. This was then used to calculate the overall best evidence synthesis across studies.
Consistent evidence: ≥ 75% of studies reported the same direction of effect (either positive or negative/no association).
Mixed evidence: < 75% of analyses reported the same direction of effect.
If consistent evidence was found, the strength of evidence was assessed:
-
(i)
Strong evidence: > 2 studies with low risk of bias.
-
(ii)
Moderate evidence: 1 study with low risk of bias. and 1 other study; or: > 2 studies with moderate or high risk of bias.
-
(iii)
Limited evidence with low risk of bias: 1 study with low risk of bias.
-
(iv)
Limited evidence: ≤ 2 studies with moderate or high risk of bias.
Results
Studies included
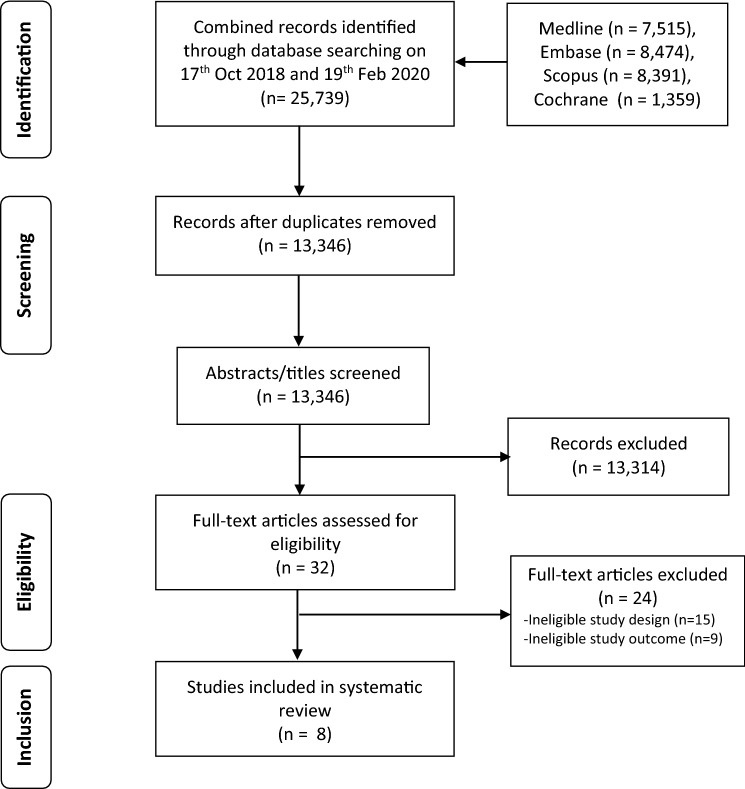
Combining results from the search in October 2018 and the updated search in February 2020, 25, 739 titles were identified through the search strategy, with 13,346 remaining after removal of duplicates. After screening titles and abstracts, the full text of 32 articles was evaluated, and eight articles met the inclusion criteria (Fig. 1). No additional articles were found by reviewing the reference lists of eligible studies.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) flowchart of study selection
Study characteristics
Eight prospective cohort studies were included [37–44] (Table 1). Five studies included men and women [40–44], whilst three studies included only men [37–39]. The smallest study included 177 participants [37], whilst the largest study included 5560 participants [40]. The shortest follow-up period was a mean of 2.28 years [39] and the longest follow-up was reported as a mean (standard deviation) of 23.5 (3.3) years [37].
Table 1.
Characteristics of studies investigating risk factors for the progression of finger interphalangeal joint osteoarthritis
| Authors | Population | Length of follow-up (years) | Age (years) (mean) | Female (%) | Inclusion criteria | Exclusion criteria | N (n) | Criteria for IPJ OA progression | Risk factor assessed |
|---|---|---|---|---|---|---|---|---|---|
| Plato et al. [35] | White middle class volunteers participated in the BLSA in the USA | Group 1: 0–3, group 2: 4–7, group 3: 8–11, group 4: 12–16 | NS | 0 | NS | NS | 478 (NS) | Increase by ≥ 1 grade from the highest KL [41] grade at baseline in any DIPJ | Older age in men |
| Kallman et al. [33] | White middle class volunteers who participated in the BLSA in the USA | ≥ 20 (age < 60 years); ≥ 14 (age ≥ 60 years) | NS | 0 | NS | Maximum KL score (4) at baseline (per patient); Not specified | 177 (177) | Increase by ≥ 1 grade from the highest KL [41, 42] grade at baseline in any PIPJ | Older age in men |
| Busby et al. [34] | White middle class volunteers who participated in the BLSA in the USA | 5–16.3 | NS | 0 | NS | Joints with KL score of 4 at baseline | 386 (NS) |
Outcome 1: increase by ≥ 1 grade from the highest KL [42] grade at baseline in any IPJ (DIPJ and PIPJ assessed separately) Outcome 2: increase in number of IPJs with KL [42] grade ≥ 2 (DIPJ and PIPJ assessed separately) |
Older age in men |
| Kalichman et al. [39] | Chuvashians; Village; Randomly recruited | 8 | Men: 45.3, Women: 49.7 | 52 | NS | NS | 263 (263) | Increase in number of IPJs with KL [42] grade ≥ 2 (DIPJ and PIPJ assessed separately) | Alcohol, anthropometric features, familial relationship, gender (female), older age in men, older age in women, smoking |
| Kalichman et al. [40] | Chuvashians; Village; Randomly recruited | 8 | Men: 47.4, women: 50.9 | 46 | NS | Bone disease, amenorrhoea, hormone replacement therapy, steroids | 557 (513) | Increase by ≥ 1 grade in a cumulative KL [38] sum score (2nd, 3rd and 4th, PIPJs) | Epiphyseal index (larger) |
| Hoeven et al. [36] | Rotterdam | 10 | Men: 67.5, women: 68.6 | 58 | ≥ 55 years, living for ≥ 1 year in Ommoord, knee, hip, hand X-rays | No X-rays, rheumatoid, fractures | 5650 (2442) | Increase by ≥ 1 KL [41] grade in ≥ 1 IPJ, if ≥ 1 IPJ had KL [41] grade ≥ 2 at baseline (DIPJ and PIPJ assessed separately) | Atherosclerosis |
| Haugen et al. [37] | USA; Hospital study sites | 4 | 58.4 | 58 | NS | Systemic inflammatory arthritis, bilateral end stage knee OA, inability to walk without aids, contraindication to MRI | 994 (994) | Increase by ≥ 1 grade in a cumulative modified KL [42, 53] sum score (DIPJ and PIPJ assessed together) | Alcohol (higher intake), BMI (higher)—at age 25, BMI (higher)—current, smoking, waist circumference (higher) |
| Marshall et al. [38] | From CASHA and CASK cohorts; GP community | 7 | 60.5 | 60 | Age 50–69 years at baseline, reported hand pain in last month | Inflammatory arthritis, all hand joints affected with KL ≥ 2 at baseline, deaths/untraceable/address unknown, severe/terminal illness | 706 (388) |
Outcome 1: Increase by ≥ 1 grade in a cumulative KL [43] sum score (DIPJ and PIPJ assessed together) Outcome 2: increase in number of IPJs with KL [43] grade ≥ 2 (DIPJ and PIPJ assessed together) |
BMI (higher)—current, diabetes type 2/impaired fasting glucose, dyslipidaemia, hypertension, number of metabolic factors (higher) |
BLSA Baltimore Longitudinal Study of Aging, BMI body mass index, CASHA Clinical Assessment Studies of the Hand, CASK Clinical Assessment Studies of the Knee, DIPJ distal interphalangeal joint, GP general practice, IPJ interphalangeal joint, KL Kellgren–Lawrence atlas, PIPJ proximal interphalangeal joint, N number at baseline, n number at follow-up, NS not specified, OA osteoarthritis, USA United States of America, X-rays plain film radiographs
Risk of bias
Seven studies were rated as having overall high risk of bias [37–39, 41–44], and one study was of moderate risk of bias [40] (Table 2). ‘Study participation’ was of high risk of bias in four studies due to studies not adequately reporting recruitment periods and places of recruitment [37, 39, 41, 44]. In the ‘Study attrition’ domain, Plato et al. and Kallman et al. did not clearly report response rates and reasons for participants with loss to follow-up [37, 39], whilst Haugen et al. and Marshall et al. had less than 80% response rates and also did not report reasons for loss to follow-up [41, 42]. When assessing the ‘Statistical analysis and reporting’ domain, it was found that Plato et al., Busby et al., and Kalichman et al. did not provide effect measures, but only reported p values or stated whether results were ‘significant or not significant’ [38, 39, 43].
Table 2.
Risk of bias for studies assessing potential risk factors for the progression of finger interphalangeal joint osteoarthritis, assessed using a modified Quality in Prognosis Studies (QUIPS) tool
| Authors | Biasesa | Overall risk of bias | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Plato et al. [35] | High | High | Moderate | Moderate | High | High |
| Kallman et al. [33] | High | High | Low | Low | Moderate | High |
| Busby et al. [34] | Moderate | Moderate | Low | Moderate | High | High |
| Kalichman et al. [39] | Moderate | Low | Moderate | Moderate | High | High |
| Kalichman et al. [40] | High | High | Moderate | Low | Moderate | High |
| Hoeven et al. [36] | Moderate | Low | Moderate | Low | Moderate | Moderate |
| Haugen et al. [37] | High | High | Moderate | Moderate | Moderate | High |
| Marshall et al. [38] | Moderate | High | Low | Moderate | Low | High |
aBiases from modified Quality in Prognosis Studies (QUIPS) tool: (1) study participation; (2) study attrition; (3) prognostic factor measurement; (4) outcome measure; (5) statistical analysis and reporting
Measurements for the progression of finger interphalangeal joint osteoarthritis
All studies assessed osteoarthritis radiographically, using a version of the Kellgren and Lawrence (KL) classification [45, 46] (Table 1). Three studies measured IPJ osteoarthritis progression as a ≥ 1 grade increase from the highest KL grade at baseline [37–39]; three studies measured it as an increase in the total number of IPJs with KL grade ≥ 2 [38, 42, 43]; one study measured progression as a ≥ 1 grade KL increase in a ≥ 1 IPJ [40]; and three studies measured it as ≥ 1 grade increase in a cumulative KL sum score [41, 42, 44]. No studies measured osteoarthritis progression through a deterioration in symptomatic scoring.
Risk factors for the progression of finger interphalangeal joint osteoarthritis
Eighteen potential risk factors were assessed, most commonly in one study only (effect measures shown in Electronic Supplementary Material 3). For potential risk factors assessed by more than one study, due to heterogeneity in the definitions of the risk factor/s, statistical tests, and osteoarthritis definitions, a best evidence synthesis was performed. Three risk factors were identified: diabetes type 2/impaired fasting glucose (IFG) [42]; and larger epiphyseal index (EI) in males [44], and in females [44] (all with limited evidence) (Table 3). Older age in men [37–39, 43] and in women [43] showed mixed results (Table 3).
Table 3.
Potential risk factors for the progression of finger interphalangeal joint osteoarthritis, assessed using a best evidence synthesis
| Consistent evidence for a risk factor | Consistent evidence for not being a risk factor | Mixed evidence | ||||||
|---|---|---|---|---|---|---|---|---|
| Strong evidence | Moderate evidence | Limited evidence with low risk of bias | Limited evidence | Strong evidence | Moderate evidence | Limited evidence with low risk of bias | Limited evidence | |
| Using all definitions of IPJ osteoarthritis progression | ||||||||
| Diabetes/impaired fasting glucose [38] | Higher alcohol intake [37, 39] | Older age in men [33–35, 39]a | ||||||
| Larger epiphyseal index in females [40] | Anthropometric features [39] | Older age in women [39]a | ||||||
| Larger epiphyseal index in males [40] | Atherosclerosis [36] | |||||||
| Larger BMI—at age 25 years [37] | ||||||||
| Larger BMI—current [37, 38] | ||||||||
| Dyslipidaemia [38] | ||||||||
| Familial relationship [39] | ||||||||
| Gender (female) [39] | ||||||||
| Gender (male) [39] | ||||||||
| Hypertension [38] | ||||||||
| Higher number of metabolic factors [38] | ||||||||
| Smoking [37, 39] | ||||||||
| Larger waist circumference [37] | ||||||||
| In DIPJs only | ||||||||
| Older age in women [39] | Higher alcohol intake [39] | Gender (female) [39] | ||||||
| Anthropometric features [39] | Older age in men [34, 39] | |||||||
| Atherosclerosis [36] | ||||||||
| Familial relationship [39] | ||||||||
| Gender (male) [39] | ||||||||
| In PIPJs only | ||||||||
| Larger epiphyseal index in females [40] | Higher alcohol intake [39] | Older age in men [33–35, 39]a | ||||||
| Larger epiphyseal index in males [40] | Anthropometric features [39] | Older age in women [39] | ||||||
| Atherosclerosis [36] | ||||||||
| Familial relationship [39] | ||||||||
| Gender (female) [39] | ||||||||
| Gender (male) [39] | ||||||||
| Smoking [39] | ||||||||
BMI body mass index, DIPJ distal interphalangeal joints, IPJ interphalangeal joint, PIPJ proximal interphalangeal joints
aConflicting results within one study
Diabetes type 2/impaired fasting glucose (IFG)
Marshall et al. assessed diabetes type 2/IFG compared to not having these conditions in a total of 474 participants [42] (effect measures shown in Electronic Supplementary Material 3). In a complete case analysis, these conditions were associated with an increase by ≥ 1 grade in a cumulative KL [47] sum score for all IPJs [adjusted mean difference (95% confidence interval) 7.78 (1.13–14.43)] [42]. However, there was no association following multiple imputation [4.50 (− 0.26 to 9.25)] [42]. Diabetes type 2/IFG was associated with an increase in the number of IPJs with KL [47] grade ≥ 2 following multiple imputation and complete case analysis [2.06 (0.25–3.87) and 3.35 (1.08–5.62), respectively] [42].
Large finger epiphyseal index (EI)
Kalichman et al. investigated larger EI in 177 participants [44] (Electronic Supplementary Material 3). A positive association was found in both males (multiple regression coefficient, β = 0.202; 95% CI not reported) and females (β = 0.325; 95% CI not reported), between larger EI and IPJ osteoarthritis progression (measured as an increase by ≥ 1 grade in a cumulative KL [38] sum score for PIPJs in the assessed digits).
DIPJ and PIPJ subgroup analysis
In the DIPJ subgroup analysis, eight potential risk factors were assessed, and only older age in women was found to be a risk factor (correlation coefficient 0.20) [43] (limited evidence) (Table 3). In the PIPJ subgroup analysis, 11 potential risk factors were assessed, and larger EI in males (β = 0.202; 95% CI not reported) and females (β = 0.325; 95% CI not reported) were identified as risk factors [44] (limited evidence for both) (Table 3).
Discussion
Osteoarthritis is one of the largest health-care burdens, and radiographic hand osteoarthritis is highly prevalent, affecting more than one out of five adult Americans [48]. Osteoarthritis is considered to be progressive in some cases. However, there is no unified method to measure the progression of hand osteoarthritis, and IPJ osteoarthritis is now considered to be a different disease subset from first CMCJ osteoarthritis. As IPJ osteoarthritis progresses, it can be treated surgically, and there are currently no disease-modifying drugs. Risk factors which increase the chance of IPJ osteoarthritis progression in patients have been studied in the literature. We identified eight studies (seven high risk of bias) investigating potential risk factors for the progression of finger IPJ osteoarthritis [37–44]. All studies measured osteoarthritis progression radiographically, using a version of the KL classification system [45, 46]. Our review found that patients with diabetes/IFG [42], and both male and females with a larger finger EI [44], are at increased risk of IPJ osteoarthritis progression (limited evidence), whilst older age in men [37–39, 43] and in women [43] showed mixed evidence. Results were largely similar when DIPJ and PIPJ osteoarthritis when assessed separately.
The KL classification system [45, 46] was used to measure osteoarthritis progression by all studies [37–44, 49]. The KL classification system [45, 46] is a sensitive method for measuring the progression of radiographic hand osteoarthritis over a 1-year time frame [20]. All of the studies included in this review were longitudinal studies, and the shortest follow-up period had a mean of 2.28 years [39]. Therefore, all studies would have adequately detected any radiographic IPJ osteoarthritis progression. However, the definitions of each measure of progression varied across studies. Some studies measured an increase in KL grade [37–40], whilst others measured it as an increase in the number of joints with a particular KL grade [38, 40, 42, 43] and still other studies measured it as an increase in a cumulative KL sum score [41, 42, 44] (which is dependent on either an increase in KL grade of already affected joints, or an increase in the number of joints with a particular KL grade). The sensitivity of the KL classification system [45, 46] in detecting IPJ osteoarthritis progression measured in these different ways has not yet been investigated. Additionally, potential risk factors that occur at a localised joint level (such as joint trauma) could also be risk factors for isolated IPJ osteoarthritis progression. However, localised risk factors were not assessed by studies in this review. Further research is required to understand whether there are any joint-specific risk factors for IPJ osteoarthritis progression, and whether these might cause osteoarthritis to progress at one joint independently of other IPJs.
Diabetes/IFG was found to be a risk factor for IPJ osteoarthritis progression [42]. Marshall et al. suggest diabetes/IFG might be a risk factor for the progression of osteoarthritis due to hyperglycaemia [42]. Hyperglycaemia has been shown to induce reactive oxygen species and the production of cytokines, which result in joint inflammation and in the production of proteolytic enzymes that degrade cartilage [50]. However, in a Delphi study consisting of a panel of hand surgeons, the use of diabetic medication and abnormal fasting glucose were not identified as risk factors for finger IPJ osteoarthritis progression [51]. This suggests that though diabetes/IFG might have a relationship with IPJ osteoarthritis on a molecular level, in a clinical context the effect is not yet well recognised. Our results also found that larger finger EI is a risk factor for IPJ osteoarthritis progression [44]. In hip and knee osteoarthritis, larger cross-sectional areas in the femoral neck and proximal femoral shaft and in the tibial plateau, respectively, have also been described [52, 53]. Additionally, in knee osteoarthritis, a loss of articular cartilage coupled with larger bone epiphyseal area results in a change of loading and force across a joint, further contributing to the progression of osteoarthritis [54]. However, in the hands, and particularly the finger IPJs, the load across the joint is much lower, suggesting that there might be other mechanisms which contribute to the relationship between EI and IPJ osteoarthritis progression. Given the limited evidence reported in our systematic review, further high-quality studies are needed to assess this relationship.
The studies included in this systematic review are limited by their moderate/high risk of bias [37–39, 41–44]. This resulted in lower levels of evidence for each potential risk factor assessed. All studies included in this review used radiographic methods to measure osteoarthritis progression. However, there is poor correlation between radiographic and symptomatic osteoarthritis [10, 11], and studies assessing risk factors for the progression of symptomatic IPJ osteoarthritis are required. The time interval between baseline and follow-up measurements of osteoarthritis varied between studies, making it difficult to quantify the exact impact of a potential risk factor on osteoarthritis progression. Similarly, study populations varied with regard to age of participants, which could moderate the effect of other potential risk factors. All studies in this review are Phase 1 or 2 prognostic factor studies. Phase 3 studies are required to more adequately understand the effect of multiple risk factors on the progression of IPJ osteoarthritis and assess prognostic pathways [55, 56]. During data extraction, it became clear that studies used a variety of definitions to measure the progression of IPJ osteoarthritis. Therefore, a secondary aim of this review was established, to describe the criteria used to define the progression of IPJ osteoarthritis. This resulted in deviation from the study protocol registered on PROSPERO [17]. However, performing this analysis showed that there is great heterogeneity in the way IPJ osteoarthritis progression is defined, and further consensus work is needed to establish common criteria for defining disease progression. This review is limited by a small number of studies assessing each potential risk factor and meta-analyses could not be performed. Future work should also focus on standardising definitions of IPJ osteoarthritis progression, enabling harmonisation of datasets and pooling of study data.
Conclusion
Few studies which assess potential risk factors for hand IPJ osteoarthritis progression exist, and most are of high risk of bias. In the literature, the progression of IPJ osteoarthritis is measured radiographically using the KL classification system [45, 46], and no studies on symptomatic osteoarthritis progression were identified. Diabetes/IFG and larger finger EI are risk factors for disease progression, though evidence is limited. A better understanding of risk factors is needed to inform the identification and management of patients with a high risk of IPJ osteoarthritis progression.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Elinor Harriss for assistance with creating the search strategies, running the searches, and de-duplicating the results.
Author contributions
KS, XY, JCEL, GSC, NKA, DF, and SRF made substantial contributions to the conception and design of the study. KS, XY, and SRF were responsible for data acquisition, analysis and interpretation. GSC provided statistical expertise. KS drafted the manuscript, and XY, JCEL, GSC, NKA, DF, and SRF revised it critically for important intellectual content. KS, XY, JCEL, GSC, NKA, DF, and SRF read and approved the final version of the manuscript to be submitted. KS, XY, JCEL, GSC, NKA, DF, and SRF agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated or resolved.
Funding
This study was funded by the Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis (Grant reference 21595). KS is funded by the British Society for Surgery of the Hand Research Fellowship. XY is funded by the China Scholarship Council. JCEL is funded by a Versus Arthritis Clinical Research Fellowship (Grant reference 21605) and the Medical Research Council Doctoral Training Fellowship (Grant reference MR/K501256/1). GSC and DF are funded by the NIHR Oxford Biomedical Research Centre. NKA is funded by the Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis. SRF is funded by the Centre for Sport, Exercise and Osteoarthritis Research Versus Arthritis (Grant reference 21595). The study sponsors had no involvement in the study design, data collection, analysis or interpretation, in writing the manuscript.
Availability of data and material
Provided in the Electronic Supplementary Material.
Compliance with ethical standards
Conflict of interest
NKA receives personal fees from Pfizer/Lily for consultancy and a grant from Merck, outside the submitted work. KS, XY, JCEL, GSC, NKA, DF, and SRF certify they have no financial or commercial association that might pose a conflict of interest in connection with the submitted article. KS, XY, JCEL, GSC, NKA, DF, and SRF certify they have no non-financial association that might pose a non-financial conflict of interest in connection with the submitted article.
External editing and information facilitation (search) support
No external editing support was provided. Information facilitation (search) support was provided by Ms Elinor Harris, Knowledge Centre Manager and Outreach Librarian and the Bodleian Health Care Libraries, University of Oxford.
Footnotes
Conference abstract presentations
Some data from this manuscript has been published as a conference abstract: Shah K et al (2020) A systematic review of risk factors and diagnostic methods for hand interphalangeal joint osteoarthritis progression. Osteoarthr Cartil 28(S86–S527): S426.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/22/2021
A Correction to this paper has been published: 10.1007/s00296-021-04823-5
Contributor Information
Karishma Shah, Email: karishma.shah@ndorms.ox.ac.uk.
Xiaotian Yang, Email: yangxiaotian0821@hotmail.com.
Jennifer C. E. Lane, Email: jennifer.lane@ndorms.ox.ac.uk
Gary S. Collins, Email: gary.collins@csm.ox.ac.uk
Nigel K. Arden, Email: nigel.arden@ndorms.ox.ac.uk
Dominic Furniss, Email: dominic.furniss@ndorms.ox.ac.uk.
Stephanie R. Filbay, Email: stephanie.filbay@unimelb.edu.au
References
- 1.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. 2013;21(9):1145–1153. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Martin J, Mankin HJ. Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect. 2000;49:481–489. [PubMed] [Google Scholar]
- 4.Zhang W, et al. EULAR evidence-based recommendations for the diagnosis of hand osteoarthritis: report of a task force of ESCISIT. Ann Rheum Dis. 2009;68(1):8–17. doi: 10.1136/ard.2007.084772. [DOI] [PubMed] [Google Scholar]
- 5.UK AR (2013) Osteoarthritis of the hand and wrist, A.R. UK, Editor
- 6.Harris CA, et al. Understanding patient preferences in proximal interphalangeal joint surgery for osteoarthritis: a conjoint analysis. J Hand Surg Am. 2018;43(7):615–624.e4. doi: 10.1016/j.jhsa.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Visser AW, et al. Radiographic scoring methods in hand osteoarthritis—a systematic literature search and descriptive review. Osteoarthr Cartil. 2014;22(10):1710–1723. doi: 10.1016/j.joca.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Maheu E, et al. Design and conduct of clinical trials in patients with osteoarthritis of the hand: recommendations from a task force of the Osteoarthritis Research Society International. Osteoarthr Cartil. 2006;14(4):303–322. doi: 10.1016/j.joca.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Egger P, et al. Patterns of joint involvement in osteoarthritis of the hand: the Chingford Study. J Rheumatol. 1995;22(8):1509–1513. [PubMed] [Google Scholar]
- 10.Kalichman L, Hernandez-Molina G. Hand osteoarthritis: an epidemiological perspective. Semin Arthritis Rheum. 2010;39(6):465–476. doi: 10.1016/j.semarthrit.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Dahaghin S, et al. Clinical burden of radiographic hand osteoarthritis: a systematic appraisal. Arthritis Rheum. 2006;55(4):636–647. doi: 10.1002/art.22109. [DOI] [PubMed] [Google Scholar]
- 12.Bozic KJ, et al. Shared decision making in patients with osteoarthritis of the hip and knee: results of a randomized controlled trial. J Bone Jt Surg Am. 2013;95(18):1633–1639. doi: 10.2106/JBJS.M.00004. [DOI] [PubMed] [Google Scholar]
- 13.Royal College of Surgeons of England (2018) Future of surgery. https://futureofsurgery.rcseng.ac.uk/. Accessed 25 Nov 2019
- 14.Kwok WY, et al. Risk factors for progression in hand osteoarthritis: a systematic review. Arthritis Care Res (Hoboken) 2013;65(4):552–562. doi: 10.1002/acr.21851. [DOI] [PubMed] [Google Scholar]
- 15.Kloppenburg M, et al. Research in hand osteoarthritis: time for reappraisal and demand for new strategies. An opinion paper. Ann Rheum Dis. 2007;66(9):1157–1161. doi: 10.1136/ard.2007.070813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 17.University of York. PROSPERO International prospective register of systematic reviews. https://www.crd.york.ac.uk/prospero/. Accessed 4 Jan 2019
- 18.Methley AM, et al. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579–579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouzzani M, et al. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maheu E, et al. Reproducibility and sensitivity to change of four scoring methods for the radiological assessment of osteoarthritis of the hand. Ann Rheum Dis. 2007;66(4):464–469. doi: 10.1136/ard.2006.060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cochrane Handbook for Systematic Reviews of Interventions. Vol. 6.0. 2019: Cochrane
- 22.Iansavichene AE, et al. Should systematic reviewers search for randomized, controlled trials published as letters? Ann Intern Med. 2008;148(9):714–715. doi: 10.7326/0003-4819-148-9-200805060-00023. [DOI] [PubMed] [Google Scholar]
- 23.Li G, et al. A scoping review of comparisons between abstracts and full reports in primary biomedical research. BMC Med Res Methodol. 2017;17(1):181. doi: 10.1186/s12874-017-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427–437. doi: 10.7326/0003-4819-144-6-200603210-00010. [DOI] [PubMed] [Google Scholar]
- 25.Huguet A, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the GRADE framework. Syst Rev. 2013;2:71. doi: 10.1186/2046-4053-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grooten WJA, et al. Elaborating on the assessment of the risk of bias in prognostic studies in pain rehabilitation using QUIPS-aspects of interrater agreement. Diagn Progn Res. 2019;3:5. doi: 10.1186/s41512-019-0050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheehan KJ, et al. Prognostic factors of functional outcome after hip fracture surgery: a systematic review. Age Ageing. 2018;47(5):661–670. doi: 10.1093/ageing/afy057. [DOI] [PubMed] [Google Scholar]
- 28.Valentin GH, et al. Prognostic factors for disability and sick leave in patients with subacute non-malignant pain: a systematic review of cohort studies. BMJ Open. 2016;6(1):e007616. doi: 10.1136/bmjopen-2015-007616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton CL, et al. Clinical course and prognostic factors in conservatively managed carpal tunnel syndrome: a systematic review. Arch Phys Med Rehabil. 2016;97(5):836–852.e1. doi: 10.1016/j.apmr.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Lobatto DJ, et al. Preoperative risk factors for postoperative complications in endoscopic pituitary surgery: a systematic review. Pituitary. 2018;21(1):84–97. doi: 10.1007/s11102-017-0839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The Cochrane collaboration . Review Manager (RevMan) Copenhagen: The Nordic Cochrane Centre; 2014. [Google Scholar]
- 32.GRADE Working Group (2013) Quality of evidence, in GRADE handbook for grading quality of evidence and strength of recommendations, H. Schünemann, et al., Editors
- 33.Lievense AM, et al. Prognostic factors of progress of hip osteoarthritis: a systematic review. Arthritis Rheum. 2002;47(5):556–562. doi: 10.1002/art.10660. [DOI] [PubMed] [Google Scholar]
- 34.Belo JN, et al. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 2007;57(1):13–26. doi: 10.1002/art.22475. [DOI] [PubMed] [Google Scholar]
- 35.Bastick AN, et al. Prognostic factors for progression of clinical osteoarthritis of the knee: a systematic review of observational studies. Arthritis Res Ther. 2015;17(1):152. doi: 10.1186/s13075-015-0670-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teirlinck CH, et al. Prognostic factors for progression of osteoarthritis of the hip: a systematic review. Arthritis Res Ther. 2019;21(1):192. doi: 10.1186/s13075-019-1969-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kallman DA, et al. The longitudinal course of hand osteoarthritis in a male population. Arthritis Rheum. 1990;33(9):1323–1332. doi: 10.1002/art.1780330904. [DOI] [PubMed] [Google Scholar]
- 38.Busby J, et al. A longitudinal study of osteoarthritis of the hand: the effect of age. Ann Hum Biol. 1991;18(5):417–424. doi: 10.1080/03014469100001712. [DOI] [PubMed] [Google Scholar]
- 39.Plato CC, Norris AH. Osteoarthritis of the hand: longitudinal studies. Am J Epidemiol. 1979;110(6):740–746. doi: 10.1093/oxfordjournals.aje.a112855. [DOI] [PubMed] [Google Scholar]
- 40.Hoeven TA, et al. Association of atherosclerosis with presence and progression of osteoarthritis: the Rotterdam Study. Ann Rheum Dis. 2013;72(5):646–651. doi: 10.1136/annrheumdis-2011-201178. [DOI] [PubMed] [Google Scholar]
- 41.Haugen IK, et al. The prevalence, incidence, and progression of hand osteoarthritis in relation to body mass index, smoking, and alcohol consumption. J Rheumatol. 2017;44(9):1402–1409. doi: 10.3899/jrheum.170026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marshall M, et al. Metabolic risk factors and the incidence and progression of radiographic hand osteoarthritis: a population-based cohort study. Scand J Rheumatol. 2019;48(1):52–63. doi: 10.1080/03009742.2018.1459831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kalichman L, et al. Repeated measurement study of hand osteoarthritis in an apparently healthy Caucasian population. Am J Hum Biol. 2005;17(5):611–621. doi: 10.1002/ajhb.20417. [DOI] [PubMed] [Google Scholar]
- 44.Kalichman L, et al. Epiphyseal expansion in hand bones: association with age, sex, and hand osteoarthritis. Osteoarthr Cartil. 2008;16(5):560–565. doi: 10.1016/j.joca.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The epidemiology of chronic rheumatism . Atlas of standard radiographs. Oxford: Blackwell Scientific; 1963. [Google Scholar]
- 47.Lawrence JS. Rheumatism in populations. London: William Heinemann Medical Books; 1977. pp. 98–155. [Google Scholar]
- 48.Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cvijetic S, Kurtagic N, Ozegovic DD. Osteoarthritis of the hands in the rural population: a follow-up study. Eur J Epidemiol. 2004;19(7):687–691. doi: 10.1023/b:ejep.0000036794.40723.8e. [DOI] [PubMed] [Google Scholar]
- 50.Courties A, et al. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthr Cartil. 2015;23(11):1955–1965. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 51.Shah K, van Santen J, Furniss D. Delphi consensus of risk factors for development and progression of finger interphalangeal joint osteoarthritis. J Hand Surg Eur. 2019;2019:1753193419865872. doi: 10.1177/1753193419865872. [DOI] [PubMed] [Google Scholar]
- 52.Arokoski JP, et al. Increased bone mineral content and bone size in the femoral neck of men with hip osteoarthritis. Ann Rheum Dis. 2002;61(2):145–150. doi: 10.1136/ard.61.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wluka AE, et al. Tibial plateau size is related to grade of joint space narrowing and osteophytes in healthy women and in women with osteoarthritis. Ann Rheum Dis. 2005;64(7):1033–1037. doi: 10.1136/ard.2004.029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cicuttini FM, et al. Comparison of tibial cartilage volume and radiologic grade of the tibiofemoral joint. Arthritis Rheum. 2003;48(3):682–688. doi: 10.1002/art.10840. [DOI] [PubMed] [Google Scholar]
- 55.Altman DG, Lyman GH. Methodological challenges in the evaluation of prognostic factors in breast cancer. Breast Cancer Res Treat. 1998;52(1–3):289–303. doi: 10.1023/a:1006193704132. [DOI] [PubMed] [Google Scholar]
- 56.Hayden JA, et al. Identifying phases of investigation helps planning, appraising, and applying the results of explanatory prognosis studies. J Clin Epidemiol. 2008;61(6):552–560. doi: 10.1016/j.jclinepi.2007.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Provided in the Electronic Supplementary Material.