Abstract
Background
The aim of this study was to explore the impact of lymphadenectomy and umbilectomy on long-term survival and progression-free survival (PFS) of patients with urachal carcinoma.
Material/Methods
We performed a retrospective analysis of 39 patients with urachal carcinoma. Clinicopathologic outcomes were evaluated, and overall survival (OS) and PFS were assessed by Kaplan-Meier method and Cox regression analysis.
Results
Thirty-four (87.2%) patients underwent partial cystectomy, and 3 (7.7%) patients underwent radical cystectomy with en bloc urachal resection. Eighteen (46.2%) patients underwent lymphadenectomy and 27 (69.2%) patients had umbilectomy. Multivariate analysis showed that tumor size (P=0.011), Mayo stage (P=0.012), and umbilectomy (P=0.007) were the independent prognostic factors for OS. The median overall survival time was 67 months. The differentiation degree of tumor (P=0.049), Mayo stage (P=0.004), and umbilectomy (P=0.046) were the independent prognostic factors for PFS. Lymph node resection was not a predictor of OS. Patients had poorer prognosis when the tumor invaded the entire wall, including the mucous layer, muscular layer, and serous layer of the bladder compared with those that invaded only the muscular layer (P=0.014).
Conclusions
Lymph node metastases and failure to undergo umbilectomy were the independent prognostic factors for OS and PFS. Lymph node resection was not a predictor of OS. Patients had poorer prognosis when the tumor invaded the entire wall of the bladder compared with those that invaded the muscular layer.
MeSH Keywords: Lymphadenectomy, Prognostic Factors, Umbilectomy, Urachal Carcinoma
Background
Urachal cancer is rare and accounts for only 0.5–2% of all bladder-associated malignancies. It has aggressive behavior and poor prognosis [1]. Up to 90% of all urachal cancers are adenocarcinomas, and 20% to 40% of bladder adenocarcinomas are of urachal origin [2,3]. Other histological types of urachal cancers are sarcomas (leiomyosarcoma, rhabdomyosarcoma, and malignant fibrous histiocytoma), small-cell carcinomas, transitional cell cancer, and mixed neoplasias [4]. Urachal carcinoma mainly affects patients 40–70 years old and has a male predilection [5]. The most frequent symptoms are hematuria (73%), abdominal pain (14%), and dysuria (13%) [2]. The median overall survival (OS) time from diagnosis for urachal cancer of all stages is 42.9–57.6 months, and the estimated 5-year OS rate is about 50% [6,7]. Urachal carcinoma is classified according to the Sheldon, Mayo, and TNM staging systems (Table 1). Surgery is the primary treatment for urachal cancer, but no standard surgical treatment has been recommended. Partial cystectomy with en bloc resection of the urachus is the main surgical option. However, the efficacy of lymphadenectomy or umbilectomy is still debatable. We retrospectively reviewed patients with urachal cancer who were treated at our institution. We explored the impact of lymphadenectomy and umbilectomy on long-term survival and progression-free survival (PFS).
Table 1.
Staging system for urachal cancer.
| Stage | Sheldon staging system | Mayo staging system | TNM staging system | |
|---|---|---|---|---|
| I | Tumor is limited to the urachal mucosa | Tumor is confined to the urachus or bladder | Tumor invades the subepithelial connective tissue | |
| II | Invasion into but not beyond the urachal muscular layer | Extension beyond the muscular layer of the urachus or bladder | Invasion of the muscular layer of the urachus or bladder | |
| III | IIIA | Local extension to the bladder | Metastasis to regional lymph nodes | Invasion of the perivesical soft tissue, prostate, uterus, or vagina |
| IIIB | Local extension to abdominal wall | |||
| IIIC | Invasion of the peritoneum | |||
| IIID | Invasion of the local viscera other than the bladder | |||
| IV | IVA | Metastases to lymph nodes | Metastases to non-regional lymph nodes or other distant sites | Invasion of the abdominal wall and metastases to lymph nodes or other distant sites |
| IVB | Distant metastases | |||
Material and Methods
We retrospectively studied 39 patients with urachal cancer who were treated at our institution from January 2009 to December 2019. All the available clinical and pathological data of each patient were reviewed. Urachal carcinoma was classified according to the Sheldon, Mayo, and TNM staging systems (Table 1). Follow-up was routinely performed every 3 months in the first 2 years after surgery, then every 6 months in the next 3 years, and once a year in the following years.
Statistical analysis
Clinical and pathological characteristics were compared by Wilcoxon test, chi-square test, or Fisher exact test. OS and PFS were analyzed by Kaplan-Meier method and log-rank test. Univariate analysis with log-rank test and multivariate analysis with Cox proportional hazards regression model were used to estimate hazard ratios (HRs) and 95% confidence intervals (CI). Statistical analyses were performed using SPSS software version 23 (SPSS, Inc.), and 2 tailed P<0.05 was considered statistically significant.
Results
The clinical characteristics of the 39 patients are shown in Table 2. The male-to-female ratio was 2: 1. The median age was 49 (range: 23–86) years. Most patients’ primary symptom was hematuria (82.1%). All patients had no preoperative metastatic evidence and received surgery as primary treatment. Thirty-four (87.2%) patients underwent partial cystectomy, 3 (7.7%) patients underwent radical cystectomy with en bloc urachal resection, and 2 (5.1%) patients only underwent observation and were found to have metastatic nodules in the peritoneum and mesenterium. Eighteen (46.2%) patients underwent lymphadenectomy. We found that 27 (69.2%) patients had umbilectomy, whereas the others had not. Most of the patients (79.5%) underwent laparoscopic surgery, whereas the others underwent open surgery or robot-assisted laparoscopic surgery.
Table 2.
Clinical characteristics of patients with urachal carcinoma.
| Variables | n (%) |
|---|---|
| Gender | |
| Male | 26 (66.7%) |
| Female | 13 (33.3%) |
| Age (years) | 49 (23–86) |
| Primary symptoms | |
| Hematuria | 32 (82.1%) |
| Dysuria | 2 (5.1%) |
| Abdominal pain | 4 (10.1%) |
| Palpable mass | 3 (7.7%) |
| Surgery | |
| Partial cystectomy | 34 (87.2%) |
| Radical cystectomy | 3 (7.7%) |
| Others | 2 (5.1%) |
| Lymphadenectomy | |
| Yes | 18 (46.2%) |
| No | 21 (53.8%) |
| Umbilectomy | |
| Yes | 27 (69.2%) |
| No | 12 (30.8%) |
| Surgical approach | |
| Laparoscopy | 31 (79.5%) |
| Robot-assisted laparoscopy | 5 (12.8%) |
| Open surgery | 3 (7.7%) |
The pathological results are shown in Table 3. Adenocarcinoma was the predominant type of tumor (89.7%). Four (22.2%) of the 18 patients who underwent lymphadenectomy had positive results.
Table 3.
Pathological characteristics of patients with urachal carcinoma.
| Variables | n (%) |
|---|---|
| Tumor size (cm) | 4.4 (1.5–9) |
| Tumor differentiation | |
| Well or moderate | 15 (38.5%) |
| Poor | 15 (38.5%) |
| Not mentioned | 9 (23.1%) |
| Histology | |
| Adenocarcinoma | 35 (89.7%) |
| Others | 4 (10.3%) |
| Lymph nodes status | |
| Positive | 4 (22.2%) |
| Negative | 14 (77.8%) |
The median follow-up time was 45 months. The median OS time for all patients was 67 months. Sixteen patients died because of the progression of urachal carcinoma. The 3- and 5-year survival rates were 68.6% and 55.2%, respectively. We used Sheldon, Mayo, and TNM staging systems to determine tumor stage (Table 4). The median survival time of each stage was also listed.
Table 4.
Tumor staging and median survival time of different staging systems.
| Stage | Sheldon staging system | Mayo staging system | TNM staging system | |||
|---|---|---|---|---|---|---|
| n (%) | Median survival time (months) | n (%) | Median survival time (months) | n (%) | Median survival time (months) | |
| I | 2 (5.1%) | 86 | 2 (5.1%) | 86 | 2 (5.1%) | 86 |
| II | 1 (2.6%) | 83 | 29 (74.4%) | 65 | 25 (64.1%) | 67 |
| III | 29 (74.4%) (III A: 28, III B: 1) | 67 | 3 (7.7%) | 15 | 4 (10.3%) | 62 |
| IV | 6 (5.4%) (IV A: 3, IV B: 3) | 15 | 5 (12.8%) | 15 | 8 (20.5%) | 15 |
Multivariate analysis showed that tumor size (HR=1.51, 95% CI=1.098–2.077, P=0.011), Mayo stage (HR=1.954, 95% CI=1.160–3.291, P=0.012), and umbilectomy (HR=0.141, 95% CI=0.034–0.591, P=0.007) were the independent prognostic factors for OS. Radical cystectomy was not superior to partial cystectomy in terms of oncological results, and lymphadenectomy had no positive effect on survival. However, among the patients who underwent lymphadenectomy, patients with lymph node metastases had significantly worse survival than those with negative results (P<0.001, Figure 1). However, they shared the same stage in Sheldon system (stage IIIA), Mayo system (stage II), and TNM system (stage II).
Figure 1.
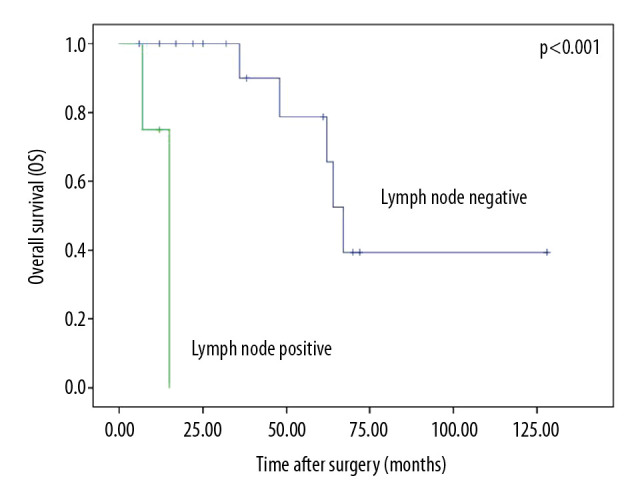
Overall survival by lymph node status.
No significant difference (p=0.435) was observed in the survival of patients whose tumor invaded perivesical soft tissue (TNM stage III) compared with those whose tumor invaded the bladder (TNM stage II). However, we found a significant difference in survival between patients whose tumor invaded the muscular layer and those whose tumor invaded the entire bladder wall including the mucous layer, muscular layer, and serous layer (P=0.014, Figure 2).
Figure 2.
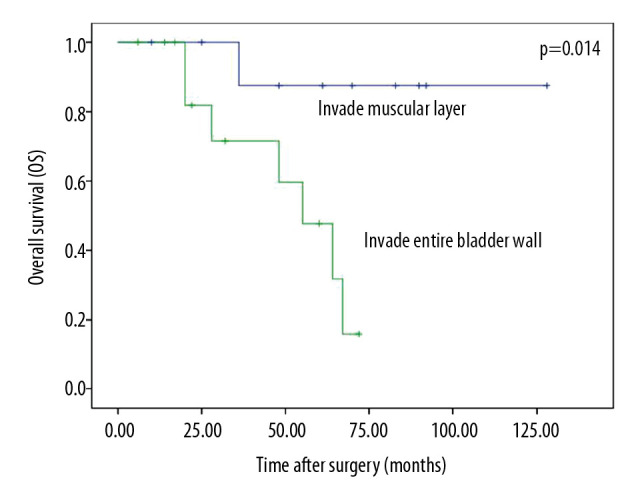
Overall survival of tumor invading muscular layer and entire bladder wall.
Sixteen (41.0%) patients had tumor progression during the follow-up period. The median progression time was 7 months (range, 0–45 months). The 3- and 5-year PFS rates were 58.5% and 48.3%, respectively. Multivariate analysis showed that the differentiation degree of the tumor (HR=1.968, 95% CI=0.982–3.943, P=0.049), Mayo stage (HR=2.248, 95% CI=1.299–3.890, P=0.004), and umbilectomy (HR=0.355, 95% CI=0.128–0.983, P=0.046) were the independent prognostic factors for PFS. Tumor size was not a prognostic factor of PFS.
Patients with disease progression had worse survival (P<0.001, Figure 3). The median OS from the diagnosis of tumor metastases was 14 months (range, 5–92 months). Patients with lymph node metastases had significantly lower PFS compared with those without lymph node metastases (P<0.001, Figure 4). The median time from the diagnosis of lymph node metastasis to other visceral metastasis was 4.5 months (range, 2–7 months).
Figure 3.
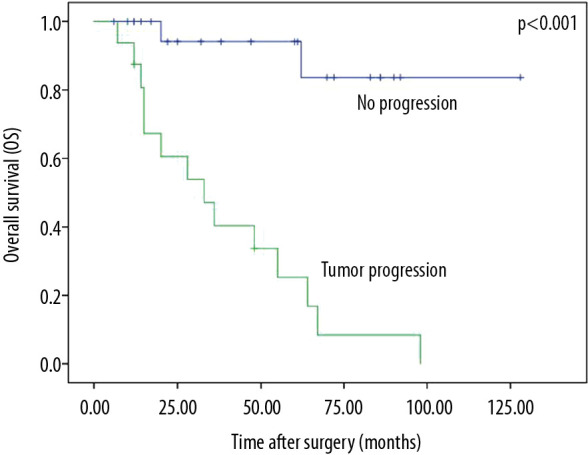
Overall survival by tumor progression.
Figure 4.
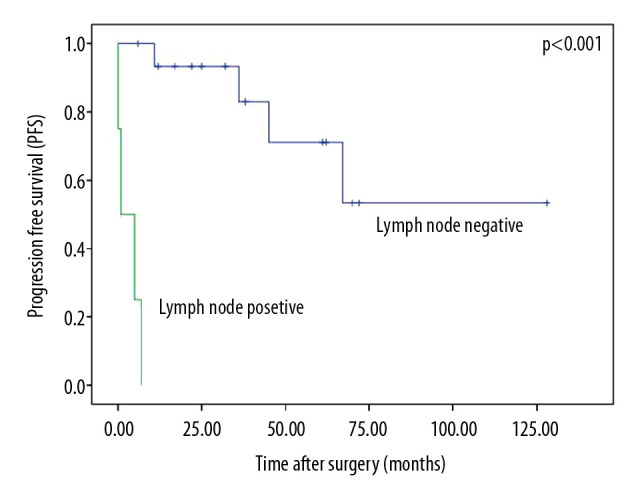
Progression-free survival by lymph node status.
The patients who underwent umbilectomy had significantly longer median survival time (87 vs. 48 months, P=0.03) and PFS (67 vs. 31 months, P=0.036) than those who did not.
Table 5 lists the 16 patients who had postoperative chemotherapies and their initial radiographic responses. Most of the patients (13/16, 81.3%) had cisplatin-based combination therapies (gemcitabine, paclitaxel, 5-fluorouracil [5-FU], and PD-1 inhibitor). The response rate and progression rate were both 30.8%. Three patients had radiotherapy to treat metastatic lesion and had no effective response. The bone pain of the patient who had metastatic bone tumor was relieved by radiotherapy.
Table 5.
Chemotherapy and radiographic response.
| Regimen | CR/PR | SD | PD | Total |
|---|---|---|---|---|
| Cisplatin-based | 4 (30.8%) | 5 (38.4%) | 4 (30.8%) | 13 |
| Cisplatin+gemcitabine | 2 (40%) | 1 (20%) | 2 (40%) | 5 |
| Cisplatin+paclitaxel | 1 (20%) | 2 (40%) | 2 (40%) | 5 |
| Cisplatin+5-FU | 1 (100%) | 0 | 0 | 1 |
| Cisplatin+PD-1 inhibitor | 0 | 2 (100%) | 0 | 2 |
| Others | 0 | 2 (66.7%) | 1 (33.3%) | 3 |
CR – complete response; PR – partial response; SD – stable disease; PD – progressive disease.
There were 5 patients in our study who received target therapies. Two of the patients treated in recent years were given a PD1 inhibitor (nivolumab). They both showed microsatellite instability (MSI), with MSH 6 loss and PD-L1 tumor proportion score (TPS) ≥1% by immunohistochemistry detection and PCR sequencing. These 2 patients all had stable disease (SD). Two patients had wild-type BRAF, ALK, EGFR, NRAS, and MET, and were given bevacizumab (VEGFR inhibitor) together with chemotherapy agents. One of them had SD and the other patient had cancer progression after having tried most first-line and second-line chemotherapy agents and still had disease progression (PD) when also receiving following bevacizumab. One patient was given afatinib (VEGFR inhibitor) along with TS-1 (tegafur, gimeracil, and oteracil) after GS (gemcitabine+cisplatin) therapy failed to control the disease and after paclitaxel+TS-1 stabilized the disease. The immunohistochemistry detection of surgical specimens showed PD-1 (−) and PD-L1 (−). The metastatic tumor was clinging to the iliac arteries, preventing use of needle biopsy for further detection. Thus, afatinib was empirically used and achieved SD. Of the 5 patients, the one who had PD died 15 months after surgery, 10 months after the diagnosis of metastasis. The median OS of the other 4 patients was 41 months (range, 20–67 months) and median the survival time after metastasis was 25 months (range, 12–48 months).
Discussion
The median OS time from the diagnosis of urachal cancer in all stages is 42.9–57.6 months, and the estimated 5-year OS rate is about 50% [5–10]. Our results were similar, as our computed 5-year survival rate was 55.2%.
The median survival time was more than 10 years for TNM stage I, 6.2–7.5 years for TNM stage II, and 1.8 years for TNM stage III and above. The OS for distant metastatic disease is less than 1 year [5,6]. In our study, the median survival time of most patients in each stage of the 3 different systems were similar to those in the literature. However, our results showed a longer survival time (62 months) for TNM stage III. All the patients with TNM stage III in our study had tumors that invaded the perivesical soft tissue without invading other adjacent organs, which might explain their better prognoses.
Bruins found no significant difference in the survival of patients whose tumor invaded the bladder only and those whose tumor invaded perivesical fat (p=0.96) [7]. We had similar results in our study. However, we found that patients whose tumor invaded the entire wall of the bladder (including the mucous, muscular, and serous layers) without invading the perivesical tissue had poorer prognoses compared with those whose tumor invaded the mucous and muscular layers only (P=0.014). Both groups of patients shared the same stage in the 3 staging systems. We believe that this factor should be taken into account when staging urachal cancer. Tumors invading the entire wall of the bladder and the perivesical tissue should be classified into the same stage.
As our study results showed, the 3 staging systems can predict the survival of patients with urachal cancer. However, each staging system has advantages and limitations. The Sheldon staging system divides urachal cancer into 8 detailed stages and is provided the most accurate staging among the 3 systems. However, some authors considered the Sheldon system over-specified and unnecessarily complicated as most patients are classified into stage III, especially stage IIIA, and far fewer patients are categorized into other stages [2,11]. In our study, 71.8% (28/39) of the patients were classified into stage IIIA. Other research indicated that the Sheldon staging system does not account for the fact that urachal cancer can present in any location along the urachus from the umbilicus to the bladder. A tumor close to the umbilicus is likely to involve the abdominal wall, and extravesical tumors close to the bladder are likely to invade the bladder [6]. The Mayo staging system is considered superior to the Sheldon staging system because of its simplicity and more balanced distribution of urachal cancer stages; thus, the Mayo staging system provides a higher prognostic value in multivariable models [11,12]. Some authors found that the applicability of the TNM classification for urachal cancer is limited as the tumor does not arise from the bladder’s surface urothelium, whereas other authors considered the TNM system a good predictor of survival for patients with urachal cancer [6,13].
Lymph node status and the presence of distant metastases are associated with poor prognosis [7–9]. Urachal cancer metastasizes into the pelvic lymph nodes by lymphatic dissemination and into distant organs, including the lungs, bones, and peritoneum, by hematogenous dissemination. Survival is not correlated with the site of metastatic disease [8].
The median time of recurrence after the resection of the primary tumor is 29 months [8]. In our study, the median progression time was 7 (range, 0–45) months and the 5-year PFS was 48.3%. The differentiation degree of the tumor, TNM stage, and umbilectomy were the independent prognostic factors for PFS.
Surgery, including en bloc excision of the umbilicus, entire urachus, and perivesical soft tissue coupled with partial cystectomy or radical cystectomy, is the primary treatment for urachal cancer [14]. Partial cystectomy and radical cystectomy provide similar oncological results, and no evidence has shown that radical cystectomy is superior to partial cystectomy [7,8,11,15,16]. However, organ-preserving partial cystectomy provides a higher quality of life and less complications and should therefore be preferred [2]. A positive surgical margin is one of the most important risk factors in urachal cancer [7,11,17]; therefore, complete tumor resection is of great importance in partial cystectomy. Our results showed a longer median OS time (67 months) than reported in most of the literature. The reason might be that we had no patients with positive surgical margin. We found that surgical approach, including open surgery and minimally invasive surgery, was not associated with OS. A previous study from our institution showed similar results and suggested that combined extraperitoneal and transperitoneal laparoscopic surgery was feasible and had similar prognosis with patients who underwent open surgery [18].
Most studies demonstrated that patients who underwent pelvic lymphadenectomy have no substantial difference in survival compared to those who did not undergo lymphadenectomy [6,11,17]. Lymph node positivity is low and is found in only 17% of cases in the literature [2], which is similar to our results (22.2%). Thus, the necessity of lymphadenectomy is debatable. Some authors suggest that lymphadenectomy is not necessary unless lymph node involvement has been confirmed by preoperative examination [5]. Preoperative computed tomography (CT) and magnetic resonance imaging might provide information about pelvic lymph node and distant metastases. However, imaging examinations have limited value in estimating tumor invasion [19]. In the present study, only 1 of the 4 patients with positive lymph nodes was found to have abnormal pelvic lymph nodes by preoperative CT. One patient with enlarged pelvic lymph nodes as detected by preoperative MRI was pathologically proven negative. We believe that removal of the pelvic lymph node may be recommended considering the fact that patients with nodal involvement discovered at surgery and proven by pathology have a similar poor prognosis (less than 20% 5-year survival) as those with distant metastases. Thus, lymphadenectomy might be beneficial [8]. Lymphadenectomy might be helpful in staging tumors, predicting prognosis, and guiding further treatment [20]. In this study, surgery with umbilectomy was an important prognostic factor for OS and PFS. In addition, patients who failed to undergo umbilectomy have poorer survival rates [5,11]. We suggest performing umbilectomy together with urachus and tumor resection for urachal cancer.
Metastatic diseases have no standard treatment strategy because of the minimal or absence of benefit of adjuvant chemotherapy or radiation [11,21]. First-line chemotherapies used in urothelial carcinomas of the bladder and colon cancer, including cisplatin-, paclitaxel-, and 5-FU-based therapies, are often used in urachal cancer [2]. In our study, most patients (81.3%) with disease progression received cisplatin-based chemotherapies, and their response rate was 30.8%, which was similar to the data reported in the literature. A meta-analysis of a large number of patients showed that the combination of cisplatin and 5-FU might be the most effective treatment because of its high response rate (43%) and low progression rate (13%) [2]. As there is no targeted therapy for urachal cancer due to its rarity, it shares the molecular profile and treatment of colorectal carcinoma [22,23]. RAS mutations with KRAS and NRAS and BRAF mutations were found in urachal cancer [24]. MSI was detected in urachal cancer [25] and is associated with poor response to 5FU [26]. Targeted therapies, such as EGFR inhibitors and PD-1/PD-L1 inhibitors, are increasingly used alone or in combination with chemotherapeutic agents for urachal cancer. However, their efficacy and clinical benefit are uncertain as only a small series of studies have been conducted [27,28]. Further research and long-term observation are still needed.
The major limitation of our study is that it is a single-center retrospective study with a small series of patients. A multicenter randomized clinical trial is needed to investigate the prognostic factors and proper indications of different surgical approaches for urachal cancer.
Conclusions
Our study showed that tumor size, TNM stage, umbilectomy, and lymph node status were the independent prognostic factors for the OS of urachal cancer. Lymph node metastases and failure to undergo umbilectomy were the indicators of poor PFS. Although lymph node resection was not a predictor of survival, we recommend lymphadenectomy in the absence of contraindications. Patients whose tumor invaded the entire wall of the bladder had poorer prognoses compared with those whose tumor invaded only the muscular layer of the bladder.
Abbreviations
- OS
overall survival
- PFS
progression-free survival
- HRs
hazard ratios
- CI
confidence intervals
- CR
complete response
- PR
partial response
- SD
stable disease
- PD
progressive disease
Footnotes
Conflict of Interest
None.
Source of support: Departmental sources
References
- 1.Paras FA, Jr, Maclennan GT. Urachal adenocarcinoma. J Urol. 2008;180(2):720. doi: 10.1016/j.juro.2008.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Szarvas T, Módos O, Niedworok C, et al. Clinical, prognostic, and therapeutic aspects of urachal carcinoma-A comprehensive review with meta-analysis of 1,010 cases. Urol Oncol. 2016;34(9):388–98. doi: 10.1016/j.urolonc.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Munichor M, Szvalb S, Cohen H, et al. Mixed adenocarcinoma and neuroendocrine carcinoma arising in the urachus. A case report and review of the literature. Eur Urol. 1995;28(4):345–47. doi: 10.1159/000475079. [DOI] [PubMed] [Google Scholar]
- 4.Ghazizadeh M, Yamamoto S, Kurokawa K. Clinical features of urachal carcinoma in Japan: Review of 157 patients. Urol Res. 1983;11(5):235–38. doi: 10.1007/BF00272286. [DOI] [PubMed] [Google Scholar]
- 5.Chen D, Li Y, Yu Z, et al. Investigating urachal carcinoma for more than 15 years. Oncol Lett. 2014;8(5):2279–83. doi: 10.3892/ol.2014.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molina JR, Quevedo JF, Furth AF, et al. Predictors of survival from urachal cancer: A Mayo Clinic study of 49 cases. Cancer. 2007;110(11):2434–40. doi: 10.1002/cncr.23070. [DOI] [PubMed] [Google Scholar]
- 7.Bruins HM, Visser O, Ploeg M, et al. The clinical epidemiology of urachal carcinoma: Results of a large, population based study. J Urol. 2012;188(4):1102–7. doi: 10.1016/j.juro.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Siefker-Radtke AO, Gee J, Shen Y, et al. Multimodality management of urachal carcinoma: The M. D. Anderson Cancer Center experience. J Urol. 2003;169(4):1295–98. doi: 10.1097/01.ju.0000054646.49381.01. [DOI] [PubMed] [Google Scholar]
- 9.Wright JL, Porter MP, Li CI, et al. Differences in survival among patients with urachal and nonurachal adenocarcinomas of the bladder. Cancer. 2006;107(4):721–28. doi: 10.1002/cncr.22059. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Yuasa T, Uehara S, et al. Clinical outcome of urachal cancer in Japanese patients. Int J Clin Oncol. 2016;21(1):133–38. doi: 10.1007/s10147-015-0866-8. [DOI] [PubMed] [Google Scholar]
- 11.Ashley RA, Inman BA, Sebo TJ, et al. Urachal carcinoma: Clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer. 2006;107(4):712–20. doi: 10.1002/cncr.22060. [DOI] [PubMed] [Google Scholar]
- 12.Kim IK, Lee JY, Kwon JK, et al. Prognostic factors for urachal cancer: A bayesian model-averaging approach. Korean J Urol. 2014;55(9):574–80. doi: 10.4111/kju.2014.55.9.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhillon J, Liang Y, Kamat AM, et al. Urachal carcinoma: A pathologic and clinical study of 46 cases. Hum Pathol. 2015;46(12):1808–14. doi: 10.1016/j.humpath.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herr HW, Bochner BH, Sharp D, et al. Urachal carcinoma: Contemporary surgical outcomes. J Urol. 2007;178(1):74–78. doi: 10.1016/j.juro.2007.03.022. discussion 78. [DOI] [PubMed] [Google Scholar]
- 15.Pinthus JH, Haddad R, Trachtenberg J, et al. Population based survival data on urachal tumors. J Urol. 2006;175(6):2042–47. discussion 2047. [Google Scholar]
- 16.Claps M, Stellato M, Zattarin E, et al. Current understanding of urachal adenocarcinoma and management strategy. Curr Oncol Rep. 2020;22(1):9. doi: 10.1007/s11912-020-0878-z. [DOI] [PubMed] [Google Scholar]
- 17.Niedworok C, Panitz M, Szarvas T, et al. Urachal carcinoma of the bladder: Impact of clinical and immunohistochemical parameters on prognosis. J Urol. 2016;195(6):1690–96. doi: 10.1016/j.juro.2015.11.067. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Li X, Ming S, et al. Combined extraperitoneal and transperitoneal laparoscopic extended partial cystectomy for the treatment of urachal carcinoma. J Endourol. 2016;30(3):280–85. doi: 10.1089/end.2015.0423. [DOI] [PubMed] [Google Scholar]
- 19.Thali-Schwab CM, Woodward PJ, Wagner BJ. Computed tomographic appearance of urachal adenocarcinomas: Review of 25 cases. Eur Radiol. 2005;15(1):79–84. doi: 10.1007/s00330-004-2408-z. [DOI] [PubMed] [Google Scholar]
- 20.Duan F, Zhai W, Zhang B, et al. Urachal carcinoma: Impact of recurrence pattern and lymphadenectomy on long-term outcomes. Cancer Med. 2020;9(12):4166–74. doi: 10.1002/cam4.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henly DR, Farrow GM, Zincke H. Urachal cancer: Role of conservative surgery. Urology. 1993;42(6):635–39. doi: 10.1016/0090-4295(93)90526-g. [DOI] [PubMed] [Google Scholar]
- 22.Riva G, Mian C, Luchini C, et al. Urachal carcinoma: from gross specimen to morphologic, immunohistochemical, and molecular analysis. Virchows Arch. 2019;474(1):13–20. doi: 10.1007/s00428-018-2467-1. [DOI] [PubMed] [Google Scholar]
- 23.Behrendt MA, van Rhijn BW. Genetics and biological markers in urachal cancer. Transl Androl Urol. 2016;5(5):655–61. doi: 10.21037/tau.2016.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Módos O, Reis H, Niedworok C, et al. Mutations of KRAS, NRAS, BRAF, EGFR, and PIK3CA genes in urachal carcinoma: Occurence and prognostic significance. Oncotarget. 2016;7(26):39293–301. doi: 10.18632/oncotarget.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sirintrapun SJ, Ward M, Woo J, et al. High-stage urachal adenocarcinoma can be associated with microsatellite instability and KRAS mutations. Hum Pathol. 2014;45(2):327–30. doi: 10.1016/j.humpath.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–57. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collazo-Lorduy A, Castillo-Martin M, Wang L, et al. Urachal carcinoma shares genomic alterations with colorectal carcinoma and may respond to epidermal growth factor inhibition. Eur Urol. 2016;70(5):771–75. doi: 10.1016/j.eururo.2016.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Wei Y, Zhang J, et al. Adjuvant chemo-radiation therapy provided good local control and survival for a young patient with advanced urachal carcinoma: A case report and literature review. Clin Genitourin Cancer. 2020;18(3):e303–8. doi: 10.1016/j.clgc.2019.12.014. [DOI] [PubMed] [Google Scholar]


