Abstract
Gliadin, a component of gluten and a known epitope, is implicated in celiac disease (CeD) and results in an inflammatory response in CeD patients when consumed. Acrylamide-based polyelectrolytes were employed as models to determine the effect of molecular weight and pendent group on non-covalent interaction modes with gliadin in vitro. Poly(sodium 2-acrylamido-2-methylpropane sulfonate) and poly(sodium 3-methylpropyl-3-butanoate) were synthesized via aqueous reversible addition fragmentation chain transfer (aRAFT) polymerization and characterized by GPC-MALLS. The polymer/gliadin blends were examined via circular dichroism, zeta potential measurements, ANS fluorescence spectroscopy, and dynamic light scattering. Acrylamide polymers containing strong anionic pendent groups had a profound effect on gliadin secondary structure and solution behavior below the isoelectric point, while polymers containing hydrophobic character only had a minor impact. The polymers had little effect on gliadin secondary structure and solution behavior at the isoelectric point.
Keywords: Celiac disease, anionic polymers, aRAFT, gliadin, non-covalent binding
Graphical Abstract
Low molecular weight low dispersity polyelectrolytes with sulfonate and carboxylate pendent groups synthesized via RAFT display structure- and pH-dependent binding behavior with gliadin. At low pH both systems alter gliadin secondary structure, with sulfonate functionality displaying the larger effect, while at neutral pH no binding is observed.

Introduction
Celiac disease (CeD) is an autoimmune disorder that arises in genetically predisposed individuals and results in damage to the small intestine after ingestion of gluten-containing products.1 Gluten is mainly comprised of two storage proteins: glutenin and gliadin.1 Gliadin is a heterogeneous mixture of proteins comprised of four subgroups: α-, β-, γ-, and ω-gliadins; of these, α-gliadin has been shown to contain the epitope that ultimately elicits an improper immune response.2,3,4,5,6 After normal digestive proteolysis, a 33 residue peptide fragment of α-gliadin is absorbed into the epithelial lining of the intestinal tract. For those suffering from CeD, the interaction between the absorbed epitope and specific cell surface receptor proteins, such as HLA-DQ2, triggers the immune response.4,7 Truncated variants of this epitope (25-mer, 18-mer, 17-mer) have also been shown to elicit immunoactivity.8 The biological process by which this occurs is well understood; nonetheless, a cure or successful treatment has yet to be formally established.
Polymer binders provide a promising approach to therapeutic agent development due to their ability to deliver, neutralize, and/or sequester compounds in the body.9,10 Natural polymers, such as enzymes, have been proposed as therapeutic agents for CeD because of their ability to bind and cleave peptides in an analogous fashion to gastrointestinal proteolysis, resulting in small fragments or individual amino acids.11,12,13,14 These enzymes, such as prolyl endopeptidases (PEPs) are endoproteolytic, cleaving at specific residues of the gliadin structure.11,12 PEPs have displayed the ability to reduce a t-cell response upon digestion of gluten in CeD patients,15 but have limitations, such as low-throughput production of enzymes during in vivo studies, which hinder broad implementation.16
Synthetic polymers have shown direct, non-covalent binding to gliadin to prevent surface modification of glutamine residues to glutamic acid and subsequent inflammatory responses in the body.17,18,19 Chen et al. reported the use of synthetic peptides, ~12 residues in length, as non-covalent binders to gliadin to prevent enzymatic modification of glutamine residues to glutamic acid;17 this site-specific deamidation reaction occurs in normal digestion and is a pivotal step in the series of biological events that results in an inflammatory response in CeD patients.20,21,22 These peptides resulted in a one-third decrease in the glutamine to glutamic acid gliadin surface modification. Pinnier et al. reported the effects on enzymatic activity of a high molecular weight anionic copolymer of hydroxyethyl methacrylate (HEMA) and styrene sulfonate (SS) (poly(HEMA-co-SS)) when complexed with gliadin.18,23 The poly(HEMA-co-SS) incorporated both hydrophobic and anionic functionality, and demonstrated biocompatibility, non-covalent binding to gluten, and a reduction in immune response.24 The mechanisms of complexation to gliadin in this system, such as contribution of hydrophobic and anionic moieties, are not well understood, and the high molecular weight of the polymer complicates the determination of specific binding interactions.
In this study, low molecular weight polymers of varying anionic character and hydrophobic content were prepared to evaluate the effects of polymer structure on binding interactions and solution behavior with gliadin. Aqueous reversible addition fragmentation chain transfer (aRAFT) polymerization was employed to achieve polymers of target molecular weight with low dispersity. Acrylamide polymers with pendent groups of sodium sulfonate or pH-responsive sodium carboxylate/carboxylic acid were utilized to determine the impact of anionic and hydrophobic character on non-covalent interactions with gliadin in varying pH environments. Acrylamide-based polymers were selected due to their water solubility, hydrolytic stability, biocompatibility, and stability across a wide range of solution conditions.25,26,27,28
Experimental Section
Materials.
Acrylonitrile was purchased from Acros Organics. Gliadin from wheat was purchased from Sigma Aldrich. Sodium 2-acrylamido-2-methyl-1-propanesulfonic acid salt solution (AMPS) was purchased from Sigma Aldrich and precipitated in acetone, followed by vacuum filtration, and dried under vacuum and stored at −20° C until experimental use. Ultra-pure grade TRIS and TRIS hydrochloride were purchased from VWR Life Science AMRESCO (USA). The radical initiator 4,4’-azobis(4-cyanopentanoic acid) (V-501) was purchased from Sigma Aldrich. All other solvents and reagents were purchased from either Sigma Aldrich (USA) or ThermoFisher Scientific (USA). All chemicals were purchased in the highest purity available and used without further purification unless otherwise indicated. The chain transfer agent 4-cyano-4-(ethylsulfanylthiocarbonyl) sulfanylpentanoic acid (CEP) was synthesized according to published literature procedures (1H-NMR spectrum, supporting information (SI) Figure S.1).29,30 Light scattering cells were high precision cylindrical cuvettes purchased from LS instruments (Switzerland). Dialysis tubing (MWCO = 500 – 1000 g/mol, 10 mL) for polymer purification was purchased from Spectrum Labs. Black flat bottom polystyrene 96 well NBS microplates were purchased from Corning.
Characterization.
Weight-average and number-average molecular weight (Mw, Mn), molecular weight distribution, and dispersity for polymer samples were determined using a gel permeation chromatography system consisting of an Agilent 1260 Infinity II system operating at 30° C with a PL aquagel MIXED-OH column, an online multiangle laser light scattering detector operating at 653 nm (Dawn Heleos II, Wyatt Technology Inc.), and an online differential refractometer (Optilab T-rEX, Wyatt Technology Inc).TRIS buffer pH 8.0 was used as the mobile phase at a flow rate of 0.5 mL/min with a sample concentration of 10 mg/ml and an injection volume of 50 μL. Detector signals were simultaneously recorded and absolute molecular weights and dispersities were calculated using ASTRA 7 software (Wyatt Technology Inc). A literature value of 0.145 mL/g was used as the dn/dc for polymer samples.31,32,33 Low molecular weight aqueous standards provided by the column manufacture (Agilent) were evaluated prior to testing of our synthesized polymers in order to ensure adequate resolution of the column.
In Vitro Polymer/Protein Interactions.
Each protein (gliadin from wheat or bovine serum albumin) (20 μM) was blended 50/50 v/v% with 60 μM of either poly(2-acrylamido-2-methylpropane sulfonate) (PAMPS, degree of polymerization (DP) 15 or 25) or poly(3-acrylamido-3-methylbutanoic acid) (PAMBA, DP 15 or 25) in order to achieve a final 3:1 molar ratio. All samples were prepared in a 65/35 v/v 20 mM TRIS buffer (pH 2 or 7)/EtOH and interactions monitored by circular dichroism, zeta potential, ANS fluorescence, and dynamic light scattering. All tests were performed at 37°C.
Circular Dichroism.
Far-UV CD measurements were obtained using a Jasco J-815 spectropolarimeter (Jasco, MD) and a 0.1 cm path-length quartz cuvette was used (Metrohm) for sample measurements. Data acquisition parameters include testing samples in continuous scan mode from 260 nm – 190 nm, 0.1 nm data pitch, 8 sec response time, 1.00 nm bandwidth, and a 50 nm/min scanning speed with data averaged over three scans.
ANS Fluorescence.
8-Anilinonaphthalene-1-sulfonic acid (ANS) fluorescence measurements were performed using a BioTek Synergy H1 plate reader by exciting at 388 nm while monitoring emission at 540 nm. Each measurement was taken by injecting 50 μL of sample into each well with 10 μL of ANS solution in buffer (600 μM stock; final concentration of 100 μM) at each pH in triplicate. Statistical significance of averages for fluorescence measurements was determined using the two-sample t-test at a p = 0.05 threshold.
Zeta Potential Measurements.
Samples were tested using a DTS-1060 folded cell (Fisher Scientific) on a Malvern Zetasizer (Nano-S). The Smoluchowski model34 was used for determining zeta potential from electrophoretic mobility.
Dynamic Light Scattering.
Variable-angle dynamic light scattering measurements were taken with a Brookhaven Instruments BI-200SM goniometer, an avalanche photodiode detector, TurboCorr correlator, and a Spectra Physics Model 127 HeNe laser using an incident light of 633 nm operating at 40 mW. Light scattering measurements for each sample were taken at five angular positions (60°, 75°, 90°, 105°, and 120°) to determine the hydrodynamic radius for each individual size distribution. A detailed procedure for determining the hydrodynamic radius from relaxation times associated with each size distribution can be found in the SI.
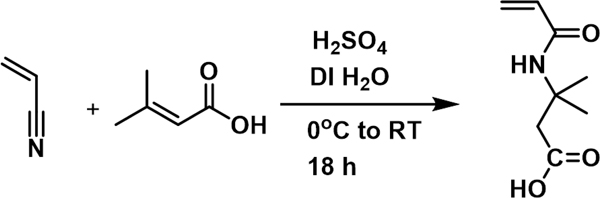
AMBA Synthesis.
The monomer 3-acrylamido-3-methylbutanoic acid (AMBA) was synthesized by modifying a literature protocol (Scheme 1).35 In the modified procedure, methylene-bis (2,6-di-tert-butylphenol) was eliminated, the molar equivalents of reactants were adjusted, and the temperatures during reaction and purification steps were altered. In summary, acrylonitrile (2.73 mL, 0.04 mol), 3,3-dimethylacrylic acid (4 g, 0.04 mol), and DI H2O (0.36 mL, 0.02 mol), were combined into a round-bottomed flask and cooled to 0 °C. Sulfuric acid (4.59 mL, 0.083 mol) was then added dropwise at 0 °C and the reaction stirred for 18 hours. After reacting for 18 hours, the mixture was recooled to 0°C before DI H2O (20 mL) was added to precipitate unreacted starting material, which was subsequently removed via vacuum filtration. The product was extracted using CHCl3, and the solvent removed via rotary evaporation. The product was then recrystallized several times using a 50/50 petroleum ether/methyl ethyl ketone, and obtained by removing residual solvent in a vacuum oven.
Scheme 1.
Reaction scheme for the synthesis of monomer 3-acrylamido-3-methylbutanoic acid.
AMBA, 1H-NMR (300 MHz, CDCl3): δ [ppm] 6.24 (d, 1H), 6.07 (d, 1H), 5.62 (d, 1H), 2.85 (d, 2H), 1.5 (d, 6H). [1H-NMR Spectrum, SI Figure S.2]
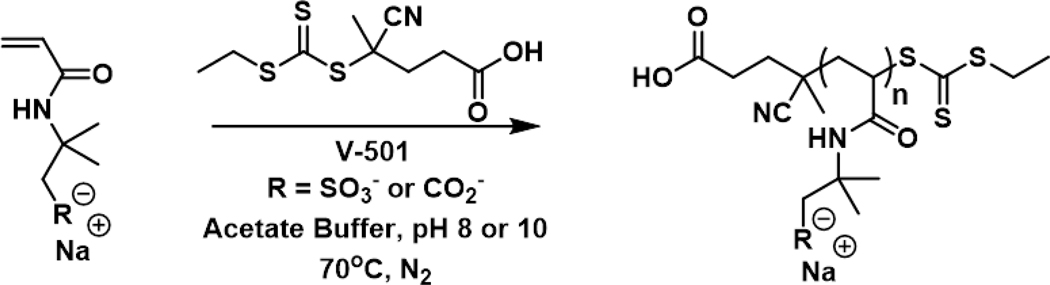
Aqueous RAFT Polymerization of Anionic Monomers.
Anionic acrylamide-based polymers with DPs of ~15 (molecular weight: PAMBA sodium salt = 3,170 g/mol, PAMPS sodium salt = 3212 g/mol) and ~25 (molecular weight: PAMBA salt = 4120 g/mol, PAMPS = 5120 g/mol) were synthesized by aRAFT polymerization following literature procedures (Scheme 2).36,37 In this work, the chain transfer agent (CTA) 4-cyano-4-(ethylsulfanylthiocarbonyl)sulfanylpentanoic acid (CEP) was used instead of 4-cyanopentanoic acid dithiobenzoate (CTP), as the synthesis of the CEP was simpler and also yielded pseudo first order polymerization kinetics. All other polymerization conditions from literature the procedure were maintained, as outlined in the SI. Molecular weight and dispersity were determined via gel permeation chromatography (GPC).
Scheme 2.
Synthetic scheme for the aRAFT polymerization of anionic monomers
In order to determine the time required to achieve DP 15 and 25, kinetic studies were performed where the disappearance of the vinyl monomer peaks was monitored by 1H-NMR. The internal standard sodium benzenesulfonate was used to determine the monomer concentration throughout the polymerization by comparing the relative integral of the benzene protons (~ 7.77 ppm, 2H) to the vinyl protons of the monomer (~5.73 ppm, 1H) (Figure S. 3). Monomer concentration was used to calculate the theoretical molecular weight and degree of polymerization (equations S. 1 and S.2, respectively).38 The kinetic plots can be found in SI, Figures S. 4 and S. 5, which display a linear increase when ln([M]0/[M]) is plotted as a function of time, indicating pseudo first order kinetics for both NaPAMPS and NaPAMBA polymerizations, and control for these polymerization conditions has been previously established by McCormick et al.36 Polymerizations were conducted in triplicate to ensure reproducibility.
Results and Discussion
Synthesis and Characterization of Acrylamide Polymers.
Water-soluble acrylamide-based monomers were selected for preparation of anionic polymers of specific structures and molecular weights, due to the availability of a variety of pendent groups with differing functionality, their facile polymerization in aqueous conditions, and hydrolytic stability. For this work, two pendent groups (sulfonate and carboxylate/carboxylic acid) and two chains lengths (DP 15 or 25) were selected in order to investigate the effects of anion strength, hydrophobic content, and polymer chain length on polymer/protein interactions in varying pH environments. Specifically, the strong polyelectrolyte PAMPS (Figure 1A) was selected because the sulfonate pendent group remains negatively charged across the pH range evaluated, while the weak polyelectrolyte PAMBA (Figure 1B) is pH responsive (AMBA units are expected to have a pKa of around 5.5),39 exists in the acid form and has hydrophobic character at low pH, and exhibits anionic character at neutral pH (PAMBA salt).40 The two polymer chain lengths were selected to span gliadin epitope fragment sizes and to allow evaluation of the polymer/protein complexes by DLS and circular dichroism. Solution environments of pH 2 and 7 were selected to mimic the conditions of the stomach and intestinal tract, respectively, for investigation of polymer/protein interactions at different points in the digestive process.
Figure 1.
Polymer structures of A) poly(sodium 2-acrylamido-2-methylpropane sulfonate) (PAMPS) and B) poly(3-acrylamido-3-methylbutanoic acid) (PAMBA).
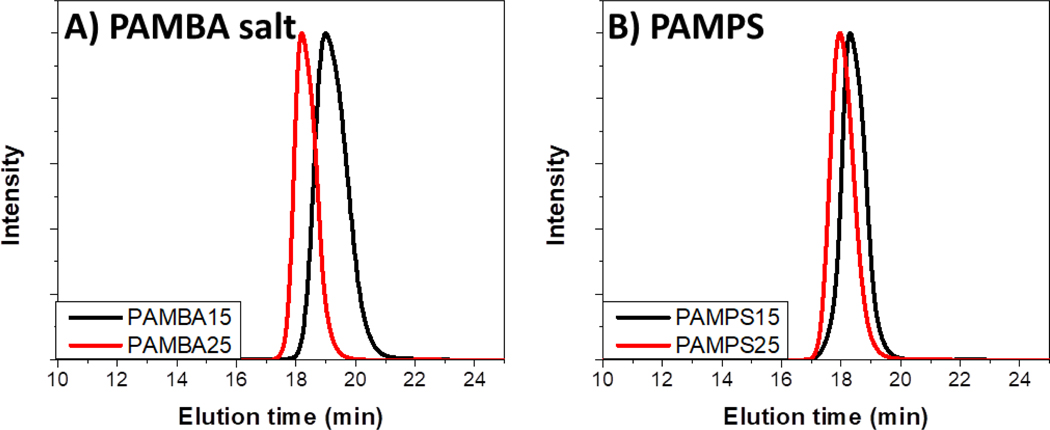
AMBA monomer was synthesized according to literature procedure35 and characterized by 1H-NMR (SI Figure S. 2). The monomers were polymerized with target DPs of 15 and 25 by aRAFT following slightly modified literature procedures,36,37 to obtain well-defined low molecular weight polymers. GPC MALLS analysis of the four polymers (PAMBA15 salt, PAMBA25 salt, PAMPS15, and PAMPS25) indicates unimodal distributions, low dispersity, and experimental Mn in close agreement with theoretical values (Figure 2 and Table 1)
Figure 2.
GPC traces for A) PAMBA salt, DP 15 (—) and DP 25 ( ) B) PAMPS, DP 15 (—) and DP 25 (
) B) PAMPS, DP 15 (—) and DP 25 ( ) in TRIS buffer pH 8 indicate unimodal distributions with low dispersities.
) in TRIS buffer pH 8 indicate unimodal distributions with low dispersities.
Table 1.
Molecular weight data for anionic polymers determined via GPC.
| Polymera | Theoretical | GPC-MALLS | ||
|---|---|---|---|---|
| Mnthb,c | Mnc,d | Mwc,d | Ð,d | |
| PAMBA15 | 3200 | 3,170 | 3,830 | 1.21 |
| PAMBA25 | 4450 | 4,120 | 4,190 | 1.02 |
| PAMPS15 | 3700 | 3,210 | 3,220 | 1.01 |
| PAMPS25 | 5530 | 5,120 | 5,300 | 1.04 |
All samples were characterized in the salt form
Determined using 1H-NMR and RAFT theoretical equations (SI Figure S.3 and equation S.1).
Values in g/mol.
Determined by GPC-MALLS
Investigation of In Vitro Interactions between Gliadin and Acrylamide Polymers.
Far-UV CD was employed to determine the influence of polymer structure and chain length on gliadin secondary structure. It is important to note that “gliadin” refers to a collective blend of α-, β-, γ-, and ω-gliadins from wheat. With an isoelectric point (pI) of 6.8, gliadin displays a net positive surface charge at pH 2 and a net neutral charge at pH 7. CD curves are characteristic of the average of the secondary structures present in a sample, while changes in CD curves indicate changes in structural elements.41 Gliadin secondary structure was analyzed in the absence and presence of each polymer at varying pH. CD plots of molar ellipticity as a function of wavelength for the gliadin control and polymer blends are shown in Figure 3, and plots of the polymer controls (showing no secondary structure) are provided in Figure S.6 (SI).
Figure 3.
Circular dichroism plots for gliadin blends at A) pH 2 and B) pH 7. Presence of polymers alters gliadin secondary structure at pH 2, while at pH 7gliadin maintains secondary structure.
At pH 2 (Figure 3A) the gliadin control and blends display distinct curves, indicating that both the presence of polymer and the nature of the pendent group impact gliadin secondary structure, presumably through non-covalent interactions. The greatest changes in structure are observed for the PAMPS blends, attributed to electrostatic interactions of the positively charged gliadin with the anionic sulfonate pendent groups. PAMBA, on the other hand, is in the protonated state at pH 2, indicating that hydrophobic or other interactions may be involved in the gliadin structural changes for this polymer. At pH 7, all solutions display similar curve shapes (Figure 3B), indicating no measurable effect of the polymer on gliadin secondary structure. The negatively charged polymers appear to have no significant interaction with the neutral gliadin at pH 7.
Qualitative analyses of the CD curves from Figure 3 reveal that at pH 2, the gliadin control has a predominant α-helical structure with a minimum at 208 nm and a shoulder at 222 nm. For gliadin/PAMBA blends, small decreases in α-helix content are observed, with a reduction in ellipticity at 208 nm. Conversely, significant structural changes are observed in gliadin/PAMPS blends, where α-helix content decreases by about 50% based on reduction in ellipticity at 208 nm. No clear differences are observed for the blends of different PAMPS molecular weight. These findings indicate that polymer structure, particularly anionic strength, has a dramatic influence on protein secondary structure below the pI of gliadin, while polymer chain length has little to no effect.
At pH 7, the gliadin control shows ellipticity similar to that of gliadin/PAMPS blends at pH 2. These findings indicate that a neutral complex is formed between the positively charged gliadin and negatively charged PAMPS at pH 2, forming a secondary structure similar to that exhibited by gliadin alone at neutral pH. Gliadin/polymer blends at pH 7 show structural percentages within 4% of the control gliadin, indicating that polymer interactions have little to no impact on gliadin secondary structure in solutions near the pI of gliadin.
These findings are further supported by zeta potential measurements, performed to establish polymer/protein complex stability and characterize anionic: cationic charge ratio42 at varying pH (Table 2). As expected, gliadin has a positive zeta potential at pH 2 since it is below the pI. The zeta potential approaches neutral when gliadin is blended with PAMPS15 or 25, indicating the polymer anions interact with the gliadin cations to form a neutral complex. Conversely, when gliadin is blended with PAMBA15 or 25, there is no change in zeta potential indicating no changes in charge ratio, or electrostatic interactions, are taking place.
Table 2.
Zeta potential measurements of gliadin blends at varying pH.
| Sample | pH 2 (mV) | pH 7 (mV) |
|---|---|---|
| Gliadin Control | 3.89 ± 0.40 | −0.66 ± 0.02 |
| Gliadin/PAMBA15 | 3.72 ± 0.12 | −12.2 ± 0.79 |
| Gliadin/PAMBA25 | 3.75 ± 0.07 | −10.5 ± 0.17 |
| Gliadin/PAMPS15 | 0.41 ± 0.11 | −8.45 ± 0.22 |
| Gliadin/PAMPS25 | −0.30 ± 0.05 | −9.75 ± 0.29 |
At pH 7 gliadin approaches a neutral zeta potential, which is expected as gliadin approaches the pI. For the gliadin blends, zeta potential values decrease upon addition of anionic polymers. Interestingly, the gliadin blends with PAMBA15 or 25 salt have a decreased zeta potential in comparison to the blends with PAMPS15 or 25. This is attributed to the strong anionic character of PAMPS, allowing it to readily interact with the positively charged sites on the gliadin surface even at a net neutral charge, whereas the weaker PAMBA salt anionic character limits such electrostatic interactions. A similar trend was observed by Hattori et al., 43 where electrostatic interactions between anionic polyelectrolytes and β-Lactoglobulin above the pI (i.e. at net negative charge) were attributed to non-uniform positive sites creating “patches” on the protein surface, allowing for attractive and repulsive forces to take place simultaneously.
ANS fluorescence was performed to evaluate hydrophobicity of the gliadin blends. ANS is a known fluorescent probe that displays an increase in fluorescence intensity upon interaction with hydrophobic surfaces.44 Additionally, literature reports have described the use of ANS to detect hydrophobic interactions between polymers and protein.45,46 ANS fluorescence data for the gliadin blends as a function of pH are shown in Figure 4, where the (*) symbol denotes statistical significance (p ≤ 0.05) of differences from the gliadin control (data for neat polymers are provided in SI, Figure S.7). Statistically significant decreases in fluorescence are observed at pH 2 for gliadin in the presence of PAMPS15 and 25, indicating the mode of interaction is not hydrophobic in nature. For gliadin/PAMBA15 and 25, differences from the control are not statistically significant. ANS fluorescence intensity is lower at pH 7 than at pH 2 for all solutions, and there is no statistically significant difference between the gliadin blends and the control. These trends are similar to those observed with CD and zeta potential measurements, where polymer structure influences gliadin blend solution behavior at acidic pH but has little to no effect at neutral pH.
Figure 4.

ANS Fluorescence of Gliadin blends at varying pH. Polymer structure influences hydrophobicity of gliadin blends at acidic pH.
Dynamic light scattering was employed to investigate the solution behavior of the polymer/protein complexes at varying pH values (Table 3). As described in the experimental section, the Rh for each size distribution is determined using the relaxation time. Figure 5 displays an example of the data obtained for the gliadin control at pH 7, which contains two size distributions. The decay rate plotted as a function of q2 for all samples can be found in SI (Figures S.8 – S.17).
Table 3.
Dynamic light scattering measurements of gliadin blends at varying pH.
| pH | Sample | Rh (nm) |
|---|---|---|
| Gliadin Control | 44 | |
| 123 | ||
| Gliadin/PAMBA15 | 18 | |
| 184 | ||
| pH 2 | Gliadin/PAMBA25 | 10 |
| 118 | ||
| Gliadin/PAMPS15 | 7 | |
| 27 | ||
| Gliadin/PAMPS25 | 3.5 | |
| 50.5 | ||
| Gliadin Control | 388 | |
| 593 | ||
| pH 7 | Gliadin/PAMBA15 | 482 |
| Gliadin/PAMBA25 | 232 | |
| Gliadin/PAMPS15 | 440 | |
| Gliadin/PAMPS25 | 513 | |
Figure 5.
Example of data obtained through light scattering method A) autocorrelation functions which are converted to B) relaxation time vs intensity plots, which are used to determine the decay rates, C) which are plotted as a function of q2 to determine the hydrodynamic radius.
Thomson et al.47 determined the radius of gyration (Rg) of α-, γ-, and ω-gliadins to be 3.55 nm, 3.8 nm, and 4.6 nm, respectively using small angle x-ray scattering, indicating the size range expected for the monomeric species. Using DLS to determine Rh, we observe two size distributions at pH 2 for the gliadin control, with Rh of 44 and 123 nm (Table 3). The size distributions are attributed to aggregates of different sizes formed through interactions between the various subgroups. Sato et al. reported that gliadin mixtures form dimers and oligomers in dilute solution.48 Similar size distributions to those obtained in the neat solution are observed for gliadin in the presence of PAMBA15 or 25, indicating that this polymer has little effect on solution aggregation behavior. In contrast, size distributions of significantly smaller size are obtained for blends of gliadin with PAMPS. PAMPS15 blends show size distributions of Rh 7 and 27 nm, while PAMPS25 blends show size distributions of Rh 3.5 and 50 nm, indicating that gliadin monomers and dimers are present in the PAMPS solutions. These findings suggest that non-covalent interactions with PAMPS reduce gliadin aggregation in solution.
At pH 7 the gliadin control contains two size distributions of much larger size, Rh 388 and 593 nm, which are again attributed to aggregates of different sizes. The large aggregate size is attributed to the reduction of protein stability near the pI. Gliadin/polymer blends show one size distribution with hydrodynamic radii several hundred nm in size. Gliadin in the presence of PAMBA25 salt shows a reduction in size by a factor of approximately 1.6; the other polymers blends appear to have little to no effect on gliadin solution behavior.
DLS analysis of polymer controls shows no signal at either pH values, because of the small size and μM concentration of the polymers, and no evidence of aggregation. Typically light scattering techniques become limited for polymers that are below 10,000 g/mol since scattered light is proportional to the concentration of a sample multiplied by the molecular weight.49
Conclusions
Acrylamide based polymers containing either sodium sulfonate or sodium carboxylate/carboxylic acid pendant moieties were synthesized to low degrees of polymerization (DP 15 and 25) via aqueous RAFT polymerization for determination of the effects of structure and molecular weight on binding interactions with gliadin. At pH below the isoelectric point of gliadin, the negatively charged polyelectrolytes have a dramatic effect on gliadin secondary structure. The strong polyelectrolyte PAMPS alters the gliadin secondary structure to a greater extent than does the weak polyelectrolyte PAMBA. The effect of molecular weight within the range tested appears to be much smaller than the effect of polymer structure, and no clear evidence of hydrophobic interactions is observed. Future studies could focus on a larger range of polymer molecular weights and explore the effect of dispersity on gliadin secondary structure. The low molecular weight model systems evaluated in this study increase the understanding of polymer binding with gliadin and provide a platform for therapeutic agent design of specific structure/binding interactions with food proteins. The platform can be extended to a broad range of biomedical investigations involving protein-related disorders where polymer binders are used to minimize undesired protein interactions in the body.
Supplementary Material
Acknowledgements
Funding was provided by the National Institutes of Health (R15GM 123431) and the National Science Foundation National Research Traineeship “Interface” (Award#1449999). The authors would also like to thank the Mississippi INBRE for use of fluorescence instrumentation.
References
- 1.Fasano A, Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiological Reviews 2011, 91 (1), 151–175. [DOI] [PubMed] [Google Scholar]
- 2.Ciccocioppo R; Di Sabatino A; Corazza GR, The immune recognition of gluten in coeliac disease. Clinical and Experimental Immunology 2005, 140 (3), 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green PHR; Lebwohl B; Greywoode R, Celiac disease. Journal of Allergy and Clinical Immunology 2015, 135 (5), 1099–1106. [DOI] [PubMed] [Google Scholar]
- 4.Anderson RP; Degano P; Godkin AJ; Jewell DP; Hill AVS, In vivo antigen challenge in celiac disease identifies a single transglutaminase-modified peptide as the dominant A-gliadin T-cell epitope. Nat Med 2000, 6 (3), 337–342. [DOI] [PubMed] [Google Scholar]
- 5.Maiuri L; Ciacci C; Ricciardelli I; Vacca L; Raia V; Auricchio S; Picard J; Osman M; Quaratino S; Londei M, Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. The Lancet 2003, 362 (9377), 30–37. [DOI] [PubMed] [Google Scholar]
- 6.van Herpen TW; Goryunova SV; van der Schoot J; Mitreva M; Salentijn E; Vorst O; Schenk MF; van Veelen PA; Koning F; van Soest LJ; Vosman B; Bosch D; Hamer RJ; Gilissen LJ; Smulders MJ, Alpha-gliadin genes from the A, B, and D genomes of wheat contain different sets of celiac disease epitopes. BMC genomics 2006, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao SW; Bergseng E; Molberg O; Xia J; Fleckenstein B; Khosla C; Sollid LM, Antigen Presentation to Celiac Lesion-Derived T Cells of a 33-Mer Gliadin Peptide Naturally Formed by Gastrointestinal Digestion. The Journal of Immunology 2004, 173 (3), 1757–1762. [DOI] [PubMed] [Google Scholar]
- 8.Camarca A; Anderson RP; Mamone G; Fierro O; Facchiano A; Costantini S; Zanzi D; Sidney J; Auricchio S; Sette A; Troncone R; Gianfrani C, Intestinal T cell responses to gluten peptides are largely heterogeneous: implications for a peptide-based therapy in celiac disease. Journal of immunology 2009, 182 (7), 4158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Connor EF, Lees I, Maclean D, Polymers as drugs - Advances in therapeutic applications of polymer binding agents. Journal of Polymer Science Part A: Polymer Chemistry 2017, 55 (18), 3146–3157. [Google Scholar]
- 10.Bertrand N, Gauthier MA, Bouvet C, Moreau P, Petitjean A, Leroux JC, Leblond J, New pharmaceutical applications for macromolecular binders. Journal of Controlled Release 2011, 155, 200–210. [DOI] [PubMed] [Google Scholar]
- 11.Sollid LM, Khosla C, Future therapeutic options for celiac disease. Nature Clinical Practice Gastroenterology & Hepatology 2005, 2 (3), 140–147. [DOI] [PubMed] [Google Scholar]
- 12.Bethune MT, Strop P, Tang Y, Sollid LM, Khosla C, Heterologous Expression, Purfication, Refolding, and Structural-Functional Characterization of EP-B2, a Self-Activating Barley Cysteine Endoprotease. Chemistry & Biology 2006, 13 (6), 637–647. [DOI] [PubMed] [Google Scholar]
- 13.McCarville JL, Caminero A, Verdu EF, Pharmacological approaches in celiac disease. Current Opinion in Pharmacology 2015, 25, 7–12. [DOI] [PubMed] [Google Scholar]
- 14.Schuppan D; Junker Y; Barisani D, Celiac Disease: From Pathogenesis to Novel Therapies. Gastroenterology 2009, 137 (6), 1912–1933. [DOI] [PubMed] [Google Scholar]
- 15.Plugis NM, Khosla C, Therapeutic approaches for celiac disease. Best Practice & Research Clinical Gastroenterology 2015, 29, 503–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bethune MT, Khosla C, Oral Enzyme Therapy for Celiac Sprue. Methods in Enzymology 2012, 502, 241–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen T, Hoffmann K, Ostman S, Sandberg AS, Olsson O, Identification of gliadin-binding peptides by phage display. BMC Biotechnology 2011, 11 (6), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinier M; Verdu EF; Nasser–Eddine M; David CS; Vézina A; Rivard N; Leroux JC, Polymeric Binders Suppress Gliadin-Induced Toxicity in the Intestinal Epithelium. Gastroenterology 2009, 136 (1), 288–298. [DOI] [PubMed] [Google Scholar]
- 19.Liang L; Pinier M; Leroux J-C; Subirade M, Interaction of α-gliadin with poly(HEMA-co-SS): Structural characterization and biological implication. Biopolymers 2009, 91 (2), 169–178. [DOI] [PubMed] [Google Scholar]
- 20.Király R; Demény M; Fésüs L, Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS Journal 2011, 278 (24), 4717–4739. [DOI] [PubMed] [Google Scholar]
- 21.Li X; Lin C; O’Connor PB, Glutamine deamidation: Differentiation of Glutamic acid and γ-Glutamic acid in peptides by electron capture dissociation. Analytical chemistry 2010, 82 (9), 3606–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y; Tramper J, Novel applications for microbial transglutaminase beyond food processing. Trends in Biotechnology 2008, 26 (10), 559–565. [DOI] [PubMed] [Google Scholar]
- 23.Pinier M; Fuhrmann G Fau - Galipeau HJ; Galipeau Hj Fau - Rivard N; Rivard N Fau - Murray JA; Murray Ja Fau - David CS; David Cs Fau - Drasarova H; Drasarova H Fau - Tuckova L; Tuckova L Fau - Leroux J-C; Leroux Jc Fau - Verdu EF; Verdu EF, The copolymer P(HEMA-co-SS) binds gluten and reduces immune response in gluten-sensitized mice and human tissues. (1528–0012 (Electronic)). [DOI] [PubMed] [Google Scholar]
- 24.Liang L; Pinier M; Leroux JC; Subirade M, Interaction of alpha-gliadin with poly(HEMA-co-SS): structural characterization and biological implication. Biopolymers 2009, 91 (2), 169–78. [DOI] [PubMed] [Google Scholar]
- 25.Ladmiral V, Melia E, Haddleton DM, Synthetic Glycopolymers: An Overview. European Polymer Journal 2004, 40, 431–449. [Google Scholar]
- 26.Gruber H, Knaus S, Synthetic polymers based on carbohydrates: preparation, properties, and applications. Macromol. Symp 2000, 152, 95–105. [Google Scholar]
- 27.Miura Y, Hoshino Y, Seto H, Glycopolymer Nanobiotechnology. Chem. Rev. 2016, 116, 1673–1692. [DOI] [PubMed] [Google Scholar]
- 28.Spain SG, Cameron NR, A spoonful of sugar: the application of glycopolymers in therapeutics. Polymer Chemistry 2011, 2 (1), 60–68. [Google Scholar]
- 29.Convertine AJ, Benoit DSW, Duvall CL, Hoffman AS, Stayton PS, Development of a novel endosomolytic diblock copolymer for siRNA delivery. J. Controlled Release 2009, 133 (3), 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le TP, Moad G, Rizzardo E, Thang SH Polymerization with living characteristics. May 11, 2010, 2010. [Google Scholar]
- 31.Theisen A, Johann C, Deacon MP, Harding SE, Refractive Increment Data-Book for Polymer and Biomolecular Scientists. Nottingham University Press: Manor Farm, Main Street, Thrumpton; Nottingham, NG11 OAX, United Kingdom, 2000. [Google Scholar]
- 32.Fisher LW, Sochor AR, Tan JS, Chain Characteristics of Poly(2-acrylamido-2-methylpropane sulfonate) Polymers. 1. Light Scattering an Intrinsic-Viscosity Studies. Macromolecules 1977, 10 (5), 949–954. [Google Scholar]
- 33.Bowman WA, Rubinstein M, Tan JS, Polyelectrolyte-Gelatin Complexation: Light Scattering Study. Macromolecules 1997, 30, 3262–3270. [Google Scholar]
- 34.Hunter RJ, The Calculation of Zeta Potential In Zeta Potential in Colloid Science, Ottewill RH, Rowell RL, Ed. Elsevier: 1981; pp 59–124. [Google Scholar]
- 35.Hoke DI, Robins RD, Preparation and polymerization of 3-acrylamido-3-methylbutanoic acid. Journal of Polymer Science Part A: Polymer Chemistry 1972, 10 (11), 3311–3315. [Google Scholar]
- 36.Sumerlin BS; Donovan MS; Mitsukami Y; Lowe AB; McCormick CL, Water-Soluble Polymers. 84. Controlled Polymerization in Aqueous Media of Anionic Acrylamido Monomers via RAFT. Macromolecules 2001, 34 (19), 6561–6564. [Google Scholar]
- 37.Morgan SE, Jones P, Lamont AS, Heidenreich A, McCormick CL, Layer-by-Layer Assembly of pH-Responsive, Compositionally Controlled (Co)polyelectrolytes Synthesized via RAFT. Langmuir 2007, 23, 230–240. [DOI] [PubMed] [Google Scholar]
- 38.Abel BA, Sims MB, McCormick CL, Tunable pH- and CO2-Responsive Sulfonamide-Containing Polymers by RAFT Polymerization. Macromolecules 2015, 48 (16), 5487–5495. [Google Scholar]
- 39.Sumerlin BS, Lowe AB, Thomas DB, McCormick CL, Aqueous Solution Properties of pH-Responsive AB Diblock Acrylamido Copolymers Synthesized via Aqueous RAFT. Macromolecules 2003, 36 (16), 5982–5987. [Google Scholar]
- 40.Bruice PY, Organic Chemistry, Fifth Edition Prentice Hall: Upper Saddle River, New Jersey: 07458, 2007. [Google Scholar]
- 41.Greenfield NJ, Analysis of Circular Dichroism Data. Methods in Enzymology 2004, 383, 282–317. [DOI] [PubMed] [Google Scholar]
- 42.Jiang J; Prausnitz JM, Molecular Thermodynamics for Protein Precipitation with a Polyelectrolyte. The Journal of Physical Chemistry B 1999, 103 (26), 5560–5569. [Google Scholar]
- 43.Hattori T, Hallberg R, Dubin PL, Roles of Electrostatic Interaction and Polymer Structure in the Binding of B-Lactoglobulin to Anionic Polyelectrolytes: Measurement of Binding Constants by Frontal Analysis Continuous Capillary Electrophoresis. Langmuir 2000, 16, 9738–9743. [Google Scholar]
- 44.Gasymov OK, Gasgow BJ, ANS Fluorescence: Potential to Augment the Identification of the External Binding Sites of Proteins. Biochim Biophys Acta 2007, 1774 (3), 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu J, Zhao C, Lin W, Hu R, Wang Q, Chen H, Li L, Chen S, Zheng J, Binding characteristics between polyethylene glycol (PEG) and proteins in aqueous solution. Journal of Materials Chemistry B 2014, 2, 2983–2992. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Zhao C, Hu R, Lin W, Wang Q, Zhao J, Bilinovich SM, Leeper TC, Li L, Cheung HM, Chen S, Zheng J, Probing the weak interaction of proteins with neutral and zwitterionic antifouling polymers. Acta Biomaterialia 2014, 10, 751–760. [DOI] [PubMed] [Google Scholar]
- 47.Thomson NH, Miles MJ, Popineau Y, Harries J, Shewry P, Tatham AS, Small angle X-ray scattering of wheat seed-storage proteins: α-, γ-, ω- gliadins and the high molecular weight (HMW) subunits of glutenin. Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology 1999, 1430 (2), 359–366. [DOI] [PubMed] [Google Scholar]
- 48.Sato N, Matsumiya A, Higashin Y, Funaki S, Kitao Y, Oba Y Inoue R, Arisaka R, Sugiyama M, Urade R, Molecular Assembly of Wheat Gliadins into Nanostructures: A Small-Angle X-Ray Scattering Study of Gliadins in Distilled Water over a Wide Concentration Range. Journal of Agricultural and Food Chemistry 2015, 63 (39), 8715–8721. [DOI] [PubMed] [Google Scholar]
- 49.Oberlerchner JT, Rosenau T, Potthast A, Overview of Methods for the Direct Molar Mass Determination of Cellulose. Molecules 2015, 20, 10313–10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.