Abstract
Introduction:
Polycystic Ovary Syndrome (PCOS), the most common endocrinological problem among women in the reproductive age, is characterized by chronic ovulatory dysfunction, hyperandrogenism, and raised Luteinizing hormone : Follicle Stimulating Hormone (LH:FSH) ratio. Obesity and insulin resistance have been linked to PCOS. However, there is recently a growing population of thin, lean women who are diagnosed with PCOS.
Aim:
This study aimed to compare normal and high Body mass index (BMI) women with PCOS and to investigate the correlation between BMI and LH/FSH ratio.
Methods:
It was a case -control study at the Department of Obstetrics and Gynaecology, Qassim University clinic, Saudi Arabia. Women with PCOS were included in the study and were classified according to their BMI. Their computerized records were retrieved for the demographic, clinical, and laboratory data. The study groups were compared by the t-test and the Spearman correlation between BMI and LH/FSH ratio was calculated.
Results:
A total of 63 women were included in this study (normal BMI group: n=30, and high BMI group: n=33). There was no difference between the two groups in terms of the LH/FSH ratio (2.76 vs. 2.79, P=0.48). There was no significant correlation between BMI and LH/FSH ratio, prolactin, or Thyroid stimulating hormone (TSH ) levels (Spearman correlation with P>0.05).
Conclusion:
The data suggests that the body mass index was not correlated with increased LH/FSH ratio. Since LH/FSH ratio was the same in normal BMI women, healthcare professionals need to think about ways to normalize this ratio beyond weight reduction.
Keywords: Polycystic Ovary Syndrome, Body Mass Index, LH, FSH
1. INTRODUCTION
Polycystic Ovary Syndrome (PCOS) is a heterogeneous disorder characterized by chronic ovulatory dysfunction and hyperandrogenism. It is considered as the most common endocrinological problem affecting women, and the most prevalent cause of their menstrual irregularities during the reproductive age. It is predicted that PCOS might affect up to 25% of women in their reproductive age. Excess androgen is the key hormonal problem in PCOS patients, which might be caused by other conditions such as obesity and insulin resistance, which commonly manifest with PCOS (1-3).
Theoretically, PCOS is a result of abnormal interaction between different behavioral, environmental, and genetic factors. The most commonly seen clinical picture of PCOS is: enlarged one or both ovaries, and theca cells are inflamed, enlarged, and secreting higher amounts of androgens than normal. The increased androgenic secretion is a result of enzymatic over activity, which is involved in steroid production. PCOS is associated with long -term metabolic abnormalities and cardiovascular risks like insulin resistance, dyslipidemia, endothelial dysfunction, and elevated serum luteinizing hormone (LH) levels (2, 4, 5).
Abnormality of the hypothalamic-pituitary-ovarian or adrenal axis has been imposed in the pathophysiology of polycystic ovarian disease. A disturbance in the secretion pattern of the gonadotrophin-releasing hormone (GnRH) results in the relative increase in LH to FSH release (6). Ovarian estrogen is responsible for causing an abnormal feedback mechanism that caused an increase in LH release (7). Usually, in healthy women, the ratio between LH and FSH usually lies between 1 and 2. In polycystic ovary disease women, this ratio becomes reversed, and it might reach as high as 2 or 3 (8).
As a result of raised LH/FSH ratio, ovulation does not occur in polycystic ovary disease patients (9). The mainstay in the treatment of PCOS includes lifestyle modification, essentially weight loss, in addition to adjuvant pharmacological treatment. Drugs used to treat PCOS include oral contraceptives (OC), antiandrogen therapy, and anti-insulin medications such as metformin, followed by ovulation induction with clomiphene citrate (CC). It is estimated that about 25% of women with PCOS would have a drug resistance against CC. Alternatively, gonadotrophins may be used as a substitute in CC-resistant (CCR) women. But unluckily, using gonadotrophins might have some drawbacks like being expensive, with a high risk of multiple pregnancies and ovarian hyperstimulation (10-13). PCOS is associated with obesity and insulin resistance. On the other hand, there is a growing population of thin slim women having PCOS as well (14).
2. AIM
This study aimed to explore the LH/FSH ratio in women with low BMI PCOS patients in comparison to women with high BMI. Women with high BMI are normally advised to lower the body weight to normalize the ratio but if this ratio is really the same in low weight women then we need to think of other ways to normalize it.
3. METHODS
Study design and setting
This was a retrospective case-control study conducted at the Department of Obstetrics and Gynecology, Qassim University clinic, Buraidah, AL Qassim, Saudi Arabia from September 2018 to May 2019. Statistical approval was taken from the local ethical committee. The anonymity and confidentiality of the subjects was followed in line with Helsinki declaration.
Inclusion criteria
The study included both married and unmarried women diagnosed with PCOS based on the Rotterdam ESHRE/ ASRM sponsored PCOS consensus criteria 2003. Patients were diagnosed with PCOS if they have two confirmed criteria out of the following three a) anovulation or oligomenorrhea; b) clinical or biological evidence of hyperandrogenism; and c) Presence of polycystic ovaries on ultrasound (15). The definitive diagnostic criteria of PCOS on using transvaginal ultrasound was around 10-12 or more ovarian follicles measuring 2-10 mm, with a pearl necklace appearance.16
Exclusion criteria
Patients with other associated disorders as Cushing disease, hypothyroidism, hyperprolactinemia, adrenal hyperplasia, or ovarian tumors were excluded. Patients whose data was not available in the hospital records were also excluded.
Study groups
The BMI was calculated as weight (kg) divided by the height in meters squared (m2). Patients were classified according to their BMI into two groups; normal BMI group (control) includes women with BMI ≤25 kg/m2, and high BMI group (cases) includes women with BMI>25 kg/m2.
Source of data
Patients’ data were retrieved from the computerized hospital records. Demographic features, clinical presentation, and blood biochemistry was noted. Hormonal profile of included patients was checked for the following measures: serum levels of LH, FSH, Prolactin, Thyroid stimulating hormone (TSH), Triiodothronine (T3), and thyroxine (T4 ) and testosterone.
Statistical analysis
Descriptive statistics were used to describe characteristics of the two groups. Data were described as mean (±SD) or frequency and percentage for continuous and categorical variables respectively. The two groups were compared by the student t-test or the Mann-Whitney test for normally and non-normally distributed continuous variables, respectively. The comparison between the two groups in terms of categorical variables was done by the chi-square test. Correlation analysis was done between the LH, FSH, LH/FSH ratio, prolactin, TSH, and BMI by using the Pearson correlation coefficient (r). A P value of <0.05 was considered for statistical significance. All analyses were conducted by the Statistical Package for Social Science, SPSS for Windows, version 23.
4. RESULTS
Characteristics of the Study Population
The study included 63 women who were diagnosed with PCOS according to Rotterdam ESHRE/ ASRM sponsored PCOS consensus criteria (normal BMI group: n=30, and high BMI group: n=33).was 25.6 (±7.4) years for the high BMI group and 24.8 (±5) years for the normal BMI group. The mean BMI was 30.3 (±4.4) kg/m2 for the high BMI group and 21.5 kg/m2 (±1.8) for the normal BMI group. Table 1 shows the characteristics of the study groups.
Table 1. The characteristics of the study population.
| Variable | High BMI group | Normal BMI group | P value† |
|---|---|---|---|
| (n=33) | (n=30) | ||
| Age (year) | 25.6 ± 7.4 | 24.8 ± 5.1 | 0.63 |
| Height (cm) | 159.5 ± 5.5 | 159.6 ± 6.5 | 0.96 |
| Weight (kg) | 77.1 ± 13.5 | 57.9 ± 12.9 | <0.0001* |
| BMI (kg/m2) | 30.3 ± 4.3 | 21.5 ± 1.7 | < 0.0001* |
| LH (IU/litre) | 10.8 ± 4.3 | 10.85 ± 5.08 | 0.48 |
| FSH (IU/litre) | 4.46 ± 2.3 | 5.0 ± 4.0 | 0.53 |
| LH/FSH Ratio | 2.79 ± 1.4 | 2.76 ± 1.52 | 0.48 |
| LH/FSH Ratio >2 | 26 (79%) | 22 (73%) | 0.6 |
| LH/FSH Ratio ≤ 2 | 7 (21%) | 8 (27%) | |
| Prolactin (ng/ml) | 30.45 ± 22.7 | 25.54 ± 15.5 | 0.99 |
| TSH (mIU/L) | 2.67 ± 1.38 | 2.75 ± 1.6 | 0.93 |
| T4 (mIU/L) | 1.18 ± 0.035 | 1.26 ± 0.01 | 0.049* |
| Testosterone (ng/ml) | 0.54 ± 0.18 | 0.38 ± 0.06 | 0.044* |
Data are presented as mean ± SD or n (%);
Mann-Whitney test or t-test for continuous variables and chi-square test for categorical variables;
statistically significant
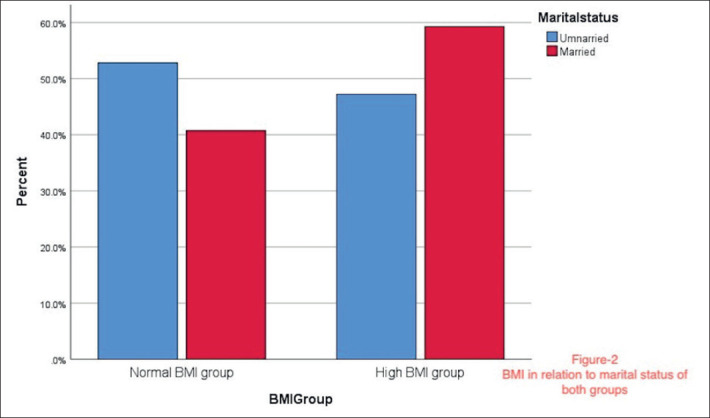
Regarding the occupation, most of the women were students or housewives, and a minority of women in both groups had jobs. More women were unmarried in the high BMI group, while nearly equal married and unmarried women were present in the normal BMI group. Undergraduates were highly represented in the study, as well as postgraduates. Most of the patients presented with oligomenorrhea, amenorrhea, or a combination of multiple PCOS characteristic symptoms. The marital status and the clinical presentation of the study groups are shown in Figure 1 and Figure 2.
Figure 1. Distribution of clinical presentation of patients.
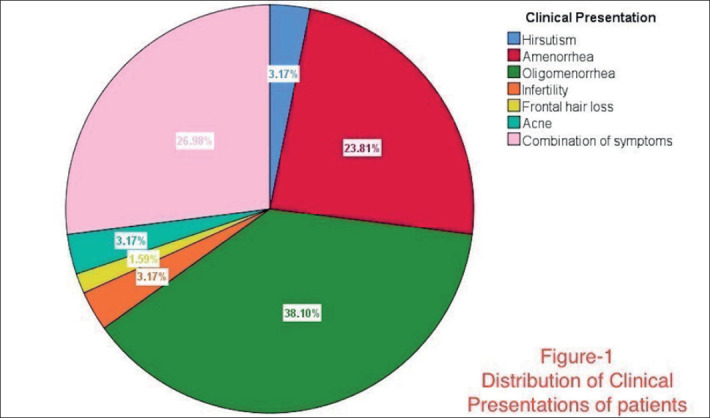
Figure 2. BMI in relation to marital status of both groups.
Comparison of the presenting symptoms between the study groups
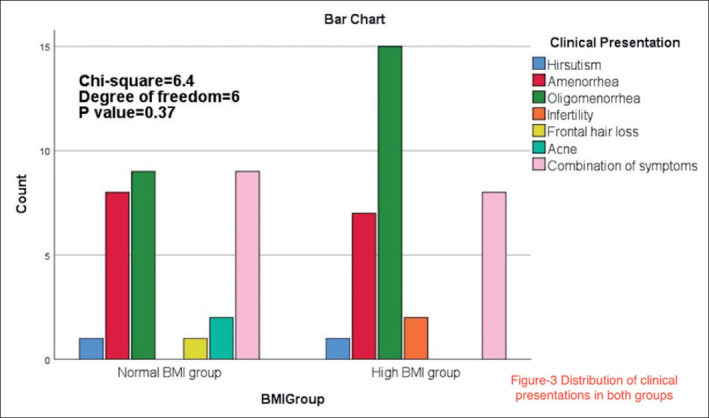
There was no statistically significant association between the BMI group and the clinical presentation of the study participants (chi-square=6.4, df=6, P=0.37). The frequency of the presenting symptom in the study groups are shown in Figure 3.
Figure 3. Distribution of clinical presentations in both groups.
Comparison of hormone levels between the study groups
Both groups had comparable serum LH level, FSH level, LH/FSH ratio, prolactin level, testosterone, and TSH level. However, patients in the high BMI group had significantly lower serum levels of T4 compared to the normal BMI group (1.18 vs. 1.26 mIU/L; P=0.049). Serum testosterone level was higher in the high BMI group compared with the normal BMI group (0.54 vs. 0.38 ng/ml, P=0.044). The LH/FSH ratio was 2.67 in the high BMI group and 2.75 in the normal BMI group. The percentage of women with LH/FSH ratio > 2 was not statistically different between the two groups. The comparison between the two groups in terms of biochemical analysis results is shown in Table 1.
Correlation between BMI and hormonal assays
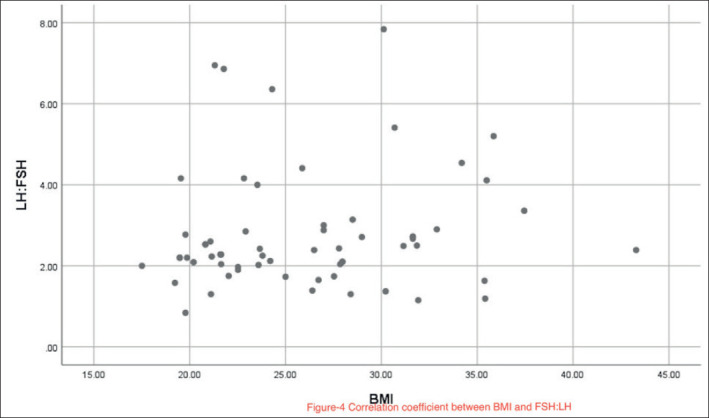
The Spearman correlation coefficient between the BMI and LH/FSH ratio was r=0.09 (P=0.44). A scatter plot of the correlation between BMI and LH/FSH ratio is shown in Figure 4. The BMI did not significantly correlate with any other serum hormone levels including the LH, FSH, prolactin, or TSH (all P>0.05).
Figure 4. Correlation coefficient between BMI and FSH:LH.
5. DISCUSSION
The results have shown that both normal BMI group and high BMI group had comparable LH/FSH ratio. In addition, study showed no significant correlation between the BMI and the LH/FSH ratio in the studied population.
The normal gonadotrophin axis is disturbed in PCOS women, therefore LH levels increase, and FSH levels decrease, leading to a reversal of LH/FSH ratio (16). The results are not concordant with the study of Lal et al. (17) who found significant differences between obese and non-obese women regarding LH, FSH, and LH/FSH ratio. Upon these findings, they advised obese women who shared in the study to try to lose weight and change their lifestyle.
A study by Alnakash et al. (18) has found no significant statistical correlation between BMI and LH/FSH Ratio (18). Kiddy et al. (19) found a negative correlation between BMI and FSH levels in obese women and found hirsutism to be more present in obese than thin women (19). Nonetheless, our study failed to show any significant correlation between BMI and the serum hormone levels of PCOS women including LH, FSH, and LH/FSH ratio.
Obesity, insulin resistance, and dyslipidemia are PCOS-related morbidities and were found to be correlated with the LH/FSH ratio in a nationally representative sample of post-menopausal women in the USA (20). A study on 175 PCOS women in Iran showed that compared with non-obese women, obese PCOS women had higher odds of having a sonographic view of their polycystic ovaries using ultrasound examination (21).
Results of this study could be explained by the relatively small sample size compared to previous studies. It is also possible that geographical or dietary variations play a role in the hormonal levels of PCOS women. Future research into the risk factors of PCOS in Saudi women is needed. I used a BMI cut off value of 25 kg/m2 which is different from some previous studies that considered the overweight population (BMI 25 to 29.9 kg/m2) to be classified in the normal BMI group.
The significant findings in this study were the slight differences between the study groups in terms of the T4 and testosterone levels (P>0.05). Although the TSH as well as T4 levels were within the normal laboratory reference (TSH 0.4-4miu/Land T4 0.4-5miu/L). T4 levels were lower in the high BMI group compared with the normal BMI group, while testosterone levels were significantly higher in the high BMI group compared with the normal BMI group. Similar findings were reported by Yuan et al. (22) who found that PCOS women with high BMI have excess androgens (22). The relationship between thyroid functions and PCOS has been found in recent studies (23, 24). This study expands this by reporting lower T4 levels in PCOS women with high BMI compared to those with normal BMI level. If we assume that PCOS in high BMI women was associated with obesity, the higher T4 level in normal BMI women might indicate that normal BMI women develop PCOS in a different way independent from obesity and thyroid activity. This might be related to presence of autoantibodies against thyroid gland and we should further investigate these women for autoimmune conditions. This aspect needs to be further researched and the findings of this study open doors for such aspect to be researched in future.
Nonetheless, this study is limited by the relatively small sample size of the population owing to the relatively less PCOS patients attending at our clinic. The study was not powered to follow up patients for several years to examine the role of hormonal changes and BMI in the reversal of LH/FSH ratio. Future well-designed studies with larger sample size and long term follow up are needed to understand the nature of interaction between the BMI and the hormonal levels in PCOS women.
6. CONCLUSION
The data suggest that the body mass index was not correlated with increased LH/FSH ratio. Since LH/FSH ratio was the same in normal and high BMI women This finding has an important implication for clinical practice and future research. Weight loss is usually advisable for women with PCOS. However, my findings showed that normal BMI women with PCOS already have hormonal imbalances and an LH/FSH ratio comparable to that of the high BMI women. This finding implies that healthcare professionals should to rethink about ways to normalize LH/FSH ratio other than weight loss. Similarly, researchers should investigate the pathogenesis of PCOS in normal BMI women and whether the clinical presentation and clinical treatment would differ based on the BMI.
Acknowledgment:
I acknowledge Mr. Waleed Alharbi from IT department who helped me in accessing the data
Author’s contribution:
Author of this article has been involved in all steps of preparation the article including, also, final proof reading.
Conflict of interest:
None declared.
Financial support and sponsorship:
Nil.
REFERENCES
- 1.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011 Apr;7(4):219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 2.Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014 Oct;171(4):P1–29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 3.Diamanti-Kandarakis E, Christakou C, Marinakis E. Phenotypes and Enviromental Factors: Their Influence in PCOS. Curr Pharm Des. 2012 Jan;18(3):270–282. doi: 10.2174/138161212799040457. [DOI] [PubMed] [Google Scholar]
- 4.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. Reprod Endocrinol. 2014;0(20):22–35. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS) Hum Reprod. 2012 Jan;27(1):14–24. doi: 10.1093/humrep/der396. [DOI] [PubMed] [Google Scholar]
- 6.SS Y. The polycystic ovary syndrome. Clin Endocrinol. 1980;12:177–183. doi: 10.1111/j.1365-2265.1980.tb02132.x. [DOI] [PubMed] [Google Scholar]
- 7.TJ M. Pathogenesis and treatment of polycystic ovary syndrome. N Engl J Med. 1988;(318):558–562. doi: 10.1056/NEJM198803033180906. [DOI] [PubMed] [Google Scholar]
- 8.Richard SL. 8th. Philadelphia: Lippincott Williams & Wilkins; 2003. Androgen excess disorders. Danforth’s Obstetrics and Gynecology; pp. 663–672. Chapter 37. RA. [Google Scholar]
- 9.Johansson J, Stener-Victorin E. Polycystic ovary syndrome: effect and mechanisms of acupuncture for ovulation induction. Evid Based Complement Alternat Med. 2013;2013:762615. doi: 10.1155/2013/762615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kazerooni T, Shojaei-Baghini A, Dehbashi S, Asadi N, Ghaffarpasand F, Kazerooni Y. Effects of metformin plus simvastatin on polycystic ovary syndrome: a prospective, randomized, double-blind, placebo-controlled study. Fertil Steril. 2010 Nov;94(6):2208–2213. doi: 10.1016/j.fertnstert.2009.11.045. [DOI] [PubMed] [Google Scholar]
- 11.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of Cardiovascular Risk and Prevention of Cardiovascular Disease in Women with the Polycystic Ovary Syndrome: A Consensus Statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010 May;95(5):2038–2049. doi: 10.1210/jc.2009-2724. [DOI] [PubMed] [Google Scholar]
- 12.Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of Simvastatin and Oral Contraceptive Agent on Polycystic Ovary Syndrome: Prospective, Randomized, Crossover Trial. J Clin Endocrinol Metab. 2007 Feb;92(2):456–461. doi: 10.1210/jc.2006-1988. [DOI] [PubMed] [Google Scholar]
- 13.Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Effects of Simvastatin and Metformin on Polycystic Ovary Syndrome after Six Months of Treatment. J Clin Endocrinol Metab. 2011 Nov;96(11):3493–3501. doi: 10.1210/jc.2011-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goyal M, Dawood AS. Debates Regarding Lean Patients with Polycystic Ovary Syndrome: A Narrative Review. J Hum Reprod Sci. 2017;10(3):154–161. doi: 10.4103/jhrs.JHRS_77_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004 Jan;19(1):41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 16.Balen AH, Laven JSE, Tan S-L, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003 Nov;9(6):505–514. doi: 10.1093/humupd/dmg044. [DOI] [PubMed] [Google Scholar]
- 17.Lal L, Bharti A, Perween A. To Study The Status of LH: FSH Ratio in Obese And Non-Obese Patients of Polycystic Ovarian Syndrome. IOSR J Dent Med Sci. 2017;16(01):20–23. [Google Scholar]
- 18.Alnakash AH, Al-Tae’ NK. Polycystic ovarian syndrome: the correlation between the LH/FSH ratio and disease manifestations. Middle East Fertility Society Journal. 2007;12(1):35–40. [Google Scholar]
- 19.Kiddy ds, sharp ps, white dm, scanlon mf, mason hd, bray cs, et al. Differences in clinical and endocrine features between obese and non-obese subjects with polycystic ovary syndrome: an analysis of 263 consecutive cases. Clin endocrinol (oxf) 1990 Feb;32(2):213–220. doi: 10.1111/j.1365-2265.1990.tb00857.x. [DOI] [PubMed] [Google Scholar]
- 20.Beydoun HA, Beydoun MA, Wiggins N, Stadtmauer L. Relationship of obesity-related disturbances with LH/FSH ratio among post-menopausal women in the United States. Maturitas [Internet] 2012 Jan;71(1):55–61. doi: 10.1016/j.maturitas.2011.10.010. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378512211003604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esmaeilzadeh S, Andarieh MG, Ghadimi R, Delavar MA. Body Mass Index and Gonadotropin Hormones (LH & FSH) Associate With Clinical Symptoms Among Women With Polycystic Ovary Syndrome. Glob J Health Sci [Internet] 2014 Sep 28;7(2) doi: 10.5539/gjhs.v7n2p101. Available from: http://www.ccsenet.org/journal/index.php/gjhs/article/view/39332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan C, Liu X, Mao Y, Diao F, Cui Y, Liu J. Polycystic ovary syndrome patients with high BMI tend to have functional disorders of androgen excess: a prospective study. J Biomed Res [Internet] 2016 May 30;30(3):197–202. doi: 10.7555/JBR.30.20140111. Available from: http://www.jbr-pub.org.cn/ch/reader/view_abstract.aspx?file_no=jbr160306&flag=1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai J, Zhang Y, Wang Y, Li S, Wang L, Zheng J, et al. High Thyroid Stimulating Hormone Level Is Associated With Hyperandrogenism in Euthyroid Polycystic Ovary Syndrome (PCOS) Women, Independent of Age, BMI, and Thyroid Autoimmunity: A Cross-Sectional Analysis. Front Endocrinol (Lausanne) [Internet] 2019 Apr 10;10 doi: 10.3389/fendo.2019.00222. Available from: https://www.frontiersin.org/article/10.3389/fendo.2019.00222/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Q, Wang J-B. Subclinical Hypothyroidism in PCOS: Impact on Presentation, Insulin Resistance, and Cardiovascular Risk. Biomed Res Int [Internet] 2016;2016:1–7. doi: 10.1155/2016/2067087. Available from: http://www.hindawi.com/journals/bmri/2016/2067087/ [DOI] [PMC free article] [PubMed] [Google Scholar]


