Abstract
Background
Rumex nepalensis(RN) Spreng has been used to treat ulcer disease in Ethiopian folk medicine. This study aimed to determine the anti-ulcer activity of hydroalcoholic root crude extract and solvent fractions of R. nepalensis in rats.
Methods
The effect of R. nepalensis crude hydromethanolic extract and solvent fractions at doses (100, 200, 400 mg/kg/day) and repeated dosing (200 mg/kg/day for 10 and 20 days) was examined on ulcers in rats in pyloric ligation-, cold restraint stress-, and acetic acid-induced ulcer models. Cimetidine (100 mg/kg/day) and/or Omeprazole (20 mg/kg/day) were used as standard drugs and served as a positive control. Data were analyzed by one-way ANOVA post hoc followed by a Tukey HSD test with SPSS software version 24.0, and P≤ 0.05 was considered as statistically significant.
Results
In the pylorus ligation-induced ulcer model, pretreatment with the crude extract significantly reduced the degree of gastric secretions, pH, total acidity, and ulcerations in a dose-dependent manner. Gastroprotection offered by the R. nepalensis 400 mg/kg test extract was comparable to that of the standard. Among fractions, the ethyl acetate fraction at 400 mg/kg had the highest protection of ulcer but the chloroform fraction was ineffective. In the cold restraint stress-induced ulcer model, R. nepalensis at 200 and 400 mg/kg reduced the lesion index significantly (P<0.01). With relevant chronic ulcer model treatment, a dose of R. nepalensis at 200 and 400 mg/kg healed ulcers significantly with a curative ratio of 53.22% and 54.59%, respectively.
Conclusion
From this study, it is concluded that hydromethanolic crude extract and solvent fractions of R. nepalensis root showed promising anti-ulcer activity. This upholds its folkloric use. Thus, it is considered as a possible source to develop a new anti-ulcer agent.
Keywords: pylorus ligation, stress ulcer, hydromethanolic extract, Rumex nepalensis
Introduction
Maintaining high resistance to wreck is feasible thanks to the presence of a variety of physiological defensives and rapid repair of injured mucosa when damage occurs.1 The mucus barrier consists of mucus, bicarbonate anions, and phospholipids forming a surface layer on the gastric mucosa. The bicarbonate maintains the pH near 7 in the mucosa. The mucous layer protects from the proteolytic actions of pepsin. Mucin units to polymerize into large mucin multimers.2 The surface epithelial cells form a “barrier” preventing back diffusion of acid and pepsin.3 Continuous cell renewal from mucosal progenitor cells maintains the structural integrity of the mucosa.4 PGI2 and NO maintain the viability of endothelial cells and stop platelet and leukocyte adherence to the micro-vascular endothelial cells, thus preventing compromise of the microcirculation.5 Peptic ulcer disease may be a break within the lining of the stomach, the upper part of the intestine, or lower esophagus.6 Gastritis is an inflammation of the gastric mucosa and a precursor of ulceration.7 There are around 6000 species of vascular plants in Ethiopia, out of which over 14% have been used as traditional plant medicines.8 Despite their important contribution, medicinal plants have received little modern research and development.9
For centuries, herbals have been used traditionally for the treatment of a large range of ailments, including gastrointestinal disorders. Medicinal plants possessing active principles like flavonoids, saponins, tannins, and terpenoids are found to have anti-ulcer activity.10 Leaf crude extract of Jasminiumgradiflorum,11 Orbignyaphalerata and Euterpe edulis,12 Azadirachta indicia,13 Papaya carica,14 and ribwort L.15 possess anti-ulcer activity. Leaf crude extract of Diodiasarmentosa (Rubiaceae), Cassia nigricans (Celsapinaceae), Ficus exasperate (Moraceae) and Synclisiascabrida (Menispermaceae) are the most popularly used anti-ulcer recipes in Nigeria. In vivo studies in mice and rats revealed their anti-ulcer activities by decreasing the ulcer index in aspirin-induced ulcerogenesis, delayed gastrointestinal transit, increased pH, and decreased volume and acidity of gastric secretion. Leaf extracts of Wilbrandiaebracteata,16 Toonaciliata roemer,17 garden rocket,18 Calligonumsomosum,19 Pedalium murex,20 Voacangaafricana, and Eremomastaxspeciosa 21 have shown anti-ulcer activity. Alkaloids from the bark of Enantiachlorantha prevent ulcerations.22,23 The effectiveness and mechanisms of action of crude herbal extracts vary, following the composition of their chemical constituents.24 Oroxylumindicum, Zingiber officinale, Alchorneaglandulosa, and Tephrosia purpurea containing phenolic compounds are used as folklore medicine.25
Clove, cinnamon, oregano, Capsicum, turmeric, ginger, and polygonum species contain phytoconstituents with antioxidative potential which give them anti-ulcer activity.26 The root and rhizome of Leguminosae contain glycyrrhizinic acid, a triterpenoid saponin having protection against ulcers.27 Twelve medicinal herbs were used as anti-ulcer (PUD) herbal formulations in a previous study.28
Rumex nepalensis belongs to the magnoliopsid family and magnoliopsid genus. The genus consists of about 250 species of herbs, and are distributed worldwide.29 R. nepalensis grows in parts of China, Afghanistan, India, Indonesia, Japan, Myanmar, Nigeria, Ethiopia, and the Republic of South Africa.30 It is commonly called “Tult” in Amharic, and “Shuultii” in Oromifa. R. nepalensis has served as the basis of traditional medicine systems in Nigeria, India, China, Indonesia, Ethiopia, and Nepal. In Ethiopia, a root decoction of R. nepalensis is given orally for five to fifteen consecutive days on an empty stomach to treat ascariasis, abdominal bleeding, and peptic ulceration. A piece of fresh root is ground in to stick and applied externally to prevent bleeding and to facilitate wound healing and applied to swollen gums, as a cure for jaundice, and with antihistaminic, anticholinergic, antibradykinin, anti-inflammatory, antibacterial, antipyretic, antiplasmodial, hypoglycemic, and analgesic activities.31–34 Medicines used for the treatment of GIT disorders comprise 8% of total prescriptions.35 The increase of resistant strains of H. pylori to different antimicrobials especially in developed countries has been given great attention.36 Patients on once-daily doses of proton pump inhibitors (PPIs) failed at a rate of 56% which indicates significant PPI drug resistance and rapid metabolism by CYP2C19 polymorphism.37,38 Several orthodox drugs employed for the treatment of the disease are associated with drug–drug and drug–food interactions, increased risk of bacterial infection, tolerance, relapse and resistance, which limit their use and require replacement drugs to be found for ulcer treatment.39
An ethnobotanical survey in Denbia (northern, Ethiopia) 40 and East Shewa Zone of Oromia Regional State reported that a root decoction of R. nepalensis Spreng. is taken orally for 15 days in an empty stomach to treat ulcer disease.31,41
The Rationale of the Study
Peptic ulcer disease is one of the serious gastrointestinal diseases in the world, having a major effect on morbidity. Medicines used for the treatment of GIT disorders comprise some 8% of total prescriptions. Drug resistance has also a strong impact on the price of curing ulcer disease. This study verified the claims of previous reports on the knowledge of native traditional medicine practitioners. The finding of this experimental study helps the scientific community to further investigate this candidate medicinal plant by initiating advanced studies on formulations and molecular mechanisms of plant source drugs and by isolating a scientific anti-ulcer compound.
Methods and Materials
Chemicals and Drugs
Absolute ethanol (96%) (Bululux lab.), phenolphthalein (RFCL; RANKEM, India), absolute methanol (ReAgentchem Ltd; India), glacial acetic acid (Lobe chemi, India), Omeprazole (Cadila Pharmaceuticals, Bengaluru, India), Cimetidine (ACILOC, Cadila, Ethiopia), 0.9% normal saline (Cadila Pharmaceuticals, Bengaluru, India), distilled water (EPharm, Ethiopia), ketamin (Fisher Scientific, UK), diazepam (Fisher Scientific, UK), chloroform (NICELab), ethyl acetate, buffered formalin (10%), H2SO4, HCL, 2% ferric chloride, benzene (Nice Laboratory Reagent, Kerala, India), 10% ammonium hydroxide, 0.01 N sodium hydroxide (Central Drug House India) were purchased. All chemicals were analytical grade and purchased from Bahirdar Pharmaceutical Fund Supply Agency and obtained from the University of Gondar School of Pharmacy laboratories.
Instruments, Apparatus, and Supplies
Filter paper (Whatman No. 1 EXACL; India), examination glove, rotary evaporator (YAMATO rotary evaporator RE301, Japan), lyophilizer (Labfreeze Instruments Group Co., Ltd., Japan), analytical grade balance (JA203P), hand lens (10×), deep freezer (DN-86W258), syringe with a needle, forceps, surgical scalpel blade, conical flask, beaker, Buchner funnel, adhesive plaster, gauze, permanent marker, surgical threads with curved needles, mortar and pestle, centrifuge (Eppendorf AG-5703DQ713856), pH meter (Adwa AD8000), oral gavages, and others have been used.
Plant Material Collection and Identification
Fresh roots of R. nepalensis were collected from the vicinity of Debre Tabor town, about 154 km southeast of Gondar town and 655 km from Addis Ababa at a longitude and latitude of 38°1′E, 11°51′N with an elevation of 2706 meters on January 10, 2019. Taxonomic identification of the plant specimen was made by a botanist at the Department of Biology, College of Natural and Computational Science, University of Gondar, Gondar, Ethiopia, and also the voucher specimen number Wor1/2018 was deposited there for future reference.
Experimental Animals
Wistar rats of either sex weighing 150–250 g inbred at the Animal House of Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia were used. Animals were kept in polypropylene cages (6 rats per cage) in environmentally controlled rooms (25 ± 2°c, 12:12 h light and dark cycle), with free access to water. Rats were kept under standard laboratory conditions and fed with a standard pellet diet (NPD). They were acclimatized to the laboratory environment for five days, and randomly divided into groups in each phase of the experiments. All procedures during this study conformed to the guiding principles for research involving animals as recommended by the guiding principles within the care and use of animals.42
Preparation of Plant Root Extract
Freshly collected roots of R. nepalensis were thoroughly washed with water to remove dirt and dried under shade at room temperature. After complete drying, the plant was pulverized by a mechanical grinder. The dried powdered root of R. nepalensis was extracted via a cold maceration technique with 80% methanol (hydromethanol).43 About 1 kg powder was soaked with a total of 8000 mL of 80% methanol with three Erlenmeyer flasks and placed on a platform shaker at 120 rpm at room temperature for 72 hours. Then, the extract was first filtered using gauze (muslin cloth) followed by Whatman filter paper No. 1. The residue was separately re-soaked with the identical solvents and in similar conditions twice to maximize the yield.44 Then, subsequent filtrates were concentrated on a rotary evaporator at a temperature of 40°C and 60 rpm. The concentrated filtrate was frozen overnight employing an electric refrigerator and freeze-dried with a lyophilizerat at –50°C and vacuum pressure (200 milliBar) to remove water. Finally, the yield of extract was calculated, labeled, and preserved in a refrigerator at −4°C until use.33
Preparation of Solvent Fractions
The hydromethanolic crude extract was further fractionated by solvent–solvent extraction in separatory funnel apparatus using solvents with differing polarity (chloroform, ethyl acetate, and distilled water). Ninety grams of the dried hydromethanolic crude extract were suspended in 400 mL water and slightly shaken to dissolve completely with solvent. The mixture was transferred into a separator funnel. Then, an equal volume of chloroform was added to it. The new mixture was shaken gently and allowed to settle for a few times until it forms two layers. The density of chloroform is higher than water so taken the lower phase so the chloroform fraction was collected. To collect the whole chloroform fraction an identical procedure was repeated twice as described above. To the remaining aqueous portion in the separating funnel, an equal volume (400 mL) of ethyl acetate was added, followed by vigorous shaking as described for the preceding solvent fractionation procedure. The upper layer was ethyl acetate, which was separated from an aqueous portion, and therefore the same procedure was repeated twice as above. The filtrate of chloroform and ethyl acetate fractions was concentrated on the rotary evaporator at 45 rpm and 40°C to get the chloroform and ethyl acetate fraction. The remaining aqueous residue was frozen in a refrigerator overnight and freeze-dried with a lyophilizer to get an aqueous fraction. The yields of the dried fractions were calculated for every solvent extract then transferred into separate vials and stored at −4°C until use.45
Preliminary Phytochemical Screening
Secondary metabolites are the classes of compounds that have curative activity against several ailments in man and thus could explain the standard use of medicinal plants for the treatment of some illnesses. There are chemical compounds (phenolic compounds, alkaloids, terpenoids, steroids, quinones, saponins) with complex structures and with more restricted distribution than primary metabolites. They are not indispensable for the plant that contains them; a minimum of their metabolic functions have not been discovered yet. Standard qualitative tests were employed to detect secondary metabolites like phenols, cardiac glycosides, saponins, sterols, anthraquinones, flavonoids, tannins, alkaloids, and terpenoids.46,47
Acute Oral Toxicity Study
Acute oral toxicity testing was conducted in healthy, nulliparous, and non-pregnant female Wistar rats using Organization for Economic Cooperation and Development (OECD, 2008) 425 guidelines. Accordingly, five female Wistar rats of 8–12 weeks were used to estimate the LD50 of R. nepalensis extract. All rats were fasted (food but not water) overnight before and 4 hours after the administration of the extract. The rat in the sighting study was given R. nepalensis hydromethanolic crude extract with a limit dose of 2000 mg/kg by oral gavage. No death was observed when monitored for the primary 24 hours. Then four other rats were sequentially treated with the identical dose of the extract. The rats were housed separately and observed continuously for 4 hours with 30 min interval and then daily for 2 weeks for any signs of toxicity, such as behavioral profile (alertness, restlessness, irritability, and fearfulness), autonomic profiles (salivation, lacrimation, perspiration (diaphoresis), piloerection, incontinence, and defecation), neurologic (CNS) profile (spontaneous activity (drowsiness), reactivity, touch response, pain response, convulsions, tremors, and gait), physical states like loss of appetite, for morbidity or mortality and other signs of toxicity.6,48
Grouping and Dosing of Experimental Animals
Wistar albino rats weighing 150–250 g of both sexes were randomly divided into five groups having six rats per group for each of the models employed within the study and was fasted in individual cages for twenty-four hours before the actual experiment. Negative controls were treated with 10 mL/kg/day of solvent which contains 3 mL of 95% ethanol + 7 mL of Tween 80 and 90 mL distilled water to make 100 mL volume, used to suspend hydromethanolic crude extract and solvent fractions. Positive control groups were treated with either Cimetidine 100 mg/kg/day or Omeprazole 20 mg/kg/day. The treatment groups received 5% (100 mg/kg/day), 10% (200 mg/kg/day) and 20% (400 mg/kg/day) of the dose of acute oral toxicity studies (2000 mg/kg) doses of the hydromethanolic crude extract. The three models employed were pyloric ligation-induced ulcer, cold restraint stress-induced ulcer, and acetic acid-induced chronic ulcer models. Meanwhile, the solvent fraction’s anti-ulcer activity was evaluated by using the model within which the crude extract revealed the most effective anti-ulcer activity.15
Pylorus Ligation-Induced Ulcer
Animals were fasted for twenty-four hours before the study but had free access to water. Negative and positive controls received solvent (10 mL/kg) and Omeprazole (20 mg/kg), respectively. A hydromethanolic crude extract of R. nepalensis was given to the animals in the treatment group (group III, IV, and V) for ten days at 100, 200, and 400 mg/kg, respectively. After one hour of last drug treatment, rats were anesthetized with 50 mg/kg of ketamine combined with 5 mg/kg of diazepam i.p.; the abdomen was opened by a tiny low midline incision below the os. The pyloric portion of the stomach was slightly lifted out and ligated with suturing material, avoiding traction to the pylorus or damage to its blood supply. The stomach was replaced carefully and also the wall was closed by interrupted sutures using Merisilk no. 2. Rats were sacrificed by cervical dislocation after four hours of pyloric ligation. The abdomen was opened, the cardiac end of the stomach dissected out, and also the contents drained into a glass tube. Each stomach was examined for lesions and indexed in line with severity.49
Cold Restraint Stress-Induced Ulcer
The hypothermic restraint stress-induced gastric-ulcer model was assessed on rats. Rats were divided into five groups of six animals each. After 24 hours of starvation, the rats have received one oral administration of hydromethanolic root crude extract of R. nepalensis at 100, 200, and 400 mg/kg, Cimetidine 100 mg/kg, and solvent 10 mL/kg/day. One hour after treatment, gastric ulceration was induced by immobilizing the rats inside a closed cylindrical cage and inside refrigerator maintained at 2–4°C. After 3 hours the rats were sacrificed by cervical dislocation, the stomach was removed, opened along the greater curvature followed by gentle washing with water to remove the gastric contents and blood clots, scanned and examined for ulcers.18,50
Acetic Acid-Induced Chronic Ulcer
The rats were fasted for twenty-four hours before the study but had free access to water. On the day of the experiment, the rats were anesthetized with 50 mg/kg of ketamine combined with 5 mg/kg of diazepam i.p. The wall was opened by laparotomy and peptic ulcer induced by injection of 20% acetic acid (50 µL) into the subserosal layer within the glandular part of the anterior wall. The stomach was bathed with saline to avoid adherence to the external surface of the ulcerated region and also the abdomen was then closed using catgut chromic number 2/0 and Merisilk no. 2. At some point after surgery, the rats were treated with an oral dose of the solvent (10 mL/kg), hydromethanolic crude extract at 100, 200, and 400 mg/kg/day, and Cimetidine 100 mg/kg for 15 days. On the 16th day, rats were sacrificed by cervical dislocation, stomach was removed, and also the mucosal damage was assessed via ulcer index and scoring ulcer parameters.49
Parameters for Evaluation of Anti-Ulcer Activity
Macroscopic Evaluation of Stomach
The stomach was opened along the greater curvature, rinsed with saline to remove gastric contents and blood clots, and examined by a 10× magnifier lens to assess the formation of ulcers. The number of ulcers is counted and scored:51 normal-colored stomach (0), red coloration (0.5), superficial mucosal injury (spot ulcer) (1), hemorrhagic streak (1.5), deep Ulcer (2), perforation (3). Ulcer index (UI) was measured by using the following formula: 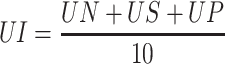
Where UI= Ulcer Index; UN = average number of ulcers per animal; US = average number of severity score; UP = percentage of animals with ulcers.
Percentage inhibition of ulceration was calculated as follows:
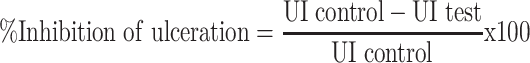 |
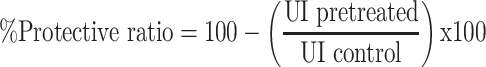 |
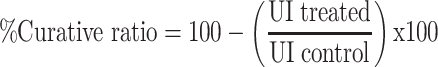 |
Determination of pH and Volume of Gastric Secretions
After the abdomen was opened the cardiac end of the stomach was dissected out and its content drained into a glass tube. The quantity of the digestive juice was measured after centrifugation at 2000 rpm for 10 minutes. From the supernatant, an aliquot (1 mL of each) was taken and diluted with 1 mL of water, and the pH of the fluid was measured using a pH meter.
Determination of Total Acidity
An aliquot of 1 mL digestive juice diluted with 9 mL of distilled water was taken into a 50 mL conical flask and two drops of phenolphthalein indicator were added thereto and titrated with 0.01 N NaOH until a permanent pink color was observed. The quantity of 0.01 N NaOH consumed was noted. The entire acidity is expressed as mEq/L by the subsequent formula.52
 |
Data Quality Control
The data quality was maintained by grouping experimental rats by simple random sampling technique, blinding the data collection system of all parameters; maintaining and applying standard procedures, and using analytically graded materials.
Statistical Analysis
Results were expressed as mean ± SEM and were analyzed using Windows SPSS version 20.0. Comparisons were made between negative control, positive control, and treatment groups of varied doses using ANOVA following by Post hoc Tukey’s HSD multiple comparison tests. At a 95% confidence interval, p< 0.05 was considered as statistical significance.
Result
Percentage Yields of Crude Extract and Solvent Fractions
From the crude hydromethanolic crude extract, 196 g were obtained with a percentage yield of 19.6%, and 90 g of the crude extract was used for solvent fractionation (see Table 1).
Table 1.
Amount of Crude Extract, Physical Description of Extracts and Percentage Yields of Crude Extract and Fractions
| Extracting Solvent | Physical Description of the Extract | Yield (g) | % Yield (W/W) |
|---|---|---|---|
| 80% Methanol | Brown powder | 196 | 19.6 |
| Chloroform fraction | Brown powder | 15.39 | 17.1 |
| Ethyl acetate fraction | Gummy dark brown | 42.12 | 46.8 |
Preliminary Phytochemical Screening
The phytochemical screening of the root hydroalcoholic crude extract of R. nepalensis showed the presence of alkaloids, anthraquinones, flavonoids, glycosides, phenolic compounds, plant steroids, saponins, tannins, and terpenoids (see Table 2).
Table 2.
Preliminary Phytochemical Screening of Hydromethanolic Crude Extract and Solvent Fractions of R. nepalensis
| Phytochemical | 80% Methanolic Crude Extract | Aqueous Fraction | Chloroform Fraction | Ethyl Acetate Fraction |
|---|---|---|---|---|
| Alkaloids | + | – | + | + |
| Anthraquinones | + | + | + | + |
| Flavonoids | + | + | – | + |
| Glycosides | + | + | + | + |
| Phenolic compounds | + | + | – | + |
| Plant steroids | – | – | – | – |
| Saponins | + | + | – | + |
| Tannins | + | + | – | + |
| Terpenoids | + | + | – | + |
Notes: - +: present, -: absent.
Acute Oral Toxicity Study
Acute oral toxicity test was performed in female rats as per OECD-425 guidelines (OECD, 2008). The study indicated that the hydromethanolic crude extract of R. nepalensis caused no mortality at 2000 mg/kg within the first 24 hours and for the following 15 days. Physical and behavioral observations of the experimental rats also revealed no visible signs of acute oral toxicity.
Effect of Hydromethanolic Crude Extract on Pylorus Ligation-Induced Ulcer
The effect of hydromethanolic root crude extract of R. nepalensis on pylorus ligation induced ulceration. Pylorus ligation caused the buildup of gastric secretions of 4.75 ± 0.075 mL with pH 1.88 ± 0.13 in a negative control group. The full acidity of the gastric secretions was found to be 56.33 ± 0.22 mEq/L. Pretreatment with the R. nepalensis hydromethanolic crude extract, significantly (P<0.001) reduced the amount of gastric secretions 2.10 ± 0.11, 2.04 ± 0.12, and 1.93 ± 0.05 mL at the doses of 100, 200, and 400 mg/kg, respectively. The pH of the digestive fluid was significantly (P<0.001) elevated to 5.97 ± 0.14, 6.03 ± 0.19, and 6.09 ± 0.10 at a dose of 100, 200, and 400 mg/kg/day of hydromethanolic crude extract R. nepalensis, respectively. Additionally, total acidity was also reduced significantly (P<0.001) as compared to the negative control. Further, it was observed that pyloric ligation caused gastric ulcerations, and pretreatment with R. nepalensis root extract had reduced them significantly in a dose-dependent manner (see Table 3).
Table 3.
Effect on GI Secretion, Total Acidity and pH on Pylorus Ligation-Induced Ulcerated Rat
| Group | VoGJ | TA(mEq/L) | % Red TA | pH |
|---|---|---|---|---|
| Solvent 10 ml/kg | 4.75 ± 0.075 | 56.33 ± 0.22 | 0.00 | 1.88 ± 0.13 |
| Omp 20 mg/kg | 1.83 ± 0.05a3 | 16.65 ± 0.304a3 | 70.44 | 6.19 ± 0.13a3 |
| RN 100 mg/kg | 2.10 ± 0.11a3 | 17.78 ± 0.19a3 | 68.44 | 5.97 ± 0.14a3 |
| RN 200 mg/kg | 2.04 ± 0.12a3 | 17.50 ± 0.43a3 | 68.93 | 6.03 ± 0.19a3 |
| RN 400 mg/kg | 1.93 ± 0.05a3 | 16.73 ± 0.15a3 | 70.3 | 6.09 ± 0.10a3 |
Notes: Each value represents mean ± SEM; n=6; a: against negative control; 3: p<0.001; Omp: Omeprazole, SEM: standard error of the mean; RN100: Rumex nepalensis 100 mg/kg, RN200: Rumex nepalensis 200 mg/kg, RN400: Rumex nepalensis 400 mg/kg, VoGJ: volume of gastric juice, %Red V: percentage reduction in volume of gastric juice, TA: total acidity, % Red TA: percentage reduction in total acidity.
In the pylorus ligation ulcer induced model the percentage inhibition of ulceration was found to be 51.17%, 59.78%, and 67.52% at 100, 200 and 400 mg/kg/day of hydromethanolic crude extract of R. nepalensis, respectively. Gastro-protection offered by the R. nepalensis 400 mg/kg test extract was comparable to that of Omeprazole (20 mg/kg) (see Table 4).
Table 4.
Effect on Ulcer Score and Ulcer Index on Pylorus Ligation-Induced Ulcerated Rat
| Group | US | % Red US | UI | % Red UI |
|---|---|---|---|---|
| Solvent 10 mL/kg | 78.00 ± 3.06 | 0.00 | 23.00 ± 0.45 | 0.00 |
| Omp 20 mg/kg | 5.92 ± 2.26a3 | 92.41 | 7.51 ± 2.38a3 | 67.35 |
| RN 100 mg/kg | 8.83 ± 1.28a3 | 88.68 | 11.23 ± 0.18a2 | 51.17 |
| RN 200 mg/kg | 6.50 ± 1.38a3 | 91.67 | 9.25 ± 1.85a3 | 59.78 |
| RN 400 mg/kg | 5.67 ± 1.87a3 | 92.7 | 7.47 ± 2.36a3 | 67.52 |
Notes: Each value represents mean ± SEM; n=6; a: against negative control; b: against standard (Omp20); c: against 200 mg/kg; d: against 400 mg/kg; 1: p<0.05, 2: p<0.01, 3: p<0.001; Omp: Omeprazole, SEM: standard error of the mean, RN: Rumex nepalensis.
Effect of Crude Extract on Cold Restraint Stress-Induced Ulcer
Observing the results obtained in the cold restraint stress-induced ulcer model, it had been found that R. nepalensis hydromethanolic crude extract at a dose of 200 and 400 mg/kg/day significantly reduced the ulcer index to 10.24 ± 2.05 (p<0.01) and 9.52 ± 1.91 (P<0.01), respectively, and ulcer score to 12.92 ± 2.61 (p<0.001) and 8.50 ± 1.82 (p<0.001), respectively, compared with the negative control group. The proportion inhibition of the ulcer was 32.1%, 48.15%, and 51.78% in the groups treated with 100, 200, and 400 mg/kg of hydromethanolic crude extract of R. nepalensis, and 60.45% in the positive controls. Although the ulcer index was reduced at a dose of R. nepalensis 100 mg/kg weight by 32.1% (13.41 ± 0.09), this reduction did not reach statistical significance as compared to the negative control (see Table 5).
Table 5.
Effect on Ulcer Score and Ulcer Index in Cold Restraint Stress-Induced Ulcer Treated with Crude Extract of R. nepalensis
| Group | Ulcer Score | Ulcer Index |
|---|---|---|
| Solvent 10 mL/kg | 60.83 ± 0.84 | 19.75 ± 0.10 |
| Cim 100 mg/kg | 7.58 ± 2.46a3 | 7.81 ± 2.47a3 |
| RN 100 mg/kg | 19.75 ± 0.56a3b2d2 | 13.41 ± 0.09 |
| RN 200 mg/kg | 12.92 ± 2.61a3 | 10.24 ± 2.05a2 |
| RN 400 mg/kg | 8.50 ± 1.82a3 | 9.52 ± 1.91a2 |
Notes: Each value represents mean ± SEM; n=6; a: against negative control; b: against standard (Omp20); d: against 400 mg/kg; 2: p<0.01, 3: p<0.001; Cim: cimetidine; RN: Rumex nepalensis, SEM: Standard error of the mean.
Effect of Crude Extract on Acetic Acid-Induced Ulcer
Hydromethanolic crude extract of R. nepalensis root used at a dose of 200 and 400 mg/kg weight significantly (P<0.01) healed ulcers after 15 days of treatment, showing the curative effect of 53.22% and 54.59%, respectively. Hydromethanolic crude extract of R. nepalensis root used at 100 mg/kg showed insignificant ulcer inhibition compared to the negative control. Hydromethanolic crude extract of R. nepalensis at 200 and 400 mg/kg/day produced a significant difference (P<0.001) compared to the negative control group (see Table 6).
Table 6.
Effects on Ulcer Score and Ulcer Index on Acetic Acid-Induced Ulcer Treated with Crude Extract of R. nepalensis
| Group | Ulcer Score | % Red US | UI | % Red UI |
|---|---|---|---|---|
| Solvent 10 mL/kg | 71.42 ± 0.79 | 0.00 | 20.48 ± 0.84 | 0.00 |
| Cim 100 mg/kg | 6.17 ± 1.99a3 | 91.36 | 7.57 ± 2.39a3 | 63.04 |
| RN 100 mg/kg | 30.25 ± 2.08a3b3c3d3 | 57.64 | 15.04 ± 0.35b1 | 26.10 |
| RN 200 mg/kg | 8.50 ± 1.83a3d3 | 88.10 | 9.58 ± 1.92a2 | 53.22 |
| RN 400 mg/kg | 6.83 ± 1.40a3 | 90.44 | 9.3 ± 1.86a2 | 54.59 |
Notes: Each value represents mean ± SEM; n=6; a: against negative control; b: against standard (Cim100); c: against 200 mg/kg; d: against 400 mg/kg; 1: p<0.05, 2: p<0.01, 3: p<0.001; Cim100: cimetidine 100 mg/kg, DW: distilled water; RN: Rumex nepalensis, SEM: standard error of the mean.
The results of the percentage ulcer inhibition effect of R. nepalensis root crude extract on hypothermic restraint stress-induced ulcer model (supplementary Figure 1). Pyloric ligation-induced gastric ulcer model (supplementary Figure 2) Hypothermic restraint stress-induced ulcer model (supplementary Figure 3). Acetic acid-induced gastric ulcer model (supplementary Figure 4).
Effect of Solvent Fractions on Pylorus Ligation-Induced Ulcer
After a crude extract study on both acute and chronic ulcer models, the effective model with the best percentage ulcer inhibition is the pylorus ligation-induced peptic ulcer model, which was selected for further study of the anti-ulcer activity of solvent fractions of hydromethanolic crude extract of R. nepalensis. Omeprazole 20 mg/kg, and chloroform fractions at 100, 200, and 400 mg/kg cause a percent reduction of ulcer score of 93.78%, 4.57%, 7.78%, and 12.16%, respectively. An ethyl acetate fraction of R. nepalensis at 100, 200, and 400 mg/kg produced a percent reduction of ulcer score of 89.12%, 90.86%, and 93.51%, respectively. An aqueous fraction R. nepalensis at 100, 200, and 400 mg/kg produced s percent reduction of ulcer score of 75.68%, 80.99%, and 85.38%, respectively. A chloroform fraction of R. nepalensis at 100 mg/kg/day was unable to reduce US and UI significantly as compared to the negative control group of rats. A significant difference was also observed between all doses of chloroform fraction as compared to the quality at P<0.001. All ethyl acetate and aqueous fractions in all doses were able to reduce ulcer index and ulcer score significantly as compared to the negative control (P<0.001). The ethyl acetate fraction at 400 mg/kg/day showed the highest protection as compared to all fractions (see Table 7).
Table 7.
Rumex nepalensis Root Solvent Fractions Effect on Ulcer Score and Ulcer Index in Pylorus Ligation-Induced Ulcerated Rats
| Group | US | % Red US | UI | % Red UI |
|---|---|---|---|---|
| Solvent 10 mg/kg | 91.17 ± 1.94 | 0.00 | 24.82 ± 0.23 | 0.00 |
| Omp 20 mg/kg | 5.67 ± 1.89a3 | 93.78 | 7.47 ± 2.36a3 | 69.9 |
| CF 100 mg/kg | 87.00 ± 2.94b3 | 4.57 | 24.19 ± 0.37b3 | 2.54 |
| CF 200 mg/kg | 84.08 ± 4.20b3 | 7.78 | 23.73 ± 0.69b3 | 4.4 |
| CF 400 mg/kg | 80.08 ± 1.58b3 | 12.16 | 22.69 ± 0.21b3 | 8.6 |
| EF 100 mg/kg | 9.92 ± 1.03a3 | 89.12 | 11.6 ± 0.23a3 | 53.26 |
| EF 200 mg/kg | 8.33 ± 1.68a3 | 90.86 | 9.58 ± 1.92a3 | 61.40 |
| EF 400 mg/kg | 5.92 ± 1.89a3 | 93.51 | 7.63 ± 2.41a3 | 69.26 |
| AF 100 mg/kg | 22.17 ± 0.90a3b2 | 75.68 | 13.48 ± 0.15a3 | 45.69 |
| AF 200 mg/kg | 17.33 ± 3.51a3b1 | 80.99 | 11.02 ± 2.20a3 | 55.60 |
| AF 400 mg/kg | 13.33 ± 2.69a3 | 85.38 | 10.37± 2.04a3 | 58.23 |
Notes: Each value represents mean ± SEM; n=6; CF: chloroform fraction, EF: ethyl acetate fraction, AF: aqueous fraction, a: against negative control, b: against standard (Omp20); 1: p<0.05, 2: p<0.01, 3: p<0.001, Omp20: Omeprazole 20 mg/kg, SEM: standard error of the mean.
The pylorus ligation-induced peptic ulcer model produced a gastric secretion of 6.22 ± 0.11 mL in the negative control group and treatment with Omeprazole at 20 mg/kg, and a chloroform fraction of R. nepalensis at 100, 200, and 400 mg/kg reduced gastric secretion 1.45 ± 0.1 mL, 6.08 ± 0.27 mL, 5.94 ± 0.38 mL, and 5.26 ± 0.23 mL, respectively. A chloroform fraction at 400 mg/kg reduced gastric secretions significantly (P<0.05) as compared to the negative control group. The ethyl acetate fraction of R. nepalensis at 100, 200, and 400 mg/kg reduced gastric secretion 1.83 ± 0.07 mL, 1.81 ± 0.05 mL, and 1.51 ± 0.13 mL, respectively. An aqueous fraction of R. nepalensis at 100, 200, and 400 mg/kg reduced gastric secretion 1.80 ± 0.05 mL, 1.78 ± 0.06 mL, and 1.64 ± 0.10 mL, respectively. All the three doses of ethyl acetate and aqueous fractions produced significant (p<0.001) differences compared to the negative control group.
In the pylorus ligation-induced peptic ulcer model, Omeprazole at 20 mg/kg, and the chloroform fraction of R. nepalensis at 100, 200, and 400 mg/kg produced 8.55 ± 0.26, 61.14 ± 2.28, 59.43 ± 1.28, and 57.83 ± 2.43 percentage reduction in total acidity of gastric secretion, respectively. The ethyl acetate fraction of R. nepalensis at 100, 200, and 400 mg/kg reduced gastric secretion by 9.91 ± 0.20, 9.63 ± 0.08, and 9.04 ± 0.53 percentage values, respectively. An aqueous fraction of R. nepalensis at 100, 200, and 400 mg/kg reduced gastric secretion 10.04 ± 0.30, 9.41 ± 0.26, and 8.93 ± 0.28 produced percentage reduction in total acidity of gastric secretions, respectively. All the three doses of chloroform, ethyl acetate, and aqueous fractions produced significant (p<0.001) difference compared to the negative control group (64.03 ± 0.6) in percentage reduction in total acidity of gastric secretions (see Table 8).
Table 8.
Solvent Fractions Antisecretory Effect on Pylorus Ligation-Induced Ulcer
| Group | VoGJ (mL) | % Red V | TA(mEq/L) |
|---|---|---|---|
| Solvent 10 mL/kg | 6.22 ± 0.11 | 64.03 ± 0.6 | 1.7 ± 0.06 |
| Omp 20 mg/kg | 1.45 ± 0.10a3 | 8.55 ± 0.26a3 | 6.73 ± 0.09a3 |
| CF 100 mg/kg | 6.08 ± 0.27b3 | 61.14 ± 2.28b3 | 1.80 ± 0.07b3 |
| CF 200 mg/kg | 5.94 ± 0.38b3 | 59.43 ± 1.28b3 | 1.91 ± 0.18b3 |
| CF 400 mg/kg | 5.26 ± 0.23a1b3 | 57.83 ± 2.43a1b3 | 2.24 ± 0.06a1b3 |
| EF 100 mg/kg | 1.83 ± 0.07a3 | 9.91 ± 0.20a3 | 6.25 ± 0.10a3 |
| EF 200 mg/kg | 1.81 ± 0.05a3 | 9.63 ± 0.08a3 | 6.36 ± 0.09a3 |
| EF 400 mg/kg | 1.51 ± 0.13a3 | 9.04 ± 0.53a3 | 6.54 ± 0.02a3 |
| AF 100 mg/kg | 1.80 ± 0.05a3 | 10.04 ± 0.30a3 | 6.19 ± 0.12a3b1 |
| AF 200 mg/kg | 1.78 ± 0.06a3 | 9.41 ± 0.26a3 | 6.30 ± 0.17a3 |
| AF 400 mg/kg | 1.64 ± 0.10a3 | 8.93 ± 0.28a3 | 6.41 ± 0.10a3 |
Notes: Each value represents mean ± SEM; n=6; CF: chloroform fraction, EF: ethyl acetate fraction, AF: aqueous fraction, a: against negative control; b: against standard (Omp20), 1: p<0.05, 2: p<0.01, 3: p<0.001, Omp20: Omeprazole 20 mg/kg, DW10: SEM: standard error of the mean, VoGJ: volume of gastric juice secretions, %Red V: percentage reduction in volume of gastric juice, TA: total acidity, %Red TA: percentage reduction in total acidity of gastric secretions.
Discussion
In our study we included three models: pylorus ligation-induced ulcer model, cold restrained induced ulcer model, and acetic acid-induced chronic ulcer model by determining the degree of gastric secretions, pH, total acidity, and ulcerations.
Therapy for gastroduodenal ulcer remains one of the key problems of modern gastroenterology. To date, no drug meets all the goals of peptic ulcer disease therapy. Drug tolerance of conventional anti-ulcer agents has been reported during the therapy of PUD. Also, these drugs have serious side effects when used for a long time; therefore, there is a need for novel and effective anti-ulcer agents with improved safety profiles. The present plant extraction yield (19.6%) is almost comparable with the previous reported yield (17.26%),33 in a study done at Addis Ababa University, Ethiopia, thus supporting that the hydroalcoholic solvent possesses a good extracting potential. The highest (46.8%) and lowest (17.1%) yields of solvent fraction were obtained from ethyl acetate fractions and chloroform fractions of the roots of R. nepalensis, respectively. From these data, it is possible to say that the roots of R. nepalensis contained more polar and semi-polar compounds. This property of methanol may be due to its medium polarity index as compared to chloroform and aqueous solvents. In the present qualitative phytochemical analysis, the 80% methanolic extract of the root of R. nepalensis was positive for flavonoids, saponins, tannins, phenols, terpenoids, anthraquinones, glycosides, and alkaloids. Even though this phytochemical analysis is in line with a report done at Addis Ababa University, Ethiopia, steroids were detectable but not alkaloids;33 on the contrary in the present study the reverse is true. This disparity may be due to environmental factors or time of plant collection and extraction difference. In this study, preliminary phytochemical analysis of chloroform fraction revealed the presence of only alkaloids, anthraquinones, and glycosides, whereas in the case of ethyl acetate solvent fractions all secondary metabolites found in the crude extract had been detected. In the aqueous solvent fractions except for alkaloids, all secondary metabolites detected in the crude extract had been detected. Similarly, another study performing a phytochemical analysis of the aqueous extracts of the roots of R. nepalensis reported the presence of anthraquinones, flavonoids, glycosides, triterpenoid, tannins, and saponins, but unlike the present study, steroids were detected.33 From the above findings it is better to assume that R. nepalensis is better extracted with more polar and semi-polar solvents than more nonpolar ones. The acute toxicity study revealed that the plant extract was safe in rats at a limit dose of 2000 mg/kg and that the median lethal dose (LD50) of the extract is above 2000 mg/kg. The finding supports the work done on rats in another study, in which 3.2 g/kg to 5 g/kg hydromethanolic root crude extract of R. nepalensis was safe.33 Pyloric ligation-induced ulcers are due to increased accumulation of gastric acid and pepsin, leading to the auto-digestion of the gastric mucosa. In the present study, R. nepalensis at 100, 200, and 400 mg/kg treatment decreased the gastric juice volume, total acidity, and increased pH significantly (P<0.001). Percentage protection of ulcerogenesis by pylorus ligation was found to be 51.17%, 59.78%, and 67.52% at a dose of R. nepalensis of 100, 200 mg/kg, and 400 mg/kg, respectively, and the gastroprotection offered by R. nepalensis at 400 mg/kg hydromethanolic crude extract was comparable to that of the standard (67.35%). This effect implies that hydromethanolic root crude extract of R. nepalensis had a dose-dependent activity in preventing gastric ulceration. From a multiple solvent point of view, ethyl acetate fraction at 400 mg/kg showed highest ulcer inhibition as compared to all fractions and crude extract, which was comparable to Omeprazole treatment and found to be effective in reducing pylorus ligation-induced gastric ulceration. But the lowest ulcer protection activity was observed by chloroform fraction at 100, 200, and 400 mg/kg groups, with a percentage reduction of ulcer index of 2.54%, 4.4%, and 8.6%, which failed to reach statistical significance. The corresponding gastroprotective effect of R. nepalensis shown in this study is attributed to the chemical makeup of the extract. Phytoconstituents extracted from anti-ulcerogenic terpenoids include triterpenes, diterpenes, and terpenic derivatives. The triterpenic derivative carbenoxolone is an excellent stimulant of mucus synthesis, maintains the prostaglandin content of gastric mucosa at high levels, and inhibits pepsin secretion. It is known that triterpenoid compounds have antisecretory and cytoprotective activity. Tannins have anti-ulcer activity and are known to “tan” the outermost layer of the mucosa. By doing so it renders the gastric mucosa less permeable and more resistant to chemical and mechanical injury or irritation.27 A study conducted on the root extract analysis of R. nepalensis showed that the chloroform fraction (IC50=10.24 µg/mL) and ethyl acetate fraction (IC50=8.7 µg/mL) had free radical scavenging activity. Based on their IC50, the ethyl acetate fraction was effective at a lower concentration as compared to chloroform.53 Unlike the other solvent fractions and crude extract, chloroform fraction was negative for saponins, flavonoids, terpenoids, tannins, and polyphenolic compounds in the present study. These might be reasons why crude extract, ethyl acetate, and aqueous fractions of R. nepalensis showed good anti-ulcer activity while chloroform fractions revealed an insignificant anti-ulcer effect. The hypothermic restraint stress-induced gastric ulcer model provides both emotional stresses and physiological stress to the animal and it mimics clinical acute gastric lesions, which may appear in the gastric mucosa as a consequence of major trauma, surgery, or sepsis, being widely accepted for studying the mechanism of stress-induced gastric lesions. It brings central nervous system factors in to play in the production of gastric pathology.54 In the present study, R. nepalensis at 200 and 400 mg/kg doses is able to reduce ulceration risk significantly. Ulcer index (19.75 ± 0.10) in the negative controls is coherent with other reports (24 ±3.24); this suggests that this study was conducted in accordance with the standard procedure.18 As compared to the other models, the R. nepalensis at 100 mg/kg extract showed the lowest efficacy of ulcer inhibition (31.2%); this might justify that R. nepalensis at 100 mg/kg as a single dose is not the right dose to prevent cold restraint stress-related gastric ulceration. This may be due to single-dose administration end up with a short time that a plasma concentration elapsed within the therapeutic window or unable to achieve minimum effective plasma concentration. The protective efficacy against cold restraint-stress induced ulcers may be due to the antioxidant, anti-secretagogue potential, anticholinergic, anti-inflammatory, and central nervous system activity of R. nepalensis,55 thereby strengthening the animals’ physiological capabilities to decrease stress ulcers. It has been reported that anti-secretory agents such as histamine H2-receptor antagonists ameliorate the decrease in gastric mucosal blood flow caused by factors that disturb the gastric mucosa. Since R. nepalensis has antihistaminic nature it may increase mucosal blood flow to gastric mucosa, thereby preventing ulceration.18 Apart from its preventive effect, the present study also evaluates the effect of R. nepalensis root extract on healing chronic gastric ulcers. The crude extract reduces both ulcer surface area and ulcer base. R. nepalensis at 200 and 400 mg/kg body weight doses significantly (P<0.01) healed ulcers after 15 days of treatment. At these doses the ulcer curative ratio was found to be 53.22% and 54.59%, respectively. This result was in line with the report of Jasminum grandiflorum hydromethanolic leaf extract on acetic acid-induced gastric ulcer in rats at a dose of 100 and 200 mg/kg for 20 days with a percentage curative ratio of 56% and 66%.11 All doses of the crude extract able to reduce the ulcer score significantly after 15 days of treatment at a significance level of P<0.001 as compared to the negative control groups. Meanwhile, the lowest dose had a significant difference to the standard drugs used as a positive control. This result might infer that crude extract had a dose-dependent ulcer healing effect. During the 15 days of treatment, R. nepalensis extract or Cimetidine did not produce any visible signs of toxicity. It is well known that gastric cytoprotective property alone in rats does not produce significant ulcer healing in humans. The results of the present study suggest that ulcer healing effect of R. nepalensis root extract observed in humans may be due to both gastric antisecretory and cytoprotective effects. The constituent(s) responsible for the anti-ulcer effect of the root is not known. Besides their action as gastroprotective, anti-secretory, and antioxidant agents, flavonoids also act in the healing of gastric ulcers. Hence, it is assumed that flavonoids may contribute at least in part to the chronic ulcer healing effect of the candidate. These secondary metabolites may stimulate important cellular mechanisms such as migration and proliferation of epithelial cells that may have a cytoprotective effect by stimulating the release of prostaglandins. Angiogenesis plays a pivotal role in the acceleration of healing of ulcers since the neovasculature promotes nutrient supply to the healing tissue. This complex sequence of events requires a high degree of coordination among different cell types, which is regulated by several factors. Among them, growth factors such as the vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), and their receptors are known to play an important role in tissue repair and the healing of ulcers. Saponins isolated from Red Ginseng exhibited ulcer healing and promoted angiogenesis via the stimulation of VEGF production. So the chronic gastric ulcer healing effect of the present extract also might benefit from its ability to upregulate growth factors in response to saponins. Therefore, the free radical scavenging potential of the plant extract might be highly helpful in protecting gastric ulcer complications and in healing ulcers.
Conclusion
From the present study, it can be concluded that crude methanolic extract and solvent fractions of Rumex nepalensis root have shown promising anti-ulcer activity on pyloric ligation, cold restraint stress, and acetic acid-induced ulcer models, which upholds its folkloric use. Thus, it could be considered as a potential source to develop new anti-ulcer agents; however, species variation would limit such a straightforward extrapolation of the findings of this study to humans.
Recommendation
This study confirmed the claim in Ethiopian traditional medicine that the plant has therapeutic value in stomach ulcers. So it needs further in-depth investigation by using other models, isolation, and characterization of the active principles responsible for the anti-ulcer activity and to elucidate the exact mechanism action of this plant. Evaluation of its anti-ulcer efficacy in case of co-morbid illness in those previously reported.
Acknowledgment
Most importantly, we would like to thank the University of Gondar College of Medicine and Health Sciences, School of Pharmacy, Department of Pharmacology for ethical approval and indispensable support, and involvement in different activities from the beginning to the finalization of the work. Secondly, we would like to thank Mr. Alemant Tafese and Mr. Abraham Degu for their support during laboratory work. Thirdly, we would like to thank Dr. Getinet Masresha for the authentication of the plant material.
Abbreviations
CYP2C19, cytochrome P450 2C19; EU, European Union; GIT, gastrointestinal tract; ICLAS, International Council for Laboratory Animal Science; LD50, median lethal dose; mEq/L, milliequivalent per liter; mg/kg, milligram per kilogram; NPR, normal pellet diet; PPIs, proton pump inhibitors; PUD, peptic ulcer disease; RN, Rumex nepalensis; SEM, standard error of mean; UI, ulcer index; μL, microliter.
Availability of Data and Material
All data generated or analyzed during this study have been attached as supplementary material.
Ethical Approval and Consent to Participate
The experimental animals were handled and cared for in the experimental procedures according to the local ethical and internationally accepted laboratory animal use, care and welfare guidelines such as Basel declaration, ICLAS Ethical Guideline, and the EU directive on the protection of animals used for scientific purposes.52 The ethical clearance was requested and obtained from the research and ethics review committee of the Department of Pharmacology, School of Pharmacy, University of Gondar by Reference number of SOP4/101/11, on March 13, 2019. The research was performed as per the agreement.
Author Contributions
Both authors contributed to designing, conducting the research, data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests to disclose.
References
- 1.Allen A, Flemström G Gastroduodenal mucus bicarbonate barrier: protection against acid and pepsin. Am J Physiol. 2005. 288(1):C1–9. 10.1152/ajpcell.00102.2004 [DOI] [PubMed] [Google Scholar]
- 2.Maggie Ham YA, Koji Takeuchi MH Montrose, and Jonathan D. Kaunitz. Gastroduodenal Mucosal Defense In: Johensonl R, editor. Physiology of Gastro Intestinal Tract. 2: Elsevier; 2006. p. 1259–1291. [Google Scholar]
- 3.Werther JL The gastric mucosal barrier. Mount Sinai j Med 2000; 67(1):41–53. [PubMed] [Google Scholar]
- 4.Leedham SJ, Brittan M, Preston SL, McDonald SA, Wright NA The stomach periglandular fibroblast sheath: Gut. 2006;55(2):295–296. [PMC free article] [PubMed] [Google Scholar]
- 5.Laine L, Takeuchi K, Tarnawski A Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135(1):41–60. 10.1053/j.gastro.2008.05.030 [DOI] [PubMed] [Google Scholar]
- 6.Abebaw M, Mishra B, Gelayee DA Evaluation of anti-ulcer activity of the leaf extract of OsyrisquadripartitaDecne.(Santalaceae) in rats. J Exp Pharmacol. 2017;9:1 10.2147/JEP.S125383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas S, Femeesh M, Nafia K, Siyad M, Shrikumar S Pharmacological review of antiulcer screening.
- 8.Alemayehu G, Asfaw Z, Kelbessa E Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora District, North Shewa Zone of Amhara Region, Ethiopia. J Med Plants Studies. 2015;3(6):01–11. [Google Scholar]
- 9.Yohannis SW, Zemede A, Ensermu K Ethnobotanical study of medicinal plants used by local people in Menz Gera Midir District, North Shewa Zone, Amhara Regional State, Ethiopia. J Med Plants Res. 2018;12(21):296–314. 10.5897/JMPR2018.6616 [DOI] [Google Scholar]
- 10.Gadekar R, Singour PK, Chaurasiya PK, Pawar RS, Patil UK A potential of some medicinal plants as an antiulcer agents. Pharmacogn Rev. 2010. 4(8):136 10.4103/0973-7847.70906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umamaheswari M, Asokkumar K, Rathidevi R, Sivashanmugam AT, Subhadradevi V, Ravi TK Antiulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J Ethnopharmacol. 2007;110(3):464–470. 10.1016/j.jep.2006.10.017 [DOI] [PubMed] [Google Scholar]
- 12.Torres OJ, Santos OJ, Moura RS, et al. Activity of orbignyaphalerata and euterpeedules in the prevention and treatment of peptic ulcer in rats. ABCD. Arquivos Brasileiros De CirurgiaDigestiva (São Paulo). 2018;31(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maity P, Biswas K, Chattopadhyay I, Banerjee RK, Bandyopadhyay U The use of neem for controlling gastric hyperacidity and ulcer. Phytother Res. 2009. 23(6):747–755. 10.1002/ptr.2721 [DOI] [PubMed] [Google Scholar]
- 14.Pinto LA, Cordeiro KW, Carrasco V, Carollo CA, Cardoso CA, Argadoña EJ, de Cássia Freitas K. Antiulcerogenic activity of Carica papaya seed in rats. Naunyn-Schmiedeberg’s Archives of Pharmacol. 2015;388(3):305–317. 10.1007/s00210-014-1069-y [DOI] [PubMed] [Google Scholar]
- 15.Melese E, Asres K, Asad M, Engidawork E Evaluation of the antipeptic ulcer activity of the leaf extract of Plantagolanceolata L. Phytother Res. 2011. 25(8):1174–1180. 10.1002/ptr.3411 [DOI] [PubMed] [Google Scholar]
- 16.Coelho RG, Gonzalez FG, Sannomiya M, Di Stasi LC, Vilegas W Gastric anti-ulcer activity of leaf fractions obtained of polar extract from Wilbrandiaebracteata in mice. Nat Prod Res. 2009;23(1):51–59. 10.1080/14786410701782544 [DOI] [PubMed] [Google Scholar]
- 17.Malairajan P, Gopalakrishnan G, Narasimhan S, Veni KJ, Kavimani S Anti-ulcer activity of crude alcoholic extract of Toonaciliata Roemer (heart wood). J Ethnopharmacol. 2007;110(2):348–351. 10.1016/j.jep.2006.10.018 [DOI] [PubMed] [Google Scholar]
- 18.Alqasoumi S, Al-Sohaibani M, Al-Howiriny T, Al-Yahya M, Rocket RS “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J Gastroenterol. 2009;15(16):1958 10.3748/wjg.15.1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XM, Zakaria MN, Islam MW, et al. Anti-inflammatory and anti-ulcer activity of Calligonumcomosum in rats. Fitoterapia. 2001;72(5):487–491. 10.1016/S0367-326X(01)00271-4 [DOI] [PubMed] [Google Scholar]
- 20.Banji D, Singh J, Banji OJ, Shanthamurthy M Scrutinizing the aqueous extract of leaves of pedalium murex for the antiulcer activity in rats. Pak J Pharm Sci. 2010. 1;23. [PubMed] [Google Scholar]
- 21.Sharifi-Rad M, Fokou PV, Sharopov F, et al. Antiulcer agents: from plant extracts to phytochemicals in healing promotion. Molecules. 2018. 23(7):1751 10.3390/molecules23071751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan PV, Nyasse B, Dimo T, Wafo P, Akahkuh BT Synergistic and potentiating effects of ranitidine and two new anti-ulcer compounds from Enantiachlorantha and Voacangaafricana in experimental animal models. Die Pharmazie. 2002. 57(6):409–412. [PubMed] [Google Scholar]
- 23.Tan PV, Nyasse B, Enow-Orock GE, Wafo P, Forcha EA Prophylactic and healing properties of a new anti-ulcer compound from Enantiachlorantha in rats. Phytomedicine. 2000;7(4):291–296. 10.1016/S0944-7113(00)80046-X [DOI] [PubMed] [Google Scholar]
- 24.Kota BP, Teoh AW, Roufogalis BD Pharmacology of traditional herbal medicines and their active principles used in the treatment of peptic ulcer, diarrhoea and inflammatory bowel disease. New Adv Basic Clin Gastroenterol. 2012. 18;14:297–310. [Google Scholar]
- 25.Sumbul S, Ahmad MA, Mohd A, Mohd A Role of phenolic compounds in peptic ulcer: an overview. J Pharm Bioallied Sci. 2011. 3(3):361 10.4103/0975-7406.84437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Mofleh IA Spices, herbal xenobiotics and the stomach: friends or foes? World J Gastroenterol 2010;16(22):2710 10.3748/wjg.v16.i22.2710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borrelli F, Izzo AA The plant kingdom as a source of anti‐ulcer remedies. Phytother Res. 2000. 14(8):581–591. [DOI] [PubMed] [Google Scholar]
- 28.Xie JH, Chen YL, Wu QH, et al. Gastroprotective and anti-Helicobacter pylori potential of herbal formula HZJW: safety and efficacy assessment. BMC Complement Altern Med. 2013. 13(1):119 10.1186/1472-6882-13-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao KN, Ch S, David Banji HS, Mahesh V A study on the nutraceuticals from the genus Rumex.
- 30.Himi H Chromosome numbers of 11 species in Japanese Rumexsubg. Rumex (Polygonaceae). J Phytogeograp Taxonomy.1999;47:121–130. [Google Scholar]
- 31.Birhanu Z Ethno-botanical survey on medicinal plants used by ethnic groups of Denbia district, north-western Ethiopia. J Nat Remed. 2011; 11(2):119–123. [Google Scholar]
- 32.Sharma PA, Rani SA, Ojha SN, Sood SK, Rana JC Indian herbal medicine as hepatoprotective and hepatocurative: a review of scientific evidence. Life Sci Leaflets. 2014. 1;49:61–115. [Google Scholar]
- 33.Habtamu A Evaluation of the Antiplasmodial and Antimicrobial Properties of the Medicinal Plants RumexnepalensisSpreng and Centellaasiatica L (Doctoral dissertation, Addis Ababa Universty).
- 34.Uyanikoğlu A, Danalioğlu A, Akyüz F, et al. Etiological factors of duodenal and gastric ulcers. Turkish J Gastroenterol. 2012. 23(2):99–103. 10.4318/tjg.2012.0435 [DOI] [PubMed] [Google Scholar]
- 35.Nugent R, Back E, Beith A The race against drug resistance. Washington (DC): Center for Global Development; 2010. 14. [Google Scholar]
- 36.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62(1):34–42. 10.1136/gutjnl-2012-302254 [DOI] [PubMed] [Google Scholar]
- 37.Cicala M, Emerenziani S, Guarino MP, Ribolsi M Proton pump inhibitor resistance, the real challenge in gastro-esophageal reflux disease. World J Gastroenterol. 2013;19(39):6529 10.3748/wjg.v19.i39.6529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson WL The role of antisecretory drugs in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1997. 11(S1):21–25. 10.1046/j.1365-2036.11.s1.4.x [DOI] [PubMed] [Google Scholar]
- 39.Ray WA, Chung CP, Stein CM, et al. Risk of peptic ulcer hospitalizations in users of NSAIDs with gastroprotective cotherapy versus coxibs. Gastroenterology. 2007; 133(3):790–798. 10.1053/j.gastro.2007.06.058 [DOI] [PubMed] [Google Scholar]
- 40.Ragunathan M, Solomon M The study of spiritual remedies in orthodox rural churches and traditional medicinal practice in Gondar Zuria district, Northwestern Ethiopia. Pharmacognosy J. 2009;1(3). [Google Scholar]
- 41.Kefalew A, Asfaw Z, Kelbessa E Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):25 10.1186/s13002-015-0014-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American PS, World MA Guiding principles for research involving animals and human beings. Am J Physiol Cell Physiol. 2002. 282(6):3–p. [DOI] [PubMed] [Google Scholar]
- 43.Ghosh L, Gayen JR, Sinha S, Pal S, Pal M, Saha BP Antibacterial efficacy of RumexnepalensisSpreng. Roots Phytother Res. 2003. 17(5):558–559. 10.1002/ptr.1162 [DOI] [PubMed] [Google Scholar]
- 44.Mohana S, Acharya BK, Madamwar D Distillery spent wash: treatment technologies and potential applications. J Hazard Mater. 2009; 163(1):12–25. 10.1016/j.jhazmat.2008.06.079 [DOI] [PubMed] [Google Scholar]
- 45.Bantie L, Assefa S, Teklehaimanot T, Engidawork E In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyusHocsht.(Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014. 14(1):79 10.1186/1472-6882-14-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chhetri SB, Phytochemical Screening KD, Total Phenolic and Flavonoid Content and Antioxidant Activity of Selected Nepalese Plants. World J Pharm Pharm Sci. 2017;6(12):951–968. [Google Scholar]
- 47.Asrade S, Mengesha Y, Moges G, Gelayee DA In vivo antiplasmodial activity evaluation of the leaves of Balanites rotundifolia (Van Tiegh.) Blatter (Balanitaceae) against Plasmodium berghei. J Exp Pharmacol. 2017;9:59 10.2147/JEP.S130491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandra P, Kishore K, Ghosh AK Assessment of antisecretory, gastroprotective, and in-vitro antacid potential of Daucus carota in experimental rats. Osong Public Health Res Perspectives. 2015;6(6):329–335. 10.1016/j.phrp.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamashiro Filho P, Olaitan BS, de Almeida DA, et al., de Oliveira Martins DT. Evaluation of antiulcer activity and mechanism of action of methanol stem bark extract of Lafoensiapacari A. St.-Hil.(Lytraceae) in experimental animals. J Ethnopharmacol. 2012;144(3):497–505. 10.1016/j.jep.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 50.de Almeida AB, Luiz-Ferreira A, Cola M, Batista LM, Di Pietro Magri L, Tiene MR, Santos LC, Vilegas W, Souza-Brito AR. Antiulcerogenic activity of aqueous fraction from leaves of ArctiumlappaL.(Asteraceae). J Med Plants Res. 2012;6(9):1764–1769. [Google Scholar]
- 51.Kulkarni SK Hand Book of Experimental Pharmacology. Vallabh prakashan; 1987. [Google Scholar]
- 52.Kaur A, Kumar S, Sharma R Assessment of anti-ulcer activity of Rheum emodii rhizomes extract. Indo Global J Pharmaceutical Sci. 2012;2(3):333–341. [Google Scholar]
- 53.Gautam R, Karkhile KV, Bhutani KK, Jachak SM Anti-inflammatory, cyclooxygenase (COX)-2, COX-1 inhibitory, and free radical scavenging effects of Rumexnepalensis. Planta Med. 2010;76(14):1564–1569. 10.1055/s-0030-1249779 [DOI] [PubMed] [Google Scholar]
- 54.Brodie DA Experimental peptic ulcer. Gastroenterology. 1968; 55(1):125–134. 10.1016/S0016-5085(19)34099-5 [DOI] [PubMed] [Google Scholar]
- 55.Grover JK, Adiga G, Vats V, Rathi SS Extracts of Benincasahispida prevents development of experimental ulcers. J Ethnopharmacol. 2001; 78(2–3):159–164 10.1016/S0378-8741(01)00334-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study have been attached as supplementary material.


