Abstract
CD47 belongs to immunoglobulin superfamily and is widely expressed on the surface of cell membrane, while another transmembrane protein SIRPα is restricted to the surface of macrophages, dendritic cells, and nerve cells. As a cell surface receptor and ligand, respectively, CD47 and SIRPα interact to regulate cell migration and phagocytic activity, and maintain immune homeostasis. In recent years, studies have found that immunoglobulin superfamily CD47 is overexpressed widely across tumor types, and CD47 plays an important role in suppressing phagocytes activity through binding to the transmembrane protein SIRPα in phagocytic cells. Therefore, targeting CD47 may be a novel strategy for cancer immunotherapy, and a variety of anti-CD47 antibodies have appeared, such as humanized 5F9 antibody, B6H12 antibody, ZF1 antibody, and so on. This review mainly describes the research history of CD47-SIRPα and focuses on macrophage-mediated CD47-SIRPα immunotherapy of tumors.
Keywords: immunotherapy, CD47, SIRPα, macrophage, tumor
Introduction
Traditional treatments for cancer mainly include surgery, radiation therapy, and chemotherapy. Surgical treatment can quickly remove tumor tissues, but it is only suitable for early lesions and may cause the proliferation and metastasis of cancer cells. Surgery is a high-risk and traumatic treatment. Few tumor types that can be completely cured by chemotherapy alone mainly include testicular seminoma, chorionic epithelial cancer, and acute lymphocytic leukemia; meanwhile, tumors, such as early nasopharyngeal cancer, laryngeal cancer, and partial skin cancer, can be cured by radiotherapy alone. For other tumors, chemoradiotherapy is an adjuvant treatment used to improve the effectiveness of surgical treatment or an alternative treatment for advanced cancer without surgical indications. In general, existing therapies fail to treat all types and stages of cancer and may reduce the quality of life for survivors. Cancer treatment remains a clinical challenge due to the limitations of traditional treatment modalities and their adverse effects. In this regard, scholars have developed methods with high efficacy and few side effects, and immunotherapy has emerged as the most promising research field.1
Considerable progress has been achieved in T cell immunotherapy, but this treatment has off-target effects and a series of toxic side effects, such as cytokine release syndrome and neurotoxicity; as such, increasing number of studies has focused on anti-CD47 immunotherapy. In recent years, scholars have reported the increased expression of CD47 on different types of tumor cells and that tumor growth and metastasis can be inhibited significantly by blocking the interaction between CD47 and signal-regulating protein alpha (SIRPα). Hence, the CD47-SIRPα pathway can be used as a therapeutic target for tumors.2
Structure
CD47 is a protein complex composed of specific integrin, G protein, and cholesterol and is widely expressed on the surface of cell membrane. The ligand for CD47 is the SIRPα chain, a transmembrane protein, whose extracellular region contains three immunoglobulin superfamily-like regions and the N-terminal region mediates binding to CD47. SIRPα is expressed on the surface of macrophages, dendritic cells, and nerve cells; regulates cell migration and phagocytic activity; and maintain immune homeostasis through the contact between cell surface receptors and ligands. The binding of CD47 to SIRPα can generate inhibitory signals, thereby reducing the activity of macrophages and suppressing the non-specific immune system.
Mechanisms of Action
The interaction of CD47 and SIRPα plays an important role in regulation of the immune system by mediating B-lymphocyte adhesion to unactivated endothelial cells, regulating B-cell aggregation, and participating in B-lymphocyte regeneration.3 In addition, the interaction of CD47 and fusion receptor SIRPα on the surface of macrophages is involved in the fusion and multinucleation of macrophages; this step is a key in differentiation of macrophages into osteoblasts and giant cells.4 The intracellular domain of SHRP has a typical immune-receptor tyrosin-based inhibitory motif (ITIM), which can be phosphorylated after the interaction of CD4 and SHRP. SIRPa with phosphorylated ITIM binds to and activates SH2-domain-containing protein tyrosine phosphatase SHP-1 or SHP-2, which inhibits the accumulation of myosin-II at the phagocytic synapse, thereby transmitting inhibitory signals and inhibiting phagocytosis of macrophages.5,6 CD47 and SIRPα also participate in migration of monocytes across cerebral endothelium in development of neuroinflammatory diseases. SIRPα-CD47-mediated transendothelial migration involves Gi protein activity, which is a known signaling component of CD47. Finally, the cross-linking of CD47 on cerebral endothelium induces the cytoskeletal reorganization of endothelium; this process does not involve the Gi protein.7
Research has reported the relatively stable interaction between CD47 and αⅡbβ3 or αⅤβ3 on platelets, αⅤβ3 on melanoma cells, and α2β1 on smooth muscle cells and platelets; this interaction promotes the activation and aggregation of platelets as well as the chemotaxis of tumor cells and smooth muscle cells.8 In addition, CD47 binds to thrombospondin-1 (TSP-1) and regulates integrin activity. TSP is an adhesion glycoprotein, and the prototype member TSP-1 is the most studied in this family.9 Therefore, besides SIRPα, CD47 can interact with a variety of molecules and regulate certain functions. It would also be helpful to explain how these functions alter tumor immunity.
Evolution
Fujioka et al5 found that SHPS-1 is a novel membrane glycoprotein and reported for the first time that SIRPα is SHPS-1. Motegi et al10 demonstrated that the expression of CD47 increased with enhanced tumor cell invasion, and CD47 is physically and functionally associated with vitronectin receptor (VnR)-αvβ3 integrin, which plays an important role in tumor cell invasion. Motegi et al proved that CD47-Fc fusion protein or anti-SHPS-1 Abs inhibits the invasion and metastasis of human melanoma cells by blocking CD47-SIRPα interactions.
Subsequent research on the interaction of CD47-SIRPα has emerged, and increasing lines of evidence showed that the blocking of CD47-SIRPα interactions could be used in cancer treatment. Kikuchi et al11 demonstrated that the ligation of CD47 antigen by two antigen-binding sites of MABL dimer is required for inducing apoptosis. MABL is a constructed single-chain antibody fragment (scFv) of murine monoclonal antibody, which specifically binds to human CD47. This apoptosis-inducing dimer appears to be a lead candidate for novel cancer treatment. Vignery12 found that macrophages are present in all tissues and that CD47 is ubiquitously expressed; the macrophage fusion receptor and its ligand CD47 may mediate the homotypic fusion of macrophages and allow for their recognition as “self” before fusion. This study is the first to elaborate CD47-SIRPα macrophage-mediated immunotherapy of tumors. Scholars have clarified the mechanism of action of CD47-SIRPα, and macrophage-mediated tumor immunotherapy has gained increasing attention. CD47 may play an inhibitory role in NK cell-mediated cytotoxicity against cancer cells, suggesting the possible mechanism of immune escape in human cancers.
Another discovery is that CD47 inhibits tumor angiogenesis and regulates long-term vascular responses in tumors by mediating the production of thrombospondin 1 (TSP1).13 A mouse experiment suggested that the blockade of CD47 signaling can delay tumor growth and that CD47 may be a target for immunotherapy in human acute myeloid leukemia.14 The interaction between CD47 and SIRPα prevents the phagocytosis of macrophages, while CD47-blocking Abs enhances the engulfment of tumor cells by antigen-presenting cells; CD47-SIRPα signaling system may be a direction for treatment of various cancers, autoimmune diseases, and skeletal diseases.15
In 2015, anti-CD47 Abs and anti-SIRPa Abs have been discovered and used in CD47-mediated immunotherapy. Humanized 5F9 antibody (Hu5F9-G4), a novel monoclonal anti-human CD47 antibody, can induce potent macrophage-mediated phagocytosis of primary human acute myelocytic leukemia (AML) cells and completely eliminate these cells in vivo. At present, Hu5F9-G4 has entered the clinical trials in patients with AML and solid tumors.16,17 In 2016, a fully human anti-CD47 blocking antibody ZF1 was reported by Zeng. ZF1 is comparable with humanized anti-CD47 blocking antibody B6H12 in terms of high specificity and affinity for CD47; ZF1 can even induce stronger phagocytosis of leukemic cancer cells by macrophages than B6H12 in vitro.18 In the same year, Gizem19 reported that the diminishing interaction of CD47-SIRPα can enhance the killing of macrophages on tumor cells. Monoclonal Abs, such as anti-SIRPα Abs, anti-CD47 BRIC126 Abs, and anti-CD47 B6H12 Abs, can promote macrophage phagocytic activity and inhibit tumor cell growth and metastasis.20
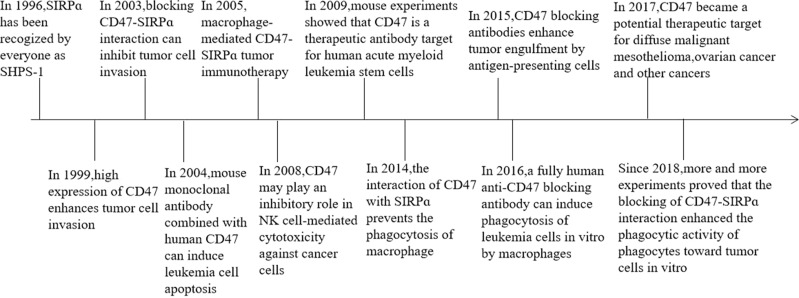
CD47 was found to be a potential therapeutic target for diffuse malignant mesothelioma, and its binding to SIRPα on macrophages can inhibit phagocytosis.21 Moreover, data on mouse research showed that CD47-CAR-T cells can kill cancer cells, such as ovarian cancer cells and digestive tumor cells.22 In 2018, 4-methylumbelliferone (4Mu) can downregulate the expression of CD47 (marker of tumor cells and cancer stem cells) in hepatocellular carcinoma, resulting in enhanced phagocytosis of antigen-presenting cells and elicited potent cytotoxic-specific T cell response.23 Recent studies on mice have shown that the blockade of CD47-SIRPα interaction not only enhances the phagocytic activity of phagocytes against tumor cells but also promotes the stimulation of tumor-specific cytotoxic T cells by macrophages or dendritic cells24 (Figure 1). Many animal experiments on CD47 immunotherapy of tumors have emerged, and researchers believe that CD47 immunotherapy could be a novel strategy for tumor treatment and metastasis prevention. The above description is the time progress of CD47-SIRPα (Table 1).
Figure 1.
The timeline of the key discoveries.
Table 1.
Targeted CD47/SIRPα Antibody Research in Recent Years
| Year | Product | Ab | Diseases |
|---|---|---|---|
| 2015 | Hu 5F9-G4 | Anti‐CD47Ab(IgG4) | Solid tumors, non-Hodgkin’s lymphoma, acute myeloid leukemia, myelodysplastic syndrome |
| 2017 | CC‐90002 | Anti‐CD47Ab(IgG4) | Solid tumors, non-Hodgkin’s lymphoma, acute myeloid leukemia, myelodysplastic syndrome |
| TTI‐621 | SIRPα‐Fc(IgG1) | Solid tumors, acute myeloid leukemia | |
| 2018 | ALX148 | Anti-SIRPα(IgG1Fc) | Solid tumors, hematopoietic malignancies |
| 2019 | A0-176 | Anti‐CD47Ab(IgG4) | Solid tumors, hematopoietic malignancies |
| 2020 | HuNb1-IgG4 | Anti‐CD47Ab(IgG4) | Lymphoma, Ovarian cancer |
Anti-Tumor-Related Research
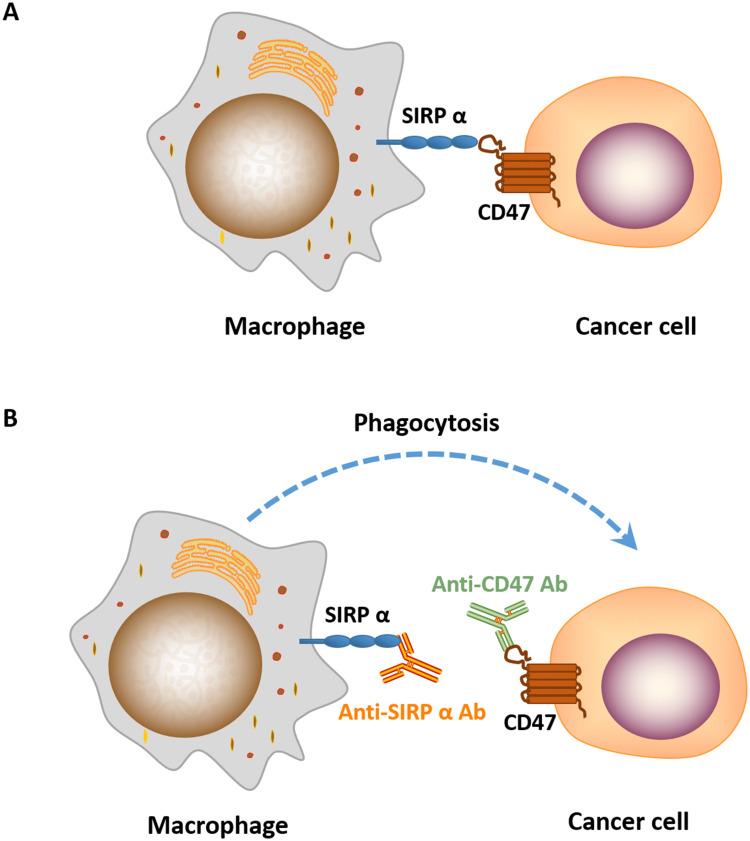
CD47 works as a “don’t eat me” signal to inhibit phagocytic function by binding to SIRPα on phagocytes. SIRPα is particularly abundant on macrophages, while the expression of CD47 is ubiquitous in normal cells and increases significantly in cancer cells to evade immune surveillance (Figure 2). Studies have shown that the blockade of CD47-SIRPα interaction enhances the phagocytic activity of macrophages and other phagocytes in tumor,24 such as microglia, the innate immune effector cell in the central nervous system, is activated after the use of CD47 inhibitors, and participates in tumor suppression. However, the mechanism of targeting CD47 is not well understood. Antibody-dependent cell-mediated cytotoxicity (ADCC) and Fc-independent CD47 intrinsic functions are the most recognized mechanisms involved in anti-CD47 therapy, but which of the two factors predominates has not reached a consensus. Given the wide expression of CD47, if ADCC is predominant, then the possibility of on-target toxicity to healthy cells increases. A tolerable toxicity in anti-CD47 therapy may be achieved with a compromise in efficacy. In addition, targeting SIRPα may provide a way to overcome on-target toxicity to healthy cells due to the limited tissue expression of SIRPα.25,26 If Fc-independent CD47 intrinsic functions predominate, then ADCC should be separated from CD47 blockade and CD47 Abs, which has minimal ADCC, should be combined with other Abs that function depending on strong ADCC effects.
Figure 2.
(A) CD47-SIRPɑ interaction blocks macrophage phagocytosis of cancer cells. (B) Treatment of cancer cells treated with anti-CD47/SIRPɑ Ab induces phagocytosis by macrophage.
Since performing bone marrow transplantation has become possible, leukemia has changed from being an incurable disease to a treatable one, and the cure rate has increased significantly. However, bone marrow resources are very scarce, and a large number of patients fail to find a suitable donor that has bone marrow that matches to human lymphocyte antigens. Even if bone marrow transplantation is successful, the recurrence rate is as high as 70% within 5 years. All these limitations on treatment of leukemia have led scholars to focus on leukemia immunotherapy; as such, CD47-mediated immunotherapy has received considerable attention in recent years. Elevated expression of CD47 has been observed in murine myeloid leukemia, human acute lymphoblastic leukemia, acute myeloid leukemia, and multiple myeloma and is considered to be related to the progression and deterioration of these diseases.25 Several experiments showed that anti-CD47 monoclonal antibodies stimulate the preferential phagocytosis of leukemia stem cells by mouse macrophages; hence, blocking of CD47-SIRPα may be a potential therapeutic approach for leukemia (especially acute myeloid leukemia).28,29 Moreover, the blockade of SIRPα may enhance the Ab-dependent cellular phagocytosis (ADCP) activity of mouse macrophages on Burkitt’s lymphoma cells.30–32 Overall, CD47-SIRPα triggers a cascade of events that inhibit phagocytosis, and using anti-CD47 Abs may become a novel approach for treatment of hematological malignancies.33
Tumors in the digestive tract of humans have become increasingly prominent given the prolonged average life span of humans and the combined effect of various factors, such as dietary habits. Approximately 300,000 people worldwide die from esophageal cancer (EC) each year; EC is a common gastrointestinal tumor and the leading cause of cancer-related death. Current standard treatments include the combination of surgery, chemotherapy, and radiation therapy; however, the five-year survival rate of patients with advanced EC remains poor.34 The use of CD47 Abs can block CD47 signaling and enhance the phagocytosis of phagocytes; as such, CD47 Abs provides new opportunities for treatment of EC.35 In addition, scholars have focused on the application of CD47-mediated immunotherapy to other digestive system tumors, such as pancreatic cancer, colon cancer, and virus-driven gastric cancer.32,36
As environmental pollution becomes increasingly serious, the incidence of skin cancer also increases. When traditional treatment is ineffective for highly malignant and metastasized skin cancer (such as cutaneous squamous cell carcinoma and cutaneous T-cell lymphoma), the blockage of CD47-SIRPα by anti-CD47 Abs or anti-SIRPα Abs has been regarded as target for therapeutic strategy.37–40
Ovarian cancer, which is the most lethal gynecological malignancy, is usually asymptomatic at the early stages of the disease but is only diagnosed at advanced stages. Ovarian cancer usually spreads intraperitoneally due to the unique anatomical characteristics of the ovary. Several experiments showed that CD47 may be a reliable biomarker for predicting the progression of ovarian precancer and ovarian cancer.41 Macrophages are silenced by CD47 molecules expressed in tumor cells, allowing them to escape the killing effect of the immune system. This phenomenon may be one of the mechanisms of immune tolerance and the reason for the occurrence and sustainable development of tumors. Therefore, breaking the immune signal between tumor cells and macrophages and improving the ability of macrophages to recognize and kill tumor cells are research hotspots and cause difficulty in tumor immunotherapy research; elucidating these processes is the key to the overall efficacy of immunotherapy.
CD47 participates in tumor immune escape by combining with SIRPα in other cancers, such as glioblastoma,42 leiomyosarcoma,43 osteosarcoma,44 malignant mesothelioma,24,45 non-Hodgkin’s lymphoma,46 cervical cancer, ovarian cancer, renal cell carcinoma,47–49 and bladder tumor.50 Given that CD47 is widely expressed in various cancer types, it represents a potential and widely applicable target for immunotherapy. Using the mechanism of CD47-SIRPα interaction has achieved some success in anti-tumor research.
Perspectives
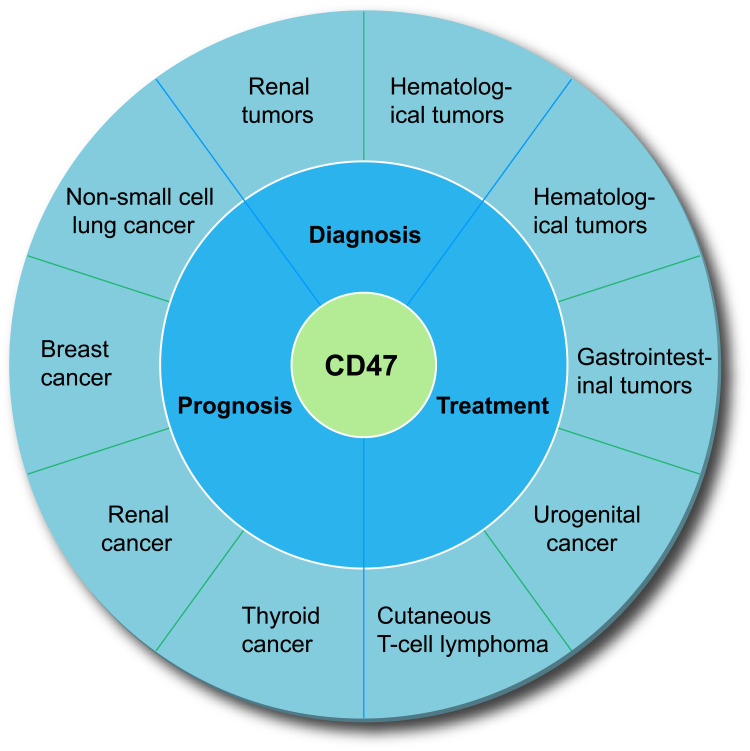
Phagocytosis requires simultaneous activation of “eat me” signals (such as calcium reticulum protein) and disruption of “don’t eat me” signals. Either of the two events is insufficient to trigger a phagocytic response against cancer. CD47, the fundamental “don’t eat me” signal, inhibits phagocytosis by binding to SIRPα in phagocytes. A large number of studies have shown that CD47 plays an important role in assessment of the diagnosis, treatment, and prognosis of many tumors (Figure 3).
Figure 3.
The value of CD47 in the diagnosis, treatment, and prognosis assessment of various tumors.
The ideal situation is the use of monoclonal Abs or similar molecules to block the CD47-SIRPα signaling pathway and destroy the “don’t eat me” signal to restore the phagocytosis of tumor cells by macrophages.51 When CD47-related therapeutic drugs kill tumor cells, they will inevitably and accidentally damage red blood cells, leading to agglutination.52 In Phase I clinical trials of CD47, anemia and thrombocytopenia have become dose-limiting toxicity factors. A large number of red blood cells in the body will become the best covering for tumor cells, and CD47 drugs will be depleted by red blood cells before reaching the tumor cells. Therefore, protecting red blood cells while maximally killing tumor cells is a problem to be solved. To overcome this problem, different companies have proposed different solutions. The mainstream practice represented by Forty-Seven,13 Celgene,29 and Surface Oncology is to develop an IgG4-type CD47 antibody at the Fc end, rather than an IgG1 type antibody that can elicit strong ADCC and CDC effects.53 This strategy will reduce the effect of the antibody on red blood cells and platelets; however, replacing IgG1 with IgG4 will greatly impair the killing ability of CD47 monoclonal antibodies against tumor cells, which may be the reason that Celgene terminated the clinical trial of CC-90002.54
The second solution is to reduce the ability of CD47 drugs to bind to red blood cells, thereby avoiding killing of such cells. The Trillium’s product, TTI-621, is a fusion protein of the SIRP protein and the Fc end of the antibody. This protein has weak binding ability to red blood cells, which may be related to conformational changes in CD47 in the erythrocyte membrane. However, TTI-621 can still bind to human platelets and leukocytes, resulting in thrombocytopenia or leukopenia.55
Another idea is to completely abandon the potent ADCC effect of CD47 antibodies and only use the biological effects of CD47-SIRPα itself to release the anti-tumor potential of macrophages. For example, ALX148 developed by ALX Oncology uses a Fc end that is not biologically active;56 AO-176 induces macrophages to engulf tumor cells by blocking CD47-SIRPα.57 HuNb1-IgG4, an innovative anti-CD47 nanobody, can enhance the macrophage-mediated phagocytosis of tumor cells.58 In this case, CD47 drugs must be used in combination with other drugs, especially drugs where the ADCC effect is the main factor that determines the phagocytosis and anti-tumor activity of macrophages and natural killer cells, or with systematic PD-1/PD-L1 inhibitor drugs that can regulate immunity. What is more, simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release.59 The evidence is the positive clinical results of using the combination of Hu5F9-G4 and Maebro announced by Forty-Seven. In addition, bispecific Abs based on CD47 is a possible development direction.
Given the specificity of the CD47 target, developing it as an effective and safe drug is difficult. With the development of nanomaterials, nanotechnology has been successfully applied in the field of medicine, especially in drug production and pharmacology. Nanotechnology can directly employ the arrangement of atoms and molecules to manufacture drugs with specific functions, leading to sophisticated pharmaceutical production. Based on the special properties of nanoparticles, their surface can be modified to form a targeted, controlled-release, and easy-to-detect sole drug delivery carrier, so the drug can be easily transferred to the human body to kill cancer cells; this strategy could be a new method for treating local lesions in the body.
Nanoparticles synthesized with CD47mAb attached to the surface have immunoglobulin targeting function and high safety. CD47mAb blocks CD47-SIRPα by binding to CD47, thereby activating macrophage immune activity and mediating nanoparticles to enter into cells. In addition, the nanoparticles are transformed into M1 type by inducing tumor-associated macrophage M2 type. The dead tumor cells release antigens, proteases, reactive oxygen species, and cytokines to further attract and activate macrophages.60
Conclusions
Collectively, CD47 is overexpressed widely across tumor types and the blockade of CD47-SIRPα interaction enhances the phagocytic activity of phagocytes on tumor cells. Targeting CD47 may be a promising cancer treatment. However, the existence of some factors has led to overestimation of the efficacy of anti-CD47 treatment in preclinical models, and due to heterogeneity, the efficacy of different tumors varies greatly. At this time, the true clinical effect may be difficult to compare with the results of animal experiments, so it is necessary to carefully select the appropriate patient based on the expression and aggregation of CD47. Currently, researchers are also facing huge challenges brought about by the CD47 target, such as the challenge of blood toxicity on drug design and the challenge of clinical dose tolerance of the target drug. Further research is still needed to explore safer and more effective anti-CD47-SIRPα antibody types.
Funding Statement
This work was supported by the National Natural Science Foundation of China [No. 81872493, 81803151], the China Postdoctoral Science Foundation [No.2017T100407], the Jiangsu Provincial Medical Talent Foundation the ‘Six Talent Peaks’ Project of Jiangsu Province (No.WSW-074).
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Hossain F, Majumder S, Ucar DA, et al. Notch signaling in myeloid cells as a regulator of tumor immune responses. Front Immunol. 2018;9:1288. doi: 10.3389/fimmu.2018.01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J, Jin S, Guo X, et al. Targeting the CD47-SIRPα signaling axis: current studies on B-cell lymphoma immunotherapy. J Int Med Res. 2018;46(11):4418–4426. doi: 10.1177/0300060518799612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida H, Tomiyama Y, Oritani K, et al. Interaction between Src homology 2 domain bearing protein tyrosine phosphatase substrate-1 and CD47 mediates the adhesion of human B lymphocytes to nonactivated endothelial cells. J Immunol. 2002;168(7):3213–3220. doi: 10.4049/jimmunol.168.7.3213. [DOI] [PubMed] [Google Scholar]
- 4.Han X, Sterling H, Chen Y, et al. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem. 2000;275(48):37984–37992. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- 5.Fujioka Y, Matozaki T, Noguchi T, et al. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol. 1996;16(12):6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai RK, Discher DE. Inhibition of “self” engulfment through deactivation of myosin-II at the phagocytic synapse between human cells. J Cell Biol. 2008;180(5):989–1003. doi: 10.1083/jcb.200708043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Vries HE, Hendriks JJ, Honing H, et al. Signal-regulatory protein alpha-CD47 interactions are required for the transmigration of monocytes across cerebral endothelium. J Immunol. 2002;168(11):5832–5839. doi: 10.4049/jimmunol.168.11.5832. [DOI] [PubMed] [Google Scholar]
- 8.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11(3):130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 9.Chung J, Wang XQ, Lindberg FP, et al. Thrombospondin-1 acts via IAP/CD47 to synergize with collagen in alpha2beta1-mediated platelet activation. Blood. 1999;94(2):642–648. doi: 10.1182/blood.V94.2.642 [DOI] [PubMed] [Google Scholar]
- 10.Motegi S, Okazawa H, Ohnishi H, et al. Role of the CD47-SHPS-1 system in regulation of cell migration. EMBO J. 2003;22(11):2634–2644. doi: 10.1093/emboj/cdg278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi Y, Uno S, Yoshimura Y, et al. A bivalent single-chain Fv fragment against CD47 induces apoptosis for leukemic cells. Biochem Biophys Res Commun. 2004;315(4):912–918. doi: 10.1016/j.bbrc.2004.01.128. [DOI] [PubMed] [Google Scholar]
- 12.Vignery A, Gilgenkrantz S. Macrophage fusion: are somatic and cancer cells possible partners? Med Sci. 2005;21(12):1070–1075. doi: 10.1051/medsci/200521121070. [DOI] [PubMed] [Google Scholar]
- 13.Kim MJ, Lee JC, Lee JJ, et al. Association of CD47 with natural killer cell-mediated cytotoxicity of head-and-neck squamous cell carcinoma lines. Tumour Biol. 2008;29(1):28–34. doi: 10.1159/000132568. [DOI] [PubMed] [Google Scholar]
- 14.Majeti R, Chao MP, Alizadeh AA, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138(2):286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murata Y, Kotani T, Ohnishi H, et al. The CD47-SIRPα signalling system: its physiological roles and therapeutic application. J Biochem. 2014;155(6):335–344. doi: 10.1093/jb/mvu017. [DOI] [PubMed] [Google Scholar]
- 16.Vonderheide RH. CD47 blockade as another immune checkpoint therapy for cancer. Nat Med. 2015;21(10):1122–1123. doi: 10.1038/nm.3965. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Wang L, Zhao F, et al. Pre-clinical development of a humanized anti-CD47 antibody with anti-cancer therapeutic potential. PLoS One. 2015;10(9):e0137345. doi: 10.1371/journal.pone.0137345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zeng D, Sun Q, Chen A, et al. A fully human anti-CD47 blocking antibody with therapeutic potential for cancer. Oncotarget. 2016;7(50):83040–83050. doi: 10.18632/oncotarget.13349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bener G, Félix A, Sánchez de Diego C, Pascual Fabregat I, Ciudad CJ, Noé V. Silencing of CD47 and SIRPα by polypurine reverse hoogsteen hairpins to promote MCF-7 breast cancer cells death by PMA-differentiated THP-1 cells. BMC Immunol. 2016;17(1):32. doi: 10.1186/s12865-016-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang C, Gao S, Zhang H, et al. CD47 is a potential target for the treatment of laryngeal squamous cell carcinoma. Cell Physiol Biochem. 2016;40(1–2):126–136. doi: 10.1159/000452530. [DOI] [PubMed] [Google Scholar]
- 21.Schürch CM, Forster S, Brühl F, et al. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology. 2017;7(1):e1373235. doi: 10.1080/2162402x.2017.1373235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golubovskaya V, Berahovich R, Zhou H, et al. CD47-CAR-t cells effectively kill target cancer cells and block pancreatic tumor growth. Cancers. 2017;9(12):139. doi: 10.3390/cancers9100139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez MM, Fiore E, Bayo J, et al. 4Mu decreases cd47 expression on hepatic cancer stem cells and primes a potent antitumor T cell response induced by interleukin-12. Mol Ther. 2018;26(12):2738–2750. doi: 10.1016/j.ymthe.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murata Y, Saito Y, Kotani T, et al. CD47-signal regulatory protein α signaling system and its application to cancer immunotherapy. Cancer Sci. 2018;109(8):2349–2357. doi: 10.1111/cas.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ring NG, Herndler-Brandstetter D, Weiskopf K, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114(49):E10578–E10585. doi: 10.1073/pnas.1710877114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer. 2017;76:100–109. doi: 10.1016/j.ejca.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Willingham SB, Volkmer JP, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109(17):6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi S. Molecular functions of SIRPα and its role in cancer. Biomed Rep. 2018;9(1):3–7. doi: 10.3892/br.2018.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Yin C, Feng L, et al. Ara-C and anti-CD47 antibody combination therapy eliminates acute monocytic leukemia THP-1 cells in vivo and in vitro. Genet Mol Res. 2015;14(2):5630–5641. doi: 10.4238/2015.May.25.15. [DOI] [PubMed] [Google Scholar]
- 30.Goto H, Kojima Y, Matsuda K, et al. Efficacy of anti-CD47 antibody-mediated phagocytosis with macrophages against primary effusion lymphoma. Eur J Cancer. 2014;50(10):1836–1846. doi: 10.1016/j.ejca.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Weiskopf K, Anderson KL, Ito D, et al. Eradication of canine diffuse large B-cell lymphoma in a murine xenograft model with CD47 blockade and anti-CD20. Cancer Immunol Res. 2016;4(12):1072–1087. doi: 10.1158/2326-6066.cir-16-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michaels AD, Newhook TE, Adair SJ, et al. CD47 blockade as an adjuvant immunotherapy for resectable pancreatic cancer. Clin Cancer Res. 2018;24(6):1415–1425. doi: 10.1158/1078-0432.ccr-17-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ A, Hua AB, Montfort WR, et al. Blocking “don’t eat me” signal of CD47-SIRPα in hematological malignancies, an in-depth review. Blood Rev. 2018;32(6):480–489. doi: 10.1016/j.blre.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26(7):1086–1092. doi: 10.1200/jco.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao CL, Yu S, Wang SH, et al. Characterization of cluster of differentiation 47 expression and its potential as a therapeutic target in esophageal squamous cell cancer. Oncol Lett. 2018;15(2):2017–2023. doi: 10.3892/ol.2017.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Sime W, Juhas M, et al. Crosstalk between colon cancer cells and macrophages via inflammatory mediators and CD47 promotes tumour cell migration. Eur J Cancer. 2013;49(15):3320–3334. doi: 10.1016/j.ejca.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Ingram JR, Blomberg OS, Sockolosky JT, et al. Localized CD47 blockade enhances immunotherapy for murine melanoma. Proc Natl Acad Sci U S A. 2017;114(38):10184–10189. doi: 10.1073/pnas.1710776114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yanagita T, Murata Y, Tanaka D, et al. Anti-SIRPα antibodies as a potential new tool for cancer immunotherapy. JCI Insight. 2017;2(1):e89140. doi: 10.1172/jci.insight.89140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folkes AS, Feng M, Zain JM, et al. Targeting CD47 as a cancer therapeutic strategy: the cutaneous T-cell lymphoma experience. Curr Opin Oncol. 2018;30(5):332–337. doi: 10.1097/cco.0000000000000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu L, Yu GT, Deng WW, et al. Anti-CD47 treatment enhances anti-tumor T-cell immunity and improves immunosuppressive environment in head and neck squamous cell carcinoma. Oncoimmunology. 2018;7(4):e1397248. doi: 10.1080/2162402x.2017.1397248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye X, Wang X, Lu R, et al. CD47 as a potential prognostic marker for oral leukoplakia and oral squamous cell carcinoma. Oncol Lett. 2018;15(6):9075–9080. doi: 10.3892/ol.2018.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li F, Lv B, Liu Y, et al. Blocking the CD47-SIRPα axis by delivery of anti-CD47 antibody induces antitumor effects in glioma and glioma stem cells. Oncoimmunology. 2018;7(2):e1391973. doi: 10.1080/2162402x.2017.1391973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edris B, Weiskopf K, Weissman IL, et al. Flipping the script on macrophages in leiomyosarcoma. Oncoimmunology. 2012;1(7):1202–1204. doi: 10.4161/onci.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu JF, Pan XH, Zhang SJ, et al. CD47 blockade inhibits tumor progression human osteosarcoma in xenograft models. Oncotarget. 2015;6(27):23662–23670. doi: 10.18632/oncotarget.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dammeijer F, Lievense LA, Kaijen-Lambers ME, et al. Depletion of tumor-associated macrophages with a CSF-1R kinase inhibitor enhances antitumor immunity and survival induced by DC immunotherapy. Cancer Immunol Res. 2017;5(7):535–546. doi: 10.1158/2326-6066.cir-16-0309. [DOI] [PubMed] [Google Scholar]
- 46.Zhang X, Fan J, Wang S, et al. Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol Res. 2017;5(5):363–375. doi: 10.1158/2326-6066.cir-16-0398. [DOI] [PubMed] [Google Scholar]
- 47.Liu F, Dai M, Xu Q, et al. SRSF10-mediated IL1RAP alternative splicing regulates cervical cancer oncogenesis via mIL1RAP-NF-κB-CD47 axis. Oncogene. 2018;37(18):2394–2409. doi: 10.1038/s41388-017-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu R, Wei H, Gao P, et al. CD47 promotes ovarian cancer progression by inhibiting macrophage phagocytosis. Oncotarget. 2017;8(24):39021–39032. doi: 10.18632/oncotarget.16547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan M, Zhu L, Zhuang H, et al. Lewis Y antigen modified CD47 is an independent risk factor for poor prognosis and promotes early ovarian cancer metastasis. Am J Cancer Res. 2015;5(9):2777–2787. [PMC free article] [PubMed] [Google Scholar]
- 50.Olcucuoglu E, Sirin ME, Aydog G, et al. Relationship between immunohistochemical staining extent of CD47 and histopathologic features of bladder tumor. Cent Eur J Urol. 2017;70(4):349–355. doi: 10.5173/ceju.2017.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chao MP, Weissman IL, Majeti R. The CD47-SIRPα pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24(2):225–232. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oldenborg PA, Zheleznyak A, Fang YF, et al. Role of CD47 as a marker of self on red blood cells. Science. 2000;288(5473):2051–2054. doi: 10.1126/science.288.5473.2051. [DOI] [PubMed] [Google Scholar]
- 53.Pietsch EC, Dong J, Cardoso R, et al. Anti-leukemic activity and tolerability of anti-human CD47 monoclonal antibodies. Blood Cancer J. 2017;7(2):e536. doi: 10.1038/bcj.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Y, Ma Y, Gao P, et al. Targeting CD47: the achievements and concerns of current studies on cancer immunotherapy. J Thorac Dis. 2017;9(2):E168–E174. doi: 10.21037/jtd.2017.02.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Petrova PS, Viller NN, Wong M, et al. TTI-621 (SIRPαFc): a CD47-blocking innate immune checkpoint inhibitor with broad antitumor activity and minimal erythrocyte binding. Clin Cancer Res. 2017;23(4):1068–1079. doi: 10.1158/1078-0432.ccr-16-1700. [DOI] [PubMed] [Google Scholar]
- 56.Kauder SE, Kuo TC, Harrabi O, et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS One. 2018;13(8):e0201832. doi: 10.1371/journal.pone.0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puro RJ, Bouchlaka MN, Hiebsch RR, et al. Development of AO-176, a next generation humanized anti-CD47 antibody with novel anti-cancer properties and negligible red blood cell binding. Mol Cancer Ther. 2019. doi: 10.1158/1535-7163.mct-19-1079. [DOI] [PubMed] [Google Scholar]
- 58.Ma L, Zhu M, Gai J, et al. Preclinical development of a novel CD47 nanobody with less toxicity and enhanced anti-cancer therapeutic potential. J Nanobiotechnology. 2020;18(1):12. doi: 10.1186/s12951-020-0571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lian S, Xie R, Ye Y, et al. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. EBioMedicine. 2019;42:281–295. doi: 10.1016/j.ebiom.2019.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ai X, Hu M, Wang Z, et al. Enhanced cellular ablation by attenuating hypoxia status and reprogramming tumor-associated macrophages via NIR light-responsive upconversion nanocrystals. Bioconjug Chem. 2018;29(4):928–938. doi: 10.1021/acs.bioconjchem.8b00068. [DOI] [PubMed] [Google Scholar]