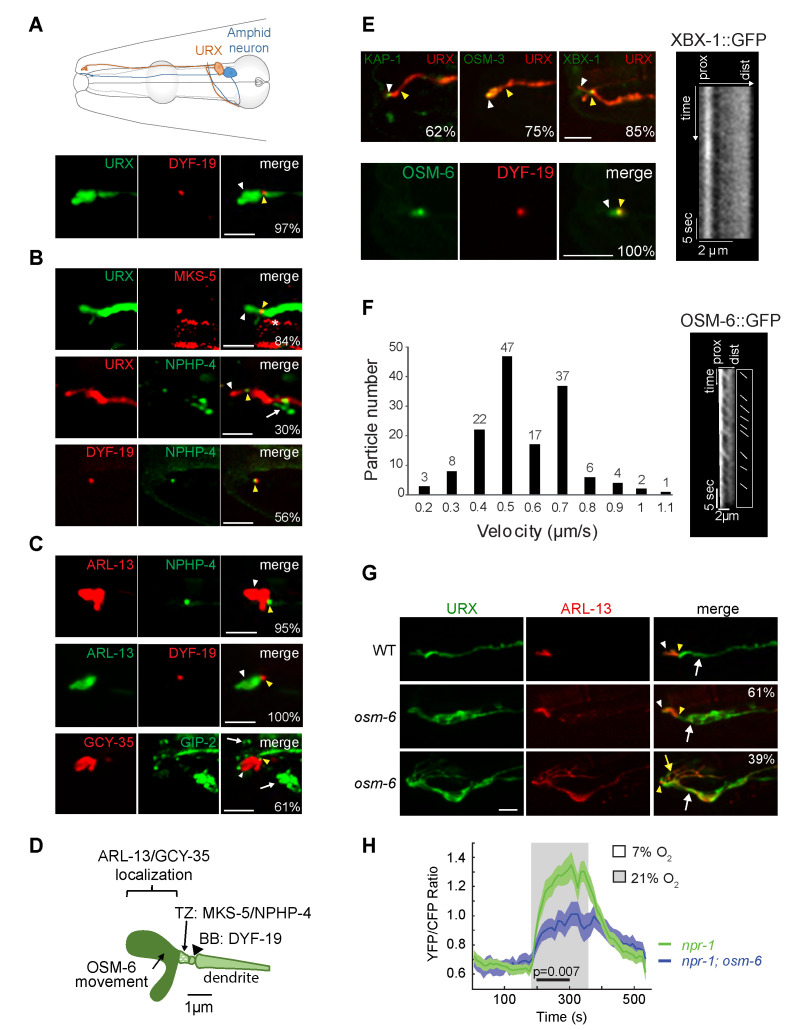
Figure 1. The URX neurons contain a cilium-like structure at their distal dendritic ends.
A) (Top) Schematic of an URX (orange) and representative amphid sensory neuron (blue) in the head of an adult C. elegans hermaphrodite. Only neurons on the left are shown. (Bottom) Localization of the basal body-associated protein DYF-19::tagRFP expressed under the gcy-36 promoter at the URX distal dendritic ends. URX is marked via expression of gcy-32p::GFP.
B) (Top two rows) Localization of the transition zone proteins MKS-5::tagRFP and NPHP-4::GFP in URX. MKS-5::tagRFP and NPHP-4::GFP were expressed under the gcy-32 and endogenous promoters, respectively. Arrow indicates localization of NPHP-4 in other cilia, asterisk indicates background fluorescence in the pharynx. URX is marked with gcy-32p::GFP or gcy-36p::RFP. (Bottom row) Colocalization of DYF-19::tagRFP and NPHP-4::GFP at the URX distal dendritic ends.
C) (Top two rows) Colocalization of ARL-13::tagRFP and ARL-13::GFP with NPHP-4::GFP and DYF-19::tagRFP, respectively, at the URX distal dendritic ends. ARL-13 was expressed under the gcy-32 promoter, NPHP-4 and DYF-19 were expressed under the gcy-36 promoter. (Bottom row) Colocalization of GCY-35::mKate with GIP-2::GFP at the URX distal dendritic ends. GCY-35::mKate was expressed under the gcy-36 promoter; GIP-2::GFP was expressed from its endogenous locus (Harterink et al. 2018); arrows indicate localization in other cells.
D) Summary of localization patterns of examined fusion proteins at the URX sensory endings. TZ: transition zone, BB: basal body.
E) Localization of KAP-1::GFP, OSM-3::GFP, XBX-1::GFP, OSM-6::GFP and DYF-19::tagRFP fusion proteins at the URX distal dendritic ends. Fusion proteins were expressed under the gcy-32 (OSM-3, KAP-1, OSM-6) or gcy-36 (XBX-1, DYF-19) promoters. URX was visualized via expression of gcy-36p::RFP. (Right) Representative kymograph of XBX-1::GFP movement in the URX sensory endings.
F) (Left) Histogram of OSM-6::GFP anterograde velocity in the URX cilium-like structure. OSM-6::GFP was expressed in URX under the gcy-32 promoter. (Right) Representative kymograph and schematic of OSM-6::GFP movement in the URX cilium-like structure. n= total of 148 particles from 20 animals.
G) Localization of ARL-13::tagRFP in URX (marked via expression of gcy-32p::GFP) in wild-type and osm-6(p811) animals. White and yellow arrows indicate the main dendrite and dendritic branches, respectively.
H) Average YFP/CFP ratio change (solid lines) in URX neurons expressing YC2.60 in animals of the indicated genotypes in response to a 7%-21% oxygen shift. Shaded areas are SEM. The black bar indicates the time intervals used for statistical comparisons (Mann-Whitney U Test). Only responding neurons were included for generating the graph and the statistical analysis (WT: 21/21; osm-6: 20/44). Since the laboratory N2 strain contains a gain-of-function variant of the NPR-1 neuropeptide Y-like receptor that inhibits some oxygen responses (Weber et al. 2010; Busch et al. 2012), imaging experiments were performed in an npr-1(ad609) loss-of-function background.
In all relevant panels, white and yellow arrowheads indicate the presumptive cilia-like structure and the basal body/transition zone, respectively. Anterior is at left in all image panels. Numbers in lower right corners indicate the percentage of animals exhibiting the observed localization pattern; n > 21 for each. Scale bars: 5 µm unless otherwise noted.
Description
A subset of sensory neurons in C. elegans contains compartmentalized sensory structures termed cilia at their distal dendritic ends (Ward et al. 1975; Perkins et al. 1986; Doroquez et al. 2014). Cilia present on different sensory neuron types are specialized both in morphology and function, and are generated and maintained via shared and cell-specific molecules and mechanisms (Perkins et al. 1986; Evans et al. 2006; Mukhopadhyay et al. 2007; Mukhopadhyay et al. 2008; Morsci and Barr 2011; Doroquez et al. 2014; Silva et al. 2017). The bilaterally symmetric pair of URX oxygen-sensing neurons in the C. elegans head (Figure 1A) is thought to be non-ciliated (Ward et al. 1975; Doroquez et al. 2014) but nevertheless exhibits intriguing morphological similarities with ciliated sensory neurons. URX dendrites extend to the nose where they terminate in large bulb-like complex structures (Ward et al. 1975; Doroquez et al. 2014; Cebul et al. 2020) (Figure 1A). These structures concentrate oxygen-sensing signaling molecules (Gross et al. 2014; Mclachlan et al. 2018) suggesting that similar to cilia, these structures are specialized for sensory functions. Microtubule growth events similar to those observed in ciliated sensory neurons were also reported at the distal dendritic regions of URX, implying the presence of a microtubule organizer such as a remodeled basal body (Harterink et al. 2018). Moreover, a subset of ciliary genes is expressed in URX (Kunitomo et al. 2005; Harterink et al. 2018; Mclachlan et al. 2018). We tested the hypothesis that URX dendrites contain cilia at their distal ends.
We first examined the localization of proteins associated with different ciliary compartments in URX. The basal body component DYF-19/FBF1 (Wei et al. 2013) was enriched at single puncta at the distal dendritic ends of URX (Figure 1A). A similar localization pattern was previously reported for the centrosome-associated protein gamma-tubulin (Harterink et al. 2018). The MKS-5::tagRFP (Williams et al. 2011) and NPHP-4::GFP (Jauregui and Barr 2005; Williams et al. 2011) transition zone fusion proteins were also localized to single puncta at the URX dendritic tips, distal to the region of DYF-19 localization (Figure 1B). In addition, ARL-13::GFP, a well-characterized ciliary membrane marker (Cevik et al. 2010; Li et al. 2010) was restricted to a region distal to the DYF-19::tagRFP and NPHP-4::GFP puncta (Figure 1C). The mKate-tagged oxygen-sensing soluble guanylyl cyclase GCY-35 was also highly enriched at the distal dendritic ends of URX in a domain that was defined proximally by the GIP-2 component of gamma-TuRC expressed from its endogenous locus (Figure 1C) (Wang et al. 2015; Harterink et al. 2018). We noted that overexpression of ARL-13 and GCY-35 resulted in an expansion of the presumptive cilium-like structure in URX; overexpression of ARL-13 has previously been reported to alter cilia morphology in other cell types (Hori et al. 2008; Larkins et al. 2011). Together, these observations imply the presence of a cilium-like structure at the distal dendritic ends of URX that houses signaling molecules, and that is delineated proximally by a transition zone and basal body (summarized in Figure 1D). We speculate that the short length of this structure may have precluded its identification in previous ultrastructural studies (Ward et al. 1975; Perkins et al. 1986; Doroquez et al. 2014).
We next investigated whether intraflagellar transport (IFT) necessary for cilia generation and maintenance (Rosenbaum and Wittman, 2014) could be detected in this cilium-like organelle in URX. Although the KAP-1 kinesin-2 motor component, OSM-3 homodimeric motor, and XBX-1 dynein light chain subunit were localized to the very distal ends of the URX dendrites, we were unable to detect movement of these proteins under standard conditions (Figure 1E). Similar to the localization of these proteins, the IFT core component OSM-6::GFP was also present in a small domain distal to that occupied by the DYF-19::tagRFP puncta (Figure 1E). However, we observed anterograde movement of OSM-6::GFP in the URX dendritic ends at an average speed of 0.61 ± 0.20 µm/sec (Figure 1F), consistent with kinesin-2-mediated transport. We were unable to detect and/or quantify retrograde movement of OSM-6::GFP. Unlike in other characterized sensory cilia which typically exhibit robust IFT, episodes of OSM-6::GFP anterograde movement in the presumptive URX cilia-like structure were infrequent and could be observed in only ~20% of examined neurons under specific conditions (see Methods).
We asked whether IFT proteins are necessary for the structure and/or function of the URX cilium-like structure. Although we were unable to definitively determine whether the very short cilium-like structure was further truncated in osm-6 mutants, we found that ARL-13::tagRFP was mislocalized to the URX dendrite in all examined osm-6 mutants (Figure 1G). Interestingly, ~40% of URX neurons in osm-6 mutants also exhibited multiple branches emanating from their distal dendritic ends; these branches contained ARL-13::GFP (Figure 1G). Similar, albeit less extensive, branches (also referred to as ‘posterior projections’) have been reported from the distal ends of a subset of ciliated sensory neuron dendrites in animals mutant for IFT genes in C. elegans (Lewis and Hodgkin 1977; Perkins et al. 1986; Fujiwara et al. 1999; Murayama et al. 2005; Kunitomo and Iino 2008; Maurya et al. 2019).
URX responds to a 7%-21% rise in oxygen with a tonic increase in intracellular calcium levels (Zimmer et al. 2009; Busch et al. 2012). We found that only 45% (20/44) of URX neurons in osm-6(p811) mutants exhibited oxygen-evoked responses as assessed via changes in YC2.60 fluorescence, as compared to 100% of neurons (21/21) in wild-type animals. Moreover, response amplitudes were significantly decreased in responding URX neurons in osm-6 mutants (Figure 1H). We conclude that a subset of IFT molecules is necessary for the morphological and functional integrity of the cilia-like compartment in URX.
The sensory compartments at the distal ends of URX dendrites exhibit several features characteristic of cilia. Basal body, transition zone, IFT, and ciliary membrane and membrane-associated markers, including sensory signaling molecules, are localized in domains whose relative organization at the URX dendritic ends is similar to those present in bona fide sensory cilia. Moreover, OSM-6 appears to undergo IFT in a subset of URX cilia-like structures and is required for correct neuronal morphology, ciliary protein localization and sensory functions. However, we did not observe movement of any additional IFT proteins including motors. It is possible that motor movement in the short URX cilium is difficult to detect due to technical considerations. Alternatively, URX may employ cell-specific mechanisms including cell-specific motors to build its cilia. Finally, environmental manipulations have been shown to alter neuronal and cilia morphology including URX dendritic morphology in C. elegans (Albert and Riddle 1983; Mukhopadhyay et al. 2008; Procko et al. 2011; Schroeder et al. 2013; Cohn et al. 2019). Thus, trafficking in URX cilia may be modulated in a context-specific manner, perhaps to regulate cilium length or protein composition in response to specific cues. In the future, it will be interesting to correlate IFT in URX cilia with neuronal responses under defined external and internal conditions.
Methods
C. elegans growth
C. elegans strains were maintained at 20°C under normoxic conditions on standard NGM agar plates seeded with E. coli OP50 unless specified otherwise (all strains used in this work are indicated in Table 1). Mutant and transgenic strains were generated using standard methods. The presence of the desired mutation was confirmed by sequencing. Plasmids were injected at 1-10 ng/µl (see Table 2) with co-injection markers (unc-122p::gfp, unc-122p::rfp or unc-122p::dsRed) injected at 30 ng/µl.
Molecular biology
All DNA constructs were generated using standard cloning techniques, subcloned into the pPD95.77 C. elegans expression plasmid (A.Fire, Stanford University) and validated by restriction enzyme digestion and sequencing. Plasmids used in this work are indicated in Table 2.
Microscopy
One day-old adults were anesthetized using 10mM tetramisole (Sigma), diluted in M9 buffer and mounted onto 10% agarose pads. Live anesthetized animals were imaged on an inverted spinning disk microscope (Zeiss Axio Observer with a Yokogawa CSU-22 spinning disk confocal head). Images of protein localization were generated by collecting optical sections (z-stack) every 0.2-0.27 µm using Plan Apochromat 100x/1.40 NA or 63X/1.20 NA oil immersion objectives. Maximum intensity projection (z-stacks) images were generated using SlideBook 6.0 software (Intelligent Imaging Innovations, 3i). Enhancement of brightness and contrast across the entire image, as well as image rotations were performed using ImageJ/Fiji (NIH) software. Each strain was imaged independently on at least two different days. Two or more transgenic lines were generated and examined for each transgenic strain.
IFT
L4 animals were picked 18-24 hours prior to imaging and maintained at 15°C on unseeded NGM plates under normoxic conditions. OSM-6 IFT movement was observed only under these conditions in very young adults. 2D timelapse videos of OSM-6 and XBX-1 were recorded using SlideBook 6.0 software (Intelligent Imaging Innovations, 3i) using an inverted spinning disk confocal microscope (Zeiss Axio Observer with a Yokogawa CSU-22 spinning disk confocal head) using a Plan Apochromat 63X NA 1.2 oil immersion objective. The kymographs were generated using the MultipleKymographs plugin in ImageJ/Fiji (NIH). Individual particle tracks were manually traced.
Calcium imaging
Imaging of the calcium response in URX neurons was carried out across 3 days in animals expressing gcy-37p::YC2.60 as previously described (Chen et al. 2017). Briefly, movies were recorded with a Nikon AZ100 microscope equipped with a Nikon ×2 AZ-Plan Fluor objective and Hamamatsu ORCA-FLASH4.0 cameras using NIS-Elements software and 500ms exposure time. Excitation light produced by a Nikon Intensilight C-HGFI was passed through a 438/24nm filter and a Semrock FF458DiO2 dichroic. The emission light was split using a Cairn Research TwinCam dual camera adapter and passed through CFP and YFP filters (483/32nm and 542/27nm respectively), and a DC/T510LPXRXTUf2 dichroic. Dermabond adhesive was used to immobilize young adults on agar pads while keeping the nose exposed. 1ml of concentrated OP50 in M9 buffer was applied to the head and allowed to dry before the slide was transferred to the imaging chamber. In the imaging chamber, animals were exposed to 7% oxygen for 2 minutes before starting the experiment in which 7% then 21% then 7% oxygen concentrations were sequentially flowed into the imaging chamber for 3 min intervals. The recorded data was processed using Neuron Analyzer, a custom-written Matlab program.
Reagents
Table 1. List of strains used in this work.
| Strain | Genotype | Source |
| PY11327 | iaIs25 [gcy-37p::gfp, unc-119(+)]; oyEx643 [gcy-36p::dyf-19::tagRfp, unc-122p::mCherry] | Inna Nechipurenko |
| PY10377-79 | Ex[gcy-32p::gfp, gcy-32p::mks-5::tagRfp; unc-122p::Rfp] Lines 2A, 6A, 21A | This work |
| PY10380 | Ex[nphp-4p::nphp-4::gfp, gcy-36p::tagRfp, unc-122p::Rfp] Line 2A | This work |
| PY10381 | Ex[gcy-32p::osm-3b::gfp, gcy-36p::tagRfp, unc-122p::Rfp] Line 10B | This work |
| PY10382-84 | Ex[gcy-32p::kap-1::gfp, gcy-36p::tagRfp, unc-122p::Rfp] Lines 7E, 11B, 16C | This work |
| PY11316 | iaIs25 [gcy-37p::gfp, unc-119(+)]; oyEx645 [gcy-32p::arl-13::tagRfp, unc-122p::mCherry] | Inna Nechipurenko |
| PY10386 | osm-6(p811); iaIs25 [gcy-37p::gfp]; Ex[gcy-32p::arl-13::tagRfp, unc-122p::dsRed] | This work |
| PY10357 | Ex[gcy-32p::osm-6::gfp, gcy-36p::tagRfp, unc-122p::Rfp] | This work |
| PY10388-90 | Ex[gcy-36p::xbx-1::gfp, gcy-36p::tagRfp, unc-122p::Rfp] Lines 13C, 13A, 4A | This work |
| PY10391-94 | Ex[gcy-32p::arl-13::tagRfp, gcy-36p::nphp-4::gfp, unc-122p::gfp] Lines 1-4 | This work |
| PY10395 | Ex[gcy-36p::nphp-4::gfp, gcy-36p::dyf-19::tagRfp, unc-122p::gfp] Line 8 | This work |
| PY10396-99 | Ex[gcy-36p::dyf-19::tagRfp; gcy-32p::osm-6::gfp, unc-122p::Rfp] Lines 2, 4, 5A, 5B | This work |
| PY10310 | gip-2(lt19[gip-2::gfp::loxP::cb-unc-119(+)::loxP]); hrtSi57[gcy-36p::gcy-35::mKate-intra] | Martin Harterink |
| PY10387 | Ex[gcy-36p::dyf-19::tagRfp, gcy32p::arl-13::gfp, unc-122p::Rfp] Line 2 | This work |
| PY10352 | osm-6(p811); npr-1 (ad609), Ex[gcy-37p::YC2.60] | This work |
Table 2. List of plasmids used in this work.
| Plasmid | Description | Source (injection concentration) |
| PSAB1231 | gcy-32p::mks-5cDNA::tagRfp | Inna Nechipurenko (5 ng/µl) |
| PSAB1224 | gcy-36p::tagRfp | This work (5 ng/µl) |
| PSAB1028 | nphp-4p::nphp-4::gfp | Maureen Barr (10 ng/µl) |
| PSAB1225 | gcy-32p::osm-3b::gfp | This work (10 ng/µl) |
| PSAB1226 | gcy-32p::kap-1::gfp | This work (10 ng/µl) |
| PSAB1215 | gcy-32p::arl-13::tagRfp | Inna Nechipurenko (10 ng/µl) |
| PSAB1227 | gcy-32p::osm-6::gfp | This work (10 ng/µl) |
| PSAB1228 | gcy-36p::xbx-1::gfp | This work (10 ng/µl) |
| PSAB1229 | gcy-36p::nphp-4::gfp | This work (10 ng/µl or 1ng/µl) |
| PSAB1216 | gcy-36p::dyf-19::tagRfp | Inna Nechipurenko (1 ng/µl or 5 ng/µl) |
| PSAB1230 | gcy-32p::arl-13::gfp | This work (10 ng/µl) |
Acknowledgments
Acknowledgments
We thank Maureen Barr, Martin Harterink, Max Heiman and Inna Nechipurenko for reagents, the Caenorhabditis Genetics Center for strains, and the Sengupta lab for comments and advice.
Funding
This work was funded in part by the NIH (R35 GM122463 – P.S., and F32 DC018453 – A.P.), and the EMBO (ALTF 302-2019 – N.A-W.).
References
- Albert, P. S., and D. L. Riddle, 1983 Developmental alterations in sensory neuroanatomy of the <i>Caenorhabditis elegans </i>dauer larva. J Comp Neurol. 219: 461-481. PMID: 6643716 [DOI] [PubMed]
- Busch, K. E., P. Laurent, Z. Soltesz, R. J. Murphy, O. Faivre<i> et al.</i>, 2012 Tonic signaling from O(2) sensors sets neural circuit activity and behavioral state. Nat Neurosci 15: 581-591. PMID: 22388961 [DOI] [PMC free article] [PubMed]
- Cebul, E. R., I. G. Mclachlan and M. G. Heiman, 2020 Dendrites with specialized glial attachments develop by retrograde extension using SAX-7 and GRDN-1. Development 147: dev180448. PMID: 31988188 [DOI] [PMC free article] [PubMed]
- Cevik, S., Y. Hori, O. I. Kaplan, K. Kida, T. Toivenon<i> et al.</i>, 2010 Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in <i>Caenorhabditis elegans</i>. J Cell Biol 188: 953-969. PMID: 20231383 [DOI] [PMC free article] [PubMed]
- Chen, C., E. Itakura, G. M. Nelson, M. Sheng, P. Laurent et al., 2017 IL-17 is a neuromodulator of <i>Caenorhabditis elegans</i> sensory responses. Nature 542: 43-38. PMID: 28099418 [DOI] [PMC free article] [PubMed]
- Cohn, J. A., E. R. Cebul, G. Valperga, M. De Bono, M. G. Heiman<i> et al.</i>, 2019 Long-term activity drives dendritic branch elaboration of a <i>C. elegans</i> sensory neuron. Dev Biol 461: 66-74. PMID: 31945343 [DOI] [PMC free article] [PubMed]
- Doroquez, D. B., C. Berciu, J. R. Anderson, P. Sengupta and D. Nicastro, 2014 A high-resolution morphological and ultrastructural map of anterior sensory cilia and glia in <i>C. elegans</i>. eLife 3: e01948. PMID: 24668170 [DOI] [PMC free article] [PubMed]
- Evans, J. E., J. J. Snow, A. L. Gunnarson, G. Ou, H. Stahlberg<i> et al.</i>, 2006 Functional modulation of IFT kinesins extends the sensory repertoire of ciliated neurons in <i>Caenorhabditis elegans</i>. J Cell Biol 172: 663-669. PMID: 16492809 [DOI] [PMC free article] [PubMed]
- Fujiwara, M., T. Ishihara and I. Katsura, 1999 A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of <i>C. elegans </i>sensory cilia. Development 126: 4839-4848. PMID: 10518500 [DOI] [PubMed]
- Gross, E., Z. Soltesz, S. Oda, V. Zelmanovich, Z. Abergel<i> et al.</i>, 2014 GLOBIN-5-dependent O2 responses are regulated by PDL-1/PrBP that targets prenylated soluble guanylate cyclases to dendritic endings. J Neurosci 34: 16726-16738.PMID: 25505325 [DOI] [PMC free article] [PubMed]
- Harterink, M., S. L. Edwards, B. De Haan, K. W. Yau, S. Van Den Heuvel<i> et al.</i>, 2018 Local microtubule organization promotes cargo transport in <i>C. elegans</i> dendrites. J Cell Sci 131: jcs223107. PMID: 30254025 [DOI] [PMC free article] [PubMed]
- Hori, Y., T. Kobayashi, Y. Kikko, K. Kontani and T. Katada, 2008 Domain architecture of the atypical Arf-family GTPase Arl13b involved in cilia formation. Biochem Biophys Res Commun 373: 119-124. PMID: 18554500 [DOI] [PubMed]
- Jauregui, A. R., and M. M. Barr, 2005 Functional characterization of the <i>C. elegans</i> nephrocystins NPHP-1 and NPHP-4 and their role in cilia and male sensory behaviors. Exp Cell Res 305: 333-342. PMID: 15817158 [DOI] [PubMed]
- Kunitomo, H., and Y. Iino, 2008 <i>Caenorhabditis elegans</i> DYF-11, an orthologue of mammalian Traf3ip1/MIP-T3, is required for sensory cilia formation. Genes Cells 13: 13-25. PMID: 18173744 [DOI] [PubMed]
- Kunitomo, H., H. Uesugi, Y. Kohara and Y. Iino, 2005 Identification of ciliated sensory neuron-expressed genes in <i>Caenorhabditis elegans</i> using targeted pull-down of poly(A) tails. Genome Biol 6: R17. PMID: 15693946 [DOI] [PMC free article] [PubMed]
- Larkins, C. E., G. D. Aviles, M. P. East, R. A. Kahn and T. Caspary, 2011 Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Mol Biol Cell 22: 4694-4703. PMID: 21976698 [DOI] [PMC free article] [PubMed]
- Lewis, J. A., and J. A. Hodgkin, 1977 Specific neuroanatomical changes in chemosensory mutants of the nematode <i>Caenorhabditis elegans</i>. J Comp Neurol 172: 489-510. PMID: 838889 [DOI] [PubMed]
- Li, Y., Q. Wei, Y. Zhang, K. Ling and J. Hu, 2010 The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. J Cell Biol 189: 1039-1051. PMID: 20530210 [DOI] [PMC free article] [PubMed]
- Maurya, A. K., T. Rogers and P. Sengupta, 2019 A CCRK and a MAK kinase modulate cilia branching and length via regulation of axonemal microtubule dynamics in <i>Caenorhabditis elegans</i>. Curr Biol 22: 1286-1300. PMID: 30955935 [DOI] [PMC free article] [PubMed]
- Mclachlan, I. G., I. Beets, M. De Bono and M. G. Heiman, 2018 A neuronal MAP kinase constrains growth of a <i>Caenorhabditis elegans</i> sensory dendrite throughout the life of the organism. PLoS Genet 14: e1007435. PMID: 29879119 [DOI] [PMC free article] [PubMed]
- Morsci, N. S., and M. M. Barr, 2011 Kinesin-3 KLP-6 regulates intraflagellar transport in male-specific cilia of <i>Caenorhabditis elegans</i>. Curr Biol 21: 1239-1244. PMID: 21757353 [DOI] [PMC free article] [PubMed]
- Mukhopadhyay, S., Y. Lu, H. Qin, A. Lanjuin, S. Shaham<i> et al.</i>, 2007 Distinct IFT mechanisms contribute to the generation of ciliary structural diversity in <i>C. elegans</i>. EMBO J 26: 2966-2980. PMID: 17510633 [DOI] [PMC free article] [PubMed]
- Mukhopadhyay, S., Y. Lu, S. Shaham and P. Sengupta, 2008 Sensory signaling-dependent remodeling of olfactory cilia architecture in <i>C. elegans</i>. Dev Cell 14: 762-774. PMID: 18477458 [DOI] [PMC free article] [PubMed]
- Murayama, T., Y. Toh, Y. Ohshima and M. Koga, 2005 The <i>dyf-3</i> gene encodes a novel protein required for sensory cilium formation in <i>Caenorhabditis elegans</i>. J Mol Biol 346: 677-687. PMID: 15713455 [DOI] [PubMed]
- Perkins, L. A., E. M. Hedgecock, J. N. Thomson and J. G. Culotti, 1986 Mutant sensory cilia in the nematode <i>Caenorhabditis elegans</i>. Dev Biol 117: 456-487. PMID: 2428682 [DOI] [PubMed]
- Procko, C., Y. Lu and S. Shaham, 2011 Glia delimit shape changes of sensory neuron receptive endings in <i>C. elegans</i>. Development 138: 1371-1381. PMID: 21350017 [DOI] [PMC free article] [PubMed]
- Rosenbaum, J.L., and G. B. Witman, 2002 Intraflagellar transport. Nat Rev Mol Cell Biol 3: 813-825. PMID: 12415299 [DOI] [PubMed]
- Schroeder, N. E., R. J. Androwski, A. Rashid, H. Lee, J. Lee<i> et al.</i>, 2013 Dauer-specific dendrite arborization in <i>C. elegans</i> is regulated by KPC-1/Furin. Curr Biol 23: 1527-1535. PMID: 23932402 [DOI] [PMC free article] [PubMed]
- Silva, M., N. Morsci, K. C. Nguyen, A. Rizvi, C. Rongo<i> et al.</i>, 2017 Cell-specific alpha-tubulin isotype regulates ciliary microtubule ultrastructure, intraflagellar transport, and extracellular vesicle biology. Curr Biol 27: 968-980. PMID: 28318980 [DOI] [PMC free article] [PubMed]
- Wang, S., D. Wu, S. Quintin, R. A. Green, D. K. Cheerambathur<i> et al.</i>, 2015 NOCA-1 functions with gamma-tubulin and in parallel to Patronin to assemble non-centrosomal microtubule arrays in <i>C. elegans</i>. Elife 4: e08649. PMID: 26371552 [DOI] [PMC free article] [PubMed]
- Ward, S., N. Thomson, J. G. White and S. Brenner, 1975 Electron microscopical reconstruction of the anterior sensory anatomy of the nematode <i>Caenorhabditis elegans</i>. J Comp Neurol 160: 313-337. PMID: 1112927 [DOI] [PubMed]
- Weber, K. P., S. De, I. Kozarewa, D. J. Turner, M. M. Babu<i> et al.</i>, 2010 Whole genome sequencing highlights genetic changes associated with laboratory domestication of <i>C. elegans</i>. PLoS One 5: e13922. PMID: 21085631 [DOI] [PMC free article] [PubMed]
- Wei, Q., Q. Xu, Y. Zhang, Y. Li, Q. Zhang<i> et al.</i>, 2013 Transition fibre protein FBF1 is required for the ciliary entry of assembled intraflagellar transport complexes. Nat Commun 4: 2750. PMID: 24231678 [DOI] [PMC free article] [PubMed]
- Williams, C. L., C. Li, K. Kida, P. N. Inglis, S. Mohan<i> et al.</i>, 2011 MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol 192: 1023-1041. PMID: 21422230 [DOI] [PMC free article] [PubMed]
- Zimmer, M., J. M. Gray, N. Pokala, A. J. Chang, D. S. Karow<i> et al.</i>, 2009 Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron 61: 865-879. PMID: 19323996 [DOI] [PMC free article] [PubMed]