Abstract
Background
Inappropriate use of antimicrobials (AM) is a major concern worldwide that leads to the propagation of antimicrobial resistance (AMR). In addition to its clinical implications, AMR imposes an economic burden on communities, especially developing countries with more infectious diseases and less available resources. Antimicrobial stewardship programs (ASPs) have been found to be effective in reducing AMR. This study was designed to evaluate the effect of implementing an ASP in reducing AM consumption, its economic burden, and AMR as a consecutive result.
Materials and Methods
Consumption of caspofungin, amphotericin B, voriconazole, colistin, linezolid, vancomycin, and carbapenems was compared in a prospective cross-sectional study between two time periods introduced as pre- and post-ASP. Drug use density presented as anatomical therapeutic chemical (ATC)/defined daily doses (DDD) and normalized per 1000 bed days, cost savings, and AMR patterns were evaluated.
Results
A total of 9400 AM prescriptions were analyzed during a 2-year period. Consumption measured in DDD/1000 bed days dropped by 24.8, 25.0, 35.3, 47.0, 39.2, 10.5, and 23.2 percent for amphotericin B, caspofungin, colistin, voriconazole, meropenem, imipenem, and vancomycin, respectively. Linezolid consumption increased by 26.8% after implementing ASP. The expenditure of target AMs in the average value of USD decreased by 41.3% after the intervention compared to the time before using ASP (P-value=0.001). Implementing ASP also increased AM susceptibility of Pseudomonas aeruginosa, while the susceptibility of methicillin-resistant Staphylococcus aureus did not change significantly.
Conclusion
The results of this study suggest that establishment of ASP can lead to a reduction in improper administration of AMs and their expenditure resulting in economic benefit and lowering AMR at hospitals with minimum resources. Clinical pharmacists’ role was critical to the success of this ASP and was uniquely empowered at our center.
Keywords: antimicrobial stewardship programs, appropriate prescribing, antimicrobial use, DDD, defined daily dose
Introduction
The introduction of antimicrobials (AM) as promising agents against infections was a major breakthrough in man’s history. Much attention was drawn to these agents that their vast, uncontrolled and inappropriate consumption soon became a major threat to all mankind by developing resistance patterns.1 Drug resistance is a strategy that infective species acquire in time to escape destruction. This process leads to development of antimicrobial resistance (AMR) and lowers cure chances of previously treatable infections resulting in high mortality and morbidity rates.2–4 Limited introduction of new and effective antimicrobials to the healthcare market has magnified the burden of AMR worldwide.5 Unfortunately in most societies the destructive impact of AMR as a result of AM misuse is underestimated showing favorable short-term outcome, neglecting its vicious long-term influence.6 This issue can be even more evident in low income countries due to their inefficient vaccination policies, higher rates of infectious diseases, the unavailability of a variety of effective antibiotics, and even laboratory techniques, instruments, and media required for the accurate identification of a pathogen.7,8 Considering that antimicrobial consumption has almost doubled in Iran during the past decade,9 and inappropriate empiric antibiotic therapy has been reported to be high,10 without doubt AMR is an issue in the country that must be looked into. In addition to significant mortality, AMR has been reported to add as high as trillions of dollars of cost burden to nations.11 The economic impact of AMR in the hospital setting can be attributed to longer hospital stays, the need for alternative AMs that are often more expensive, and even higher doses of anti-infective medication.1,12 Special policies have been thought upon regarding AMR worldwide. Antibiotic stewardship programs (ASPs) were introduced as a global action plan (GAP) by the WHO in 2015, in order to help save the current status of the antibiotics and raise a chance for their future effectiveness.13 Authorizing use of AMs can occur by developing guidelines and restricting their use with the help of the core members of the ASP team.14 According to the latest essential medicines list (EML) presented by WHO, antibiotics have been categorized according to their priority in use; Antibiotics such as linezolid and colistin have been reserved as “last-resort” options and vancomycin, meropenem, and imipenem have high potential for resistance.15 This puts forth the necessity of controlling use of high-cost broad spectrum AMs. While the impact of ASPs has been repeatedly demonstrated in developed and some developing countries, a paucity of literature examining ASP interventions in our country is evident and this conveys the lack of such programs being implemented or are possibly performed in an unstructured manner.16 In this study we decided to evaluate the impact of performing ASP in a large, teaching tertiary care center in southern Iran on AM consumption and its related cost.
Materials and Methods
Study Site
This cross-sectional single-center prospective study was performed at a large referral university-affiliated, 497-bed hospital with emergency, internal medicine, surgery, cardiology, obstetrics/gynecology, and dermatology wards.
Ethical Approval
This study was approved by the institutional review board and ethics committee of Shiraz University of Medical Sciences (approval code: IR.SUMS.REC.1398.749) and was conducted according to the Declaration of Helsinki regarding ethical principles for medical research.
Study Time Periods
Two time periods were designated for comparison, the pre-ASP phase (April 2016–April 2017) and the post-ASP phase (May 2017–May 2018).
Intervention
In the pre-ASP phase, data regarding consumption of the AMs selected based on their wide spectrum of activity, high cost, and relevant high risk of resistance (caspofungin, amphotericin B, voriconazole, colistin, linezolid, vancomycin, meropenem, and imipenem) were obtained from the Hospital Information System (HIS) and medical records of all patients that had administered one of the listed antimicrobials were reviewed. In May 2017 (post-ASP phase) a formal ASP consisting of two infectious disease (ID) specialists, two clinical pharmacists, a hospital administrator, a microbiologist, and an IT specialist was started which included four components: guideline revision and “AM order forms” development, respectively, performed and prepared by the ID specialists and clinical pharmacists’ collaboration, information and education provided by the clinical pharmacists, regular ward rounds by the clinical pharmacists, and intensified infectious disease consultations and feedback provided by the ID specialists. Microorganism susceptibilities and their resistance patterns were evaluated by the microbiologist before and after implementing ASP, as this information primarily helped towards selection of AMs for which restriction was planned to be performed. The IT specialist helped in organizing an electronic program towards informing the clinical pharmacists of an AM prescription and restricting the AM order by requiring the ID specialist consultation and confirmation. At last the hospital administrator supported the ASP by establishing an institutional policy towards making this program happen.
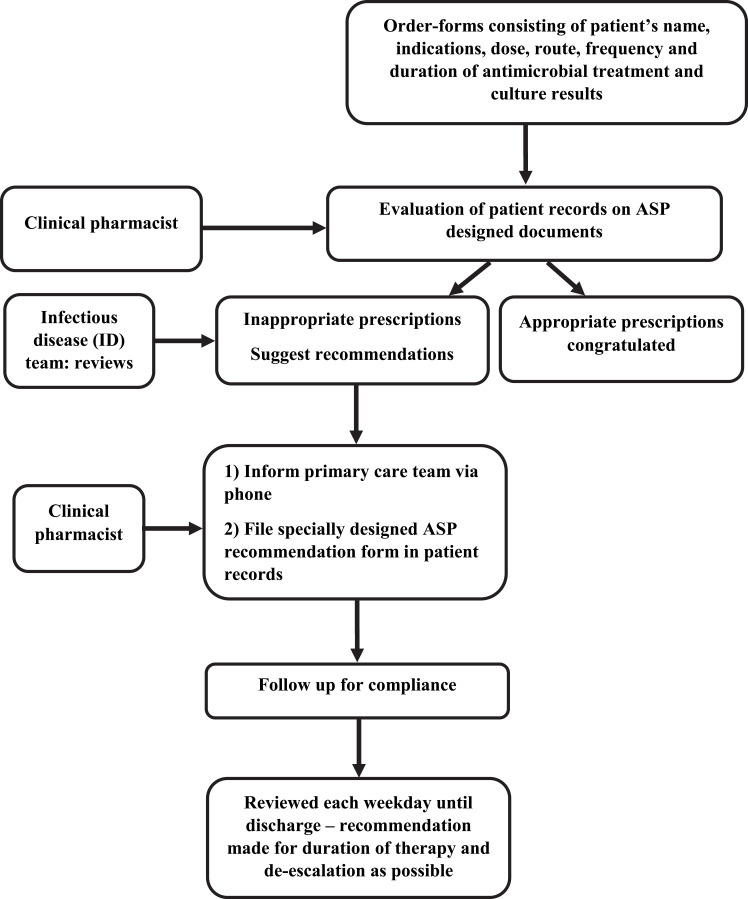
Briefly, an initial focus of the ASP was to develop treatment protocols that follow local susceptibility patterns along with national guidelines focusing on post-prescriptive audit with feedback and intervention. ASP education included initial short division-specific team briefings, summarizing the revised guidelines, and explaining the overall strategy. Workflow demonstrating the process of the ASP audit is shown in Figure 1.
Figure 1.
Antimicrobial stewardship program (ASP) workflow for audit and review.
For caspofungin, amphotericin B, voriconazole, colistin, and linezolid, order-forms consisting of the patient’s name, indications according to guidelines, dose, route, frequency, and duration of antimicrobial treatment and culture results were prepared. This form was to be filled out by the treating physician and validated by a clinical pharmacist/ID specialist to confirm accuracy of prescription. The ASP team reviewed clinical charts every weekday. Appropriate use was encouraged with positive feedback to prescribers. Inappropriate use was discussed with prescribers and coupled with a stewardship recommendation, which was filed in the patient’s record and discussed with the care providers on the phone. The hospital pharmacy checked the availability and completeness of the filled-out forms before confirming the dispense of the mentioned AMs to the ward for each patient.
Considering that restriction on the use of selected broad spectrum AMs might shift physicians to ordering other AMs, another restraint was applied for vancomycin, meropenem, and imipenem as other broad spectrum AMs available at the hospital. This restraint was applied as a level of prescription audit, requiring a specialist physician’s order, knowing that the hospital that the study took place in is a teaching hospital and medical interns and residents are also able to order AMs.
Study Design and Data Collection
All patients’ medical records, including their demographic data, length of hospital stay, duration of AM use, lab data (chemical and microbial), mortality rates, and monthly direct drug expense for each AM charged for patients based on its value provided to the ASP team by the hospital pharmacy, were evaluated before and after implication of intervention.
Drug use density expressed according to the WHO definition presented as anatomical therapeutic chemical (ATC)/defined daily doses (DDD) and normalized per 1000 bed days, cost savings, and AMR patterns were also evaluated.
The primary objective of the study was to compare data of AM use which were expressed as DDD between the pre-ASP and the post-ASP period. Secondary objectives were to compare the total cost of AMs and also change in AM resistance patterns in the two study phases at our institution. The cost data evaluated were based on purchasing costs provided by the hospital pharmacy.
Bacterial Resistance and Antibiotic Use
To evaluate the impact of changes in antibiotic use on bacterial resistance, all isolates from patients’ samples received from different wards were collected including blood, pus/wound swabs, sputum, drain fluids, and urine. Criteria for antimicrobial susceptibility testing were carried out according to Clinical Laboratory Standard Institute (CLSI) guidelines.17 Antimicrobial sensitivity testing was done on Muller Hinton Agar (MHA) by Kirby-Bauer’s disc diffusion method. Changes in the resistance pattern were compared between the two study periods.
Statistical Analysis
After gathering all data, SPSSvs16 software was used to perform the statistical analysis. Smirnov-Kolmogorov test was used to evaluate the normal distribution of data, and according to their distribution, t-test, Mann–Whitney, or Wilcoxon tests were utilized for statistical analysis. P-values<0.05 were considered significant for all tests.
Results
A total of 14,820 patients who received at least one of the target antibiotics during their inpatient stay at the two time periods were included in the study, with 7320 and 7500 subjects representing the pre-ASP and post-ASP groups, respectively. No significant differences in age and sex distribution were found between the two groups (Table 1).
Table 1.
Baseline Data of the Study Population
| Variables | Study Phase | P-value | |
|---|---|---|---|
| Pre-ASP | Post-ASP | ||
| Sex n (%) | |||
| Male | 3677 (50.2) | 3638 (48.5) | 0.15 |
| Female | 3643 (49.8) | 3862 (51.5) | |
| Age (years; mean±SD) | 58.1±19.84 | 57.9±18.5 | 0.53 |
| Ward n (%) | |||
| Intensive Care Unit | 549 (7.5) | 548 (7.3) | 0.64 |
| Internal medicine | 1954 (26.7) | 2047 (27.3) | 0.41 |
| General Surgery | 2064 (28.2) | 2063 (27.5) | 0.34 |
| Cardiac Surgery | 608 (8.3) | 683 (9.1) | 0.08 |
| Gynecology | 696 (9.5) | 652 (8.7) | 0.09 |
| Dermatology | 351 (4.8) | 390 (5.2) | 0.26 |
| Neurology | 600 (8.2) | 585 (7.8) | 0.37 |
| Coronary Care Unit | 498 (6.8) | 532 (7.1) | 0.47 |
Abbreviation: ASP, antimicrobial stewardship program.
A total number of 9400 AM prescriptions were evaluated. An overall 26.3% decrease in consumption was observed between the pre-ASP and post-ASP phases measured in DDD/1000 bed days for target AMs. The most significant reduction was observed in the use of voriconazole (−47%) and colistin (−35.3%) amongst the restricted high cost audited AMs including amphotericin B, caspofungin, voriconazole, colistin, and linezolid; However, an increasing rate of consumption was observed for linezolid (+26.8%). Also reduction in carbapenem and vancomycin consumption was observed following audit performed at the level of prescription; the highest rate of decrease was observed for meropenem (−39.2%) (Table 2).
Table 2.
Defined Daily Dose (DDD) per 1000 Patient-Days for Selected Antimicrobials During the Two Phases of the Study (Pre- and Post-Intervention)
| Antimicrobial Agent | DDD/1000 Patient Days | Percent of Change | |
|---|---|---|---|
| Pre-ASP | Post-ASP | ||
| Antifungal | |||
| Amphotericin B | 16.5 | 12.4 | −24.8% |
| Caspofungin | 1.2 | 0.9 | −25.0% |
| Voriconazole | 19.8 | 10.5 | −47.0% |
| Antibacterial (spectrum of activity) | |||
| Colistin (G−) | 45.1 | 29.2 | − 35.3% |
| Linezolid (G+) | 16.9 | 23.1 | +26.8% |
| Meropenem (G+, G−) | 22.1 | 13.1 | −39.2% |
| Imipenem (G+, G−) | 1.9 | 1.7 | −10.5% |
| Vancomycin (G+) | 5.6 | 4.3 | −23.2% |
| Total | 129.1 | 95.2 | −26.3% |
Abbreviations: ASP, antimicrobial stewardship program; DDD, defined daily dose.
The mean monthly cost for restricted AMs significantly dropped by 41.3% in the post-implementation phase of ASP in comparison with the pre-implementation phase (P-value<0.001). The average monthly cost saving during the intervention phase was $183,052. As shown in Table 3, the highest cost saving belonged to voriconazole consumption, showing a 60.6% reduction in mean monthly hospital cost attributed to use of the mentioned AM. Linezolid consumption showed an increasing trend of 10.3% in terms of its mean monthly cost burden parallel to its increased consumption rate. Table 4 demonstrates the amount of AM prescribed in terms of average vial per patient, and as shown once again voriconazole has the highest rate of reduction during the two study phases. The least reduction is accredited to amphotericin B.
Table 3.
Comparison of Mean Monthly Costs of Antimicrobial Administration in the Average Value of USD During the Two Phases of the Study (Pre- and Post-Intervention)
| Antimicrobial Agent | Study Phase | Percent of Change | P-value | |
|---|---|---|---|---|
| Pre-ASP | Post-ASP | |||
| Antifungal | ||||
| Amphotericin | 123,300 | 66,940 | −45.7% | <0.001 |
| Caspofungin | 7000 | 3700 | −47.1% | <0.001 |
| Voriconazole | 11,160 | 4400 | −60.6% | <0.001 |
| Antibacterial (spectrum of activity) | ||||
| Colistin (G−) | 26,312 | 13,500 | −48.7% | <0.001 |
| Linezolid (G+) | 18,100 | 20,170 | +10.3% | 0.002 |
| Carbapenems (G+, G−) | 182,600 | 99,270 | −45.6% | <0.001 |
| Vancomycin (G+) | 69,100 | 46,540 | −32.6% | <0.001 |
| Total | 437,572 | 254,520 | −41.8% | <0.001 |
Abbreviations: ASP, antimicrobial stewardship program; USD, United States dollars.
Table 4.
Amount of Prescribed Antimicrobial in Average Value of Vial per Patient (Mean±SD) During the Two Phases of the Study (Pre- and Post-Intervention)
| Antimicrobial Agent | Study Phase | P-value | |
|---|---|---|---|
| Pre-ASP | Post-ASP | ||
| Antifungal | |||
| Amphotericin B | 28.68±13.32 | 25.35±11.6 | 0.013 |
| Caspofungin | 13.94±4.28 | 11.32±5.12 | <0.001 |
| Voriconazole | 18.25±5.38 | 11.50±2.60 | <0.001 |
| Antibacterial (spectrum of activity) | |||
| Colistin (G−) | 45.60±12.49 | 39.33±15.76 | <0.001 |
| Linezolid (G+) | 21.84±4.33 | 23.44±5.17 | 0.003 |
| Meropenem (G+, G−) | 33.27±14.09 | 29.79±11.23 | 0.009 |
| Imipenem (G+, G−) | 23.17±19.89 | 19.51±11.66 | 0.25 |
| Vancomycin (G+) | 20.74±16.69 | 15.93±11.5 | 0.001 |
Abbreviation: ASP, antimicrobial stewardship program.
During the pre- and post-ASP phases, a total of 280 and 295 samples were received from different wards of the hospital, respectively, and were further processed for susceptibility testing. Two hundred and nine (74.64%) and 215 (72.88%) organisms were isolated in each study phase, respectively. A total number of organisms isolated from various clinical samples and their resistance patterns are shown in Table 5.
Table 5.
Antibiotic Sensitivity Pattern for Different Microorganisms Before and After Implementing ASP
| Microorganism | Antimicrobial agent | Pre-ASP | Post-ASP | P-value | ||
|---|---|---|---|---|---|---|
| Sensitivity Rate (%) | n (%) | Sensitivity Rate (%) | n (%) | |||
| Pseudomonas aeruginosa | Colistin | 90.5 | 42 (20.10) | 100 | 44 (20.47) | <0.001 |
| Cefepime | 32 | 50 | <0.001 | |||
| Amikacin | 42 | 55 | <0.001 | |||
| Ciprofloxacin | 60 | 65 | 0.047 | |||
| Carbapenems | 82.4 | 86.2 | 0.044 | |||
| Piperacillin/Tazobactam | 90 | 92 | 0.178 | |||
| Klebsiella pneumoniae | Imipenem | 45 | 23 (11.0) | 58 | 22 (10.23) | <0.001 |
| Amikacin | 70 | 77.8 | <0.001 | |||
| Ciprofloxacin | 11.1 | 27.5 | <0.001 | |||
| Cefepime | 18.3 | 25 | 0.001 | |||
| Gentamicin | 35 | 45.6 | <0.001 | |||
| Colistin | 98 | 100 | <0.001 | |||
| Staphylococcus aureus | Oxacillin | 54.2 | 36 (17.23) | 57 | 40 (18.60) | 0.278 |
| Vancomycin | 68 | 72 | 0.093 | |||
| Linezolid | 91.3 | 90 | 0.685 | |||
| Clindamycin | 12.5 | 25 | 0.005 | |||
| Cefazolin | 75 | 56.9 | <0.001 | |||
| Escherichia coli | Gentamicin | 65 | 40 (19.14) | 82.3 | 41 (19.07) | <0.001 |
| Ciprofloxacin | 30 | 40.4 | 0.0483 | |||
| Amikacin | 89.5 | 94.1 | 0.108 | |||
| Cefepime | 40 | 43.8 | 0.481 | |||
| Ampicillin/Sulbactam | 33.3 | 38.9 | 0.288 | |||
| Enterococcus spp. | Ampicillin | 3.6 | 18 (8.61) | 6.1 | 22 (10.23) | 0.202 |
| Amikacin | 4.6 | 5.4 | 0.740 | |||
| Meropenem | 1.5 | 3.5 | 0.270 | |||
| Vancomycin | 50 | 48.6 | 0.797 | |||
| Linezolid | 92 | 96 | 0.103 | |||
| Enterobacteriaceae spp. | Cefepime | 6.3 | 13 (6.22) | 15 | 11 (5.12) | 0.0145 |
| Piperacillin/Tazobactam | 66.7 | 70 | 0.514 | |||
| Ceftriaxone | 33.3 | 42 | 0.102 | |||
| Imipenem | 75 | 76.2 | 0.797 | |||
| Amikacin | 75 | 81 | 0.177 | |||
| Ciprofloxacin | 45 | 59.2 | 0.009 | |||
| Acinetobacter spp. | Imipenem | 2.2 | 37 (17.70) | 8.5 | 35 (16.28) | 0.0148 |
| Cefepime | 1.5 | 6.6 | 0.0304 | |||
| Piperacillin/Tazobactam | 4.2 | 11.5 | 0.0166 | |||
| Amikacin | 13.6 | 20.6 | 0.0968 | |||
| Ciprofloxacin | 8.7 | 10.7 | 0.542 | |||
Abbreviation: ASP, antimicrobial stewardship program.
Susceptibility of Pseudomonas aeruginosa showed a significant difference between the two study phases (P-value<0.05), demonstrating increased sensitivity towards mentioned AMs in the post-ASP phase. Staphylococcus aureus sensitivity did not change significantly for most antibiotics (Table 5).
Mortality rates were evaluated in patients that had received the target antimicrobials in the pre- and post-ASP phase. Total mortality rate decreased from 16.8% to 15.2%; however, this decrease in rate was not statistically significant (P-value=0.725).
Length of hospital stay (mean±SD) showed a significant decrease (P-value=0.0201) in post-ASP phase (10.94±5.42) compared to the pre-ASP phase (12.24±6.75).
Discussion
AMR is known as a global threat to mankind causing mortality and morbidity. The goal of preserving AM effectiveness has reached its utmost importance during the recent decade.18,19 Following this major worldwide concern, in this study we decided to develop and carry out a program that restricts the use of high-cost, broad spectrum AMs at our institution. This plan was performed with the help of the core members of an ASP team, clinical pharmacists, and ID specialists. Necessary approval of five high-cost broad spectrum AMs by an ID specialist before their administration for admitted patients at all our hospital wards was implemented. Our results indicate great reduction in the consumption of four of the studied AMs; however, linezolid showed an increase in approval and administration after the ongoing program. As it has been previously described by researchers, different cultural, contextual, and behavioral attitude exists towards the management of an infection. AM prescription has been associated to higher levels of therapeutic power for the physician and the patient, overcoming the physician’s fear of uncertainty in managing a patient.1 Therefore it is obvious that lack of authority and knowledge in the management of infectious diseases can lead to higher and inappropriate AM consumption.20 In this study we decided to take the first step by preparing order-forms according to guidelines that can help the ID specialist make a better decision on approving or disapproving the administration of an AM medication. Previous reports also approve ID consultation as a way of increasing rational antibiotic use.21,22 Knowledge of pathogens’ susceptibility, resistance patterns at the institutions, and the spectrum of AM activity are all crucial knowledge required in the management of an infection that not every physician might be trained for.1 Inappropriate AM prescription and administration has been reported as high as 79% for severe infections.23 This fact highlights the role of an ID specialist, clinical pharmacist with ID training, and an overall AM prescription audit performed by preparing guidelines and order forms for AM prescription in a healthcare institution.24
Similar to other reports,21,25 the total consumption of high-cost AMs following prescription audit was reduced except for linezolid. This reduction presented as DDD/1000 patient days was considerable (26.3%) compared to recent ASP reports from US acute-care hospitals (15.8%).26 An increase in linezolid consumption could be due to expansion of vancomycin resistant Enterococcus spp (VRE) that are a main issue in the resistance patterns at our institution. Relevant reports have shown very high consumption of linezolid at their institutions as well due to its great activity against MRSA.27 However, the inadvertent excessive use of linezolid has been reported to cause resistant patterns in the gram positive microorganisms.28,29 It has also been concluded that vancomycin usage as an anti-MRSA agent has not fully been substituted by linezolid and the latter has been added on top of previous antibiotic managements, although MRSA burden was consistent.30 This fact can add to resistance patterns;31 therefore following this study use of linezolid at our institution should be carefully assessed to control the emergence of linezolid resistant microorganisms.
In our study population the most approved AM prescriptions were based on culture results (Figure 1), considering the fact that the applied ASP policy required justification for the approval of the relevant AM medication. We should take into account that disagreement with established guidelines is a common attitude of AM prescribers that can be controlled by such restriction policies.1 In our report the highest colistin consumption was for Acinetobacter spp, linezolid was mainly used in patients with vancomycin resistant Enterococci (VRE) positive cultures, and high rates of caspofungin had been used for resistant Candida non-albicans positive cultures that are all examples of appropriateness of AM prescription approval. Culture based therapy has been highly approved for severe infections rather than empiric therapy that has been reported to increase mortality rates and length of hospital stay.23,32
Our data indicate financial savings after implementing the restriction policy. Although carrying out these programs are costly themselves, many previous reports have confirmed an overall economic benefit in restricting unapproved use of high cost medication.33–35 The overall cut in the hospital’s cost regarding AMs to 41.3% in this report is higher than a similar study reporting a 32% decline in expenditures.25 The need for earlier intervention in AM therapy rather than just focusing on its length of administration has been evident in this study and also previous reports helping towards reducing imposed financial burden.36
The increase in antibiotic susceptibility of microorganisms is a result of implementing restriction on AM use and reduction in AMR. In our study, this benefit of ASP was evident in changing Pseudomonas aeruginosa’s sensitivity towards antibiotics that is comparable with previous ASP studies,37–38 although compared to available literature,37 susceptibility of other microorganisms did not change significantly. Limiting use of antibiotics that have anti-pseudomonal activity, may be the reason for change in susceptibility of the mentioned microorganism.
Duration of hospital stay and AM administration can be affected by correct AM therapy. In our study the overall length of stay at the hospital was decreased after implementing the restriction policy. Reduced hospital stay has been reported following application of antibiotic policies in healthcare institutions, most probably due to the correct AM selection and its prompt anti-infective action consequently.33,39 Some researchers have on the contrary confirmed the non-inferiority of implementing ASP policies by reporting that hospital stay is not prolonged although antibiotic use is restricted.25,36
According to this study’s results we must highlight the fact that mortality rates did not significantly change after restriction of AM use. Considering that a part of the audit was restriction on the use of carbapenems and vancomycin as other available broad spectrum AM options, we could at least be certain that restricting primary use of AMs did not impact patients’ outcome with respect to mortality rates. This fact has also been supported previously26 and can help reduce the bias on presuming that excessive AM use can improve safety and outcome.
Limitations of this study could be the probable bias towards positive results of implementing ASP as it has been also reported as a downside of such studies. On the other hand, focusing only on AM consumption of the study population and not considering their baseline clinical status that may have affected mortality and outcome, could be another limitation of this study. Considering that this was a single center study, future multi-center studies are mandated for auditing AM consumption. Also a future study is recommended to study the rise in consumption of linezolid at our institution and its probable causes.
Conclusion
In this study we could demonstrate the significant reduction in use of high-cost broad spectrum AMs and their cost burden by performing restriction policies on their prescription at our institution. It was shown that the obligation for an ID specialist’s approval before administration of such AMs did not affect mortality rates and patient outcome. According to this single center study, performing ASPs may help towards correct clinical practice, reduction of AMR, and cost savings that may be beneficial for low income countries with a budget deficit.
Acknowledgments
This study was financially supported by Shiraz University of Medical Sciences (99-01-05-23280) and as a part of the Pharm.D thesis of Sepasian A.40
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Considering that in this study only cumulative data were evaluated and data specific to each patient was not reported, therefore confidentiality of data was respected and patient consent was not required for performing the study. This study was approved by the institutional review board and ethics committee of Shiraz University of Medical Sciences (approval code: IR.SUMS.REC.1398.749;).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hulscher ME, Grol RP, van der Meer JW. Antibiotic prescribing in hospitals: a social and behavioural scientific approach. Lancet Infect Dis. 2010;10(3):167–175. doi: 10.1016/S1473-3099(10)70027-X [DOI] [PubMed] [Google Scholar]
- 2.Gong J, Wang C, Shi S, et al. Highly drug-resistant Salmonella enterica serovar Indiana clinical isolates recovered from broilers and poultry workers with diarrhea in China. Antimicrob Agents Chemother. 2016;60(3):1943–1947. doi: 10.1128/AAC.03009-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byarugaba D. Antimicrobial resistance in developing countries and responsible risk factors. Int J Antimicrob Agents. 2004;24(2):105–110. doi: 10.1016/j.ijantimicag.2004.02.015 [DOI] [PubMed] [Google Scholar]
- 4.Bonnet V, Dupont H, Glorion S, et al. Influence of bacterial resistance on mortality in intensive care units: a registry study from 2000 to 2013 (IICU Study). J Hosp Infect. 2019;102(3):317–324. doi: 10.1016/j.jhin.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 5.Smith RA, M’ikanatha NM, Read AF. Antibiotic resistance: a primer and call to action. Health Commun. 2015;30(3):309–314. doi: 10.1080/10410236.2014.943634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiazGranados CA, Cardo DM, McGowan JE Jr. Antimicrobial resistance: international control strategies, with a focus on limited-resource settings. Int J Antimicrob Agents. 2008;32(1):1–9. doi: 10.1016/j.ijantimicag.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 7.Laxminarayan R, Heymann DL. Challenges of drug resistance in the developing world. BMJ. 2012;344:e1567. doi: 10.1136/bmj.e1567 [DOI] [PubMed] [Google Scholar]
- 8.Cox J, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low-and middle-income countries: the same but different? Clin Microbiol Infect. 2017;23(11):812–818. doi: 10.1016/j.cmi.2017.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Abbasian H, Hajimolaali M, Yektadoost A, Zartab S. Antibiotic utilization in Iran 2000–2016: pattern analysis and benchmarking with organization for economic co-operation and development countries. J Res Pharm Pract. 2019;8(3):162. doi: 10.4103/jrpp.JRPP_19_42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadatsharifi A, Davarpanah M-A, Namazi S, Mottaghi S, Mahmoudi L. Economic burden of inappropriate empiric antibiotic therapy: a report from Southern Iran. Risk Manag Healthc Policy. 2019;12:339. doi: 10.2147/RMHP.S222200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’neill J. Review on Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. London: HM Government; 2016. [Google Scholar]
- 12.Founou RC, Founou LL, Essack SY, Butaye P. Clinical and economic impact of antibiotic resistance in developing countries: a systematic review and meta-analysis. PLoS One. 2017;12(12):e0189621. doi: 10.1371/journal.pone.0189621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Organization WH. Global Action Plan on Antimicrobial Resistance. 2015:2017 [Google Scholar]
- 14.Mahmoudi L, Ghouchani M, Mahi-Birjand M, Bananzadeh A, Akbari A. Optimizing compliance with surgical antimicrobial prophylaxis guidelines in patients undergoing gastrointestinal surgery at a referral teaching hospital in southern Iran: clinical and economic impact. Infect Drug Resist. 2019;12:2437. doi: 10.2147/IDR.S212728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharland M, Pulcini C, Harbarth S, et al. Classifying antibiotics in the WHO essential medicines list for optimal use—be AWaRe. Lancet Infect Dis. 2018;18(1):18–20. doi: 10.1016/S1473-3099(17)30724-7 [DOI] [PubMed] [Google Scholar]
- 16.Firouzabadi D, Mahmoudi L. Knowledge, attitude, and practice of health care workers towards antibiotic resistance and antimicrobial stewardship programmes: a cross‐sectional study. J Eval Clin Pract. 2020;26(1):190–196. doi: 10.1111/jep.13177 [DOI] [PubMed] [Google Scholar]
- 17.CLSI. CLSI supplement M100. Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 18.Wernli D, Jørgensen PS, Harbarth S, et al. Antimicrobial resistance: the complex challenge of measurement to inform policy and the public. PLoS Med. 2017;14(8):e1002378. doi: 10.1371/journal.pmed.1002378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13(11):e1002184. doi: 10.1371/journal.pmed.1002184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson SA, Wood F, Butler CC. General practitioners’ perceptions of antimicrobial resistance: a qualitative study. J Antimicrob Chemother. 2006;59(2):292–296. doi: 10.1093/jac/dkl467 [DOI] [PubMed] [Google Scholar]
- 21.Ozkurt Z, Erol S, Kadanali A, Ertek M, Ozden K, Tasyaran MA. Changes in antibiotic use, cost and consumption after an antibiotic restriction policy applied by infectious disease specialists. Jpn J Infect Dis. 2005;58(6):338. [PubMed] [Google Scholar]
- 22.Lemmen SW, Becker G, Frank U, Daschner FD. Influence of an infectious disease consulting service on quality and costs of antibiotic prescriptions in a university hospital. Scand J Infect Dis. 2001;33(3):219–221. doi: 10.1080/00365540151060923 [DOI] [PubMed] [Google Scholar]
- 23.Marquet K, Liesenborgs A, Bergs J, Vleugels A, Claes N. Incidence and outcome of inappropriate in-hospital empiric antibiotics for severe infection: a systematic review and meta-analysis. Crit Care. 2015;19(1):63. doi: 10.1186/s13054-015-0795-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis. 2016;62(10):e51–e77. doi: 10.1093/cid/ciw118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White, Jr. AC, Atmar RL, Wilson J, Cate TR, Stager CE, Greenberg SB. Effects of requiring prior authorization for selected antimicrobials: expenditures, susceptibilities, and clinical outcomes. Clin Infect Dis. 1997;25(2):230–239. doi: 10.1086/514545 [DOI] [PubMed] [Google Scholar]
- 26.Kabbani S, Baggs J, Hicks LA, Srinivasan A. Potential impact of antibiotic stewardship programs on overall antibiotic use in adult acute-care hospitals in the United States. Infect Control Hosp Epidemiol. 2018;39(3):373–376. doi: 10.1017/ice.2017.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saxena S, Priyadarshi M, Saxena A, Singh R. Antimicrobial consumption and bacterial resistance pattern in patients admitted in ICU at a tertiary care center. J Infect Public Health. 2019;12(5):695–699. doi: 10.1016/j.jiph.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 28.Scheetz MH, Knechtel SA, Malczynski M, Postelnick MJ, Qi C. Increasing incidence of linezolid-intermediate or-resistant, vancomycin-resistant Enterococcus faecium strains parallels increasing linezolid consumption. Antimicrob Agents Chemother. 2008;52(6):2256–2259. doi: 10.1128/AAC.00070-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramírez E, Gómez-Gil R, Borobia AM, et al. Improving linezolid use decreases the incidence of resistance among Gram-positive microorganisms. Int J Antimicrob Agents. 2013;41(2):174–178. doi: 10.1016/j.ijantimicag.2012.10.017 [DOI] [PubMed] [Google Scholar]
- 30.Meyer E, Schwab F, Schroeren-Boersch B, Gastmeier P. Increasing consumption of MRSA-active drugs without increasing MRSA in German ICUs. Intensive Care Med. 2011;37(10):1628. doi: 10.1007/s00134-011-2335-9 [DOI] [PubMed] [Google Scholar]
- 31.Bai B, Hu K, Zeng J, et al. Linezolid consumption facilitates the development of linezolid resistance in Enterococcus faecalis in a tertiary-care hospital: a 5-year surveillance study. Microb Drug Resist. 2019;25(6):791–798. doi: 10.1089/mdr.2018.0005 [DOI] [PubMed] [Google Scholar]
- 32.Bizo PT, Dumitras D, Popa A. Evaluation of restricted antibiotic use in a hospital in Romania. Int J Clin Pharm. 2015;37(3):452–456. doi: 10.1007/s11096-015-0096-1 [DOI] [PubMed] [Google Scholar]
- 33.Dik J-WH, Hendrix R, Friedrich AW, et al. Cost-minimization model of a multidisciplinary antibiotic stewardship team based on a successful implementation on a urology ward of an academic hospital. PLoS One. 2015;10(5):e0126106. doi: 10.1371/journal.pone.0126106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durvasula R, Kelly J, Schleyer A, Anawalt BD, Somani S, Dellit TH. Standardized review and approval process for high-cost medication use promotes value-based care in a large academic medical system. Am Health Drug Benefits. 2018;11(2):65. [PMC free article] [PubMed] [Google Scholar]
- 35.Lanbeck P, Tennvall GR, Resman F. A cost analysis of introducing an infectious disease specialist-guided antimicrobial stewardship in an area with relatively low prevalence of antimicrobial resistance. BMC Health Serv Res. 2016;16(1):311. doi: 10.1186/s12913-016-1565-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagiwara D, Sato K, Miyazaki M, et al. The impact of earlier intervention by an antimicrobial stewardship team for specific antimicrobials in a single weekly intervention. Int J Infect Dis. 2018;77:34–39. doi: 10.1016/j.ijid.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 37.Kimura T, Uda A, Sakaue T, et al. Long-term efficacy of comprehensive multidisciplinary antibiotic stewardship programs centered on weekly prospective audit and feedback. Infection. 2018;46(2):215–224. doi: 10.1007/s15010-017-1099-8 [DOI] [PubMed] [Google Scholar]
- 38.Stultz JS, Arnold SR, Shelton CM, Bagga B, Lee KR. Antimicrobial stewardship impact on Pseudomonas aeruginosa susceptibility to meropenem at a tertiary pediatric institution. Am J Infect Control. 2019;47(12):1513–1515. doi: 10.1016/j.ajic.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 39.Fernández-Morato J, Moro L, Sancho J, et al. An antimicrobial stewardship program reduces antimicrobial therapy duration and hospital stay in surgical wards. Rev Esp Quimioter. 2016;29(3):119–122. [PubMed] [Google Scholar]
- 40.Sepasian A The Impact of Infectious Disease Specialist’s Consultation on Antibiotic Prescribing in Shahid Faghihi Hospital [PharmD Thesis]. Shiraz: Shiraz University of Medical Sciences, Faculty of Pharmacy; 2019. [Google Scholar]