Abstract
Background
Obesity is a risk factor for many adulthood cancers, but its role in childhood, adolescent, and young adult (AYA) cancer is unknown. Childhood and AYA acute lymphoblastic leukemia (ALL) incidence and obesity prevalence have shown concurrent increases. We sought to identify whether obesity may be a risk factor for childhood and AYA ALL.
Methods
Characteristics from individuals with ALL, aged 2‐30 years, diagnosed 2004‐2017 and treated on Children's Oncology Group (COG) protocols with available pre‐treatment anthropometric data (N = 4726) were compared to National Health and Nutrition Examination Survey controls (COG AALL17D2). Body mass index (BMI) was defined using standard CDC definitions. Multivariate conditional logistic regression assessed associations between BMI and ALL with additional analyses stratified by sex and race/ethnicity.
Results
Among cases (72% high‐risk (HR) B‐ALL, 28% T‐ALL), 5% had underweight, 58% normal weight, 17% overweight, and 20% obesity. Underweight (OR 2.11, 95% CI 1.56‐2.85) and obesity (OR 1.32, 95% CI 1.15‐1.53) were associated with B‐ALL diagnosis. Specifically, obesity was associated with B‐ALL among males (OR 1.57, 95% CI 1.30‐1.91) and Hispanic children (OR 1.78, 95% CI 1.39‐2.29). Obesity was also associated with central nervous system (CNS) involvement.
Conclusion
Pre‐treatment obesity is associated with HR B‐ALL among males and Hispanics, as well as with CNS involvement, suggesting common physiology between obesity and leukemogenesis. An association between underweight and ALL was confirmed, likely due to cancer‐associated wasting. These results have important public health implications for obesity prevention and treatment in children and adolescents to reduce cancer risk.
Keywords: childhood ALL, obesity, risk factors
Deviations in body mass index (underweight and obesity) at the time of diagnosis were significantly associated with a diagnosis of pediatric high‐risk (HR) B‐cell acute lymphoblastic leukemia (B‐ALL) compared to normal weight. Specifically, obesity was associated with HR B‐ALL in males and Hispanics. Obesity was also significantly associated with ALL central nervous system (CNS) involvement.

1. INTRODUCTION
Acute lymphoblastic leukemia (ALL) is the most common form of malignancy among children, adolescents, and young adults (AYA). 1 Since the 1970s, the incidence of ALL has steadily increased without a clear etiology. 1 Aside from inherited syndromes and certain perinatal factors including cesarean delivery, advanced maternal age, and higher birthweight, 2 , 3 , 4 few endogenous or environmental risk factors have been identified that explain the temporal changes in the incidence of ALL among this age group. 5
Over the same time frame that childhood and AYA ALL has increased, a concurrent rise in the prevalence of obesity has been observed. 6 There is growing evidence in adulthood cancers associating multiple host characteristics and lifestyle exposures with cancer risk. 7 Obesity has been strongly linked to 13 adulthood cancers 8 and is potentially associated with others, including hematologic malignancies. 9 , 10 , 11 Obesity now accounts for up to 20% of all adulthood cancers 12 and obesity‐associated cancers are increasingly being diagnosed in younger adult populations. 13 , 14 Despite this, associations between obesity and childhood cancer risk have not been well studied. Recent studies of children and AYA with obesity and ALL have focused on disease outcomes rather than etiology, and have shown increased risk for end of induction residual disease and relapse. 15 Given the associations between adult‐onset cancers and obesity, the inferior ALL outcomes in children and AYA with obesity, and the parallel increases in ALL incidence and obesity prevalence in children and AYA, we hypothesized that pre‐treatment obesity in children and AYA is associated with the development of high‐risk (HR) ALL and that it is associated with HR ALL features, such as higher initial white blood cell count, CNS disease, and prognostic cytogenetic characteristics.
2. MATERIALS AND METHODS
2.1. Study population
Children and AYA aged 2‐30 years with newly diagnosed B‐cell precursor or T‐cell ALL (B‐ALL or T‐ALL) enrolled on the five Children's Oncology Group (COG) frontline treatment studies that collected pre‐treatment height and weight were included in the analysis (COG ALL17D2, Figure 1, Table S1). Height and weight at diagnosis were not available for AALL0932, the recently completed large standard‐risk (SR) B‐ALL protocol.
FIGURE 1.
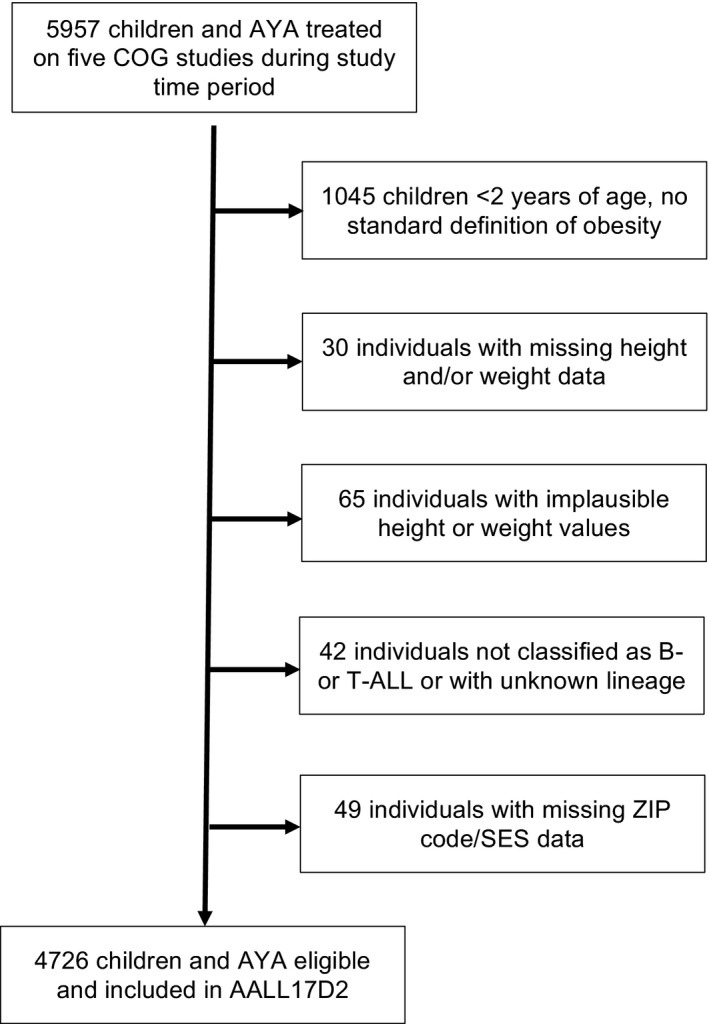
CONSORT diagram depicting exclusion of cases due to i) specific criteria of age < 2 y due to no standard definition of obesity, ii) missing anthropometric data, iii) implausible anthropometric data, iv) lack of B‐ALL or T‐ALL classification, or unknown lineage, v) and missing ZIP code/SES data. ALL, acute lymphoblastic leukemia; AYA, adolescent and young adult; COG, Children's Oncology Group; SES, socioeconomic status
The study population distribution by COG treatment protocol included: 2516 participants from AALL0232 (53.2%), 1322 participants from AALL0434 (28.0%), 29 participants from AALL0622 (0.6%), 153 participants from AALL07P4 (3.2%), and 706 participants from AALL1131 (14.9%). Controls were selected from the National Health and Nutrition Examination Survey (NHANES), 16 a nationally representative sample of US adults and children. Controls were matched 1:1 to ALL cases by age (within 1 year), sex, and race/ethnicity, given these biologic factors are associated with both obesity and leukemia risk. Year of sampling/diagnosis (within 10 years) was also matched to control for secular trends in obesity prevalence. All data were de‐identified.
2.2. Variables of interest
Anthropometric data for cases and controls were used to calculate body mass index (BMI; kg/m2). For cases, these measures were taken at the time of diagnosis prior to receiving treatment. For individuals < 20 years of age, BMI‐percentiles were determined based on sex and age using the Center for Disease Control and Prevention (CDC) standards, and for individuals ≥ 20 years of age calculated BMI was used. Pediatric (2‐<20 years of age) and adult (20‐30 years of age) definitions were used to classify individuals as underweight, normal weight, overweight, and obesity (Table 1A).
TABLE 1.
Definitions of variables of interest. (A) BMI categories; (B) SES; (C) Leukemia characteristics
| (A) | ||
|---|---|---|
| Pediatric (<20 y of age) (based on BMI for sex and age) | Adult (≥20 y of age) (BMI, kg/m2) | |
| Underweight | <5th percentile | <18.5 |
| Normal Weight | 5th‐<85th percentile | 18.5‐24.9 |
| Overweight | 85th‐<95th percentile | 25.0‐29.9 |
| Obesity | >95th percentile | >30.0 |
| (B) | ||
|---|---|---|
| Cases | Controls | |
| Low SES | >20% of the population in a ZIP code living at or below the federal poverty threshold | Poverty Income Ratio < 1.00 |
| High SES | <20% of the population in a ZIP code living at or below the federal poverty threshold | Poverty Income Ratio > 1.00 |
| (C) |
|---|
| ALL lineage (B‐ or T‐cell) |
| CNS status at diagnosis (CNS 1, 2, or 3) |
| CNS 1: Absence of leukemic blasts in CSF on cytospin preparation, regardless of WBC number |
|
CNS 2: Less than 5/uL WBCs in CSF and cytospin‐positive for blasts (or ≥5/uL WBCs but negative by Steinherz/Bleyer algorithm) |
|
CNS 3: At least 5/uL WBCs in CSF and cytospin‐positive for blasts and/or clinical signs of CNS leukemia |
| WBC count at diagnosis (<50 or >50 × 109/L) |
| Hypodiploidy (<44 chromosomes, DNA index <0.81) |
| BCR‐ABL1 fusion |
| ETV6‐RUNX1 translocation |
| KMT2A (MLL) rearrangement |
| Double Trisomy (Trisomies of chromosomes 4 and 10) |
Abbreviations: ALL, acute lymphoblastic leukemia; BMI, body mass index; CNS, central nervous system; CSF, cerebrospinal fluid; SES, socioeconomic status; WBC, white blood cell.
Demographic data including age (month and year of birth), sex, race/ethnicity, and socioeconomic status (SES) (Table 1B) were collected for cases and controls. ZIP code analysis for SES was used for cases, based on available data in case files. Additional SES data were not available through the COG. Poverty Income Ratio (PIR) was collected by NHANES and is used by the US Census to evaluate families’ income status compared to the poverty threshold. PIR was used as the measure of SES for controls. Leukemia characteristics were collected for ALL cases, including lineage (B‐ or T‐cell), centrally‐reviewed cytogenetics, DNA ploidy, white blood cell (WBC) count at diagnosis, and central nervous system (CNS) status at diagnosis (Table 1C).
2.3. Statistical analysis
Demographic characteristics as well as distribution of BMI categories were compared between controls (NHANES) and ALL cases (COG) using chi‐square testing or Fisher's exact test. Univariate analyses were performed to examine associations between BMI and ALL characteristics. Multivariable conditional logistic regression models, adjusting for SES, sampling year, and age at sampling/diagnosis were used to calculate odds ratios (OR) to examine associations between obesity and specific ALL disease characteristics with additional analyses stratified by sex and race/ethnicity. Statistical analyses were performed using SAS, version 9.4. For all analyses, P < .05 was considered statistically significant.
3. RESULTS
Among 4726 eligible cases (Figure 1), 38% were female, median age at diagnosis was 11.3 years (range 2.0‐30.0 years), 72% had B‐cell lineage disease, and low SES was present in 14%. Underweight was observed in 5% of cases, 58% were normal weight, 17% were overweight, and 20% were obese (Table 2). Difference in sampling year distribution among cases and controls (P < .0001) as well as difference in age groups between cases and controls (P = .001) were noted despite matching due to a limited number of available controls and differing enrollment periods of the COG studies, though the absolute differences between cases and controls for these factors were small. Cases were more likely to be classified as having high SES than controls (P < .0001) and BMI category distributions differed between cases and controls (P < .0001).
TABLE 2.
Descriptive characteristics of the study population
| Factor |
COG cases N = 4726 (%) |
NHANES controls N = 4726 (%) |
Χ2 P‐value |
|---|---|---|---|
| Sex | — | ||
| Male | 2909 (61.6) | 2909 (61.6) | |
| Female | 1817 (38.5) | 1817 (38.5) | |
| Race/Ethnicity | — | ||
| Non‐Hispanic, white | 2750 (58.2) | 2750 (58.2) | |
| Non‐Hispanic, black | 419 (8.9) | 419 (8.9) | |
| Hispanic | 1140 (24.1) | 1140 (24.1) | |
| Multiple/Other/Unknown | 417 (8.8) | 417 (8.8) | |
| Sampling year | <.0001 | ||
| 2003‐2004 | 170 (3.6) | 351 (7.4) | |
| 2005‐2006 | 774 (16.4) | 633 (13.4) | |
| 2007‐2008 | 1046 (22.1) | 785 (16.6) | |
| 2009‐2010 | 1255 (26.6) | 850 (18.0) | |
| 2011‐2012 | 554 (11.7) | 705 (14.9) | |
| 2013‐2014 | 435 (9.2) | 731 (15.5) | |
| 2015‐2016 | 492 (10.4) | 671 (14.2) | |
| Age (mo) | .006* | ||
| Mean (SD) | 135.7 (64.8) | 139.3 (65.7) | |
| Range | 24.0‐360.0 | 24.0‐360.0 | |
| Age (y) | .001 | ||
| Children (2‐<10) | 1753 (37.1) | 1665 (35.2) | |
| Adolescent (10‐<20) | 2794 (59.1) | 2813 (59.5) | |
| Young adult (20‐30) | 179 (3.8) | 248 (5.3) | |
| Therapeutic study | |||
| AALL0232 (B‐cell) | 2516 (53.2) | — | |
| AALL0434 (T‐cell) | 1322 (28.0) | — | |
| AALL07P4 (B‐cell) | 153 (3.2) | — | |
| AALL0622 (B‐cell) | 29 (0.6) | — | |
| AALL1131 (B‐cell) | 706 (14.9) | — | |
| SES status a | <.0001 | ||
| Low | 655 (13.9) | 1309 (27.7) | |
| High | 4071 (86.1) | 3417 (72.3) | |
| BMI category b , * | <.0001 | ||
| Underweight | 242 (5.1) | 148 (3.1) | |
| Normal weight | 2752 (58.2) | 2891 (61.2) | |
| Overweight | 784 (16.6) | 758 (16.0) | |
| Obesity | 948 (20.1) | 929 (19.7) |
Abbreviations: AYA, adolescent and young adult; BMI, body mass index; COG, Children's Oncology Group; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation; SES, socioeconomic status.
SES defined as follows: For controls—Low SES was defined as PIR ≤ 1.00; High SES was defined as PIR > 1.00; For cases—Low SES was defined as ≥ 20% of the population in a zip code living at or below the federal poverty threshold High SES was defined as < 20% of the population in a zip code living at or below the federal poverty threshold
BMI categorized as follows: For subjects < 20 y old: Underweight: <5th BMI percentile; Normal weight: 5th to < 85th BMI percentile; Overweight: ≥85th to < 95th BMI percentile; Obesity: ≥95th BMI percentile.For subjects ≥ 20 y old: Underweight: <18.5 kg/m2; Normal weight: 18.5‐<25 kg/m2; Overweight: ≥25 ‐ <30 kg/m2; Obesity: ≥30 kg/m2.
P‐value from t Test
Associations between prognostic ALL characteristics and BMI categories were assessed among cases (Table 3). CNS status was associated with BMI category (P = .01), with patients with obesity experiencing a higher proportion of CNS 2 and 3 status compared to other BMI categories. BMI category was also associated with ALL lineage (P = .006), as well as the presence of trisomies of chromosomes 4 and 10 (P < .0001). Other prognostic characteristics were not statistically associated with BMI category.
TABLE 3.
ALL prognostic characteristics by BMI category for all cases
| Factor |
Underweight N = 242 |
Normal Weight N = 2752 |
Overweight N = 784 |
Obesity N = 948 |
Χ 2 P‐value b , * |
|---|---|---|---|---|---|
| CNS status | .02 | ||||
| 1 | 201 (83.1) | 2234 (81.2) | 613 (78.2) | 729 (76.9) | |
| 2 | 27 (11.2) | 407 (14.8) | 136 (17.4) | 164 (17.3) | |
| 3 | 12 (5.0) | 105 (3.8) | 33 (4.2) | 52 (5.5) | |
| Unknown | 2 (0.8) | 6 (0.2) | 2 (0.3) | 3 (0.3) | |
| Immunophenotype | .003 | ||||
| B‐Precursor | 179 (74.0) | 1929 (70.1) | 574 (73.2) | 721 (76.1) | |
| T‐Cell | 63 (26.0) | 823 (29.9) | 210 (26.8) | 227 (24.0) | |
| Hypodiploid | .58 | ||||
| Yes | 4 (1.7) | 67 (2.4) | 21 (2.7) | 29 (3.1) | |
| No | 236 (97.5) | 2673 (97.1) | 757 (96.6) | 915 (96.5) | |
| Unknown | 2 (0.8) | 11 (0.4) | 5 (0.6) | 3 (0.3) | |
| Indeterminate | ‐ | 1 (0.04) | 1 (0.1) | 1 (0.1) | |
| BCR_ABL1 | .09 | ||||
| Yes | 16 (6.6) | 105 (3.8) | 30 (3.8) | 29 (3.1) | |
| No | 205 (84.7) | 2419 (87.9) | 681 (86.9) | 824 (86.9) | |
| Unknown | 21 (8.7) | 228 (8.3) | 73 (9.3) | 95 (10.0) | |
| ETV6_RUNX1 | .35 | ||||
| Yes | 16 (6.6) | 219 (8.0) | 56 (7.1) | 60 (6.3) | |
| No | 161 (66.5) | 1877 (68.2) | 549 (70.0) | 667 (70.4) | |
| Unknown | 65 (26.9) | 656 (23.8) | 179 (22.8) | 221 (23.3) | |
| MLL | .10 | ||||
| Yes | 2 (0.8) | 84 (3.1) | 22 (2.8) | 36 (3.8) | |
| No | 208 (86.0) | 2319 (84.3) | 648 (82.7) | 775 (81.8) | |
| Unknown | 32 (13.2) | 349 (12.7) | 114 (14.5) | 137 (14.5) | |
| Double trisomy | <.0001 | ||||
| Yes | 43 (17.8) | 270 (9.8) | 54 (6.9) | 69 (7.3) | |
| No | 162 (66.9) | 2118 (77.0) | 616 (78.6) | 732 (77.2) | |
| Unknown | 37 (15.3) | 364 (13.2) | 114 (14.5) | 147 (15.5) | |
| WBC | .21 | ||||
| <50 | 135 (56.0) | 1548 (56.3) | 454 (58.1) | 569 (60.0) | |
| ≥50 | 106 (44.0) | 1202 (43.7) | 327 (41.9) | 378 (39.9) |
Abbreviations: ALL, acute lymphoblastic leukemia; BMI, body mass index; CNS, central nervous status; WBC, white blood cell count.
Unknown and Indeterminate categories not included in Χ 2 test.
Multivariable conditional logistic regression was performed to investigate the association between BMI category and ALL diagnosis, using normal weight as the reference group and adjusting for SES, sampling year, and age at sampling/diagnosis. Underweight at diagnosis was associated with ALL (B‐ and T‐cell) diagnosis (OR 1.71, 95% CI 1.3 −2.21). An association was also identified between obesity and ALL (B‐ and T‐cell) diagnosis (OR 1.20, 95% CI 1.06‐1.36; Figure 2). When B‐ALL and T‐ALL were considered separately, BMI category was only associated with B‐ALL (underweight, OR 2.11, 95% CI 1.56‐2.85 and obesity, OR 1.32, 95% CI 1.15‐1.53; Figure 2). As such, further analyses were focused on B‐ALL.
FIGURE 2.
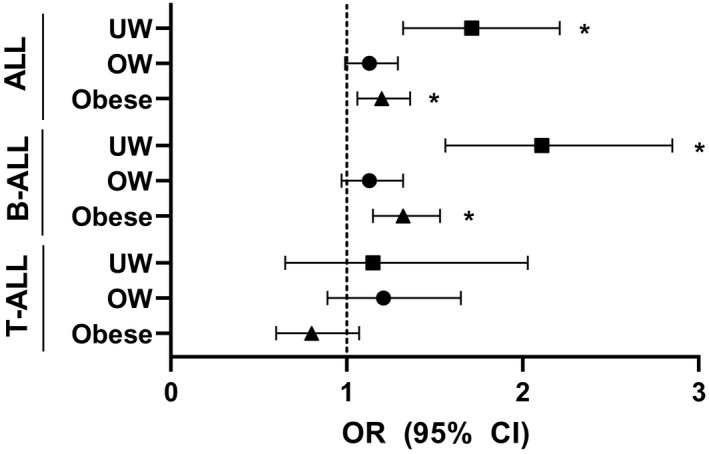
Odds of ALL by BMI Category. Calculated by multivariable conditional logistic regression. Model adjusted for: SES (High, Low), sampling year and age at sampling/diagnosis. Normal weight used as reference. Asterisks (*) indicates significant findings. Abbreviations: ALL, Acute Lymphoblastic Leukemia; OW, Overweight; UW, Underweight
Subsequent multivariable conditional logistic regression analyses stratified by sex with sex‐specific controls, identified associations between B‐ALL diagnosis among males (Figure 3A) with underweight (OR 2.30, 95% CI 1.54‐3.44) and obesity (OR 1.57, 95% CI 1.30‐1.91). Stratification by age group (2 to <10, 10 to <20, and 20‐30 years; Figure S1) showed an association between males with obesity and B‐ALL among the 2 to <10 years age group (OR 1.61, 95% CI 1.12‐2.31) and 10 to <20 years age group (OR 1.47, 95% CI 1.16‐1.87). An association was also seen between males with underweight status and ALL among the 10 to <20 years age group (OR 2.48, 95% CI 1.49 ‐ 4.13). Associations were not identified for females or for males aged 20‐30 years.
FIGURE 3.
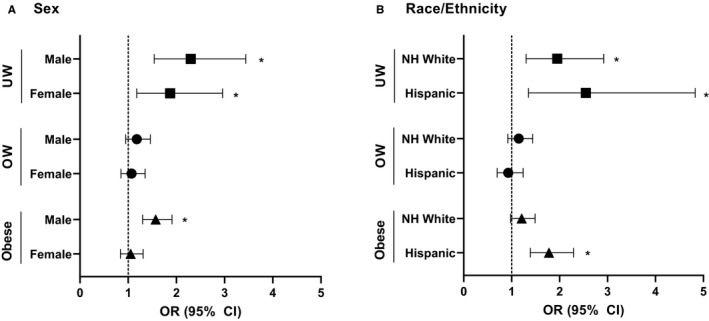
A, Odds of B‐ALL by BMI category by sex. Calculated by multivariable conditional logistic regression. Model adjusted for: SES (High, Low), sampling year and age at sampling/diagnosis. Normal weight used as reference. Asterisks (*) indicates significant findings. Abbreviations: ALL, Acute Lymphoblastic Leukemia; OW, Overweight; UW, Underweight. B, Odds of B‐ALL by BMI category by race/ethnicity. Calculated by multivariable conditional logistic regression. Model adjusted for: SES (High, Low), sampling year and age at sampling/diagnosis. Normal weight used as reference. Asterisks (*) indicates significant findings. Abbreviations: ALL, Acute Lymphoblastic Leukemia; NH, Non‐Hispanic; OW, Overweight; UW, Underweight
Additional multivariable conditional logistic regression analyses were stratified by race/ethnicity (Figure 3B). Analyses could not be stratified for Non‐Hispanic (NH) Black given limited numbers. A significant association was identified between B‐ALL diagnosis and NH Whites with underweight status (OR 1.95, 95% CI 1.30‐2.92), but not with overweight or obesity. Among Hispanic ethnicity, B‐ALL diagnosis was associated with obesity (OR 1.78, 95% CI 1.39‐2.29) and underweight (OR 2.55, 95% CI 1.35‐4.83). When further stratified by age group (Figure S2), associations with B‐ALL diagnosis were seen in those aged 10 to <20 years for NH White with underweight (OR 2.19, 95% CI 1.27) and for Hispanics with underweight (OR 2.19, 95% CI 1.01‐4.73) and Hispanics with obesity (OR 1.74, 95% CI 1.29‐2.34). No significant associations were seen in other age groups when analyses were stratified by race/ethnicity.
Further multivariable analyses assessed associations between BMI category and B‐ALL characteristics, again stratified by sex and race/ethnicity. Males with obesity had a stronger association with moderate to high levels of CNS involvement (CNS 2‐3) at diagnosis (OR 2.03, 95% CI 1.30‐3.17) compared to no CNS involvement (CNS 1) (OR 1.49, 95% CI 1.20‐1.85) (Figure 4A). These associations were not seen in females. Among Hispanic ethnicity (Figure 4B), obesity was more strongly associated with CNS 2‐3 (OR 3.21, 95% CI 1.73‐5.95) compared to CNS 1 (OR 1.60, 95% CI 1.21‐2.11). These associations were not seen in NH White. Associations between other prognostic B‐ALL characteristics and BMI category were assessed and are available in Tables S2 and S3.
FIGURE 4.
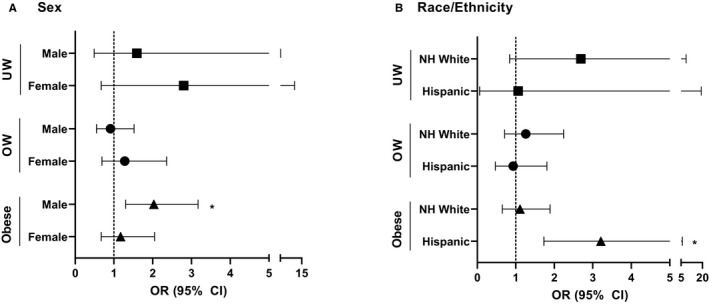
A, Odds of B‐ALL CNS Involvement by BMI category and sex. Calculated by multivariable conditional logistic regression. Model adjusted for: SES (High, Low), sampling year and age at sampling/diagnosis. Normal weight used as reference. Asterisks (*) indicates significant findings. Abbreviations: ALL, Acute Lymphoblastic Leukemia; OW, Overweight; UW, Underweight. B, Odds of B‐ALL CNS Involvement by BMI category and race/ethnicity. Calculated by multivariable conditional logistic regression. Model adjusted for: SES (High, Low), sampling year and age at sampling/diagnosis. Normal weight used as reference. Asterisks (*) indicates significant findings. Abbreviations: ALL, Acute Lymphoblastic Leukemia; NH, Non‐Hispanic; OW, Overweight; UW, Underweight
4. DISCUSSION
Here we demonstrate the novel findings that pre‐treatment obesity among children is associated with HR B‐ALL diagnosis, specifically among males and Hispanics, particularly within the adolescent age group, and that obesity is also associated with risk for ALL CNS involvement. This study also confirms the previously identified association between underweight status and ALL, 17 which is felt to be secondary to detrimental physiologic effects of untreated ALL (ie weight loss prior to diagnosis resulting in underweight status at diagnosis) and may result in an underestimation of BMI status prior to the development of ALL.
The association between pre‐treatment obesity and HR B‐ALL diagnosis suggests potential common physiology in both obesity and leukemogenesis. Inflammation has been theorized to play a role in linking obesity and oncogenesis across a variety of cancers as it alters adipokine release, cytokine expression, insulin resistance, and results in other adverse metabolic adaptations. 18 , 19 , 20 , 21 , 22 For leukemia, multiple epidemiologic studies have demonstrated an association between obesity and development of leukemia in adults. 23 The biologic relationship between obesity and leukemogenesis is likely multifactorial. Diet‐induced obesity accelerated ALL progression in murine models, which showed accompanying elevated serum levels of insulin, leptin and IL‐6. 24 Insulin is a potent growth factor that can increase ALL cell proliferation in vivo, 25 and adults with diabetes are at increased risk for many cancers, including leukemia. 26 , 27 Given the comorbidity of diabetes and obesity, hyperinsulinemia and insulin resistance may therefore be important mediators of leukemogenesis in the setting of obesity. Additionally, leptin is a pro‐inflammatory, pro‐angiogenic, and pro‐tumorigenic adipokine, which has been shown to stimulate multiple leukemia cell types. 18 , 28
Obesity‐associated inflammation results in tissue remodeling and increases free fatty acids leading to increased oxidative stress and recruitment of pro‐inflammatory macrophages and upregulation of inflammatory cytokines IL‐1, IL‐6, and TNF‐α. 29 , 30 IL‐6 plays a role in B cell proliferation and differentiation, which may impact B‐lineage leukemogenesis. 24 Obesity‐associated changes in the inflammatory microenvironment may lead to molecular and genetic alterations, resulting in leukemia development or progression. Inflammation is of interest for leukemia etiology, as well as therapy, particularly given the increasing use of immunotherapies for B‐ALL treatment. An important opportunity for future study will be assessing the effect of increasing BMI or severe obesity on leukemogenesis; unfortunately, we were not sufficiently powered to evaluate this in the present study.
The male sex‐specific association between obesity and B‐ALL also suggests a role for sex hormones in leukemogenesis. Males have a higher leukemia incidence than females, 1 which was also seen in our study population. The association seen in this study was present among pre‐adolescents and adolescents (10 to <20 years). Adult studies have demonstrated a link between estrogen and cancer, and adipose tissue can increase circulating levels of estrogen by conversion of androgens. 18 , 20 Sex hormones also regulate cellular differentiation, proliferation, and apoptosis. 18 , 20 The identified association between obesity and B‐ALL diagnosis among pre‐pubertal and pubertal males may be a result of excess of sex hormones as they enter puberty resulting in higher levels of estrogen leading to leukemogenesis. While this suggests females should be at higher risk for leukemia, it is possible females have a greater tolerance for circulating estrogen given higher baseline levels compared to males. Additionally, a recent study of metabolomic profiles in youth with obesity demonstrated a unique profile among post‐pubertal males, suggesting an additional avenue for further study. 31 The identified association between obesity and B‐ALL diagnosis in pubertal and post‐pubertal males may indicate leukemia in adolescents is more biologically similar to that seen in adults compared to children.
This study did not include SR B‐ALL cases, which include the majority of pre‐pubertal children (<10 years of age); thus, the opportunity to assess associations between obesity and leukemia in pre‐pubertal children is limited. It is also critical to point out that BMI does not measure body fatness and is a poor proxy for body fatness unless children and adolescents have severe obesity. 32 Although males and females have similar body fat until puberty onset with similar increases during the entire pubertal window, later changes result in females having greater fat mass as adults. 33 Higher fat deposition in female adults may explain why some adult studies have found obesity is associated with leukemia diagnosis in both sexes. 23
Obesity was associated with B‐ALL diagnosis in Hispanic children and adolescents in our study. Obesity rates are highest among Hispanic children and they are at higher risk for obesity‐related complications. 16 , 34 , 35 , 36 Over the past two decades ALL rates have increased in Hispanic children at a greater rate than NH children, 37 , 38 , 39 particularly for adolescents aged 15 to 19 years, 37 which is the age group in which our study identified an association of B‐ALL diagnosis and obesity among Hispanic youth. Different ethnic groups have different percent body fat with similar BMIs. 40 , 41 Hispanics, particularly Mexican Americans, have higher body fat percentage compared to NH White and NH Black counterparts, which may contribute to oncogenesis. 41 Given similar temporal trends in obesity rates and ALL incidence in Hispanic youth and higher rates of adiposity and worse outcomes for ALL among Hispanic children, it is possible there is an underlying biologic mechanism placing Hispanic children at risk for B‐ALL in the setting of obesity.
Pre‐treatment obesity was associated with CNS B‐ALL involvement at diagnosis. Several prior studies have linked obesity with inferior outcomes in childhood ALL. 15 , 42 , 43 It is possible that the CNS, in the setting of obesity may provide a niche for ALL cells to escape immune surveillance and chemotherapy. Moderate CNS involvement with ALL (CNS 2) in the setting of obesity may be due to technical difficulty during a diagnostic lumbar puncture (ie obtaining peripheral blood elements in the sample), the biologic gradient seen in our study (though not statistically significant due to the limited number of CNS positive cases) suggests this finding is real and not purely due to technique. Additionally, other studies have shown obesity can impact neuro‐inflammation and the permeability of the blood brain barrier 44 , 45 , 46 , 47 suggesting a biological mechanism for ALL penetration into the CNS in patients who are obese.
Important limitations of this study must be considered. The COG studies with anthropometric data were only for HR ALL, resulting in an unavoidable sampling bias and limiting the generalizability of our findings to SR B‐ALL, which is the most common type of ALL seen in young children. Additionally, sampling years for the COG ALL studies was not perfectly matched to those of controls, which is important given the secular trends of obesity, although these trends often span decades. Similarly, the controls had a larger proportion of individuals with low SES. This may be secondary to selection bias that is inherent in the NHANES participant selection; however, prior studies using both the Surveillance, Epidemiology, and End Results program and Minnesota Cancer Surveillance System have demonstrated positive, although smaller, associations between higher SES and ALL diagnosis. 48 , 49 Notably, different methods were used for assessing SES in cases (ZIP code) and controls (PIR), which may have resulted in slightly different SES distributions in the two groups. Additionally, we were unable to compare clinical characteristics, including BMI, of COG clinical trial participants and non‐participants; however, nearly 70% of children and adolescents diagnosed with ALL participate in a COG clinical trial. 50
Additional limitations include limited availability of cytogenetic data for this analysis, precluding assessments for associations between Philadelphia‐like cytogenetics and obesity. Many of the cytogenetic abnormalities considered in this study are more common in SR B‐ALL, which was not evaluated in this study. Similarly, there were a limited number of T‐ALL cases, so it is possible an association between T‐ALL and obesity at diagnosis was not detected due to lack of power. There were limited participants ≥ 20 years of age, limiting adequate assessment of the association between obesity and ALL among young adults. Lastly, we were unable to assess or control for underlying germline mutations or inherited predisposition syndromes, which can impact both leukemogenesis and obesity risk. Despite these limitations, a positive association between HR B‐ALL and obesity was found.
In conclusion, the present study demonstrated a novel association between obesity at diagnosis and new diagnosis of HR B‐ALL, particularly in males and Hispanics. It suggests opportunities for future research to determine whether other pediatric malignancies are also associated with obesity. It also could have important public health implications for obesity prevention and treatment efforts in children and adolescents to reduce future cancer risk. Additionally, this study has implications for the CNS microenvironment in pediatric patients with obesity and ALL. Further studies addressing sex hormones and pro‐inflammatory cytokines and their roles in leukemogenesis are needed, as are studies to assess underlying genetic changes that may contribute to obesity and leukemogenesis among Hispanic children. Additional mechanistic studies could lead to novel therapeutic strategies in the future that would benefit children with obesity and HR B‐ALL.
Funding Contributions: This work was supported by the University of Minnesota Department of Pediatrics Vikings Award and by a grant from the National Cancer Institute (K08CA234232 [LMT]).
CONFLICT OF INTEREST
The authors of this manuscript certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent‐licensing arrangements), or non‐financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
AUTHOR CONTRIBUTIONS
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated sufficiently in the work to take public responsibility for the content, including participation in the concept, design, analysis, writing, or revision of the manuscript.
ETHICAL REVIEW STATEMENT
Ethical approval was not sought from our institutional review board or ethics committee prior to commencing this study.
Supporting information
Fig S1
Fig S2
Table S1
Table S2
Table S3
ACKNOWLEDGMENTS
The authors thank the University of Minnesota Department of Pediatrics and the National Cancer Institute for providing funding support for this study. The authors thank the Children's Oncology Group for providing access to previously collected data used in this study. We would like to acknowledge Jeannette M. Sample, MPH for her assistance in providing additional statistical analysis and her contributions to this paper.
Ghosh T, Richardson M, Gordon PM, Ryder JR, Spector LG, Turcotte LM. Body mass index associated with childhood and adolescent high‐risk B‐cell acute lymphoblastic leukemia risk: A Children’s Oncology Group report. Cancer Med. 2020;9:6825–6835. 10.1002/cam4.3334
[Correction added on 10 August 2020, after first online publication: The Funding information has been corrected in this version.]
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Howlader NNA, Krapcho M, Miller D, et al.(Eds). SEER cancer statistics review, 1975–2017. Bethesda, MD: National Cancer Institute; 2020. [Google Scholar]
- 2. Marcotte EL, Thomopoulos TP, Infante‐Rivard C, et al. Caesarean delivery and risk of childhood leukaemia: a pooled analysis from the Childhood Leukemia International Consortium (CLIC). Lancet Haematol. 2016;3(4):e176‐e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petridou ET, Georgakis MK, Erdmann F, et al. Advanced parental age as risk factor for childhood acute lymphoblastic leukemia: results from studies of the Childhood Leukemia International Consortium. Eur J Epidemiol. 2018;33(10):965‐976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. O’Neill KA, Murphy MFG, Bunch KJ, et al. Infant birthweight and risk of childhood cancer: international population‐based case control studies of 40 000 cases. Int J Epidemiol. 2015;44(1):153‐168. [DOI] [PubMed] [Google Scholar]
- 5. Kratz CP, Stanulla M, Cavé H. Genetic predisposition to acute lymphoblastic leukemia: overview on behalf of the I‐BFM ALL Host Genetic Variation Working Group. Eur J Med Genet. 2016;59(3):111‐115. [DOI] [PubMed] [Google Scholar]
- 6. Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr. 2014;168(6):561‐566. [DOI] [PubMed] [Google Scholar]
- 7. Stein CJ, Colditz GA. Modifiable risk factors for cancer. Br J Cancer. 2004;90(2):299‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lauby‐Secretan B, Scoccianti C, Loomis D, et al. Body fatness and cancer‐viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Psaltopoulou T, Sergentanis TN, Ntanasis‐Stathopoulos I, Tzanninis IG, Riza E, Dimopoulos MA. Anthropometric characteristics, physical activity and risk of hematological malignancies: a systematic review and meta‐analysis of cohort studies. Int J Cancer. 2019;145(2):347‐359. [DOI] [PubMed] [Google Scholar]
- 10. Patel AV, Diver WR, Teras LR, Birmann BM, Gapstur SM. Body mass index, height and risk of lymphoid neoplasms in a large United States cohort. Leuk Lymphoma. 2013;54(6):1221‐1227. [DOI] [PubMed] [Google Scholar]
- 11. Teras LR, Patel AV, Carter BD, Rees‐Punia E, McCullough ML, Gapstur SM. Anthropometric factors and risk of myeloid leukaemias and myelodysplastic syndromes: a prospective study and meta‐analysis. Br J Haematol. 2019;186(2):243‐254. [DOI] [PubMed] [Google Scholar]
- 12. Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. United States Cancer Statistics Working Group . United States Cancer Statistics: 1999–2014 incidence and mortality web‐based report. Atlanta: U.S. Department of Health and Human Services Center for Disease Control and Prevention; 2017. [Google Scholar]
- 14. Berger NA. Young adult cancer: influence of the obesity pandemic. Obesity (Silver Spring). 2018;26(4):641‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orgel E, Tucci J, Alhushki W, et al. Obesity is associated with residual leukemia following induction therapy for childhood B‐precursor acute lymphoblastic leukemia. Blood. 2014;124(26):3932‐3938. [DOI] [PubMed] [Google Scholar]
- 16. National Health and Nutrition Examination Survey Data. Hyattsville, MD: Department of Health and Human Services; https://wwwn.cdc.gov/nchs/nhanes/. Accessed January 16, 2020 [Google Scholar]
- 17. Schraw JM, Henson AT, Scheurer ME, Forman MR. The associations of height‐for‐age, weight‐for‐age, and weight‐for‐height with pediatric acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2017;39(5):376‐381. [DOI] [PubMed] [Google Scholar]
- 18. Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol. 2016;29:77‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4(8):579‐591. [DOI] [PubMed] [Google Scholar]
- 20. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3(9):565‐574. [DOI] [PubMed] [Google Scholar]
- 21. Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci. 2012;1271:37‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tahergorabi Z, Khazaei M, Moodi M, Chamani E. From obesity to cancer: a review on proposed mechanisms. Cell Biochem Funct. 2016;34(8):533‐545. [DOI] [PubMed] [Google Scholar]
- 23. Lichtman MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15(10):1083‐1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yun JP, Behan JW, Heisterkamp N, et al. Diet‐induced obesity accelerates acute lymphoblastic leukemia progression in two murine models. Cancer Prev Res (Phila). 2010;3(10):1259‐1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neely EK, Rosenfeld RG, Illescas A, Smith SD. Mitogenic effects of human recombinant insulin on B‐cell precursor acute lymphoblastic leukemia cells. Leukemia. 1992;6(11):1134‐1142. [PubMed] [Google Scholar]
- 26. Ohkuma T, Peters SAE, Woodward M. Sex differences in the association between diabetes and cancer: a systematic review and meta‐analysis of 121 cohorts including 20 million individuals and one million events. Diabetologia. 2018;61(10):2140‐2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta‐analysis. Endocr Pract. 2011;17(4):616‐628. [DOI] [PubMed] [Google Scholar]
- 28. Mouzaki A, Panagoulias I, Dervilli Z, et al. Expression patterns of leptin receptor (OB‐R) isoforms and direct in vitro effects of recombinant leptin on OB‐R, leptin expression and cytokine secretion by human hematopoietic malignant cells. Cytokine. 2009;48(3):203‐211. [DOI] [PubMed] [Google Scholar]
- 29. Saxena NK, Sharma D. Multifaceted leptin network: the molecular connection between obesity and breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(3–4):309‐320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Balistreri CR, Caruso C, Candore G. The role of adipose tissue and adipokines in obesity‐related inflammatory diseases. Mediators Inflamm. 2010;2010:802078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saner C, Harcourt BE, Pandey A, et al. Sex and puberty‐related differences in metabolomic profiles associated with adiposity measures in youth with obesity. Metabolomics. 2019;15(5):75. [DOI] [PubMed] [Google Scholar]
- 32. Ryder JR, Kaizer AM, Rudser KD, Daniels SR, Kelly AS. Utility of body mass index in identifying excess adiposity in youth across the obesity spectrum. J Pediatr. 2016;177(255–261):e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mostazir M, Jeffery A, Hosking J, Metcalf B, Voss L, Wilkin T. Evidence for energy conservation during pubertal growth. A 10‐year longitudinal study (EarlyBird 71). Int J Obes (Lond). 2016;40(11):1619‐1626. [DOI] [PubMed] [Google Scholar]
- 34. Isasi CR, Rastogi D, Molina K. Health issues in hispanic/latino youth. J Lat Psychol. 2016;4(2):67‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goran MI, Ventura EE. Genetic predisposition and increasing dietary fructose exposure: the perfect storm for fatty liver disease in Hispanics in the U.S. Dig Liver Dis. 2012;44(9):711‐713. [DOI] [PubMed] [Google Scholar]
- 36. Goran MI. Ethnic‐specific pathways to obesity‐related disease: the Hispanic vs. African‐American paradox. Obesity (Silver Spring). 2008;16(12):2561‐2565. [DOI] [PubMed] [Google Scholar]
- 37. Barrington‐Trimis JL, Cockburn M, Metayer C, Gauderman WJ, Wiemels J, McKean‐Cowdin R. Rising rates of acute lymphoblastic leukemia in Hispanic children: trends in incidence from 1992 to 2011. Blood. 2015;125(19):3033‐3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McNeil DE, Coté TR, Clegg L, Mauer A. SEER update of incidence and trends in pediatric malignancies: acute lymphoblastic leukemia. Med Pediatr Oncol. 2002;39(6):554‐557; discussion 552–553. [DOI] [PubMed] [Google Scholar]
- 39. Siegel DA, Henley SJ, Li J, Pollack LA, Van Dyne EA, White A. Rates and trends of pediatric acute lymphoblastic leukemia ‐ United States, 2001–2014. In. Vol 66. MMWR Morb Mortal Wkly. 2017;950‐954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deurenberg P, Deurenberg‐Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141‐146. [DOI] [PubMed] [Google Scholar]
- 41. Heymsfield SB, Peterson CM, Thomas DM, Heo M, Schuna JM. Why are there race/ethnic differences in adult body mass index‐adiposity relationships? A quantitative critical review. Obes Rev. 2016;17(3):262‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Butturini AM, Dorey FJ, Lange BJ, et al. Obesity and outcome in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2007;25(15):2063‐2069. [DOI] [PubMed] [Google Scholar]
- 43. Meenan CK, Kelly JA, Wang L, Ritchey AK, Maurer SH. Obesity in pediatric patients with acute lymphoblastic leukemia increases the risk of adverse events during pre‐maintenance chemotherapy. Pediatr Blood Cancer. 2019;66(2):e27515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rhea EM, Salameh TS, Logsdon AF, Hanson AJ, Erickson MA, Banks WA. Blood‐brain barriers in obesity. AAPS J. 2017;19(4):921‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Dyken P, Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood‐brain barrier. Front Neurosci. 2018;12:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Salameh TS, Mortell WG, Logsdon AF, Butterfield DA, Banks WA. Disruption of the hippocampal and hypothalamic blood‐brain barrier in a diet‐induced obese model of type II diabetes: prevention and treatment by the mitochondrial carbonic anhydrase inhibitor, topiramate. Fluids Barriers CNS. 2019;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gustafson DR, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid‐life adiposity factors relate to blood‐brain barrier integrity in late life. J Intern Med. 2007;262(6):643‐650. [DOI] [PubMed] [Google Scholar]
- 48. Wang L, Gomez SL, Yasui Y. Racial and ethnic differences in socioeconomic position and risk of childhood acute lymphoblastic leukemia. Am J Epidemiol. 2017;185(12):1263‐1271. [DOI] [PubMed] [Google Scholar]
- 49. Kehm RD, Spector LG, Poynter JN, Vock DM, Osypuk TL. Socioeconomic status and childhood cancer incidence: a population‐based multilevel analysis. Am J Epidemiol. 2018;187(5):982‐991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children's oncology group. J Clin Oncol. 2012;30(14):1663‐1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1
Table S2
Table S3
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


