Abstract
Breast cancer is the most common cancer in women, and approximately 71% of carcinomas are hormone receptor‐positive (HR+) and human epidermal growth factor receptor 2‐not‐amplified (HER2‐negative). Pathogenesis of breast cancer is associated with dysregulation of several signaling pathways, including the phosphatidylinositol‐3‐kinase (PI3K) pathway. PIK3CA, the gene encoding PI3K catalytic subunit p110α, is mutated in 20%‐40% of breast cancer patients. Several PI3K inhibitors have been developed and one, alpelisib, was recently approved for use in PIK3CA‐mutated, HR+, HER2‐negative advanced breast cancer. There are numerous types of assays and methods used in clinical studies to determine PIK3CA status in cancers. Additionally, there are several factors to consider for PIK3CA testing in clinical practice, including choice of assay, source of sample, and test timing. In this review, we discuss the use of PIK3CA as a biomarker to guide treatment decisions in patients with HR+, HER2‐negative advanced breast cancer, as well as practical considerations and recommendations for testing.
Keywords: advanced breast cancer, alpelisib, biomarker, companion diagnostic, PIK3CA
PIK3CA may be used as a biomarker to guide treatment decisions in patients with HR+, HER2‐negative ABC. PIK3CA mutation may be assessed through either tissue or liquid biopsy using PCR‐ or NGS‐based assays.
![]()
1. INTRODUCTION
Breast cancer is the most common cancer diagnosed in women and is the leading cause of cancer‐related deaths worldwide. 1 In the United States, there will be an estimated 168 292 patients living with metastatic breast cancer in 2020. 2 Approximately 71% of patients with breast cancer have hormone receptor‐positive (HR+), human epidermal growth factor receptor 2‐not‐amplified (HER2‐negative) disease, which is associated with a favorable short‐term prognosis. 3 Current treatment options for HR+, HER2‐negative advanced breast cancer (ABC) include endocrine therapies (eg, tamoxifen and aromatase inhibitors), targeted therapies (eg, cyclin‐dependent kinase 4/6 [CDK4/6] inhibitors), and chemotherapy. 3 , 4
The HR+, HER2‐negative tumors are less sensitive to chemotherapy and, despite demonstrating improved clinical outcomes, patients eventually develop resistance to CDK4/6 inhibitors and endocrine therapies—especially in the advanced setting. 3 , 4 Other targeted therapies, such as phosphatidylinositol‐3‐kinase (PI3K) inhibitors, have been developed to overcome resistance to existing therapies. 4 , 5 , 6
2. THE PI3K SIGNALING PATHWAY
The most frequently mutated signaling pathway in all breast cancers is the PI3K pathway. 7 The PI3Ks are a family of lipid and serine/threonine kinases that integrate extracellular stimuli into intracellular signals to regulate various pathways that control several physiological functions including cellular proliferation, growth, survival, differentiation, and metabolism. 7 , 8 , 9 Phosphatidylinositol‐3‐kinases are divided into 3 classes (I, II, III) based on substrate specificity and structure. 9 Of the 3 classes, class I PI3Ks are the most extensively studied and most established as a cause of many cancer types. 9 , 10
Class I PI3Ks are subdivided into subclasses IA and IB in mammals based on their mode of regulation. Class IA PI3Ks are composed of a p110 catalytic subunit and a p85 inhibitory adaptor/regulatory subunit. 8 , 9 The genes PIK3CA, PIK3CB, and PIK3CD encode class IA catalytic isoforms p110α, p110β, and p110δ, respectively. Class IA and IB PI3Ks phosphorylate phosphatidylinositides (PtdIns(4,5)P2) in vivo, whereas class III PI3Ks phosphorylate PtdIns. Some evidence suggests that class II PI3Ks may also preferentially phosphorylate PtdIns in vivo. 9
3. RATIONALE FOR TARGETING PIK3CΑ IN ABC
Constitutive activation of the PI3K pathway is commonly related to oncogenesis. 9 Class I PI3Ks, which include PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ, are aberrantly activated in breast cancer. 7 The most common mechanism leading to constitutive activation of the PI3K pathway is the somatic loss of phosphatase and tensin homologue (PTEN) by genetic or epigenetic alterations. Other mechanisms of PI3K pathway activation include the activation of receptor tyrosine kinases (RTKs) and PI3K isoform mutations, duplications, and/or overexpression. 9
In breast cancer, PIK3CA mutations are the most frequent alterations in the PI3K pathway, with at least 80% occurring within the helical (E542K and E545K) and kinase (H1047R) domains of p110α. 11 Mutations in PIK3CA have been reported in 20%‐40% of breast cancer cases, and their incidence also varies across different breast cancer molecular subtypes. 11 , 12 , 13 The incidence of PIK3CA mutations has been estimated at 36%‐45% of HR+, HER2‐negative breast cancers; up to 40% of HER2‐positive breast cancers; and 9%‐14% of triple‐negative breast cancers. 12 , 14 , 15
Regarding PIK3CA mutation as a prognostic factor, there are conflicting results on the association of PIK3CA mutations and breast cancer outcome. 16 A pooled analysis showed that the presence of PIK3CA mutations is a negative prognostic factor (pooled overall survival [OS], disease‐free survival [DFS], and progression‐free survival [PFS]) in breast cancer. 13 It has been suggested that PIK3CA may offer a differing prognostic role in early versus advanced or metastatic BC. Data from 10 319 early breast cancer patients with known PIK3CA genotype showed that PIK3CA mutations were associated with better invasive DFS, distant DFS, and OS. However, it should be noted that after adjusting for other factors including treatment, estrogen receptor/HER2 status, and tumor grade, the effect only remained significant for invasive DFS. 17 In a recent subgroup analysis of the SAFIR02 study, patients with PIK3CA‐mutated, HR+, HER2‐negative metastatic breast cancer were found to be less sensitive to chemotherapy and presented with shorter survival than patients without PIK3CA alterations (OS hazard ratio [HR] 1.44; 95% CI, 1.02‐2.03; P = .039). 18 In agreement with this, a systematic review of 12 studies conducted in HR+, HER2‐negative metastatic breast cancer reported worse prognosis in patients whose cancers had PIK3CA mutations compared with wild‐type when treated with non‐PI3K inhibitors. Notably, in patients treated with PI3K inhibitors, improved PFS was observed in the PIK3CA‐mutant cohort. 19 However, other meta‐analyses have reported the presence of PIK3CA mutations to be associated with better relapse‐free survival. 20 Overall, the prognostic significance of PIK3CA alterations in breast cancer remains inconclusive.
Mutations in PIK3CA may be found alongside HER2 amplification and PTEN loss, which also enhance PI3K signaling activity. 20 Phosphatase and tensin homologue protein loss is found in 35% of triple‐negative; 11% of HR+, HER2‐negative; 5% of HR+, HER2‐positive; and 6% of HR–, HER2‐positive breast cancers. 21
Loss of PTEN is also thought to be associated with trastuzumab resistance. Preclinical studies have shown that PIK3CA‐activating mutations and/or PTEN loss diminishes the growth inhibitory effects of trastuzumab, and trastuzumab‐resistant cell lines have been shown to respond to a selective PI3K inhibitor. In clinical studies, PTEN loss and/or PIK3CA‐activating mutations have been associated with poor clinical outcomes and were not associated with outcomes related to treatment with trastuzumab. Despite worsening clinical outcomes, patients with PTEN loss still demonstrated benefit from trastuzumab treatment. 21
4. PI3K INHIBITOR PHASE 3 CLINICAL TRIAL TESTING METHODS AND OUTCOMES
4.1. Pan‐PI3K inhibitor: Buparlisib
Buparlisib is an oral pan‐PI3K inhibitor that targets all four isoforms of class I PI3K. BELLE‐2 was a phase 3 trial that evaluated the safety and efficacy of buparlisib in combination with fulvestrant in postmenopausal women with aromatase inhibitor‐resistant HR+, HER2‐negative ABC. The status of PI3K in archival tumor tissue was determined during a 14‐day run‐in treatment phase using Sanger sequencing of the PIK3CA gene and immunohistochemistry of PTEN protein expression. Patients were classified into three categories: (a) PI3K pathway activated—if Sanger sequencing detected a mutation in PIK3CA exons 1, 7, 9, or 20, or if there was a loss of PTEN expression; (b) PI3K pathway non‐activated; and (c) PI3K pathway unknown—if the assessment was uninterpretable. Plasma samples for circulating tumor DNA (ctDNA) analysis were collected at study entry for all randomized patients. Analysis was done using beads, emulsification, amplification, and magnetics (BEAMing) based on a predefined panel of 15 PIK3CA mutations in exons 1, 7, 9, and 20 (Arg88Gln, Arg93Trp/Gln, Lys111Glu/Asn, Gly118Asp, Glu365Lys, Cys420Arg, Glu542Lys, Glu545Gly/Lys, Gln546Lys, and His1047Arg/Leu/Tyr) (Table 1). 5 , 22 , 23 , 24 , 25 , 26 , 27 Patients treated with buparlisib (n = 576) had significantly improved median PFS (mPFS) compared with placebo (n = 571; 6.9 vs 5.0 months; HR 0.78; P = .00021). A significant improvement in mPFS was also observed in buparlisib‐treated patients with known PI3K status and patients with an activated PI3K pathway (Table 1). Exploratory analysis using ctDNA showed that patients with PIK3CA mutations (n = 200) had longer PFS when treated with buparlisib compared with placebo (Table 1). This was not observed in the ctDNA PIK3CA nonmutant cohort (n = 387). 22
Table 1.
PIK3CA mutation detection assay and sample types in phase 3 PI3K inhibitor trials
| Investigational agent | Clinical trial | Method of detection | PIK3CA coverage | Type of sample | PIK3CA‐mutated | ||||
|---|---|---|---|---|---|---|---|---|---|
| Group | n | mPFS, mo | HR (95% CI) | P value | |||||
| Buparlisib |
BELLE‐2 22 (N = 1147) |
Sanger sequencing | Exons 1, 7, 9, 20 (+PTEN IHC) | Archival tumor tissue | BUP | 188 a | 6.8 | 0.76 (0.60‐0.97) | .014 |
| PBO | 184 a | 4.0 | |||||||
| BEAMing | 15 point mutations on exons 1, 7, 9, 20 | Plasma ctDNA at baseline | BUP | 87 | 7.0 | 0.58 (0.41‐0.82) | .001 | ||
| PBO | 113 | 3.2 | |||||||
|
BELLE‐3 23 (N = 432) |
BEAMing (Inostics) | 8 point mutations on exons 9, 20 | Plasma ctDNA at screening or at day 1 of cycle 1 | BUP | 100 | 4.2 | 0.46 (0.29‐0.73) | .00031 | |
| PBO | 35 | 1.6 | |||||||
| PCR (Roche cobas assay) | 17 point mutations on exons 7, 9, 20 | New or archival tumor tissue | BUP | 75 | 4.7 | 0.39 (0.23‐0.65) | <.0001 | ||
| PBO | 34 | 1.4 | |||||||
| Taselisib |
SANDPIPER 24 (N = 631) |
PCR (Roche cobas assay) | 17 point mutations on exons 7, 9, 20 | Not disclosed | TAS | 340 | 7.4 | 0.70 (0.56‐0.89) | .0037 |
| PBO | 176 | 5.4 | |||||||
| Alpelisib |
(N = 572) |
PCR (QIAGEN therascreen PIK3CA RGQ) | 11 point mutations on exons 7, 9, 20 | New or archival tissue b | ALP | 169 | 11.0 | 0.65 (0.50‐0.85) | .00065 |
| PBO | 172 | 5.7 | |||||||
| FoundationOne CDx 324‐gene NGS | All encoding exons | New or archival tissue b | ALP | 121 | 11.0 | 0.59 (0.43‐0.82) | NS | ||
| PBO | 118 | 5.5 | |||||||
Abbreviations: ALP, alpelisib; BEAMing, beads, emulsification, amplification, magnetics; BUP, buparlisib; CI, confidence interval; ctDNA, circulating tumor DNA; HR, hazard ratio; IHC, immunohistochemistry; mPFS, median progression‐free survival; NGS, next‐generation sequencing; NS, not specified; PBO, placebo; PCR, polymerase chain reaction; PI3K, phosphatidylinositol‐3‐kinase; PIK3CA, phosphatidylinositol‐3‐kinase p110α isoform; PTEN, phosphatase and tensin homologue; TAS, taselisib.
Patients were categorized as PI3K pathway‐activated (any mutation detected in PIK3CA or loss of PTEN expression).
New and archival tissues refer to samples taken ≤3 months and >3 months prior to randomization, respectively.
In BELLE‐2, the most common grade 3/4 adverse events (AEs) in the buparlisib group (n = 573) were elevated alanine aminotransferase (ALT; 25%), elevated aspartate aminotransferase (AST; 18%), hyperglycemia (15%), and rash (8%). Serious AEs occurred more frequently in the buparlisib group (23%) compared with placebo (16%). Mood disorders such as depression and anxiety were more common in the buparlisib group (buparlisib vs placebo: 26% vs 9% for depression and 22% vs 8% for anxiety). Suicidal ideation was noted in three patients in the buparlisib group and two in the placebo group, but no suicide attempts were reported. There were no noted occurrences of treatment‐related deaths. 22
The phase 3 BELLE‐3 trial evaluated the safety and efficacy of buparlisib in combination with fulvestrant in postmenopausal women with HR+, HER2‐negative ABC who progressed on/after an aromatase inhibitor and resistant to endocrine therapy plus everolimus. Plasma samples collected at screening or at cycle 1, day 1 were analyzed for PIK3CA mutations status at exons 9 (Glu542Lys, Glu545Lys, Glu545Gly, Gln546Lys) and 20 (Met1043Ile, His1047Tyr, His1047Arg, His1047Leu) using the Inostics BEAMing assay for ctDNA analysis. Mutation status of PIK3CA was also analyzed via the Roche cobas® PIK3CA assay that covers exons 7, 9, and 20, using new or archival primary or metastatic tumor sample (Table 1). 5 , 22 , 23 , 24 , 25 , 26 , 27 Patients enrolled in the trial (N = 432) who were treated with buparlisib (n = 289) had significantly longer mPFS compared with placebo (n = 143; 3.9 vs 1.8 months; HR 0.67; P = .0003). Consistent with the results of BELLE‐2, patients with PIK3CA mutation by ctDNA treated with buparlisib (n = 100) also had significantly longer mPFS compared with placebo (Table 1). 23
The safety profile of buparlisib plus fulvestrant in the BELLE‐3 trial was consistent with that of the BELLE‐2 trial. Serious AEs were reported in 22% and 16% of buparlisib and placebo groups, respectively. Adverse events that led to dose interruptions were more frequent in the buparlisib group than in the placebo group (36% vs 9%), as were dose reductions (31% vs 8%) or discontinuation (21% vs 5%). The most common reasons (all‐grade AEs) for permanent discontinuation of treatment with buparlisib were elevated ALT (6%), elevated AST (4%), and depression (2%). Suicidal ideation was noted in both treatment groups (2% vs 1%), and three suicide attempts were reported in the buparlisib group. Most on‐treatment deaths were due to metastatic breast cancer, but two were considered treatment‐related (cardiac failure [n = 1] in the buparlisib group and unknown reason [n = 1] in the placebo group). 23
Efficacy results from BELLE‐2 and BELLE‐3 demonstrated that patients with PIK3CA‐mutant, HR+, HER2‐negative ABC benefited more from treatment with a PI3K inhibitor than patients without PIK3CA mutations. However, the toxicity associated with buparlisib did not support further development of the combination of buparlisib and fulvestrant. 22 , 23
4.2. Beta‐sparing PI3K inhibitor: Taselisib
The efficacy and safety of taselisib, a beta‐sparing 28 PI3K inhibitor with enhanced activity in PIK3CA mutated breast cancer cell lines, were evaluated in the phase 3 SANDPIPER trial. PIK3CA mutation status was identified using centralized Roche cobas test (which detects the following mutations: R88Q, N345K, C420R, E542K, E545A/G/K/D, Q546K/R/E/L, M1043I, H1047L/R/Y, G1049R) (Table 1). 5 , 22 , 23 , 24 , 25 , 26 , 27 A total of 631 postmenopausal patients with ER+, HER2‐negative ABC who had disease recurrence or progressed on aromatase inhibitor were included, 516 of whom were PIK3CA‐mutant. Taselisib in combination with fulvestrant improved investigator‐assessed mPFS compared with fulvestrant alone (Table 1). The most frequent AEs of any grade in the taselisib group were diarrhea (60%), hyperglycemia (40%), stomatitis (33%), nausea (34%), decreased appetite (26%), and rash (25%). Grade 3/4 AEs occurred in 50% of patients in the taselisib‐plus‐fulvestrant group and only 16% in the placebo‐plus‐fulvestrant group. Serious AEs were also more common in the taselisib group, occurring in 32% compared with 9% in the placebo group. Adverse events led to taselisib discontinuation, dose interruption, and dose reduction in 17%, 41%, and 37% of patients, respectively. Overall, investigators concluded that taselisib had a modest benefit but an unfavorable safety profile. 24
4.3. Alpha‐specific PI3K inhibitor: Alpelisib
Alpelisib is an alpha‐specific PI3K inhibitor used in combination with fulvestrant for the treatment of postmenopausal women and men with HR+, HER2‐negative, PIK3CA‐mutated ABC. In the phase 3 SOLAR‐1 trial (NCT02437318), the safety and efficacy of alpelisib in combination with fulvestrant were evaluated in patients with HR+, HER2‐negative ABC who received prior endocrine therapy. 5 Mutation status of PIK3CA in SOLAR‐1 was determined by using a validated clinical trial assay and was transitioned to QIAGEN therascreen® PIK3CA RGQ polymerase chain reaction (PCR) kit to detect mutation hotspots in the C2 (C420R), helical (E542K, E545A, E545D, E545G, E545K, E545X, Q546E, Q546R, Q546X), and kinase domains (H1047L, H1047R, H1047X, H1047Y) of PI3K (exons 7, 9, and 20, respectively). 25 Formalin‐fixed, paraffin‐embedded tumor samples were used for testing of samples obtained at initial diagnosis or at the most recent biopsy. In addition, ctDNA was obtained at baseline and analyzed by PCR to evaluate PFS by PIK3CA mutation status 26 (Table 1). 5 , 22 , 23 , 24 , 25 , 26 , 27
A total of 572 patients were included in the trial, 341 of whom had PIK3CA‐mutated ABC by tissue analyses, while 231 were included in the PIK3CA‐nonmutated cohort. 5 , 26 The primary endpoint of PFS in the PIK3CA‐mutant cohort was met. Progression‐free survival in the PIK3CA‐mutant cohort was significantly longer in the alpelisib‐plus‐fulvestrant group compared with the placebo‐plus‐fulvestrant group (11.0 vs 5.7 months; HR 0.65; P = .00065). Notably, the proof‐of‐concept criteria to determine if treatment benefit was obtained in the PIK3CA‐nonmutant cohort were not met. 5
The most common AEs of any grade that occurred in the alpelisib‐plus‐fulvestrant group were hyperglycemia (64%), diarrhea (58%), nausea (45%), decreased appetite (36%), and rash (36%). Patients who discontinued treatment due to AEs with alpelisib and placebo were 25% and 4.2%, respectively. Hyperglycemia was the most common AE leading to alpelisib discontinuation, occurring in 6.3% of patients. 5 , 29 In patients treated with alpelisib, diabetic and prediabetic patients demonstrated greater mean increases in fasting plasma glucose from baseline than patients with normal glucose at baseline. 26
In patients with PIK3CA mutations detected in ctDNA (n = 186), PFS was found to be significantly improved in alpelisib‐treated patients compared with placebo (10.9 vs 3.7 months; HR 0.55; P value not specified), consistent with the PFS results in PIK3CA‐mutant tissue samples. 26 Retrospective testing (n = 415) using the FoundationOne CDx® 324‐gene panel next‐generation sequencing (NGS) assay was done to further characterize PIK3CA alterations observed in patients from SOLAR‐1. 25 The PIK3CA mutation status was generally consistent between PCR used at screening to classify patients and the NGS assay. 25 Patients with altered PIK3CA status (as determined by NGS) showed a very similar improvement in PFS when alpelisib was added to fulvestrant treatment 27 (Table 1). 5 , 22 , 23 , 24 , 25 , 26 , 27 Overall, the results of SOLAR‐1 suggest that PIK3CA mutation status predicts response to alpelisib.
5. PRACTICAL CONSIDERATIONS FOR PIK3CA TESTING
In clinical practice, breast cancer patients may undergo core biopsy, providing adequate tissue for diagnosis and biomarker testing, with an option for subsequent excisional biopsy or mastectomy. 30 As such, there is typically sufficient tissue available. However, it can be challenging to implement biomarker testing across samples in clinical trials—especially when there is a limited amount of tumor sample. 31 As a recent example, 27% of patients in the SOLAR‐1 trial did not undergo NGS testing due to insufficient quantity or quality of the tissue samples. 25
Another important issue is tissue preservation and preparation, which can affect the sample quality. Delays in fixation may lead to degradation of RNA and protein, which may cause alteration of immunohistochemical results; however, the American Society of Clinical Oncology—College of American Pathologists (ASCO‐CAP) guideline recommendations for biomarker testing have practically eliminated this as an issue with requirements for immediate fixation for a duration of 6 hours and not to exceed 72 hours and mandatory recording of the times in the surgical pathology report. 32 , 33 , 34 , 35 , 36 , 37 Other factors that should be taken into consideration include the type of test required, concordance between different tests, and testing centers. Communication between the clinicians and pathologists is essential to determine the type of sample, method of processing, and tests required. 31
Tumor heterogeneity and evolution should also be considered. For some types of alterations, increased rates of cell proliferation and mutation combined with genomic instability lead to significant genetic variations and intratumor heterogeneity; however, such heterogeneity for standard breast cancer biomarkers is observed in only approximately 1% of primary breast cancer biopsies. 31 , 38 Incidence of tumor heterogeneity within metastases may also occur more frequently relative to primary sites—including occurrence of ESR1 mutations in metastases that lead to acquired resistance to antiestrogen therapy (such as aromatase inhibitors). 39 , 40 , 41 Hence, it has been suggested that biomarker testing should be done in both the primary and metastatic/recurrent lesions to account for tumor heterogeneity. 42 However, it may be difficult to obtain metastatic specimens of adequate quality since core biopsy specimens tend to be smaller, which can result in insufficient sample for molecular analysis and lead to impurities due to stromal contamination. Re‐biopsies may not always be feasible due to the locations of the metastatic site, and the procedure may be associated with increased morbidity. Furthermore, a comparative genomic analysis done comparing the primary tumors and matched metastatic lesions from 23 patients with breast cancer showed that mutational profiles were concordant except for one patient. 43 Thus, overall, the choice of targeted therapy may change relatively infrequently when the metastatic lesion is profiled versus the primary lesion.
Although there are multiple types of assays available for screening, two of the most commonly used assays in clinical trials for breast cancer are PCR and NGS. Polymerase chain reaction assays are low‐cost, sensitive, rapid tests that can detect specific target mutations but do not provide a comprehensive analysis of genes being investigated. Next‐generation sequencing assays are highly sensitive and also have the ability to assay all mutations in the genome, including rare sequences and complex mutations. 31 , 44 In the retrospective NGS analysis of PIK3CA mutation in SOLAR‐1, NGS was able to detect 60 different mutations across multiple exons and five copy number variations, in contrast to point mutations across three exons by PCR. In addition, out of 175 patients assigned to the nonmutant cohort by PCR, NGS testing revealed that 28 (16%) of these patients had a PIK3CA alteration. Also, NGS detected multiple PIK3CA mutations in 44 patients, six of whom had no detected mutations by PCR. 25 Despite increased sensitivity and wider coverage, NGS does have some drawbacks, including higher cost and longer turnaround time. 31 Currently, two assays have been approved by the US Food and Drug Administration for PIK3CA testing in advanced breast cancer. The therascreen PIK3CA RGQ PCR Kit (QIAGEN GmbH) is a companion diagnostic approved for use with alpelisib in combination with fulvestrant for the treatment of HR+, HER2‐negative PIK3CA‐mutated advanced or metastatic breast cancer (following progression on or after an endocrine‐based regimen). 6 , 21 , 45 The assay is capable of detecting 11 frequently observed mutations in the PIK3CA gene (exon 7: C420R; exon 9: E542K, E545A, E545D [1635G>T only], E545G, E545K, Q546E, Q546R; and exon 20: H1047L, H1047R, H1047Y). 45 , 46 The FoundationOne® CDx assay is also approved as a companion diagnostic for detection of those mutations. 47
Tissue‐based testing is considered standard for diagnosis and treatment selection because of its widespread use and the availability of substantial evidence to support its use in early and advanced cancers. 48 , 49 , 50 Molecular characterization of tumor tissue samples remains the current gold standard of personalized medicine. 49 However, tissue‐based testing is invasive, may be associated with complications of biopsy, and may not be feasible for all patients (patient too ill, or site not accessible to biopsy). 48 , 51 In recent years focus has shifted to DNA‐based biomarkers. In contrast to tissue‐based testing, ctDNA assays are minimally invasive, making them ideal for serial monitoring. 52 Compared with protein‐based biomarkers, ctDNA has a greater dynamic range and shorter half‐life (<2.5 hours), which allows it to be a more sensitive indicator of tumor progression and treatment response. Genetic alterations leading to treatment resistance may also be identified using ctDNA. Studies have demonstrated that ctDNA can be used to detect early recurrence in asymptomatic breast cancer patients who have undergone curative surgery. However, detection of early recurrence via biomarker analysis has not yet been shown to improve patient outcomes. Further, disease recurrence may occur without an increase in biomarker levels. Studies also suggest that in addition to identifying mechanisms of acquired resistance, these assays can be used to monitor treatment response in patients with ABC. 52 Serial plasma specimens collected from 30 women with ABC receiving systemic therapy were analyzed for ctDNA to monitor tumor burden using assays that were designed to detect somatic genomic alterations (including point mutations in PIK3CA and TP53) previously identified using targeted or whole genome sequencing. In addition, cancer antigen 15‐3 (CA 15‐3) and circulating tumor cells (CTC) were measured at the same time points. 53 CA 15‐3 is a serum biomarker that can be used to monitor treatment and prognosis of patients with breast cancer, but it has low sensitivity and specificity. 52 , 53 ctDNA, CA 15‐3, and CTC were then compared to radiographic imaging, which is the standard for noninvasive monitoring of treatment response in ABC. ctDNA was identified in 97% (29/30) of samples, while CA 15‐3 and CTC were detected in 78% (21/27) and 87% (26/30) of women, respectively. ctDNA was found to have a greater correlation with changes in tumor burden than CA 15‐3 and CTC and also provided the earliest measure of treatment response in 53% (10/19) of women. 53 Studies are also mostly retrospective; hence, further clinical validity and utility studies using ctDNA‐based monitoring are still needed. 48 ctDNA assays are also relatively expensive, labor intensive, and not widely available. Examples of ctDNA biomarkers in breast cancer include ESR1, PIK3CA, and TP53. 52 For the alpelisib PCR companion diagnostic, ctDNA derived from plasma of breast cancer patients or genomic DNA extracted from tumor tissue can be used. 46
6. RECOMMENDED PIK3CA TESTING PRACTICES
The results of phase 3 trials have been informative and support the utility of PIK3CA mutation testing to guide treatment decisions in patients with HR+, HER2‐negative ABC. These results also demonstrate the importance of stratification based on genetic testing at randomization (particularly the BELLE‐3 trial). 23
Overall, the reverse transcriptase PCR‐based companion diagnostic for alpelisib is likely sufficient for most clinicians’ needs. However, the assay is limited to the detection of specific mutations and does not account for less commonly observed mutations in PIK3CA, PIK3CA copy number alterations, or alterations in other genes relevant to PI3K signaling activity such as PTEN. 46 When adequate resources are available, clinicians may want to consider supplemental testing to detect a wider array of PI3K pathway aberrations.
As discussed above, there is substantial agreement between primary and metastatic lesions, so either source would be acceptable for informing treatment decisions. 43 To address potential concerns with sample quality and degradation as well as potential tumor evolution over time, it is recommended to coordinate efforts and use fresh samples for testing when possible. 31 In terms of timing, it is recommended to test for PIK3CA mutation at diagnosis of metastatic disease to see if the patient is suitable for alpelisib treatment (Figure 1).
Figure 1.
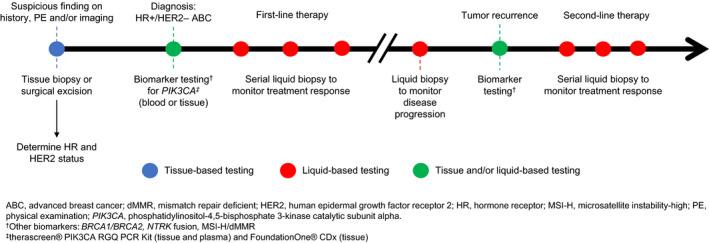
Potential timepoints for tissue and liquid biopsy testing in HR+/HER2–ABC. ABC, advanced breast cancer; dMMR, mismatch repair deficient; HER2, human epidermal growth factor receptor 2; HR, hormone receptor; MSI‐H, microsatellite instability‐high; PE, physical examination; PIK3CA, phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic subunit alpha. †Otherbiomarkers:BRCA1/BRCA2, NTRK fusion, MSI‐H/dMMR. 30 ‡ therascreen ® PIK3CA RGQ PCR Kit (tissue and plasma) and FoundationOne® CDx(tissue). 47 , 55
Although liquid biopsies are increasingly utilized, caution is warranted. Most patients have concordant tissue and liquid biopsy results, but liquid biopsies have a lower sensitivity and discordant results can occur. In most cases of discordance, the tissue sample is positive while the liquid biopsy does not detect a mutation. Hence, it is recommended that a reflex tumor tissue biopsy is done when no mutation is detected via liquid biopsy. When liquid biopsy does not detect a mutation, this may mean either the absence of the mutation in the tumor or a low amount of ctDNA in the sample. When a mutation is detected in liquid biopsy but not in tissue, it may be due to either temporal (archival tumor specimen) or spatial (subclonal mutation) heterogeneity. 48 A tissue sample only represents a single tumor region (one primary or metastatic site) and provides a snapshot of the time the sample was taken. Thus, it would not be reflective of the entire tumor molecular landscape. 48 , 50 In contrast, liquid biopsy may capture tumor sample ctDNA arising from all metastatic sites and can be done sequentially. 48 , 50 Patients with tumor‐undetected and liquid biopsy‐positive results are less likely to benefit from targeted therapy due to tumor heterogeneity. Lastly, an assay error may also occur that could give false‐negative tissue genotyping or a false‐positive ctDNA genotyping. It is also important to consider that there is no consensus regarding the timing of biomarker analysis. For early‐stage cancer, the clinical utility of using ctDNA for diagnosis is limited because mutations are generally detected at a lower rate than in advanced cancers. In advanced cancers, literature supports the use of ctDNA for treatment selection during disease progression rather than when the patient is still responding to prior therapy. When a tumor is responding to therapy, ctDNA levels may be low, thus decreasing odds of mutation detection (if present). 48
Although NGS‐based assays can detect genomic variations that may respond to targeted therapy, clinical utility in breast cancer outside of clinical trials has yet to be established. 48 , 54 A retrospective study was done in 44 metastatic breast cancer patients who underwent targeted NGS testing (FoundationOne) using the Illumina HiSeq 2000 platform. Sixteen patients were ER+, 4 were HER2‐positive, and 24 were triple‐negative. Almost all patients (n = 42, 95%) had actionable mutations, but only 55% (n = 23) of those with actionable mutations initiated targeted therapy, which suggests a lack of access to targeted therapies with approved indications and a lack of enrolling clinical trials. 54 However, as suggested by results from the SOLAR‐1 study, a number of patients were initially classified as not having altered PIK3CA by reverse transcriptase PCR analysis who were later reclassified after NGS testing. Further studies are needed to determine the incidence of rare PIK3CA mutations and to understand the impact of these mutations on clinical outcomes in the HR+, HER2‐negative ABC population.
7. SUMMARY AND CONCLUDING REMARKS
In a disease wherein molecularly targeted therapies have been limited to HER2 and estrogen receptor, PI3K inhibitors are a new class for which PIK3CA mutations predict response. Although CDK4/6 inhibitor‐based regimens have become the standard of care for advanced HR+, HER2‐negative ABC, the only biomarker that appears to predict response for that class of drugs is ESR1, which is not the molecular target. Although further studies are needed to establish the prognostic significance of PIK3CA mutations in advanced breast cancer, PIK3CA mutations predict a favorable response to PI3K inhibitor treatment.
When testing for PIK3CA mutations in ABC, various factors should be considered by pathologists and oncologists, including choice of assay, sample availability, sample collection and preparation, time of screening, and tumor heterogeneity and evolution. 14 , 16 , 31 , 44 Liquid biopsy technology is evolving but requires further evaluation. As previously noted, reflex tumor tissue biopsy is recommended for negative results due to considerable rates of discordance with tumor genotyping. 48 Next‐generation sequencing‐based assays can be considered but may have limited utility or availability in most clinical settings depending on expertise and reimbursement considerations. However, as additional molecular targets are identified in breast cancer, it is possible that NGS may become the standard of care for testing similar to what has occurred for stage IV lung cancer. With the approval of alpelisib along with therascreen PIK3CA RGQ PCR Kit (QIAGEN GmbH) and FoundationOne® CDx as companion diagnostics, PIK3CA testing practices may become standardized and widespread, and real‐world use should provide more information about the incidence and pathology of various PIK3CA alterations in HR+, HER2‐negative ABC.
CONFLICT OF INTEREST
The authors have disclosed the following potential conflicts of interest: Toppmeyer: Spouse employed by Merck; Press: Research grants to author's institution: Cepheid, Eli Lilly & Company, Novartis Pharmaceuticals, F. Hoffmann‐La Roche Ltd, Zymeworks; Consulting or advisory role with honoraria: Karyopharm Therapeutics, Puma Biotechnology, Biocartis, Eli Lilly & Company, Novartis Pharmaceuticals, F. Hoffmann‐La Roche Ltd; Expert testimony: Amgen; Travel, accommodations or expenses: Novartis.
AUTHOR CONTRIBUTIONS
Both authors contributed to the manuscript equally: conceptualization, writing ‐ original draft, and writing ‐ review and editing.
ACKNOWLEDGMENTS
Medical editorial assistance was provided by Audrey So, MD, from Healthcare Consultancy Group LLC and was funded by Novartis Pharmaceuticals Corporation. Shridar Ganesan, MD, PhD, provided his expert insights during the development of this review. M. F. Press was supported in part by grants from the Breast Cancer Research Foundation, Tower Cancer Research Foundation (Jessica M. Berman Senior Investigator Award), a gift from Dr Richard Balch and the Entertainment Industry Foundation, as well as an endowed chair, the Harold E. Lee Chair for Cancer Research.
Toppmeyer DL, Press MF. Testing considerations for phosphatidylinositol-3-kinase catalytic subunit alpha as an emerging biomarker in advanced breast cancer. Cancer Med. 2020;9:6463–6472. 10.1002/cam4.3278
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the number of women living with metastatic breast cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017;26(6):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Cancer Society . Breast Cancer Facts and Figures 2017–2018. Atlanta: American Cancer Society, Inc.; 2017. [Google Scholar]
- 4. Portman N, Alexandrou S, Carson E, Wang S, Lim E, Caldon CE. Overcoming CDK4/6 inhibitor resistance in ER positive breast cancer. Endocr Relat Cancer. 2019;26(1):R15‐R30. [DOI] [PubMed] [Google Scholar]
- 5. André F, Ciruelos EM, Rubovszky G, et al. Alpelisib (ALP) + Fulvestrant (FUL) for Advanced Breast Cancer (ABC): Results of the Phase III SOLAR‐1 Trial. Presented at: European Society for Medical Oncology 2018 Congress; October 19–23, 2018; Munich, Germany. Abstract LBA3‐PR. [Google Scholar]
- 6. Piqray [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2019. [Google Scholar]
- 7. Qin H, Liu L, Sun S, et al. The impact of PI3K inhibitors on breast cancer cell and its tumor microenvironment. PeerJ. 2018;6:e5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988‐1004. [DOI] [PubMed] [Google Scholar]
- 9. Thorpe LM, Yuzugullu H, Zhao JJ. PI3K in cancer: divergent roles of isoforms, modes of activation and therapeutic targeting. Nat Rev Cancer. 2015;15(1):7‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3‐kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3‐kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13(6):224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sobhani N, Roviello G, Corona SP, et al. The prognostic value of PI3K mutational status in breast cancer: a meta‐analysis. J Cell Biochem. 2018;119(6):4287‐4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mollon L, Aguilar A, Anderson E, et al. A systematic literature review of the prevalence of PIK3CA mutations and mutation hotspots in HR+/HER2‐ metastatic breast cancer. Cancer Res. 2018;78(13 Suppl). Abstract 1207. [Google Scholar]
- 15. Razavi P, Chang MT, Xu G, et al. The genomic landscape of endocrine‐resistant advanced breast cancers. Cancer Cell. 2018;34(3):427‐438 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Troxell M. PIK3CA/AKT1 mutations in breast carcinoma: a comprehensive review of experimental and clinical studies. J Clin Exp Pathol. 2012;02(01). 10.4172/2161-0681.S1-002 [DOI] [Google Scholar]
- 17. Zardavas D, Te Marvelde L, Milne RL, et al. Tumor PIK3CA genotype and prognosis in early‐stage breast cancer: a pooled analysis of individual patient data. J Clin Oncol. 2018;36(10):981‐990. [DOI] [PubMed] [Google Scholar]
- 18. Mosele F, Verret B, Lusque A, et al. Natural History and Outcome of Patients Presenting a Metastatic Breast Cancer (mBC) with PIK3CA Mutation. Presented at: American Association for Cancer Research 2019 Annual Meeting; March 29‐April 3, 2019; Atlanta, GA. Abstract 4895. [Google Scholar]
- 19. Anderson EJ, Mollon L, Dean JL, et al. A systematic literature review of the clinical prognosis of HR+/HER2‐ advanced or metastatic breast cancer with and without PIK3CA mutation. J Clin Oncol. 2018;36(15_suppl):e13037. [Google Scholar]
- 20. Mukohara T. PI3K mutations in breast cancer: prognostic and therapeutic implications. Breast Cancer. 2015;7:111‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stern HM, Gardner H, Burzykowski T, et al. PTEN loss is associated with worse outcome in HER2‐amplified breast cancer patients but is not associated with trastuzumab resistance. Clin Cancer Res. 2015;21(9):2065‐2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baselga J, Im S‐A, Iwata H, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor‐positive, HER2‐negative, advanced breast cancer (BELLE‐2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2017;18(7):904‐916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Di Leo A, Johnston S, Lee KS, et al. Buparlisib plus fulvestrant in postmenopausal women with hormone‐receptor‐positive, HER2‐negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE‐3): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Oncol. 2018;19(1):87‐100. [DOI] [PubMed] [Google Scholar]
- 24. Baselga J, Dent SF, Cortés J, et al. Phase III Study of Taselisib (GDC‐0032) + Fulvestrant (FULV) v FULV in Patients (pts) With Estrogen Receptor (ER)‐Positive, PIK3CA‐Mutant (MUT), Locally Advanced or Metastatic Breast Cancer (MBC): Primary Analysis from SANDPIPER. Presented at: American Society of Clinical Oncology Annual Meeting; June 1‐5, 2018; Chicago, IL. Abstract LBA 1006. [Google Scholar]
- 25. Rugo HS, Mayer I, Conte P, et al. Prevalence of PIK3CA Mutations in Patients with Hormone Receptor‐Positive, Human Epidermal Growth Factor Receptor‐2‐Negative Advanced Breast Cancer from the SOLAR‐1 trial. Presented at: American Association for Cancer Research 2019 Annual Meeting; March 29‐April 3, 2019; Atlanta, GA. Abstract CT 142. [Google Scholar]
- 26. Juric D, Ciruelos E, Rubovszky G, et al. Alpelisib + Fulvestrant for Advanced Breast Cancer: Subgroup Analyses from the Phase III SOLAR‐1 trial. Presented at: 2018 San Antonio Breast Cancer Symposium; December 4–10, 2018; San Antonio, TX. Abstract GS3‐08. [Google Scholar]
- 27. Juric D, André F, Singer CF, et al. Clinical Outcomes of Alpelisib in Hormone Receptor‐Positive, Human Epidermal Growth Factor Receptor‐2–Negative Advanced Breast Cancer by Next‐Generation Sequencing‐Detected PIK3CA Alteration Status and Phosphatase and Tensin Homolog Loss: Biomarker Analysis from the SOLAR‐1 Study. Presented at: 2019 San Antonio Breast Cancer Symposium; December 10–14, 2019; San Antonio, TX. Poster P4–10‐04. [Google Scholar]
- 28. Zumsteg ZS, Morse N, Krigsfeld G, et al. Taselisib (GDC‐0032), a potent beta‐sparing small molecule inhibitor of PI3K, radiosensitizes head and neck squamous carcinomas containing activating PIK3CA alterations. Clin Cancer Res. 2016;22(8):2009‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA‐mutated, hormone receptor‐positive advanced breast cancer. N Engl J Med. 2019;380(20):1929‐1940. [DOI] [PubMed] [Google Scholar]
- 30. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology ‐ Breast Cancer. March 6, 2020.
- 31. Han HS, Magliocco AM. Molecular testing and the pathologist's role in clinical trials of breast cancer. Clin Breast Cancer. 2016;16(3):166‐179. [DOI] [PubMed] [Google Scholar]
- 32. Srinivasan M, Sedmak D, Jewell S. Effect of fixatives and tissue processing on the content and integrity of nucleic acids. Am J Pathol. 2002;161(6):1961‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Apple S, Pucci R, Lowe AC, Shintaku I, Shapourifar‐Tehrani S, Moatamed N. The effect of delay in fixation, different fixatives, and duration of fixation in estrogen and progesterone receptor results in breast carcinoma. Am J Clin Pathol. 2011;135(4):592‐598. [DOI] [PubMed] [Google Scholar]
- 34. Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice guideline focused update. J Clin Oncol. 2018;36(20):2105‐2122. [DOI] [PubMed] [Google Scholar]
- 35. Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784‐2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolff AC, Hammond MEH, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118‐145. [DOI] [PubMed] [Google Scholar]
- 37. Wolff AC, Hammond MEH, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update. J Clin Oncol. 2013;31(31):3997‐4013. [DOI] [PubMed] [Google Scholar]
- 38. Press MF, Villalobos I, Santiago A, et al. Assessing the new American Society of Clinical Oncology/College of American Pathologists Guidelines for HER2 testing by fluorescence in situ hybridization: experience of an academic consultation practice. Arch Pathol Lab Med. 2016;140(11):1250‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toy W, Shen Y, Won H, et al. ESR1 ligand‐binding domain mutations in hormone‐resistant breast cancer. Nat Genet. 2013;45(12):1439‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Robinson DR, Wu Y‐M, Vats P, et al. Activating ESR1 mutations in hormone‐resistant metastatic breast cancer. Nat Genet. 2013;45(12):1446‐1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Oesterreich S, Davidson NE. The search for ESR1 mutations in breast cancer. Nat Genet. 2013;45(12):1415‐1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eccles SA, Aboagye EO, Ali S, et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013;15(5):R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bertucci F, Finetti P, Guille A, et al. Comparative genomic analysis of primary tumors and metastases in breast cancer. Oncotarget. 2016;7(19):27208‐27219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vnencak‐Jones CM, Berger M, Pao W. Types of Molecular Tumor Testing. https://www.mycancergenome.org/content/molecular‐medicine/types‐of‐molecular‐tumor‐testing/. Accessed July 7, 2020.
- 45. US FDA . List of Cleared or Approved Companion Diagnostic Devices (In Vitro and Imaging Tools). https://www.fda.gov/medical‐devices/vitro‐diagnostics/list‐cleared‐or‐approved‐companion‐diagnostic‐devices‐vitro‐and‐imaging‐tools. Accessed July 7, 2020.
- 46. US FDA . Premarket Approval (PMA) Therascreen PIK3CA RGQ PCR Kit. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfPMA/pma.cfm?id=P190004. Accessed July 7, 2020.
- 47. Foundation Medicine Inc . FoundationOne®CDx Technical Information. Cambridge, MA: Foundation Medicine, Inc; 2019. [Google Scholar]
- 48. Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J Clin Oncol. 2018;36(16):1631‐1641. [DOI] [PubMed] [Google Scholar]
- 49. Bratulic S, Gatto F, Nielsen J. The translational status of cancer liquid biopsies. Regen Eng Transl Med. 2019. 10.1007/s40883-019-00141-2 [DOI] [Google Scholar]
- 50. Alimirzaie S, Bagherzadeh M, Akbari MR. Liquid biopsy in breast cancer: a comprehensive review. Clin Genet. 2019;95(6):643‐660. [DOI] [PubMed] [Google Scholar]
- 51. Tay TKY, Tan PH. Liquid biopsy in breast cancer: a focused review. Arch Pathol Lab Med. 2020. 10.5858/arpa.2019-0559-RA [DOI] [PubMed] [Google Scholar]
- 52. Duffy MJ, McDermott EW, Crown J. Blood‐based biomarkers in breast cancer: from proteins to circulating tumor cells to circulating tumor DNA. Tumor Biol. 2018;40(5). 10.1177/1010428318776169 [DOI] [PubMed] [Google Scholar]
- 53. Dawson S‐J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199‐1209. [DOI] [PubMed] [Google Scholar]
- 54. Yuan Y, Yost SE, Yuan Y‐C, et al. Genomic mutation‐driven metastatic breast cancer therapy: a single center experience. Oncotarget. 2017;8(16):26414‐26423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. therascreen® PIK3CA RGQ PCR Kit Instruction for Use (Handbook). QIAGEN N.V. Hilden, Germany: QIAGEN GmbH; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


