Abstract
Antipsychotics have been utilized as the standard treatment for schizophrenia regardless of illness phase where antipsychotic monotherapy (APM) is routinely recommended as the gold standard rather than antipsychotic polypharmacy (APP). However, approximately 20 to 40% of patients with schizophrenia do not respond to APM based on randomized controlled clinical trials and large practical clinical trials indicating that the subgroup of patients with schizophrenia would need differential treatment approaches beyond traditional treatment strategies such as APM. Numerous studies have supported the use of APP in particular for patients with certain clinical situations including: failure to show efficacy or tolerability from treatment with APM, need for different treatment for targeting specific symptom domains, severe illness, failure to treatment with clozapine, skepticism about following treatment guidelines, or cross titration periods. Furthermore, recent large cohort studies and practical clinical trials have proposed more benefits of APP rather than APM in terms of rehospitalization, mortality, and specific symptoms. APP has recently become more widely utilized and recognized as one of the next treatment strategies to clinicians for patients with schizophrenia. Some experts have already proposed the revision of treatment guidelines incorporating APP as evidence-based treatment option for certain patients with schizophrenia. Taken together, APP now deserves an evidence-based and acceptable treatment strategy, not an empirical or preferential treatment approach for treatment of schizophrenia in contemporary clinical practice.
Keywords: Antipsychotic Agents, Polypharmacy, Schizophrenia, Cohort Studies
INTRODUCTION
Schizophrenia is a chronic and devastating mental illness needing a maintenance treatment for prevention of relapse and recurrence in a naturalistic practice setting. A number of antipsychotics have been developed and used to treat patients with schizophrenia, but the clinical outcomes of schizophrenia after proper treatment with antipsychotic agents are still unsatisfactory today.1,2,3,4,5 Antipsychotic monotherapy (APM) has been the gold standard for schizophrenia treatment,2,6,7,8,9 however, empirically 10 to 60% of schizophrenia patients respond poorly or only partially to APM in real practice.10,11,12,13,14 Based on recent individual patient data from randomized controlled trials (RCTs),15 approximately 2 out of 10 patients (19.8%) starting APM failed to show any symptom improvement after acute phase treatment for more than 4 weeks, however, it increased up to 4–7 out of 10 patients (43–67%) when applying different criteria for response to AP therapy (25/50% more reduction in Positive And Negative Syndrome Scale, PANSS).
Antipsychotic polypharmacy (APP) has been widely utilized for compensation of inadequate AP treatment response in real practice. APP is defined as the use two or more AP for treatments for schizophrenia for any reasons.16 Clinicians try APP in the treatment of schizophrenia for a a number of reasons including; pharmacodynamic synergy expecting differential neurotransmitter receptor affinity and occupancy broadening the range of receptor activity in combination with primary AP (positive symptoms rather than negative symptoms),11 pharmacokinetic aspects, lack of initial improvement from primary AP, avoidance of high dose AP therapy, pressure to find a rapid cure, reduction of hospitalization lengths, hastening of therapeutic response, severe psychopathology, cross titration, clozapine intolerance, evidence from RCTs, to counteract specific adverse events, treatment of comorbid conditions, economic concerns, and skepticism toward the use of treatment guidelines.
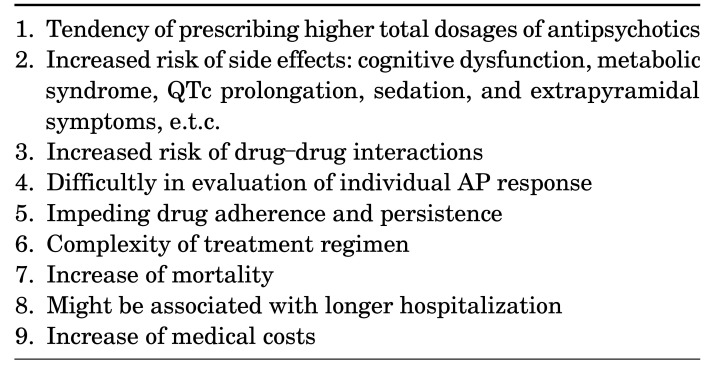
However, we need to consider numerous unexpected and unwanted adverse events from APP in the treatment of schizophrenia, which are described in Table 1. Indeed most practice guidelines do not recommend APP as the first line treatment for schizophrenia patients even if initial treatment effects were inadequate; they propose APP as one of next few available treatment options after several failures of APM, of course, some guidelines do not deal with any clinical viewpoints and opinions in relation with APP because of lack of efficacy and safety data till today.6,7,8
TABLE 1. Concerns about antipsychotic polypharmacy.
SEARCH OF DATA
The objective of the present paper was to elicit narrative and comprehensive review providing useful clinical information on the use of antipsychotic polypharmacy to clinicians by which rigorous systematic data search such as PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analyses) was not utilized. However, extensive and careful data search and review were performed by the author to produce unbiased data on the use of APP. Published articles were identified from PubMed using the key words ‘antipsychotic,’ ‘polytherapy,’ ‘schizophrenia’, ‘combination’, or ‘polypharmacy.’ There are a countless studies regarding the subject of this paper, which could not all be included here due to space limitations. Hence, largescale observational studies, claim data studies, RCTs, reviews, and meta-analyses were mainly retrieved and reviewed for the present review.
PREVALENCE OF APP
APP has been known to be utilized for 10 to 20% of schizophrenia cases in an outpatient basis, while 40% of schizophrenia cases in an inpatient basis. However, there has been wide variety of uses in epidemiological findings of APP due to a lack of established criteria on the number of APs, duration of AP combination, and other clinical situations related with AP use in treatment of schizophrenia.
According to the recent systematic review based on operational criteria using 147 studies,17 there were substantial differences in the prevalence and yearly trends of APP in North America (16%) with the lowest APP rates compared to those from Oceania (16.4%), Asia (32%) and Europe (23%).
Specifically for Asian regions, the recent Asian collaborative schizophrenia study18 including 15 countries (n=3,357) found that approximately 43% of participants were on APP which was substantially higher rates compared to that (32%) of previous systematic study.17 The mean dose of AP by chlorpromazine equivalents (CPZeq) was 424 mg/d. Among participating countries, Vietnam and Japan had the highest rates of APP usage, 59.2% and 57.4%, respectively. Such high prevalences of APP has been also consistently found in numerous independent studies in different geographic regions such as Korea, China, Brazil, Canada, America, and Japan.18,19,20,21,22,23
There has been no clear determination of how many number of APs are commonly used in APP, which is found to create a variable range of AP numbers. According to the extensive meta-analysis,17 17.8% were taking two APs and 0.2% were taking ≥3 antipsychotics. In a multicenter study24 using real world data (n=851), 19.2% of patients were on two APs, while 1.2% of patients were on three APs. In another recent study25 investigating the AP usage pattern (n=280), 44.1%, 24.4%, 1.4% and 0.7% of patients were on two, three, four and five APs, respectively. Currently available independent studies and meta-analysis tentatively showed that two APs were most frequently used in APP.
WHY DO CLINICIANS UTILIZE APP IN ROUTINE PRACTICE?
APP is not clearly defined by any consensus among experts as well as in treatment guidelines utilized for clinicians over the world and thereby it has been still one of major debates in the treatment of schizophrenia.
In general polypharmacy is defined to use five or more therapeutic agents for treating certain illness under indication,26 however, the specific criteria of APP yet not yet clearly been established. According to the recent study, it was found that APP was mostly defined simply as the use of two or more APs for treatment of schizophrenia.8 Indeed there should be numerous and diverse reasons for clinicians to utilize APP for treating their patients with schizophrenia in routine practice.
Given inadequate efficacy of APs for treatment of patients with schizophrenia, clinicians frequently and mainly use APP to ameliorate positive and/or negative symptoms, especially positive symptoms. APP is also utilized for treat and target specific comorbid symptoms such as anxiety, cognitive dysfunction, impulsive/aggressive behaviors, and sleep disturbance, e.t.c.7 Some clinicians are not willing to adhere to the recommended treatment guidelines proposing the use of APM due to insufficient persuasive reasons for the limited use of APP since inadequate clinical evidence exists from RCTs. Other reasons are also diverseincluding; cross titration, pressured feelings of clinicians themselves, treatment resistance cases, medical cost issues, prevention of relapse and recurrence, reduction of hospitalization, avoidance of high dose APM, preference of clinicians/patients, use of different pharmacological profile of individual AP for synergistic effects in treatment or counteracts against side effects, longstanding habits of clinicians for using cocktail therapy, differential choice of treatment strategy in acute/maintenance treatment phase, faster treatment response, and combination of different formulation of APs. Fig. 1 illustrates the various reasons for choosing APP in routine practice.
FIG. 1. Various reasons for choosing APP in routine practice.
WHAT BENEFITS DO WE EXPECT FROM APP?
Despite many concerns about APP, we can also expect and consider its benefits in the treatment of schizophrenia.27 In the most recent meta-analysis using data of RCTs (n=31) comparing APP vs. APM in schizophrenia,28 overall psychotic symptom reduction, and study-defined responses after AP treatment were compared between APP and APM. Based on the results, APP was found to be superior to APM regarding total symptom reduction with a large effect size difference (SMD=−0.53), while it failed to show superiority over APM regarding study-defined multiple response rates (≥20% PANSS/BPRS reduction, ≥25% PANSS reduction, and ≥20% PANSS reduction or CGI-I of 1 or 2), which is in contrast to that of their previous meta-analysis.29 Indeed, a significantly greater response rate (at least 50% reduction of the PANSS score or BPRS score or CGI-I of 1 or 2; RR=0.76 and NNT=7) was found in the previous meta-analysis including only 6 relevant studies,29 while it was not replicated in the recent meta-analysis.28 In detail, APP was superior in inpatient only studies (n=4), Chinese studies (n=4) and non-North American/European studies (n=5), while the number of high-quality studies was too inadequate to address separate analysis. Interestingly, this superiority of APP over AMP regarding both total symptom reduction and study-defined responses also became nonsignificant when analyzing only high-quality studies, indicating that such efficacy differences between APP and APM were mostly found in low-quality studies rather than by high-quality studies, expectation and selection biases in the studies included, and still lack of high-quality evidence comparing for efficacy between APP vs. APM.
According to the recent large-scale, non-interventional, retrospective-prospective parallel arm study comparing APP (addition of a second AP after >60 days of APM, n=7,901) vs. APM (switch to a new AP after >60 days of APM, n=5,480) in Hungary, significantly more psychiatric hospitalizations were found with APM than APP (hazard ration, HR=1.69).30 Further, the most recent nationwide cohort study compared the risk of psychiatric rehospitalization between APP vs APM including 62,250 schizophrenia patients analyzing 29 different APM and APP treatment types for 10 years.31 Based on the results, clozapine plus aripiprazole polypharmacy ranked as the lowest risk of psychiatric rehospitalization in the total cohort, compared to that of clozapine monotherapy, with a difference of 14%. Also, such differences between clozapine plus aripiprazole polypharmacy vs clozapine monotherapy were more evident in a subgroup analysis regarding the first episode schizophrenia showing a difference of 22% favoring APP over APM. In addition, aggregated data analysis also showed that any APP had 7% to 13% lower risks of psychiatric rehospitalization as compared with any APM.
Likewise a recent, large naturalistic study including acute-phase schizophrenia in Japan (n=1,543)21 also showed the clinical benefit of APP when the primary and secondary APM strategies failed in clinical practice where approximately 59% of the patients overall were responders to an initial or a second APM. As a next step treatment, APP (first or third AP combination to the second AP) was administered to the non-responders (n=581, 37.7%) where 522 (89.8%) showed a CGI-I score ≤3, while only 10.2% of the remaining patients showed a CGI-I score of ≥4. The responder rate of 89.8% observed in APP was much higher than the reported response rate to clozapine (40%) in the previous meta-analysis including treatment-resistant schizophrenia (TRS).32
Interestingly, a 6-month, recent RCT (n=127)33 tried to evaluate clinical benefits and risks between the continuation of APP and switching to APM in an outpatient clinic. The primary endpoint was time to all-cause discontinuation and it was shorter for patients in the APM switching group than in the APP continuation group and the APM switching group showed more frequent treatment discontinuation than the APP continuation group. Overall, 86% (n=48) in the APP continuation group were still on both APs, while 69% (n=40) in the APM switching group were still on same treatment indicating a 17% difference in AP change favoring APP over APM. Furthermore, those who stopped one AP were more associated with earlier change of their treatment in the APM switching group than in the APP continuation group; it was also notable that a significant portion of these individuals resumed their previous APP regimen. Despite a failure to show superiority of APP over APM since two thirds of patients successfully switched to APM, it clearly demonstrated that a subgroup of patients may experience practical benefits from APP rather than APM and that patients should be allowed to recover the previous APP if an adequate trial with APM comes to an end with unsatisfactory results or no utility.
WHAT CONCERNS SHOULD WE HAVE IN THE USE OF APP IN ROUTINE PRACTICE?
1. APP is strongly associated with high-dose antipsychotic treatment
The proposed rationales for the use of APP includes scientific perspectives on differential PD of currently available SGAs. A combination of two or more APs with different PD would bring about optimal occupancy of dopamine D2 receptor in certain portion of patients needing higher levels of D2 occupancy or result in a diverse range of multiple receptor activities beyond D2 receptor.34 These are associated with the augmentation of therapeutic effect in patients with TRS, hastening of treatment response, or targeting some specific comorbid conditions such as anxiety, sleep disturbance, and cognitive dysfunction, e.t.c.
Another aspect is more practical and persuasive to clinicians in the use of APP. Combinations of low-dose, two or more, APs possessing differential affinities and occupancy of multiple receptors is expected to achieve significant reduction of SEs which are susceptible to high dose APM and to attain already existing efficacy. However, currently available evidence suggests that APP is strongly associated with high/excessive dosing trends in routine practice.35
According to the previously mentioned large 10-year cohort study,16 the daily doses of APs were substantially increased in both calculations “DDD” and “CPZeq” during the 10 year periods. The yearly DDD in AMP patients also significantly increased, however, it was found to double in APP patients indicating that APP is mainly utilized for augmenting inadequate efficacy rather than counteracting SEs. Such huge differences in total AP dose between APM and APP might be possibly explained by several aspects; prior inadequate or under-treatment of first-episode schizophrenia in 1996 and conversion to a sufficient level of treatment in 2005, trends toward aggressive SGA use based on increasing clinical experiences with SGAs since high-dose FGA use is vulnerable to SEs, intensive marketing of SGAs by manufacturers since it was the early period of SGAs launch in the market, and earlier control of psychotic symptoms to reduce hospitalization for compensation of insufficient admission capacity.
The recent study analyzed the claim-data system regarding community mental health outpatients who had been treated with the same pharmacologic regimen for at least 3 months in Canada (n=435).23 The mean prescribed daily dose (PDD)/DDD ratio was significantly higher in the APP group than in the APM group (1.94 vs 0.94), further to say the PDD/DDD ratio was also extremely excessive in the APP group compared to the APM group since a 1.5 PDD/DDD ratio is considered to be a cutoff for excessive dosing. The proportion of excessive dosing was also approximately three times higher in the APP group than in the APM group irrespective of primary diagnosis. It was more profound in patients who were treated with SAG plus SGA polypharmacy and when the dose of initial AP was high. More interestingly the mean PDD/DDD ratio for individual SGA increased when it was a part of APP regardless of primary diagnosis as well. A previous study36 also found a strong association between persistent excessive dosing and APP on admission. In another previous study,37 the PDD/DDD ratio was 2.6 in the APP group, while it was 1.3 in the APM group, which is in line with recent and previous similar studies.16,23 According to the recent East Asian study investigating the medication trend in 2005 (n=194) and 2010 (n=201),38 it was found that the rate of high-dose APM significantly decreased from 30.4 to 18.4% across the year, while the rate of high-dose APP significantly increased from 34.0 in 2005 to 45.3% in 2010, indicating a replacement of high doses APM treatment trends by a high-dose APP approach. In a regression analysis, APP was confirmed to be strongly associated with high doses of AP prescription compared to APM (odds ratio=18.6).38 Such high dose trends in APP (888.3 mg/d of CPZeq) compared to APM (445.1 mg/d of CPZeq) was also replicated in another Asian study.39
2. High doses of AP in APP is strongly associated with cognitive dysfunction
Currently, a large number of studies have shown possible differences in cognitive functions between FGA and SGA where findings suggested that FGAs are mainly effective in controlling positive psychotic symptoms due to their affinity for and occupancy of D2 receptors, while SGAs are also effective to ameliorate or even improve some domains of cognitive dysfunction, not only positive symptoms. Such differences have also replicated in a number of well-designed, recent, systematic meta-analyses.40,41
A previous Japanese study (n=136)39 investigated whether APM would have better a cognitive influence than APP irrespective of combinations of FGAs or SGAs. In the study, a significant negative correlation was found between cognitive function measured by composite scores of the Brief Assessment of Cognition in Schizophrenia (BACS) and the CPZeq dose of APs (BACS Z-score of difference was almost double favoring APM over APP). Such negative correlation between BACS scores and CPZeq doses was also significant in most individual cognitive components including verbal memory, motor speed, verbal fluency, attention, and speed of processing. Interestingly, the SGA APM group showed better cognitive function even when the APP was composed of SGA plus SGA. There were also no differences in cognitive function in the APP group, regardless of the classes of combined APs. Such data clearly indicates the high dose AP results in substantial deterioration of cognitive functions whether or not the APP regimen is SGAs or FGAs added to the first APs. In a previous study,42 it was also found that the average daily dose (ADD) of APs was significantly associated with the development of notorious cognitive impairment in schizophrenia patients. In fact, APP was strongly associated with high daily doses (12.1 mg/d of risperidone equivalent dose, RISeq) compared to APM (4.2 mg/d RISeq) as well as with poor cognitive functioning measured by BACS z-score. In the study, another important finding was that the ADD to create substantially detrimental cognitive dysfunction measured by the BACS score was found to be approximately RISeq 5 mg/d or more and additional increase of RISeq 2.3 mg/d would also cause a decrease of 0.5 standard deviations of the BACS score. Intriguingly, previous research43 found that dose-reduction of AP may lead to significant improvements in cognitive function measured by the Wisconsin card sorting test (WCST, 19.9% increase in total correct answers and 34.9% decrease in perseverative errors) in schizophrenia patients who were exposed to high-doses of APP, indicating the critical role of AP total dose in modification of cognitive dysfunction in schizophrenia patients.
3. Increase of metabolic syndrome (MS) risk with APP
MS is very important clinical issue in daily practice since we cannot avoid SE when we prescribe FGAs or SGAs for treating psychotic symptoms and it is highly associated with metabolic complications leading to increased cardiovascular mortality.44 MS is well-known to significantly increase the risks to developing Diabetes Mellitus (DM), stroke, coronary heart diseases and mortality.45 After 3 years of treatment with Aps, the trend of development of MS is gradually increasing, especially, SGAs are more associated with MS rather than FGAs.46
A recent meta-analysis45 investigated 126 analyses in 77 publications (n=25,692) regarding the association of APP and MS. According to the results, the overall rate of MS was 32.5%, giving only minor differences in accordance with different methodologies of studies included in the meta-analysis (i.e., criteria of MS definition, treatment setting and sample characteristics). According to another independent study using data from the records of 458 psychiatric inpatients to compare MS between APP and APM,47 the MS rate was significantly different between the two groups favoring APM (34.3%) over APP (50.0%). Some lipid markers such as HDL <40 mg/dL or Triglyceride/HDL-cholesterol >3.5 were also higher in APP group than in APM group. Such higher rates of MS in APP vs. APM have been consistently reported in a number of previous studies.42,48 A recent Japanese study48 has also proposed the association of APP (odds ratio=2.4) with the development of pre-metabolic syndrome, while APM was associated with neither premetabolic syndrome nor MS. This study strongly suggests that an adjustment of patients' lifestyle and modulation of APP regimen could modulate or prevent the development of MS. Another interesting point was that the visceral fat obesity group was associated with higher AP total daily dose.
However, there have been also mixed findings as to whether or not APP truly increase the risk of MS in comparison with APM, possibly proposing insufficient evidence to clearly answer the clinical question on this potential weak point regarding APP yet.49,50,51 In such studies,52 the significant baseline associations of increase in weight, body mass index, and other lipid parameters with APP compared to those with APM were not maintained at the end of the follow up period, particularly, in schizophrenia patients following their first-episode, indicating that naturalistic clinical course and time effect, should be also considered in the development and attaining of MS associated with the use of APP during treatment period.
4. QTc prolongation risk in the use of APP
Traditionally AP use has been proposed to be associated with prolongation of the QT interval corrected for heart rate (QTc). Recently a cross-sectional survey (n=725)53 was conducted to investigate the relationship between APP and QTc interval. Among included patients, 186 (26%) were on APP and the mean cumulative AP dose was significantly higher in the APP group (PDD/DDD ratio=2.9) than APM (PDD/DDD ratio=0.8). As being expected, the mean QTc interval was significantly longer in the APP group (mean=420.9) than the APM group (mean=413.4). According to the large Italian network study54 based on routine practice (n=2,411), the APP treatment was positively associated with QTc prolongation despite heterogenous samples included in the study. A number of previous studies55,56 suggested that APP is potentially associated with the prolongation of QTc which is also relevant and speculative since the number of APs is a proxy of total AP dose, which is an established risk factor of QTc prolongation.57 Despite supporting evidence that APP may prolong the QTc interval, the previous meta-analysis58 failed to show clear evidence that APP significantly prolonged the QTc interval compared to APM due to a dearth of data regarding this research area and such research has been confined to the use of specific high-risk APs in the combinations such as ziprasidone, sertindole, or clozapine.
5. Poor treatment adherence in APP
Poor adherence is one of major barriers in achieving optimal clinical outcomes in schizophrenia patients and thus APP could pose a problem for maintenance of treatment since polypharmacy is clearly and consistently found to be associated with poor treatment adherence for many reasons.59 Poor adherence usually results in high rates of recurrence/relapse within a few years of recovery from the first episode which is strongly associated with poor clinical outcomes, functional impairment, high medical costs, increased rehospitalization, and unnecessary antipsychotic prescription.60,61
In a recent study,62 the non-adherence rate was 41.0% among schizophrenia patients, in which APP was found to increase the risk of non-adherence approximately twice as much compared to those with APM. There have been some debates about whether the number of APs are directly associated with poor adherence, however, a number of studies have clearly indicated that APP is strongly associated with increased risk of diverse SEs leading to poor adherence and persistence.63,64 APP was also found to be significantly associated with increased hospitalization.65 Hence it should be reasonable that APP could, at least indirectly, increase the risk of non-adherence and non-persistence, ultimately influences on the poor clinical course and treatment outcomes.
DISCUSSION
Based on currently available data from RCTs, small-scale open trials, and large cohort studies and findings from meta-analysis, APP has become closer to being a part of routine practice and not being a part of unacceptable practice in the last few decades. We have to consider practical points for proper use of APP in our routine practice.
According to intense analysis based on a large patient-level dataset of randomized multicenter trials, the rates of nonresponse and nonremission from APM for acute treatment for schizophrenia were notably high regardless of criteria for response and remission.15 APP can be actively considered if patients do not respond to adequate trials of APM with proper doses and durations of treatment or they cannot tolerate APM for any reasons (i.e., SE due to high-dose therapy). There have been no established adequate trial durations or numbers of APM. However, most treatment guidelines propose that at least two or more APMs should be tried before moving toward further treatment steps including APP. Furthermore, at least 8 weeks up to 4 months be used for evaluation of treatment effects coming from one APM trial based on treatment guidelines and existing literature.9,66
According to the recent large cohort study that followed patients for 20-years,49 clozapine plus aripiprazole polypharmacy was associated with the best outcome regarding psychiatric rehospitalization among all the 29 different APM and APP types, giving 14% to 23% lower risk of rehospitalization than clozapine monotherapy which showed the best outcome as APM. Interestingly, the clozapine doses were 426 mg/d and 399 mg/d in APM and APP, respectively, indicating that the reduced dose cannot be a main reason for better outcomes since the difference was slight, so diverse receptor activities from different APs (i.e., partial D2 receptor agonist effect of aripiprazole) may exert favorable treatment outcomes while ameliorating tolerability concerns. Any APP presented a 7% to 13% lower risk of psychiatric rehospitalization compared with any APM, indicating that rational APP should be considered proper and feasible particularly with the use of two different APs possessing different types of receptor profiles. The following could be also good examples: D2 receptor antagonist+partial agonist; D2 receptor tight binding agent+loose D2 receptor binding agent, e.t.c.
The most recent, largest and longest cohort study has clearly stated the superiority of any APP over any APM, in terms of rehospitalization and mortality.31 Furthermore, improper switch to APM was vulnerable to increasing the risk of relapse and recurrence compared to staying on APP in chronic and stabilized patients with schizophrenia.67 Indeed Dr. Stahl suggested that the changing trend toward APP in the treatment of schizophrenia has already started and thereby treatment guidelines should properly incorporate the wise and wide usage of APP in certain clinical situations and for the subgroup of patients with schizophrenia, providing 12 sensible recommendations.56,66
Currently available data from large practical clinical trials, meta-analysis, and open trials, potentially demonstrated that APP appears to no longer be an eminencebased treatment approach but now it should be immersed as an evidence-based, acceptable treatment strategy in clinical practice. APP is no longer a dirty secret for clinicians as an option for treatment of schizophrenia in clinical practice since we cannot evenly apply APM for treating patients with schizophrenia.66 The naturalistic treatment settings are quite different compared to those of RCTs, more severe and selected patients are included in such controlled trials, while clinicians should meet very heterogenous patients with diverse psychotic and comorbid conditions not easily responding to APM. Table 2 suggests practical points to consider in the use of APP in routine practice.
TABLE 2. Practical points in the use of antipsychotic polypharmacy in routine practice.
Best-match and properly-balanced APP may be one of the best-available treatment next steps and practical treatment options for patients with multiple treatment failures and tolerability issues today. It is the right time for intensive explorations and debates on the proper time and duration of APP, who should be the right patients for APP, wise shifting time/clinical situations from APP to APM, how to optimize the combination of APs in APP, clear benefits and risks of APP vs APM in individualized naturalistic treatment settings, and the appropriate revision of practice guidelines for APP which can promote secure APP and help policy makers to accept APP as one of routine treatment practices for the treatment of schizophrenia. Definitely, more adequately-powdered and well-designed APP clinical trials should be attempted to help us determine the clinical benefits, best-available APP agents, disadvantages, pharmaco-economic aspects, and individualization of APP in routine practice.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Pae CU, Han C, Bahk WM, Lee SJ, Patkar AA, Masand PS. Effectiveness and tolerability of switching to aripiprazole once monthly from antipsychotic polypharmacy and/or other long acting injectable antipsychotics for patients with schizophrenia in routine practice: a retrospective, observation study. Clin Psychopharmacol Neurosci. 2020;18:153–158. doi: 10.9758/cpn.2020.18.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang SM, Han C, Lee SJ, Jun TY, Patkar AA, Masand PS, et al. Investigational dopamine antagonists for the treatment of schizophrenia. Expert Opin Investig Drugs. 2017;26:687–698. doi: 10.1080/13543784.2017.1323870. [DOI] [PubMed] [Google Scholar]
- 3.Fountoulakis KN, Panagiotidis P, Theofilidis AT, Nimatoudis I. One-year outcome of first vs. later episode schizophrenia: a real-world naturalistic study. Clin Psychopharmacol Neurosci. 2020;18:434–444. doi: 10.9758/cpn.2020.18.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee Y, Lee MS, Jeong HG, Youn HC, Kim SH. Medication adherence using electronic monitoring in severe psychiatric illness: 4 and 24 weeks after discharge. Clin Psychopharmacol Neurosci. 2019;17:288–296. doi: 10.9758/cpn.2019.17.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pae CU. Role of the cholinesterase inhibitors in the treatment of schizophrenia. Expert Opin Investig Drugs. 2013;22:293–298. doi: 10.1517/13543784.2013.762355. [DOI] [PubMed] [Google Scholar]
- 6.Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. 2017;62:604–616. doi: 10.1177/0706743717720448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 Suppl):1–56. [PubMed] [Google Scholar]
- 8.Hasan A, Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, et al. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Schizophrenia, part 1: update 2012 on the acute treatment of schizophrenia and the management of treatment resistance. World J Biol Psychiatry. 2012;13:318–378. doi: 10.3109/15622975.2012.696143. [DOI] [PubMed] [Google Scholar]
- 9.Lee JS, Yun JY, Kang SH, Lee SJ, Choi JH, Nam B, et al. Korean Medication Algorithm for Schizophrenia 2019, second revision: treatment of psychotic symptoms. Clin Psychopharmacol Neurosci. 2020;18:386–394. doi: 10.9758/cpn.2020.18.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Schizophrenia relapse and the clinical usefulness of once-monthly aripiprazole depot injection. Neuropsychiatr Dis Treat. 2014;10:1605–1611. doi: 10.2147/NDT.S52486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SM, Han C, Lee SJ, Patkar AA, Masand PS, Pae CU. Asenapine, blonanserin, iloperidone, lurasidone, and sertindole: distinctive clinical characteristics of 5 novel atypical antipsychotics. Clin Neuropharmacol. 2013;36:223–238. doi: 10.1097/WNF.0b013e3182aa38c4. [DOI] [PubMed] [Google Scholar]
- 12.Oltra JAE. Improving therapeutic interventions of schizophrenia with advances in stem cell technology. Clin Psychopharmacol Neurosci. 2020;18:352–361. doi: 10.9758/cpn.2020.18.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee D, Lee BC, Choi SH, Kang DH, Jon DI, Jung MH. Effects of paliperidone palmitate on healthcare utilization and costs for patients with schizophrenia: a claim-based mirror-image study in South Korea. Clin Psychopharmacol Neurosci. 2020;18:303–310. doi: 10.9758/cpn.2020.18.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamei H, Homma Y, Takeuchi I, Hajitsu G, Tozawa K, Hatano M, et al. Acceptance of the deltoid muscle injection of aripiprazole long-acting injectable in the patients with schizophrenia. Clin Psychopharmacol Neurosci. 2020;18:49–57. doi: 10.9758/cpn.2020.18.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samara MT, Nikolakopoulou A, Salanti G, Leucht S. How many patients with schizophrenia do not respond to antipsychotic drugs in the short term? An analysis based on individual patient data from randomized controlled trials. Schizophr Bull. 2019;45:639–646. doi: 10.1093/schbul/sby095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Correll CU, Gallego JA. Antipsychotic polypharmacy: a comprehensive evaluation of relevant correlates of a long-standing clinical practice. Psychiatr Clin North Am. 2012;35:661–681. doi: 10.1016/j.psc.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138:18–28. doi: 10.1016/j.schres.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong M, Zeng LN, Zhang Q, Yang SY, Chen LY, Najoan E, et al. Prescription of antipsychotic and concomitant medications for adult Asian schizophrenia patients: findings of the 2016 Research on Asian Psychotropic Prescription Patterns (REAP) survey. Asian J Psychiatr. 2019;45:74–80. doi: 10.1016/j.ajp.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Hou CL, Wang SB, Wang F, Xu MZ, Chen MY, Cai MY, et al. Psychotropic medication treatment patterns in community-dwelling schizophrenia in China: comparisons between rural and urban areas. BMC Psychiatry. 2019;19:242. doi: 10.1186/s12888-019-2217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costa JO, Ceccato MDGB, Melo APS, Acurcio FA, Guimarães MDC. Gender differences and psychotropic polypharmacy in psychiatric patients in Brazil: a cross-sectional analysis of the PESSOAS Project. Cad Saude Publica. 2017;33:e00168915. doi: 10.1590/0102-311X00168915. [DOI] [PubMed] [Google Scholar]
- 21.Hatta K, Hasegawa H, Imai A, Sudo Y, Morikawa F, Katayama S, et al. Real-world effectiveness of antipsychotic monotherapy and polytherapy in 1543 patients with acute-phase schizophrenia. Asian J Psychiatr. 2019;40:82–87. doi: 10.1016/j.ajp.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Faries D, Ascher-Svanum H, Zhu B, Correll C, Kane J. Antipsychotic monotherapy and polypharmacy in the naturalistic treatment of schizophrenia with atypical antipsychotics. BMC Psychiatry. 2005;5:26. doi: 10.1186/1471-244X-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Procyshyn RM, Honer WG, Wu TK, Ko RW, McIsaac SA, Young AH, et al. Persistent antipsychotic polypharmacy and excessive dosing in the community psychiatric treatment setting: a review of medication profiles in 435 Canadian outpatients. J Clin Psychiatry. 2010;71:566–573. doi: 10.4088/JCP.08m04912gre. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Lee HW, Jung SH, Kang MH, Bae JN, Lee JS, et al. Prescription patterns for patients with schizophrenia in Korea: a focus on antipsychotic polypharmacy. Clin Psychopharmacol Neurosci. 2014;12:128–136. doi: 10.9758/cpn.2014.12.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yazici E, S Cilli A, Yazici AB, Baysan H, Ince M, Bosgelmez S, et al. Antipsychotic use pattern in schizophrenia outpatients: correlates of polypharmacy. Clin Pract Epidemiol Ment Health. 2017;13:92–103. doi: 10.2174/1745017901713010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guillot J, Maumus-Robert S, Bezin J. Polypharmacy: a general review of definitions, descriptions and determinants. Therapie. 2019 doi: 10.1016/j.therap.2019.10.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Hong JS, Kim SM, Min KJ, Chung US, Han DH. Effects of amisulpride adjunctive therapy on working memory and brain metabolism in the frontal cortex of patients with schizophrenia: a preliminary positron emission tomography/computerized tomography investigation. Clin Psychopharmacol Neurosci. 2019;17:250–260. doi: 10.9758/cpn.2019.17.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galling B, Roldán A, Hagi K, Rietschel L, Walyzada F, Zheng W, et al. Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis. World Psychiatry. 2017;16:77–89. doi: 10.1002/wps.20387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Correll CU, Rummel-Kluge C, Corves C, Kane JM, Leucht S. Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull. 2009;35:443–457. doi: 10.1093/schbul/sbn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katona L, Czobor P, Bitter I. Real-world effectiveness of antipsychotic monotherapy vs. polypharmacy in schizophrenia: to switch or to combine? A nationwide study in Hungary. Schizophr Res. 2014;152:246–254. doi: 10.1016/j.schres.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Tiihonen J, Taipale H, Mehtälä J, Vattulainen P, Correll CU, Tanskanen A. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76:499–507. doi: 10.1001/jamapsychiatry.2018.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siskind D, Siskind V, Kisely S. Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can J Psychiatry. 2017;62:772–777. doi: 10.1177/0706743717718167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Essock SM, Schooler NR, Stroup TS, McEvoy JP, Rojas I, Jackson C, et al. Effectiveness of switching from antipsychotic polypharmacy to monotherapy. Am J Psychiatry. 2011;168:702–708. doi: 10.1176/appi.ajp.2011.10060908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freudenreich O, Goff DC. Antipsychotic combination therapy in schizophrenia. A review of efficacy and risks of current combinations. Acta Psychiatr Scand. 2002;106:323–330. doi: 10.1034/j.1600-0447.2002.01331.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee LHN, Procyshyn RM, White RF, Woodward TS, Honer WG, Barr AM. Antipsychotic prescribing patterns on admission to and at discharge from a tertiary care program for treatment-resistant psychosis. PLoS One. 2018;13:e0199758. doi: 10.1371/journal.pone.0199758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barbui C, Biancosino B, Esposito E, Marmai L, Donà S, Grassi L. Factors associated with antipsychotic dosing in psychiatric inpatients: a prospective study. Int Clin Psychopharmacol. 2007;22:221–225. doi: 10.1097/YIC.0b013e3281084ea8. [DOI] [PubMed] [Google Scholar]
- 37.John AP, Dragovic M. Antipsychotic polypharmacy is not associated with reduced dose of individual antipsychotics in schizophrenia. J Clin Psychopharmacol. 2015;35:193–195. doi: 10.1097/JCP.0000000000000280. [DOI] [PubMed] [Google Scholar]
- 38.Roh D, Chang JG, Kim CH, Cho HS, An SK, Jung YC. Antipsychotic polypharmacy and high-dose prescription in schizophrenia: a 5-year comparison. Aust N Z J Psychiatry. 2014;48:52–60. doi: 10.1177/0004867413488221. [DOI] [PubMed] [Google Scholar]
- 39.Hori H, Yoshimura R, Katsuki A, Hayashi K, Ikenouchi-Sugita A, Umene-Nakano W, et al. Several prescription patterns of antipsychotic drugs influence cognitive functions in Japanese chronic schizophrenia patients. Int J Psychiatry Clin Pract. 2012;16:138–142. doi: 10.3109/13651501.2011.631018. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen RE, Levander S, Kjaersdam Telléus G, Jensen SO, Østergaard Christensen T, Leucht S. Second-generation antipsychotic effect on cognition in patients with schizophrenia--a meta-analysis of randomized clinical trials. Acta Psychiatr Scand. 2015;131:185–196. doi: 10.1111/acps.12374. [DOI] [PubMed] [Google Scholar]
- 41.Zhang JP, Gallego JA, Robinson DG, Malhotra AK, Kane JM, Correll CU. Efficacy and safety of individual second-generation vs. first-generation antipsychotics in first-episode psychosis: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16:1205–1218. doi: 10.1017/S1461145712001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elie D, Poirier M, Chianetta J, Durand M, Grégoire C, Grignon S. Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J Psychopharmacol. 2010;24:1037–1044. doi: 10.1177/0269881108100777. [DOI] [PubMed] [Google Scholar]
- 43.Kawai N, Yamakawa Y, Baba A, Nemoto K, Tachikawa H, Hori T, et al. High-dose of multiple antipsychotics and cognitive function in schizophrenia: the effect of dose-reduction. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1009–1014. doi: 10.1016/j.pnpbp.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Lochmann van Bennekom MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol. 2013;27:327–336. doi: 10.1177/0269881113477709. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders--a systematic review and meta-analysis. Schizophr Bull. 2013;39:306–318. doi: 10.1093/schbul/sbr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammoudeh S, Al Lawati H, Ghuloum S, Iram H, Yehya A, Becetti I, et al. Risk factors of metabolic syndrome among patients receiving antipsychotics: a retrospective study. Community Ment Health J. 2020;56:760–770. doi: 10.1007/s10597-019-00537-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Correll CU, Frederickson AM, Kane JM, Manu P. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89:91–100. doi: 10.1016/j.schres.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Misawa F, Shimizu K, Fujii Y, Miyata R, Koshiishi F, Kobayashi M, et al. Is antipsychotic polypharmacy associated with metabolic syndrome even after adjustment for lifestyle effects?: a cross-sectional study. BMC Psychiatry. 2011;11:118. doi: 10.1186/1471-244X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aly El-Gabry DM, Abdel Aziz K, Okasha T, Azzam H, Okasha A. Antipsychotic polypharmacy and its relation to metabolic syndrome in patients with schizophrenia: an Egyptian study. J Clin Psychopharmacol. 2018;38:27–33. doi: 10.1097/JCP.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 50.Santini I, Stratta P, D'Onofrio S, De Lauretis I, Santarelli V, Pacitti F, et al. The metabolic syndrome in an Italian psychiatric sample: a retrospective chart review of inpatients treated with antipsychotics. Riv Psichiatr. 2016;51:37–42. doi: 10.1708/2168.23452. [DOI] [PubMed] [Google Scholar]
- 51.Krane-Gartiser K, Breum L, Glümrr C, Linneberg A, Madsen M, Køster A, et al. Prevalence of the metabolic syndrome in Danish psychiatric outpatients treated with antipsychotics. Nord J Psychiatry. 2011;65:345–352. doi: 10.3109/08039488.2011.565799. [DOI] [PubMed] [Google Scholar]
- 52.Bioque M, García-Portilla MAP, García-Rizo C, Cabrera B, Lobo A, González-Pinto A, et al. Evolution of metabolic risk factors over a two-year period in a cohort of first episodes of psychosis. Schizophr Res. 2018;193:188–196. doi: 10.1016/j.schres.2017.06.032. [DOI] [PubMed] [Google Scholar]
- 53.Barbui C, Bighelli I, Carrà G, Castellazzi M, Lucii C, Martinotti G, et al. Antipsychotic dose mediates the association between polypharmacy and corrected QT interval. PLoS One. 2016;11:e0148212. doi: 10.1371/journal.pone.0148212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nosè M, Bighelli I, Castellazzi M, Martinotti G, Carrà G, Lucii C, et al. Prevalence and correlates of QTc prolongation in Italian psychiatric care: cross-sectional multicentre study. Epidemiol Psychiatr Sci. 2016;25:532–540. doi: 10.1017/S2045796015000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biancosino B, Barbui C, Marmai L, Donà S, Grassi L. Determinants of antipsychotic polypharmacy in psychiatric inpatients: a prospective study. Int Clin Psychopharmacol. 2005;20:305–309. doi: 10.1097/00004850-200511000-00004. [DOI] [PubMed] [Google Scholar]
- 56.Zink M, Kuwilsky A, Krumm B, Dressing H. Efficacy and tolerability of ziprasidone versus risperidone as augmentation in patients partially responsive to clozapine: a randomised controlled clinical trial. J Psychopharmacol. 2009;23:305–314. doi: 10.1177/0269881108089593. [DOI] [PubMed] [Google Scholar]
- 57.Sala M, Vicentini A, Brambilla P, Montomoli C, Jogia JR, Caverzasi E, et al. QT interval prolongation related to psychoactive drug treatment: a comparison of monotherapy versus polytherapy. Ann Gen Psychiatry. 2005;4:1. doi: 10.1186/1744-859X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Takeuchi H, Suzuki T, Remington G, Uchida H. Antipsychotic polypharmacy and corrected QT interval: a systematic review. Can J Psychiatry. 2015;60:215–222. doi: 10.1177/070674371506000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang JG, Roh D, Kim CH. Association between therapeutic alliance and adherence in outpatient schizophrenia patients. Clin Psychopharmacol Neurosci. 2019;17:273–278. doi: 10.9758/cpn.2019.17.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ascher-Svanum H, Faries DE, Zhu B, Ernst FR, Swartz MS, Swanson JW. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67:453–460. doi: 10.4088/jcp.v67n0317. [DOI] [PubMed] [Google Scholar]
- 61.Janssen B, Gaebel W, Haerter M, Komaharadi F, Lindel B, Weinmann S. Evaluation of factors influencing medication compliance in inpatient treatment of psychotic disorders. Psychopharmacology (Berl) 2006;187:229–236. doi: 10.1007/s00213-006-0413-4. [DOI] [PubMed] [Google Scholar]
- 62.Tareke M, Tesfaye S, Amare D, Belete T, Abate A. Antipsychotic medication non-adherence among schizophrenia patients in Central Ethiopia. S Afr J Psychiatr. 2018;24:1124. doi: 10.4102/sajpsychiatry.v24i0.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto Y, Uno J, Miwa T, Kurihara M, Tanifuji H, Tensho M. Effects of antipsychotic polypharmacy on side-effects and concurrent use of medications in schizophrenic outpatients. Psychiatry Clin Neurosci. 2012;66:405–410. doi: 10.1111/j.1440-1819.2012.02376.x. [DOI] [PubMed] [Google Scholar]
- 64.Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26:446–452. doi: 10.1097/YCO.0b013e3283642da4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun F, Stock EM, Copeland LA, Zeber JE, Ahmedani BK, Morissette SB. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. 2014;71:728–738. doi: 10.2146/ajhp130471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stahl SM. Emerging guidelines for the use of antipsychotic polypharmacy. Rev Psiquiatr Salud Ment. 2013;6:97–100. doi: 10.1016/j.rpsm.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 67.Constantine RJ, Andel R, McPherson M, Tandon R. The risks and benefits of switching patients with schizophrenia or schizoaffective disorder from two to one antipsychotic medication: a randomized controlled trial. Schizophr Res. 2015;166:194–200. doi: 10.1016/j.schres.2015.05.038. [DOI] [PubMed] [Google Scholar]