Abstract
Numerous clinical studies have reported neurological symptoms in COVID-19 patients since the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), apart from the atypical signs of pneumonia. Angiotensin-converting enzyme-2 (ACE-2), a potential receptor for SARS-CoV-2 entry, is expressed on various brain cells and cerebral parts, i.e., subfornical organ, paraventricular nucleus, nucleus of the tractus solitarius, and rostral ventrolateral medulla, as well as in non-cardiovascular areas such as the motor cortex and raphe. The resident CNS cells like astrocytes and microglia also express ACE-2, thus highlighting the vulnerability of the nervous system to SARS-CoV-2 infection. Additionally, transmembrane serine protease 2 (TMPRSS2) and furin facilitate virus entry into the host. Besides, the probable routes of virus entry into the nervous system include the hematogenic pathway, through the vagus, the olfactory nerve, or the enteric nervous system. However, the trajectory of SARS-CoV-2 to the brain needs investigation. Furthermore, a Th17-mediated cytokine storm is seen in COVID-19 cases with higher levels of IL-1β/2/7/8/9/10/17, GM-CSF, IFN-γ, TNF-α, CXCL-10, MCP1, and MIP1α/β. Some cytokines can cross the blood-brain barrier and activate the brain’s immune cells to produce neural cytokines, leading to neuronal dysfunctions. Nonetheless, most of the neurological conditions developed due to viral infections may not have effective and registered treatments. Although, some antivirals may inhibit the virus-mediated pathogenesis and prove to be suitable in COVID-19 treatment. Therefore, clinicians’ and researchers’ collective expertise may unravel the potential of SARS-CoV-2 infection to prevent short-term and long-term CNS damage.
Keywords: SARS-CoV-2, COVID-19, ACE-2, Nervous system, Cytokine storm
Introduction
The initial cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection appeared in December 2019 in Hubei province, China [1]. Since then, it has become a global threat. Besides systemic and respiratory ailments, 36.4% of coronavirus disease of 2019 (COVID-19) patients developed neurological symptoms [2]. Additionally, taste, smell, and visual impairments are reported in several cases of COVID-19 [2]. SARS-CoV-2, a human CoV (HCoV) belongs to β-coronaviruses, and various clinical and pre-clinical studies have reported potential neurovirulent properties of these viruses [3]. Furthermore, the presence of SARS-CoV-2 in cerebrospinal fluid (CSF) of COVID-19 patients is confirmed through genome sequencing [4]; however, experimental evidence is needed to validate virus-mediated neurological damage. Moreover, acute necrotizing hemorrhagic encephalopathy (ANE) was observed in brain computed tomography and magnetic resonance imaging of a COVID-19 patient [5]. This rare complication is often associated with intracranial cytokine storms and points towards the indirect mode of SARS-CoV-2 influence on the brain [5]. Also, a detailed study of brain tissue distribution of angiotensin-converting enzyme-2 (ACE-2), a potential receptor for SARS-CoV-2 entry [6], may shed light on potential SARS-CoV-2-induced neurological alterations. Elaborate ACE-2 expression studies state that the receptor is preferentially expressed in the endothelium, vascular smooth muscle cells, and on the surface of a variety of the central nervous system (CNS) and peripheral nervous system (PNS) cells [7–9]. Additional plausible entry routes to the brain may include the hematogenic pathway, transmission through the vagus, the olfactory nerve, or the enteric neuron (Fig. 1a) [10]. In brief, here we recapitulate varied aspects of COVID-19-related neurological manifestations.
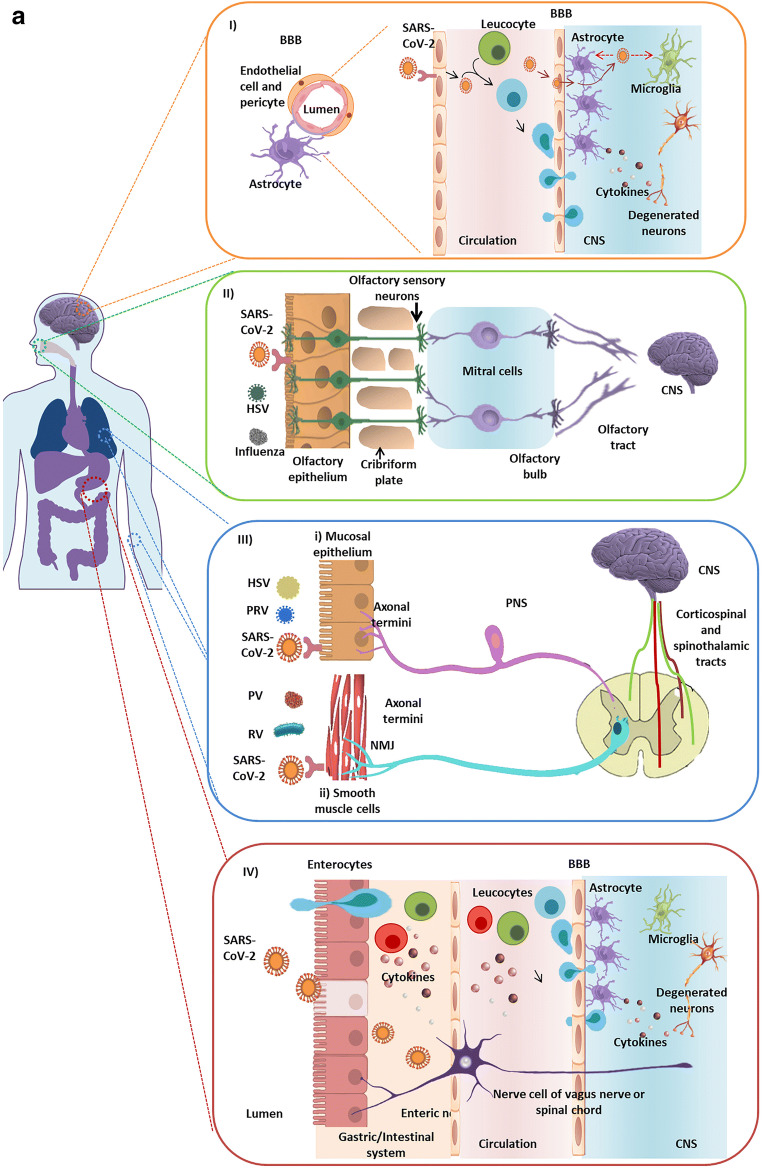
Fig. 1.
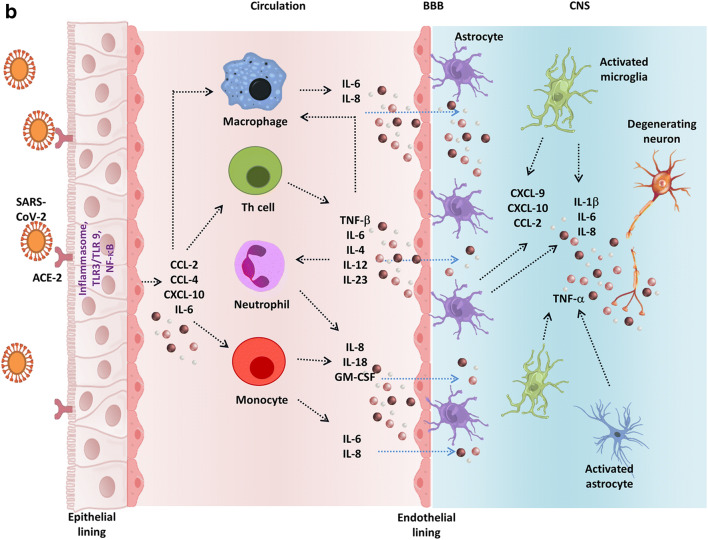
a Virus entry routes into the central nervous system (CNS). (I) The virus in the bloodstream may infect the peripheral immune cells. These infected leukocytes may traverse the blood-brain barrier (BBB) composed of specialized tight junctions, endothelial cells, pericytes, and astrocytes. In addition, the virus may also cross the BBB which could be severed due to the action of the cytokines or may enter the cerebrospinal fluid (CSF) by direct interaction with the brain microvascular endothelium cells. Both the mechanisms result in alterations in the brain homeostasis and aggravate cytokine production within the CNS (II) Several viruses like HSV and influenza viruses are known to infect the olfactory epithelial membrane. SARS-CoV-2 may also infect and damage olfactory sensory neurons (OSNs) in the epithelium lining. The damage may be direct or due to the production of cytokines produced by the accessory cells in the olfactory system. The virus may anterogradely reach the olfactory bulb through the cribriform plate. Finally, the virus may potentially gain entry into the CNS through the mitral cells along the olfactory tract. (III) Alpha herpesviruses (e.g. HSV-1, PRV) and polio virus (PV) along with rabies viruses (RV) may migrate to the CNS through the peripheral nerves. (i) Viruses may infect the mucosal epithelium following infection of the axonal termini of the peripheral nerves. The virus may spread to the spinal cord through retrograde axonal transport. (ii) Viruses infect the smooth muscle cells and spread through the neuromuscular junctions (NMJ) from muscles into the sensory/motor neurons of PNS ganglia. (IV) The gastrointestinal epithelium expresses ACE-2 receptors. Therefore, the cells may be easily infected by the virus. The virus may directly invade the enteric nervous system or indirectly it may prime the immune cells which may result in delayed neurological impairment. b SARS-CoV-2-mediated cytokine storm. After attachment and entry into the epithelial cells through ACE-2 receptor, the virus may activate the pro-inflammatory pathway through TLR or NF-κB signaling followed by the formation of inflammasome. Various pro-inflammatory cytokines and chemokines released due to this autonomous intrinsic defense mechanism include CCL-2, CCL-4, CXCL-10, and IL-6. These proteins attract various immune cells in the circulation like the monocytes, macrophages, T cells, and neutrophils at the site of infection. Additionally, the situation is worsened by production of TNF-β, IL-6, IL-4, IL-12, and IL-23 by the T lymphocytes, which further accumulate the immune cells establishing a pro-inflammatory feed-back loop. These cytokines may damage the BBB and activate astrocytes and microglia, the CNS resident immune cells. In response, the activated microglia and astrocytes produce IL-1β, IL-6, TNF-α, and IL-8. Elevated levels of these inflammatory cytokines can impart neurotoxic effects leading to neuronal dysfunction and various CNS disease–associated pathologies
Clinical Outcomes of Virus-Mediated Brain Dysfunction: More Prevalent Than Acknowledged?
The association of viruses with neural disorders is widely popular, although the relativity is still disputed. Neurodegenerative diseases, affecting approximately 37 million people worldwide, include degenerative ailments of the nervous system—the brain, spinal cord, and nerves [11]. Numerous genomic and proteomic studies unravel the similarities between virus-mediated and classical neurodegeneration or neuropathies [12, 13]. Viruses introduce alterations in the functioning of neurons directly or indirectly. The neurotropic viruses afflict neurons through cell lysis, necrosis, or apoptosis [14]. Indirectly, the viruses damage the neurons by manipulating or attacking the host immune responses. In the CNS, the virus can activate both the adaptive and innate immune responses [15]. Common pathways involved in the activation of the immune responses include the TLR mostly 3, 7, and 8 mediated damage, the release of free radicals, and inflammation [15]. Although, not always does the CNS immune response lead to detrimental outcomes as they usually assist in repair and regeneration [15].
Multiple studies mention the corroboration of infectious respiratory organisms as causative agents of various neurological diseases [16]. Respiratory syncytial virus (RSV) is known to infect the lower respiratory tract, cause infections in the immunocompromised patients, and target the CNS [16]. Often the virus is detected in the CSF samples of the patients exhibiting symptoms like seizures and convulsions, along with signs of ataxia, hormonal dysfunction, and encephalopathies [16]. Also, in vivo studies demonstrate the movement of the virus intranasally to the CNS [10]. Another respiratory virus that affects the infants and has neurovirulent abilities is the human metapneumovirus (hMPV) [17]. The virus is substantially detected in encephalopathic patients’ CSF samples, although studies demonstrating the virus’ neuroinvasive properties are to be conducted [18]. Furthermore, Hendra virus (HeV) and Nipah virus (NiV) affect humans and cause lung damage, pneumonia, along with hemorrhagic and necrotizing alveolitis [19]. Typical signs of neurological disturbance, including convulsions, seizures with motor deficits, and febrile encephalitic syndrome, are observed due to infection caused by these zoonotic viruses [19]. Animal studies show the olfactory nerve to be the main route to the CNS [20].
Also, the flu-causing influenza viruses account for numerous seasonal epidemics with a severe lethality rate, approximately a million cases per year [21]. Additionally, the viruses also affect the brain and are linked to encephalitis, febrile seizure, acute necrotizing encephalopathy, and syndromes like the Reye syndrome and Guillain–Barré syndrome [22]. According to some animal studies, the influenza virus can alter the brain homeostasis by traveling to the brain through the vagus nerve or the olfactory route [23, 24]. Intriguing, its association with Parkinson’s disease (PD) and multiple sclerosis (MS) is also mentioned [25]. Many encephalitis lethargica and postencephalitic parkinsonism cases followed by the 1918 “Spanish” flu pandemic, caused by influenza A (H1N1), make the involvement of the flu virus evident [26]. Viruses like the enteroviruses polioviruses (PV), coxsackieviruses (CV), echoviruses, and human rhinoviruses (HRV) are known to invade the CNS [27]. Studies describe HRV-induced meningitis and cerebellitis [27]. EV-A71 (hand–foot–mouth disease (HFMD)) and D68 outbreaks are associated with neurological complexities like myelitis (AFM), meningitis, and encephalitis [27].
The HCoVs can aggravate various neuropathologies. HCoVs are related to the neuroinvasive animal CoVs like porcine hemagglutinating encephalomyelitis virus, feline CoV, and the mouse hepatitis virus, which is used to generate MS models [28, 29]. Furthermore, a study conducted to demonstrate the relation between the HCoVs (229E and OC43) with MS and other neurological disorders involves identifying viral RNA in human brain autopsies [30]. Importantly, CoV-OC43 and CoV-229E are found in the CSF of PD patients [31]. However, detailed studies are needed to differentiate the mere presence and virus-associated disease alterations. In addition, association of SARS-CoV is not just limited to the lungs; instead, it is known to infect many organs, including the CNS [7, 32–34]. The real-time quantitative PCR assay targeting the polymerase (orf1ab) and nucleocapsid region of the SARS-CoV confirmed the presence of SARS-CoV in CSF and serum of the infected patients [35, 36]. A report suggests the association of status epilepticus with SARS [35]. Hospitalized children with the acute encephalitis-like syndrome were positive for anti-SARS-CoV IgM [37]. SARS-CoV is associated with demyelinating pathology and found in the brain parenchyma of MS patients [5, 28]. Neurological symptoms are also associated with MERS-CoV [38]. These examples impress on the connection of HCoVs with neurological dysfunctions. Therefore, the association of neurological complications with SARS-CoV-2 is not surprising.
According to a case report of SARS-CoV-2 infection, virus RNA was determined in the patient’s CSF; however, the nasopharyngeal swabs tested negative [4]. Currently, evidence to state the neuropathogenesis of the SARS-CoV-2 in COVID-19 remains scarce. Nevertheless, reports suggest that SARS-CoV-2 can cause meningitis and encephalitis [4]. Variable neurological symptoms are displayed by the COVID-19 patients like PNS symptoms, including hypogeusia, hyposmia, hypoplasia, and neuralgia vertigo, and CNS dysfunctions like cephalgia, impaired consciousness, seizures, ataxia, and acute cerebrovascular disease, with headache and dizziness being the most common [39, 40]. Neurological manifestations are common in many COVID-19 patients like anosmia, an early COVID-19 symptom [2, 41–43]. Though seizures are seldom reported in COVID-19 patients, and usually indicate an ischemic stroke, meningitis, or cerebral hypoxia, its association with comorbidities like hypocalcemia or drugs remains elusive [44].
The neurological alterations caused by the virus may result from direct CNS/PNS attack or indirect influence on various organs that later affect the nervous system. For example, hypertension, common COVID-19 comorbidity, results in blood-brain barrier (BBB) impairment and may enhance the risk of COVID-19-related cerebral complexities [45, 46]. A hypothesis relates neuronal damage to the respiratory stress from deteriorated lung conditions [47]. The oxygen deprivation may result in multiple organ failure and may affect the brain [47]. Besides, patients considered during the earlier studies of the SARS-CoV pandemic displayed axonal motor sensory neuropathy and myopathy [48]. However, it remains unclear if the illness was virus-mediated or an outcome of high drug doses [48]. Nevertheless, the effect of the SARS-CoV-2 on PNS is noteworthy as Guillain-Barre, Miller-Fisher syndrome, and polyneuritis cranialis are reported in COVID-19 [49–54]. Development of rhabdomyolysis, neuralgia, and myalgia in SARS-CoV-2-infected patients further support the virus’ ability to affect PNS [52–54]. A study reported elevated creatinine kinase and muscle pain in 10.7% of patients with severe COVID-19 [2]. Furthermore, some COVID-19 patients with neurological symptoms might have a prior history of neurological complications or maybe treated for viral infections. Hence, it is necessary to treat such cases using drugs with properties of high bioavailability in the brain. We have summarized information like the mode of action and brain or CSF/plasma ratio of a few antivirals, which have shown promising outcomes in COVID-19 treatment (Table 1) [70, 71]. The use of efficient BBB penetrating drugs may be preferred during this pandemic to minimize the onset of neurological consequences of SARS-CoV-2 infection.
Table 1.
Antiviral drugs proposed in COVID-19 treatment along with their mechanism of action, associated complications, and CSF to plasma ratio
| Drug name | Mechanism | Viruses affected by the drug | Brain/plasma ratio | Neurological complications the drug is active against |
|---|---|---|---|---|
| Lopinavir/ ritonavir | Inhibit the viral proteases | HIV | 0.02%/1.23% [55] | HAND [56] |
| Darunavir | Inhibit the viral proteases | HIV | 0.88% [57] | HAND [57] |
| Favipiravir | Inhibit the viral proteases | Influenza A and B | Low [58] | – |
| Remdesivir | Nucleotide analog - blocks viral nucleotide synthesis to stop viral replication | Ebola virus | < 5% [59] | – |
| Ribavirin | Inhibit viral polymerase | RSV, hepatitis C virus | 70% [60] | Nipah virus–associated encephalitis [61], neurocognitive conditions[62] |
| Oseltamivir | Inhibit viral neuraminidase | Influenza A and B | 2.1%[63] | Influenza-associated encephalitis [64, 65], PD [66] |
| Amantadine | Inhibits viral M2 protein (an ion channel) | Influenza A | 76% [67]* | Influenza-associated encephalitis [68], PD [69] |
*CSF/serum ratio. HAND HIV-associated neurocognitive disorders, PD Parkinson’s disease, RSV respiratory syncytial virus. The brain to plasma ratio or CSF to plasma ratio has been denoted for each drug assuming that brain penetration is similar between rodents, non-human primates, and human patients
Neurological Alterations Due to Cytokine Storm: a Result of Host Immunity and SARS-CoV-2 Combat
Indirectly the viruses may damage the neurons by manipulating or attacking the host immunity [15]. In the CNS, SARS-CoV-2 can activate both the adaptive and innate immunity [15]. T helper cell 17 (Th17)–mediated cytokine storm, evident in virus infections, is seen in COVID-19 with neurological manifestations (Fig. 1b) [72, 73]. Clinical studies report systemic inflammation involving enhanced cytokines, particularly IL-1β, IL-6, IL-10, granulocyte colony-stimulating factor, granulocyte-monocyte colony-stimulating factor, C-X-C motif chemokine ligand 10 (CXCL10), MCP-1, macrophage inflammatory proteins 1-α, and tumor necrosis factor α in COVID-19. Additionally CD4+ and CD8+ T cell lymphopenia and decreased secretion of IFN-γ in severe cases of COVID-19 are reported (Fig. 1a) [28, 37, 72]. Intriguingly, a study suggests that an MS patient undergoing ocrelizumab (an immunosuppressive drug) therapy diagnosed positive for COVID-19 does not display serious complications [74]. The increased levels of cytokines may escalate vascular and BBB permeability and inflammation [74, 75]. This information supports the hypothesis that increased BBB permeability allows virus entry into the CNS, leading to COVID-19-related neurological complexities. Some cytokines released in the circulation can cross the BBB and activate the resident brain immune cells like microglia and astrocytes to produce neural cytokines, further worsening the condition (Fig. 1b) [76]. Astrocytes regulate a wide variety of functions, which may aggravate neuroinflammation. Microglia mature into macrophages and may engulf the neighboring neurons on activation [77, 78]. Furthermore, microglia are the primary source of pro-inflammatory cytokines, nitric oxide, prostaglandin E2, and reactive oxygen and nitrogen species [77]. Microglia express ACE-2, along with ACE and AT1 [79]. These receptors play a significant role in microglia activation and balance the pro-inflammatory or anti-inflammatory effects [80]. More specifically, SARS-CoV-2 infection can hamper the ACE-2-mediated signaling, creating a glitch in the AT1 receptor-mediated path, thereby inducing a pro-inflammatory response [80]. In vivo studies suggest induction of pro-inflammatory cytokines in microglia and the mouse brain and spinal cord [81]. The situation becomes dreadful when the pro-inflammatory substances produced by astrocytes and microglia fenestrate the BBB [77, 78].
Besides, SARS-CoV infects the myeloid cells and manipulates the innate immune system to ease its propagation to other tissues (Fig. 1a) [82]. These persistently infected leukocytes act as reservoirs of the neuroinvasive HCoV and can be held responsible for long-term neurological sequelae [83]. Therefore, the possibility of such cases of persistent SARS-CoV-2 infection may appear in the future. Notably, peripheral inflammatory reactions observed in COVID-19 may result in symptoms of neurological disorders [84]. Cytokine storms may influence the CNS and enhance the severity of COVID-19 patients to develop ANE, meningitis, and hemorrhage [5, 85]. Therefore, it is necessary to identify the mechanism behind SARS-CoV-2-induced cytokine storms and the course of release of the cytokines during the infection. The contribution of the pro-inflammatory cytokines alone and the direct tissue damage caused by the virus needs to be addressed. The indirect influence of systemic inflammation on the CNS by targeting the pro-inflammatory mediators will be worth investigating.
ACE-2 Dependent and Independent Infection of the Nervous System in COVID-19
It is found that ACE-2 is expressed in various brain regions, like the subfornical organ, the nucleus of the tractus solitarius, and rostral ventrolateral medulla, as well as in non-cardiovascular areas such as the motor cortex and raphe [86]. According to a spatial distribution analysis, ACE-2 is expressed in substantia nigra and brain ventricles [87–89]. The protein’s cell type distribution revealed both excitatory and inhibitory neurons, pericytes and endothelial cells, and glial cells like astrocytes and oligodendrocytes in human middle temporal gyrus and posterior cingulate cortex express ACE-2, unlike the cells in the region of the prefrontal cortex [9]. Additionally, the hippocampus has few ACE-2 expressing cells [9]. Studies report that angiotensin II downregulates the expression of ACE-2 in neonatal rat cerebellar or medullary astrocytes [90]. Therefore, the predominant expression of ACE-2 in the brain hints towards the virus’s potential to infect the CNS.
Furthermore, brain endothelial and smooth muscle cells of the blood vessels express ACE-2 [7]. The virus may enter into the CNS through the hematogenic pathway, subsequently crossing the BBB [91]. A post-mortem study of the frontal lobe of a COVID-19 patient reports virus presence in neurons and capillary endothelial cells [92]. Infection of endothelial cells may allow the virus to pass from the respiratory tract to the blood. The virus in the peripheral system can move into the cerebral circulation, where the blood’s sluggish movement may facilitate the viral S protein interaction with the ACE-2 expressed on the endothelial lining of the brain (Fig. 1a) [93]. Another speculated entry route for SARS-CoV-2 may be through the enteric nervous system upon infection of enterocytes [94, 95]. Enterocytes express high magnitudes of ACE-2 [7]. Once inside the brain, the virus can infect the neural cells, astrocytes, and microglia. These cells express ACE-2, thus initiating the viral budding cycle followed by neuronal damage and inflammation (Fig. 1a) [96].
Moreover, multiple transcriptome studies show and validate ACE-2 expression levels in various non-neuronal cells of olfactory mucosa [97]. Studies support the viral susceptibility of the mucosal cells, sustentacular cells, Bowman’s cells, and olfactory stem cells [98, 99]. Loss of smell in COVID-19 is marked by potential deterioration of olfactory stem cells and other accessory cells [98]. Also, a high-throughput single-cell expression study mentioned no ACE-2 expression in olfactory covering glia, microvillar cells, and immature or mature olfactory sensory neurons [100]. It is speculated that SARS-CoV-2 on binding may stimulate olfactory receptor neurons (ORNs) to exert an exaggerated immune response. Earlier studies with SARS-CoV have established infection of the brain through ORNs [101]. Studies describing the transneuronal/transsynaptic movement of the SARS-CoV already exist. Rabies viruses can take over the vesicular axonal transport machinery to disseminate in the brain (Fig. 1a) [102]. Human herpesvirus-6 (HHV-6) propagates in olfactory endothelial (OE) cells before invading the brain [93]. These studies enable to predict and support the movement of SARS-CoV-2 through the vesicular axonal pathway in an anterograde fashion through the olfactory nerve and facilitate brain infection [102] (Fig. 1a). Also, the virus may directly reach the CSF around the olfactory nerve fibers from OE cells [82]. A probable trajectory of SARS-CoV-2 to the brain may be via high-ACE-2-expressing non-neuronal OE cells to low-ACE-2-expressing mature ORNs along the olfactory axons. This mechanism highlights the ACE-2 independent process of virus spread.
Lastly, the expression of transmembrane serine protease 2 (TMPRSS2) in human olfactory mucosa may further worsen the case of SARS-CoV-2 infection [97]. A study demonstrates that respiratory epithelial cells express TMPRSS2 without ACE-2 [103]. The mosaic distribution of TMPRSS2 in mature ORNs is reported [104]. Therefore, the virus can preferentially gain entry into the PNS through one of the two epithelial cell types in the nose, either the goblet cells or the ciliated cells. TMPRSS2, in collaboration with furin, accelerates SARS-CoV-2 entry [105]. Furin, a host serine endoprotease, is particularly of neurological relevance. In general, furin can activate neuronal growth factors and influence CNS homeostasis [106]. However, upon attachment of SARS-CoV-2 with ACE-2, the enzyme generates an active S protein through irreversible cleavage of the precursor protein [105]. The protein S1/S2 subunits separate, which subsequently facilitate virus entry into the host [105]. Thus, exploring the possible avenues of SARS-CoV-2 entry and impact on CNS is the need of the hour.
Conclusion
Various clinical reports have made the association of SARS-CoV-2 with neurological dysfunction prominent. COVID-19-associated neurological severity is primarily associated with cytokine storms. The earlier identified SARS-CoV is already known to suppress the host antiviral response and activate the pro-inflammatory pathways. Briefly, it would be crucial to analyze the IFN-antagonizing and inflammasome-activating properties of SARS-CoV-2. Furthermore, the interaction of SARS-CoV-2 and ACE-2-expressing neuronal/glial cells may facilitate virus entry into the nervous system through different routes. Thus, the nervous system’s involvement in COVID-19 may be more than the current situation apprehends, therefore referring to the virus as an underestimated pathogen. Medical expert clinicians and researchers’ collaboration may address the enhanced incidents of neural dysfunctions in infected individuals. After identifying initial neurological damages, careful monitoring of COVID-19 patients in the long term is also necessary.
Acknowledgments
We thank the Ministry of Human Resource and Development and Department of Biotechnology, Govt. of India for fellowship to Shweta Jakhmola and Omkar Indari, respectively, in the form of a research stipend. We appreciate our lab colleagues for insightful discussions and advice. We gratefully acknowledge the Indian Institute of Technology Indore for providing facilities and support.
Authors’ Contributions
Conceptualization, data curation, formal analysis, investigation: SJ, OI, SC. Methodology, project administration, resources, supervision: SJ, HCJ. Validation: HCJ. Visualization, roles/writing - original draft: SJ. Writing- review and editing: SJ, HCJ, OI.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Disclaimer
The funding organization has not played any role in the study design or the preparation of the manuscript.
Footnotes
This article is part of the topical collection on Covid-19
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed]
- 3.Steardo L, Steardo L Jr, Zorec R, Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020;e13473. 10.1111/apha.13473 [DOI] [PMC free article] [PubMed]
- 4.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. 2020;201187. 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed]
- 6.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh C-L, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamming I, Timens W, Bulthuis MLC, Lely AT, Navis GJ, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brain tissue expression of ACE2 - summary - the human protein atlas. [cited 2020 Sep 4]. https://www.proteinatlas.org/ENSG00000130234/brain
- 10.Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–393. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karim S, Mirza Z, Kamal MA, Abuzenadah AM, Azhar EI, Al-Qahtani MH, et al. The role of viruses in neurodegenerative and neurobehavioral diseases. CNS Neurol Disord Drug Targets. 2014;13:1213–1223. doi: 10.2174/187152731307141015122638. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Diefenbach E, Crossett B, Tran SL, Ng T, Rizos H, et al. First evidence of overlaps between HIV-associated dementia (HAD) and non-viral neurodegenerative diseases: proteomic analysis of the frontal cortex from HIV+ patients with and without dementia. Mol Neurodegener. 2010;5:27. doi: 10.1186/1750-1326-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi C-S, Nabar NR, Huang N-N, Kehrl JH. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101. doi: 10.1038/s41420-019-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Miranda-Saksena M, Saksena NK. Viruses and neurodegeneration. Virol J. 2013;10:172. doi: 10.1186/1743-422X-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology [Internet]. 2010;129:154–69. Available from: 10.1111/j.1365-2567.2009.03225.x [DOI] [PMC free article] [PubMed]
- 16.Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards KM, Zhu Y, Griffin MR, Weinberg GA, Hall CB, Szilagyi PG, et al. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013;368:633–643. doi: 10.1056/NEJMoa1204630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peiris JSM, Tang W-H, Chan K-H, Khong P-L, Guan Y, Lau Y-L, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broder CC, Weir DL, Reid PA. Hendra virus and Nipah virus animal vaccines. Vaccine. 2016;34:3525–3534. doi: 10.1016/j.vaccine.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhondt KP, Horvat B. Henipavirus infections: lessons from animal models. Pathogens. 2013;2:264–287. doi: 10.3390/pathogens2020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Influenza (Seasonal). [cited 2020 Sep 4]. https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- 22.Hayase Y, Tobita K. Influenza virus and neurological diseases. Psychiatry Clin Neurosci. 1997;51:181–184. doi: 10.1111/j.1440-1819.1997.tb02580.x. [DOI] [PubMed] [Google Scholar]
- 23.Park CH, Ishinaka M, Takada A, Kida H, Kimura T, Ochiai K, et al. The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol. 2002;147:1425–1436. doi: 10.1007/s00705-001-0750-x. [DOI] [PubMed] [Google Scholar]
- 24.Mori I, Nishiyama Y, Yokochi T, Kimura Y. Olfactory transmission of neurotropic viruses. J Neurovirol. 2005;11:129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- 25.Blackmore S, Hernandez J, Juda M, Ryder E, Freund GG, Johnson RW, et al. Influenza infection triggers disease in a genetic model of experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2017;114:E6107–E6116. doi: 10.1073/pnas.1620415114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Henry J, Smeyne RJ, Jang H, Miller B, Okun MS. Parkinsonism and neurological manifestations of influenza throughout the 20th and 21st centuries. Parkinsonism Relat Disord. 2010;16:566–571. doi: 10.1016/j.parkreldis.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rhoades RE, Tabor-Godwin JM, Tsueng G, Feuer R. Enterovirus infections of the central nervous system. Virology. 2011;411:288–305. doi: 10.1016/j.virol.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das SJ. A mechanism of virus-induced demyelination. Interdiscip Perspect Infect Dis. 2010;2010:109239. doi: 10.1155/2010/109239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12. 10.3390/v12010014. [DOI] [PMC free article] [PubMed]
- 30.Cristallo A, Gambaro F, Biamonti G, Ferrante P, Battaglia M, Cereda PM. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol. 1997;20:105–114. [PubMed] [Google Scholar]
- 31.Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Mov Disord. 1992;7:153–158. doi: 10.1002/mds.870070210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203:622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202:415–424. doi: 10.1084/jem.20050828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine Mig in pathogenesis. Clin Infect Dis. 2005;41:1089–1096. doi: 10.1086/444461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung ECW, Chim SSC, Chan PKS, Tong YK, Ng EKO, Chiu RWK, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng EKO, Hui DS, Chan KCA, Hung ECW, Chiu RWK, Lee N, et al. Quantitative analysis and prognostic implication of SARS coronavirus RNA in the plasma and serum of patients with severe acute respiratory syndrome. Clin Chem. 2003;49:1976–1980. doi: 10.1373/clinchem.2003.024125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59:163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finsterer J, Stollberger C. Update on the neurology of COVID-19. J Med Virol. 2020. 10.1002/jmv.26000. [DOI] [PMC free article] [PubMed]
- 41.Heidari F, Karimi E, Firouzifar M, Khamushian P, Ansari R, Mohammadi Ardehali M, et al. Anosmia as a prominent symptom of COVID-19 infection. Rhinology. 2020. 10.4193/Rhin20.140. [DOI] [PubMed]
- 42.Solomon GS. Anosmia in Alzheimer disease. Percept Mot Skills. 1994;79:1249–1250. doi: 10.2466/pms.1994.79.3.1249. [DOI] [PubMed] [Google Scholar]
- 43.Tarakad A, Jankovic J. Anosmia and ageusia in Parkinson’s disease. Int Rev Neurobiol. 2017;133:541–556. doi: 10.1016/bs.irn.2017.05.028. [DOI] [PubMed] [Google Scholar]
- 44.Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61:e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020;43:824–831. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buttler L, Jordão MT, Fragas MG, Ruggeri A, Ceroni A, Michelini LC. Maintenance of blood-brain barrier integrity in hypertension: a novel benefit of exercise training for autonomic control. Front Physiol. 2017;8:1048. doi: 10.3389/fphys.2017.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cormier Z. How Covid-19 can damage the brain. [cited 2020 Jul 1]. https://www.bbc.com/future/article/20200622-the-long-term-effects-of-covid-19-infection
- 48.Tsai L-K, Hsieh S-T, Chang Y-C. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. 2005;14:113–119. [PubMed] [Google Scholar]
- 49.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases. Elsevier; 2020 [cited 2020 Jul 1];20:e00771. https://www.sciencedirect.com/science/article/pii/S2214250920300792/pdfft?md5=b9ff1f0fee45c428e06eca17f11b5035&pid=1-s2.0-S2214250920300792-main.pdf [DOI] [PMC free article] [PubMed]
- 51.Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020. 10.1212/WNL.0000000000009619. [DOI] [PubMed]
- 52.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26:1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lippi G, Wong J, Henry BM. Myalgia may not be associated with severity of coronavirus disease 2019 (COVID-19) World J Emerg Med. 2020;11:193–194. doi: 10.5847/wjem.j.1920-8642.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu K, Pan M, Xiao Z, Xu X. Neurological manifestations of the coronavirus (SARS-CoV-2) pandemic 2019-2020. J Neurol Neurosurg Psychiatry. 2020;91:669–670. doi: 10.1136/jnnp-2020-323177. [DOI] [PubMed] [Google Scholar]
- 55.Ene L, Duiculescu D, Ruta SM. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life. J Med Life; 2011;4. https://pubmed.ncbi.nlm.nih.gov/22514580/ [PMC free article] [PubMed]
- 56.Santos JR, Muñoz-Moreno JA, Moltó J, Prats A, Curran A, Domingo P, et al. Virological efficacy in cerebrospinal fluid and neurocognitive status in patients with long-term monotherapy based on lopinavir/ritonavir: an exploratory study. PLoS One. 2013;8:e70201. doi: 10.1371/journal.pone.0070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bartels H, Decosterd L, Battegay M, Marzolini C. Darunavir concentrations in CSF of HIV-infected individuals when boosted with cobicistat versus ritonavir. J Antimicrob Chemother. 2017;72:2574–2577. doi: 10.1093/jac/dkx165. [DOI] [PubMed] [Google Scholar]
- 58.Bixler SL, Bocan TM, Wells J, Wetzel KS, Van Tongeren SA, Garza NL, et al. Intracellular conversion and in vivo dose response of favipiravir (T-705) in rodents infected with Ebola virus. Antiviral Res. 2018;151:50–54. doi: 10.1016/j.antiviral.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Connor E, Morrison S, Lane J, Oleske J, Sonke RL, Connor J. Safety, tolerance, and pharmacokinetics of systemic ribavirin in children with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1993;37:532–539. doi: 10.1128/aac.37.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chong HT, Kamarulzaman A, Tan CT, Goh KJ, Thayaparan T, Kunjapan SR, et al. Treatment of acute Nipah encephalitis with ribavirin. Ann Neurol. 2001;49:810–813. doi: 10.1002/ana.1062. [DOI] [PubMed] [Google Scholar]
- 62.Kraus MR, Schäfer A, Teuber G, Porst H, Sprinzl K, Wollschläger S, et al. Improvement of neurocognitive function in responders to an antiviral therapy for chronic hepatitis C. Hepatology. 2013;58:497–504. doi: 10.1002/hep.26229. [DOI] [PubMed] [Google Scholar]
- 63.Jhee SS, Yen M, Ereshefsky L, Leibowitz M, Schulte M, Kaeser B, et al. Low penetration of oseltamivir and its carboxylate into cerebrospinal fluid in healthy Japanese and Caucasian volunteers. Antimicrob Agents Chemother. 2008;52:3687–3693. doi: 10.1128/AAC.00327-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alsolami A, Shiley K. Successful treatment of influenza-associated acute necrotizing encephalitis in an adult using high-dose oseltamivir and methylprednisolone: case report and literature review. Open Forum Infect Dis. 2017;4:ofx145. doi: 10.1093/ofid/ofx145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newland JG, Romero JR, Varman M, Drake C, Holst A, Safranek T, et al. Encephalitis associated with influenza B virus infection in 2 children and a review of the literature. Clin Infect Dis. 2003;36:e87–e95. doi: 10.1086/368184. [DOI] [PubMed] [Google Scholar]
- 66.Kadowaki T, Komagamine T, Suzuki K, Hirata K. Oseltamivir-induced dyskinesia in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:133–134. doi: 10.1016/j.parkreldis.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Kornhuber J, Quack G, Danysz W, Jellinger K, Danielczyk W, Gsell W, et al. Therapeutic brain concentration of the NMDA receptor antagonist amantadine. Neuropharmacology. 1995;34:713–721. doi: 10.1016/0028-3908(95)00056-C. [DOI] [PubMed] [Google Scholar]
- 68.Miura M, Sugaya N, Hayashi E. Use of amantadine for influenza A encephalopathy. Kansenshogaku Zasshi. 1998;72:840–844. doi: 10.11150/kansenshogakuzasshi1970.72.840. [DOI] [PubMed] [Google Scholar]
- 69.Crosby N, Deane KH, Clarke CE. Amantadine in Parkinson’s disease. Cochrane Database Syst Rev. 2003;CD003468. 10.1002/14651858.CD003468 [DOI] [PMC free article] [PubMed]
- 70.Costanzo M, De Giglio MAR, Roviello GN. SARS-CoV-2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020. 10.2174/0929867327666200416131117. [DOI] [PubMed]
- 71.Smieszek SP, Przychodzen BP, Polymeropoulos MH. Amantadine disrupts lysosomal gene expression: a hypothesis for COVID19 treatment. Int J Antimicrob Agents. 2020;55:106004. doi: 10.1016/j.ijantimicag.2020.106004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Winter PM, Dung NM, Loan HT, Kneen R, Wills B, Thu LT, et al. Proinflammatory cytokines and chemokines in humans with Japanese encephalitis. J Infect Dis. 2004;190:1618–1626. doi: 10.1086/423328. [DOI] [PubMed] [Google Scholar]
- 74.Novi G, Mikulska M, Briano F, Toscanini F, Tazza F, Uccelli A, et al. COVID-19 in a MS patient treated with ocrelizumab: does immunosuppression have a protective role? Mult Scler Relat Disord. 2020;42:102120. doi: 10.1016/j.msard.2020.102120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klein RS, Garber C, Funk KE, Salimi H, Soung A, Kanmogne M, et al. Neuroinflammation during RNA viral infections. Annu Rev Immunol. 2019;37:73–95. doi: 10.1146/annurev-immunol-042718-041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tjalkens RB, Popichak KA, Kirkley KA. Inflammatory activation of microglia and astrocytes in manganese neurotoxicity. Adv Neurobiol. 2017;18:159–181. doi: 10.1007/978-3-319-60189-2_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mariani MM, Kielian T. Microglia in infectious diseases of the central nervous system. J Neuroimmune Pharmacol. 2009;4:448–461. doi: 10.1007/s11481-009-9170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahmoud S, Gharagozloo M, Simard C, Gris D. Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells. 2019;8. 10.3390/cells8020184. [DOI] [PMC free article] [PubMed]
- 79.Cui C, Xu P, Li G, Qiao Y, Han W, Geng C, et al. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: role of renin-angiotensin system. Redox Biol. 2019;26:101295. doi: 10.1016/j.redox.2019.101295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19. 10.3390/ijms19030876. [DOI] [PMC free article] [PubMed]
- 81.Li Y, Fu L, Gonzales DM, Lavi E. Coronavirus neurovirulence correlates with the ability of the virus to induce proinflammatory cytokine signals from astrocytes and microglia. J Virol. 2004;78:3398–3406. doi: 10.1128/jvi.78.7.3398-3406.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang M, Li CK, Li K, Hon KLE, Ng MHL, Chan PKS, et al. Hematological findings in SARS patients and possible mechanisms (review) Int J Mol Med. 2004;14:311–315. [PubMed] [Google Scholar]
- 83.Desforges M, Miletti TC, Gagnon M, Talbot PJ. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130:228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12:170–175. doi: 10.1007/s11906-010-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alenina N, Bader M. ACE2 in brain physiology and pathophysiology: evidence from transgenic animal models. Neurochem Res. 2019;44:1323–1329. doi: 10.1007/s11064-018-2679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cardin JA. Inhibitory interneurons regulate temporal precision and correlations in cortical circuits. Trends Neurosci. 2018;41:689–700. doi: 10.1016/j.tins.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020. 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed]
- 90.Gowrisankar YV, Clark MA. Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J Neurochem. 2016;138:74–85. doi: 10.1111/jnc.13641. [DOI] [PubMed] [Google Scholar]
- 91.Bostancıklıoğlu M. SARS-CoV2 entry and spread in the lymphatic drainage system of the brain. Brain Behav Immun. 2020;87:122–123. doi: 10.1016/j.bbi.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J Med Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 94.Esposito G, Pesce M, Seguella L, Sanseverino W, Lu J, Sarnelli G. Can the enteric nervous system be an alternative entrance door in SARS-CoV2 neuroinvasion? Brain Behav Immun. 2020;87:93–94. doi: 10.1016/j.bbi.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lamers MM, Beumer J, van der Vaart J, Knoops K, Puschhof J, Breugem TI, et al. SARS-CoV-2 productively infects human gut enterocytes. Science. 2020;369:50–54. doi: 10.1126/science.abc1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020. 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed]
- 97.Butowt R, Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci. 2020;11:1200–1203. doi: 10.1021/acschemneuro.0c00172. [DOI] [PubMed] [Google Scholar]
- 98.Durrant DM, Ghosh S, Klein RS. The olfactory bulb: an immunosensory effector organ during neurotropic viral infections. ACS Chem Neurosci. 2016;7:464–469. doi: 10.1021/acschemneuro.6b00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.LeMessurier KS, Tiwary M, Morin NP, Samarasinghe AE. Respiratory barrier as a safeguard and regulator of defense against influenza A virus and. Front Immunol. 2020;11:3. doi: 10.3389/fimmu.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Durante MA, Kurtenbach S, Sargi ZB, Harbour JW, Choi R, Kurtenbach S, et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat Neurosci. 2020;23:323–326. doi: 10.1038/s41593-020-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82:7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ruiz García S, Deprez M, Lebrigand K, Cavard A, Paquet A, Arguel M-J, et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development. 2019;146. 10.1242/dev.177428. [DOI] [PMC free article] [PubMed]
- 104.Saraiva LR, Ibarra-Soria X, Khan M, Omura M, Scialdone A, Mombaerts P, et al. Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Sci Rep. 2015;5:18178. doi: 10.1038/srep18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78:779–84.e5. 10.1016/j.molcel.2020.04.022 [DOI] [PMC free article] [PubMed]
- 106.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]