Abstract
Background
Myocardial involvement in the course of coronavirus disease 2019 (COVID-19) pneumonia has been reported, though not fully characterized yet. The aim of the present study is to undertake a joint evaluation of hs-Troponin and natriuretic peptides (NP) in patients hospitalized for COVID-19 pneumonia.
Methods
In this multicenter observational study, we analyzed data from n = 111 patients. Cardiac biomarkers subgroups were identified according to values beyond reference range.
Results
Increased hs-Troponin and NP were found in 38 and 56% of the cases, respectively. As compared to those with normal cardiac biomarkers, these patients were older, had higher prevalence of cardiovascular diseases (CVD) and had more severe COVID-19 pneumonia by higher CRP and d-dimer and lower PaO2/FIO2. Two-dimensional echocardiography performed in a subset of patients (n = 24) showed significantly reduced left ventricular ejection fraction in patients with elevated NP (p = 0.02), whereas right ventricular systolic function (tricuspid annular plane systolic excursion) was significantly reduced both in patients with high hs-Troponin and NP (p = 0.022 and p = 0.03, respectively). Both hs-Troponin and NP were higher in patients with in-hospital mortality (p = 0.001 and p = 0.002, respectively). On multivariable analysis, independent associations were found of hs-Troponin with age, PaO2/FIO2 and d-dimer (B = 0.419, p = 0.001; B = − 0.212, p = 0.013; and B = 0.179, p = 0.037, respectively) and of NP with age and previous CVD (B = 0.480, p < 0.001; and B = 0.253, p = 0.001, respectively).
Conclusions
Myocardial involvement at admission is common in COVID-19 pneumonia. Independent associations of hs-Troponin with markers of disease severity and of NP with underlying CVD might point toward existing different mechanisms leading to their elevation in this setting.
Electronic supplementary material
The online version of this article (10.1007/s11739-020-02498-7) contains supplementary material, which is available to authorized users.
Keywords: COVID-19, Hs-troponin, Natriuretic peptide, d-dimer, PaO2/FIO2
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory infection caused by a novel type of betacoronavirus, 2019-nCov, later renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1], that emerged in China and later spread worldwide leading to a global pandemic. In some countries, this required the creation of dedicated multidisciplinary “COVID units” for the management of affected patients [2–4]. COVID-19 is a highly contagious infection [5], associated with a significant rate of severe interstitial pneumonia and related complications including respiratory failure, thrombosis, multiorgan failure and death [6]. Systemic implications include, among others, coagulopathy with increased d-dimer levels [7, 8], marker of more severe disease affecting prognosis [9]. Several reports also described the common occurrence of myocardial involvement in patients affected by COVID-19, as revealed by detection of increased levels of high-sensitivity troponin (hs-Troponin) [10, 11] and natriuretic peptides (NP) [12] which both showed to have prognostic relevance [10, 13–15]. Reasons for myocardial injury in this setting span from cytokine or hypoxia-related injury to myocarditis and myocardial infarction [16–19]. A full understanding of mechanisms leading to myocardial involvement in the course of COVID-19 pneumonia might help gaining insights into disease pathogenesis and aid daily clinical management in these patients, in which downstream testing is usually complex and constitutes a risk for the safety of both patients and healthcare personnel [20]. Hence, the aim of the present study is to undertake a joint assessment of hs-Troponin and NP value at admission in a cohort of consecutively hospitalized COVID-19 pneumonia patients, in order to describe the incidence and the determinants of their rise.
Methods
This is a multicenter observational study on COVID-19 pneumonia patients (n = 118) consecutively admitted to dedicated medical units at Madre Giuseppina Vannini Hospital, Rome, Italy (n = 39, admission time between 1 and 30 April) and St. Andrea Hospital-Sapienza University of Rome, Italy (n = 79, admission time between 15 March and 30 April). Clinical care within these units, recently established in order to provide dedicated assistance to COVID-19 patients, was managed by full-time dedicated internal medicine or cardiology specialists.
Diagnosis of COVID-19 infection was confirmed before admission by means of nasopharyngeal swab and subsequent quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) [6], while pulmonary involvement was demonstrated in all patients by means of chest computed tomography. Symptoms at presentation and clinical history were assessed by the accepting physician. History of previous cardiovascular disease (CVD) was defined by the presence of either atrial fibrillation, coronary artery disease, heart failure or stroke. All patients underwent blood gas analysis, routine blood examination and 12-lead ECG within 24 h upon admission. Patients (n = 7) who did not have timely recorded blood gas analysis and assessment of either hs-Troponin or NP were excluded from the analysis. The final sample size consisted of n = 111 patients of whom n = 103 had hs-Troponin and n = 82 had natriuretic peptide evaluation. No routine withdrawal of any previous treatment with cardiovascular drug was undertaken, while we recorded the presence of ongoing treatment with angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB). Blood gas examination was performed with standard commercially available machines. Arterial oxygen concentration (PaO2) was normalized to the fractional volume of the inspired oxygen to calculate the PaO2/FIO2 ratio. Venous blood samples were analyzed by standard techniques within 2 h from collection. Hs-Troponin T (normal value < 14 pg/ml) and N-terminal-pro-natriuretic peptide (NT-pro-BNP) were evaluated at Madre Giuseppina Vannini Hospital. Hs-Troponin I (normal value < 35 pg/ml) and B-type natriuretic peptide (BNP) were evaluated at St. Andrea Hospital. d-dimer results were reported as fibrinogen equivalent units [21]. In order to provide unified analysis of different NPs at our institutions, we referred to specific upper range for normality (URN) as suggested by guidelines (NT-pro-BNP: 300 pg/ml; BNP: 100 pg/ml) [22]. We then reported data from our population as factor of URN increase as described in [23]. Data regarding hs-Troponin, NP, d-dimer and C-reactive protein (CRP) were all log10 transformed to yield approximately normal distribution when performing analysis of relationships as well as multivariable analysis. Outcome data are updated at May 31, 2020.
All analysis was performed using SPSS software 25 (SPSS Inc., Chicago, IL). Data are presented as mean ± standard deviation, counts (percentages) or median (interquartile range, IQR), as appropriate. Comparisons between groups were performed using Chi-squared test, Fisher’s exact test, Student t-test for independent samples or Mann–Whitney U test as appropriate. Analysis of relationships was performed using bivariate correlation and providing corresponding Pearson’s or Spearman correlation coefficients as appropriate. Log-transformed values for serum biomarkers (hs-Troponin, NP, d-dimer, CRP) were used. Multivariable linear regression was performed to investigate factors independently associated with hs-Troponin and NP, and using age, hemoglobin, creatinine, history of previous CVD, PaO2/FIO2 and CRP and d-dimer as independent variables. All tests were two-tailed, and p value of < 0.05 was considered statistically significant. All patients provided informed consent for the use of their record for research purpose, and the study complied with the content of the Declaration of Helsinki. This study received the approval from Sapienza University Ethic Committee no. CE_5773_2020.
Results
Baseline characteristics
Baseline clinical and demographic findings in n = 111 patients included in the study are summarized in Table 1. Mean age was 72 ± 17 years and 46% were male. Comorbidities were common in our population, more than half of the patients had known hypertension, approximately one-third had preexisting CVD, and 22% were taking an ACE inhibitor or ARB before hospital admission. Venous blood samples analysis revealed increase of median CRP and d-dimer. We detected raised troponin (identified by value > URN) in 38% of the cases, whereas in 16% of the patients the increase was beyond three times the respective URN. NP values were elevated in 56% of the cases. On blood gas analysis, mean PaO2 was 75 ± 22 mmHg and mean PaO2/FIO2 308 ± 99. In-hospital mortality rate in our population was 21% (n = 23).
Table 1.
Characteristics of study population overall and according to cardiac biomarkers levels at admission
| Variable | Overall (n = 111) | Hs-Troponin (n = 103) | Natriuretic peptide (n = 82) | ||||
|---|---|---|---|---|---|---|---|
| Hs-Troponin ≤ URN (n = 64) | Hs-Troponin > URN (n = 39) | P | NP ≤ URN (n = 36) | NP > URN (n = 46) | P | ||
| Age | 72 ± 17 | 66 ± 17 | 79 ± 13 | < 0.001 | 61 ± 17 | 81 ± 9 | < 0.001 |
| Sex (male) | 51 (46%) | 28 (44%) | 19 (49%) | 0.623 | 21 (58%) | 21 (46%) | 0.254 |
| Coexistent conditions | |||||||
| Hypertension | 62 (56%) | 32 (50%) | 26 (67%) | 0.098 | 15 (42%) | 31 (67%) | 0.02 |
| Dyslipidemia | 15 (14%) | 8 (12%) | 6 (15%) | 0.679 | 2 (6%) | 9 (20%) | 0.065 |
| Diabetes | 21 (19%) | 12 (19%) | 8 (20%) | 0.826 | 6 (17%) | 9 (20%) | 0.736 |
| Previous CVD | 35 (31%) | 13 (20%) | 18 (46%) | 0.006 | 4 (11%) | 26 (56%) | < 0.001 |
| Atrial fibrillation | 21 (19%) | 6 (9%) | 12 (31%) | 0.006 | 1 (3%) | 17 (37%) | < 0.001 |
| Coronary artery disease | 12 (11%) | 5 (8%) | 7 (18%) | 0.120 | 0 (0%) | 11 (24%) | 0.002 |
| Heart failure | 8 (7%) | 1 (2%) | 5 (13%) | 0.028 | 0 (0%) | 7 (100%) | 0.016 |
| Stroke | 8 (7%) | 3 (5%) | 4 (10%) | 0.422 | 3 (8%) | 5 (11%) | > 0.99 |
| CKD | 7 (6%) | 2 (3%) | 5 (13%) | 0.101 | 0 (0%) | 6 (13%) | 0.024 |
| COPD | 26 (23%) | 18 (28%) | 6 (15%) | 0.138 | 6 (17%) | 10 (22%) | 0.565 |
| Cancer | 8 (7%) | 6 (9%) | 1 (3%) | 0.249 | 1 (3%) | 5 (11%) | 0.223 |
| Autoimmune disease | 6 (5%) | 4 (6%) | 2 (5%) | > 0.99 | 4 (11%) | 2 (4%) | 0.397 |
| ACE inhibitor/ARB therapy | 24 (22%) | 12 (19%) | 9 (23%) | 0.597 | 8 (22%) | 13 (28%) | 0.534 |
| Laboratory tests | |||||||
| Hb (g/dl) | 11.8 ± 2 | 12.1 ± 2 | 11.6 ± 2 | 0.181 | 12.7 ± 2 | 11.2 ± 1.7 | 0.002 |
| WBC (per µL) | 7839 ± 4777 | 8147 ± 5430 | 7880 ± 3546 | 0.786 | 6876 ± 3187 | 8132 ± 6201 | 0.274 |
| Neutrophil (per µL) | 4857 ± 3034 | 4597 ± 2746 | 5582 ± 3412 | 0.148 | 3705 ± 1972 | 4719 ± 2909 | 0.141 |
| Lymphocyte (per µL) | 1402 ± 1196 | 1575 ± 1415 | 1220 ± 836 | 0.192 | 1473 ± 887 | 990 ± 550 | 0.011 |
| Creatinine (mg/dl) | 1.13 ± 1 | 0.96 ± 0.9 | 1.43 ± 1.9 | 0.025 | 0.78 ± 0.2 | 1.51 ± 1.4 | 0.002 |
| ALT (U/L) | 32 ± 52 | 26 ± 13 | 45 ± 87 | 0.098 | 25 ± 15 | 41 ± 80 | 0.238 |
| AST (U/L) | 30 ± 39 | 26 ± 18 | 42 ± 65 | 0.083 | 24 ± 12 | 38 ± 60 | 0.294 |
| CRP (mg/dl) | 4.8 (1.3, 9) | 4 (1.1, 7.4) | 9 (1.3, 14.3) | 0.042 | 2.6 (0.6, 6.9) | 5.3 (2.2, 11.4) | 0.011 |
| D-dimer (ng/ml FEU) | 566 (293, 1554) | 442 (244, 778) | 1269 (560, 2759) | < 0.001 | 338 (248, 1058) | 990 (463, 2054) | 0.004 |
| Hs-Troponin (pg/ml) | 17 (5, 47) | 6.8 (4, 15) | 60 (35, 94) | NA | 6.2 (4, 16) | 37.6 (19, 82) | < 0.001 |
| Hs-Troponin > URN | 39/103 (38%) | 0 (0%) | 39 (100%) | NA | 6 (18%) | 27 (64%) | < 0.001 |
| Hs-Troponin (> 3 × URN) | 17/103 (16%) | 0 (0%) | 17 (43%) | NA | 0 (0%) | 16 (42%) | < 0.001 |
| NP (factor × URN) | 1.35 (0.35, 3.56) | 0.7 (0.1, 1.8) | 3 (1.3, 15) | 0.003 | 0.3 (0.1, 0.6) | 3.3 (1.8, 15) | NA |
| NP > URN | 46/82 (56%) | 15/42 (36%) | 27/33 (82%) | < 0.001 | 0 (0%) | 46 (100%) | NA |
| Blood gas analysis | |||||||
| pH | 7.44 ± 0.08 | 7.43 ± 0.07 | 7.46 ± 0.08 | 0.045 | 7.45 ± 0.07 | 7.44 ± 0.08 | 0.571 |
| pO2 (mmHg) | 75 ± 22 | 75 ± 20 | 75 ± 24 | 0.856 | 74 ± 19 | 76 ± 21 | 0.630 |
| pCO2 (mmHg) | 38 ± 7 | 39 ± 6 | 37 ± 7 | 0.108 | 39 ± 6 | 38 ± 7 | 0.375 |
| PaO2/FIO2 | 308 ± 99 | 325 ± 97 | 276 ± 92 | 0.012 | 342 ± 91 | 293 ± 100 | 0.024 |
| Echocardiograpghic findings* | |||||||
| LVEF (%) | 55 (50, 60) | 60 (53, 60) | 55 (40, 55) | 0.112 | 60 (55, 60) | 55 (40, 55) | 0.01 |
| TAPSE (mm) | 21 (19, 23) | 23 (22, 24) | 20 (18, 22) | 0.022 | 22 (20, 24) | 19 (17, 22) | 0.03 |
| In-hospital outcome | |||||||
| In-hospital death | 23 (21%) | 7 (11%) | 12 (31%) | 0.012 | 5 (14%) | 15 (33%) | 0.05 |
Bold values indicates p < 0.05
NP Natriuretic peptide, URN upper reference of normality, CVD cardiovascular disease, CKD chronic kidney disease, COPD chronic obstructive pulmonary disease, ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, Hb hemoglobin, WBC white blood cells, AST aspartate transaminase, ALT alanine transaminase, CRP C-reactive protein, FEU fibrinogen equivalent unit, LVEF left ventricular ejection fraction, TAPSE tricuspid annular plane systolic excursion, NA not applicable
*Available in 24 out of 111 patients
Characteristics of population according to cardiac biomarkers
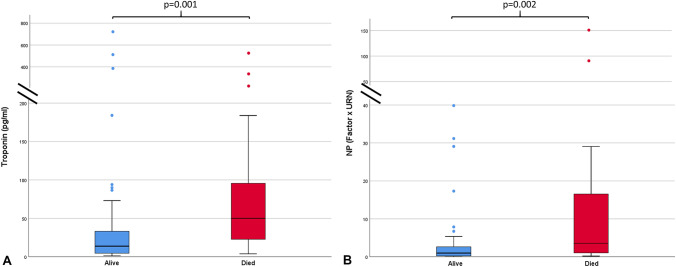
On subgroups analysis, patients who had raised hs-Troponin at admission were older, with higher prevalence of previous CVD (46% vs 20%) and NP beyond URN (82% vs 36%), higher CRP (9 (1.3, 14.3) mg/dl vs 4 (1.1, 7.4) mg/dl) and creatinine (1.43 ± 1.9 mg/dl vs 0.96 ± 0.9 mg/dl)values, whereas they had lower PaO2/FIO2(276 ± 92 vs 325 ± 97) as compared to those with normal hs-Troponin. Of note, d-dimer was significantly increased in patients with high hs-Troponin at admission (1269 (560, 2759) ng/ml vs 442 (244, 778) ng/ml). These findings were similarly represented in patients with NP beyond cut-off levels. d-dimer was significantly elevated in patients with high hs-Troponin, irrespective of whether they had normal or high NP (Fig. 1). Rate of (ACE) inhibitor or ARB intake before hospital admission was similar between cardiac biomarkers subgroups. Both patients with hs-Troponin and NP beyond normal values experienced higher rate of in-hospital mortality (31% vs 11%, p = 0.012, and 33% vs 14%, p = 0.05, respectively). In patients with worse in-hospital outcome, we detected higher levels of both hs-Troponin and NP (p = 0.001 and p = 0.002, respectively, depicted in Fig. 2).
Fig. 1.
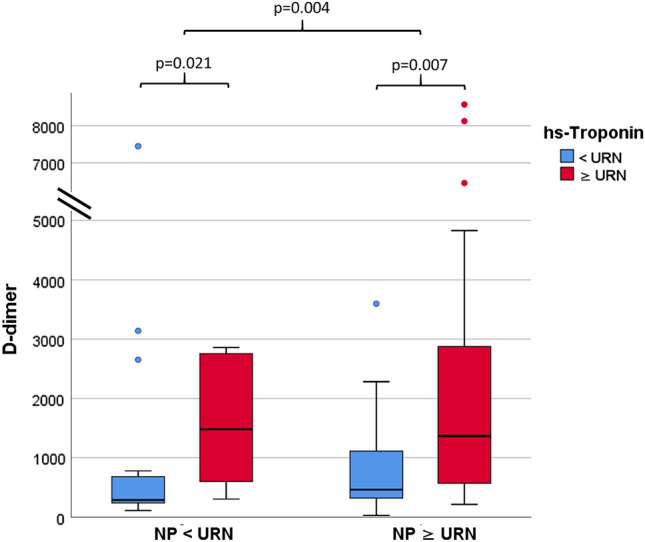
Boxplot shows significantly higher D-dimer values in patients with hs-Troponin beyond URN, both in patients with normal (p = 0.021) and elevated (p = 0.007) NP
Fig. 2.
Boxplots showing significantly higher cardiac biomarkers levels in patients with poor in-hospital outcome. Hs-Troponin (a) and NP (b) with p = 0.001 and p = 0.002, respectively
Echocardiographic findings
Transthoracic echocardiography was performed in 24 patients (22%) within the overall population. Median left ventricular ejection fraction (LVEF) and tricuspid annular plane systolic excursion (TAPSE) were 55% (50, 60) and 21 mm (19, 23), respectively. LVEF was significantly lower in patients with elevated NP, but not in those with elevated hs-Troponin (p = 0.02 and p = 0.112, respectively). TAPSE was significantly lower in both groups of elevated cardiac biomarkers (hs-Troponin: 20 mm (18, 22) vs 23 mm (22, 24), p = 0.022; NP: 19 mm (17, 22) vs 22 mm (20, 24), p = 0.03).
Analysis of relationships
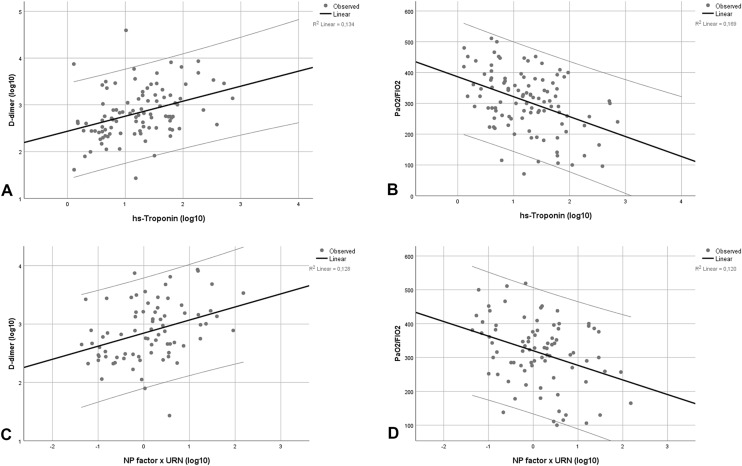
Analysis of relationships results are summarized in Table 2. We observed a significant and close correlation between hs-Troponin and NP values (r = − 0.730, p < 0.001; Supplementary Fig. 1). Both hs-Troponin and NP were associated with age (r = 0.484 and r = 0.696, respectively, both p < 0.001) and history of previous CVD (r = 0.332 and r = 0.489 with p = 0.001 and p < 0.001, respectively), as well as with hemoglobin and creatinine levels. Moreover, we detected significant associations between both cardiac biomarkers on one side and indexes of COVID-19 pneumonia severity on the other (depicted in Fig. 3). These relationships were significant for hs-Troponin (CRP: r = 0.396, p < 0.001; d-dimer: r = 0.366, p < 0.001: PaO2/FIO2: r = − 0.411, p < 0.001) as well as NP (CRP: r = 0.293, p = 0.008; d-dimer: r = 0.358, p = 0.001: PaO2/FIO2: r = − 0.347, p = 0.001).
Table 2.
Bivariate correlations of hs-Troponin and NP with patients’ characteristics and laboratory results
| Variable | Troponin (Log10) | NP (Log10 (factor × URN)) | ||
|---|---|---|---|---|
| R | P | R | P | |
| Age | 0.484 | < 0.001 | 0.696 | < 0.001 |
| Sex (male) | 0.012 | 0.907 | − 0.180 | 0.106 |
| Previous CVD | 0.332 | 0.001 | 0.489 | < 0.001 |
| ACE inhibitor/ARB therapy | 0.021 | 0.833 | 0.156 | 0.162 |
| Creatinine (mg/dl) | 0.288 | 0.003 | 0.244 | 0.027 |
| Hb (g/dl) | − 0.213 | 0.031 | − 0.412 | < 0.001 |
| CRP (Log10) | 0.396 | < 0.001 | 0.293 | 0.008 |
| D-dimer (Log10) | 0.366 | < 0.001 | 0.358 | 0.001 |
| Hs-Troponin (Log10) | NA | NA | 0.730 | < 0.001 |
| NP factor x URN (log10) | 0.730 | < 0.001 | NA | NA |
| pH | 0.063 | 0.535 | − 0.040 | 0.728 |
| pO2 | 0.030 | 0.766 | − 0.086 | 0.443 |
| pCO2 | − 0.059 | 0.560 | − 0.049 | 0.667 |
| PaO2/FIO2 | − 0.411 | < 0.001 | − 0.347 | 0.001 |
Pearson’s and Spearman (rho) coefficient, as appropriate for the type of the data; p value < 0.05 was considered significant. Bold values indicates p < 0.05
NP Natriuretic peptide, CVD cardiovascular disease, ACE angiotensin-converting enzyme, ARB angiotensin receptor blocker, Hb hemoglobin, CRP C-reactive protein, NA not applicable
Fig. 3.
Scatter plots showing correlations between hs-Troponin with D-dimer (a) and PaO2/FIO2 (b) with r = 0.366, p < 0.001 and r = − 0.411, respectively, both p < 0.001, as well as between NP with D-dimer (c) and PaO2/FIO2 (d) with r = 0.358 and r = − 0.347, respectively, both p = 0.001
On multivariable analysis (Table 3) including age, creatinine, hemoglobin, CRP, d-dimer and PaO2/FIO2, we observed independent associations between hs-Troponin and age (B = 0.419, p = 0.001), PaO2/FIO2 (B = − 0.212, p = 0.013) and d-dimer (B = 0.179, p = 0.037), whereas NP remained associated with age (B = 0.480, p < 0.001) and history of previous CVD (B = 0.253, p = 0.001).
Table 3.
Multivariable linear regression of predictive associations with hs-Troponin and NP
| Hs-Troponin (Log10) | NP (Log10 (factor × URN)) | ||||||
|---|---|---|---|---|---|---|---|
| Adj-R2 | Variable | Beta | P | Adj-R2 | Variable | Beta | P |
| 0.335 | Age | 0.419 | 0.001 | 0.442 | Age | 0.480 | < 0.001 |
| PaO2/FIO2 | − 0.212 | 0.013 | Previous CVD | 0.253 | 0.001 | ||
| D-dimer (Log10) | 0.179 | 0.037 | |||||
Independent variables included in both models: age, hemoglobin, creatinine, history of previous CVD, PaO2/FIO2 and CRP and D-dimer. Bold values indicates p < 0.05
NP Natriuretic peptide, CVD cardiovascular disease, Hb hemoglobin, CRP C-reactive protein
Discussion
Results from our multicenter observational study highlight the common occurrence of myocardial involvement in patients with COVID-19 pneumonia. Cardiac biomarkers increase was associated with both patients’ preexisting conditions and markers of COVID-19 disease severity, as well as worse in-hospital outcome. Moreover, we described significant independent correlations between hs-Troponin and PaO2/FIO2 and d-dimer on one side and between NP and history of CVD on the other. Increasing age remained an independent determinant of both cardiac biomarkers increase.
Incidence
In our analysis, more than one-third of patients had increased hs-Troponin level, though an elevation that exceeded more than three times the URN was observed only in 16% of the population, while NP elevation beyond URN was present in more than a half of our sample. Though multiple studies described lower incidence of myocardial injury in patients with COVID-19 pneumonia [6, 24], our findings are in keeping with what observed by others [12, 25], likely in relation to older age and higher prevalence of comorbidities in these cohorts. Results from our population could be related to the peculiar epidemiology of COVID-19 pneumonia in Lazio region during the index period, which was in several cases related to clusters within nursing homes and rehabilitation clinics (partial nationwide data available at [26]). This highlights the heterogeneity of COVID-19 populations described by different studies, which in turn might explain existing differences in terms of patients’ characteristics and outcomes [6, 12].
Potential mechanisms underlying myocardial involvement
We observed hs-Troponin and NP to be associated with both preexisting factors leading to higher vulnerability of myocardium (age, anemia, renal failure, previous CVD) as well as markers of COVID-19 pneumonia disease severity (d-dimer [9], CRP [24] and PaO2/FIO2 [27]). This result reiterates the view of myocardial injury in the course of COVID-19 pneumonia as mainly caused by virus independent factors, including higher myocardial oxygen consumption, increased inflammatory driver and hypoxemia and favored by underlying comorbidities such as heart failure [18]. Of note, in the course of COVID-19 pneumonia [13, 16, 17], these factors have been described as potential drivers of both hs-Troponin and NP increase, which, as expected, had parallel trend in our population.
Notwithstanding, results from our study might point toward some existing differences in mechanisms leading to hs-Troponin and NP elevation in COVID-19 pneumonia. Indeed, we observed an independent association between both d-dimer and PaO2/FIO2 with hs-Troponin, but not with NP. Interestingly, significantly elevated d-dimer concentration has been reported in COVID-19 patients with myocardial injury [10], but not in those with preexisting CVD [12]. Conversely, history of previous CVD was independently associated only with NP increase in our cohort, suggesting that preexisting underlying conditions such as heart failure and atrial fibrillation might be the major determinants of NP rather than hs-Troponin increase in these patients.
Reasons for reported findings cannot be discerned from data from our study as correlations we observed do not promptly imply the existence of any causative link. However, several hypotheses could be made to explain our observations. PaO2/FIO2 ratio is a key parameter for defining acute respiratory distress syndrome [28] and in patients with COVID-19 pneumonia has been used as marker of disease severity [27] as well as end point in studies testing novel treatments [29]. Additionally, PaO2/FIO2 may decrease with higher oxygen consumption and lower cardiac output [30]: both elements can contribute to myocardial injury. High d-dimer is commonly found in patients with COVID-19 pneumonia [7], albeit not necessarily representing the occurrence of pulmonary embolism but inflammation, hypoxia and disseminated intravascular coagulation [9, 31], factors that can be all related to myocardial injury [11]. Furthermore, thrombosis of small vessels within lung and kidney has been reported by multiple studies (reviewed in [32, 33]), possibly favored by coexistent endotheliitis found in several organs, including the heart [34]. In this context, microvascular thrombosis in coronary vessels [35, 36] is an adjunctive potential mechanism consistent with our results, already anecdotally reported [37].
Clinical implications
Our findings could potentially carry clinical implications. The therapeutic management of COVID-19 pneumonia is currently based on supportive therapy and experimental approaches [38, 39]. To provide a reliable discrimination of phenotypes within COVID-19 population could be helpful in order to plan targeted treatment [40], as hypothesized for patients with marked d-dimer elevation who might require higher anticoagulant dosage administration [41]. Based on our results, it is tempting to speculate whether increase of hs-Troponin might help identifying the subset of patients with higher systemic inflammatory response to COVID-19 infection, whereas NP the subgroup those in which preexisting CVD contributes more to the severe clinical course [12]. Finally, irrespective of the underlying mechanisms and in accordance with previous studies [10, 13], we observed higher in-hospital mortality in patients with either high hs-Troponin or NP within our population, strengthening the hypothesis that cardiac biomarkers assessment might provide useful information to aid COVID-19 pneumonia risk stratification.
Limitations
The present study should be read in light of several limitations. Though presenting a timely report regarding an ongoing medical emergency, the sample size is small, and results should be interpreted as hypothesis generating only, in need of further studies to ascertain the existence of any cause–effect relationship regarding the observed associations. Enrollment took place including patients consecutively admitted to medical units dedicated to COVID-19 assistance. However, it cannot be excluded that patients (especially males [42]) who were more severely affected when presenting to the emergency department were sent directly to intensive care units, thus being missed in our analysis. Due to safety reasons, and according to current specific guidelines [20], transthoracic echocardiography was performed only in a subset of patients within the whole population; hence, we could not provide comprehensive data regarding echocardiographic findings; notwithstanding, our results are in line with previous research linking right ventricular dysfunction to increased levels of cardiac biomarkers in COVID-19 pneumonia [43]. Individuals affected by COVID-19 represent a heterogeneous group of patients, in which the time and location of data collection may lead to significant variation among different samples; hence, our results cannot be immediately translated to study populations characterized by largely different demographic and clinical characteristics.
Conclusions
Cardiac biomarkers elevation was common in our cohort of patients with COVID-19 pneumonia and associated with worse prognosis. Independent association of hs-Troponin with PaO2/FIO2 and d-dimer on one side, and of NP with history of CVD on the other, would require further studies to fully assess whether these biomarkers could effectively contribute to a reliable differentiation of phenotypes and subsequent treatment guidance in COVID-19 pneumonia patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Data availability
Data will be made available upon request.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study received the approval from Sapienza University Ethic Committee no. CE_5773_2020.
Consent to participate
All patients provided informed consent for the use of their record for research purpose.
Consent for publication
All authors have participated in the work and have reviewed and agree with article contents.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pennica A, Conforti G, Falangone F, Martocchia A, Tafaro L, Sentimentale A, Marini V, Pezzuto A, Spuntarelli V, Martelletti P. Clinical management of adult coronavirus infection disease 2019 (COVID-19) positive in the setting of low and medium intensity of care: a short practical review. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00333-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baraboutis IG, Gargalianos P, Aggelonidou E, Adraktas A. Initial real-life experience from a designated COVID-19 centre in Athens, Greece: a proposed therapeutic algorithm. SN Compr Clin Med. 2020 doi: 10.1007/s42399-020-00324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cogliati C, Ceriani E, Brambilla AM. When internal and emergency medicine speak to each other: organization in the time of COVID. Intern Emerg Med. 2020;15:891–892. doi: 10.1007/s11739-020-02380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, Ren R, Leung KSM, Lau EHY, Wong JY, Xing X, Xiang N, Wu Y, Li C, Chen Q, Li D, Liu T, Zhao J, Liu M, Tu W, Chen C, Jin L, Yang R, Wang Q, Zhou S, Wang R, Liu H, Luo Y, Liu Y, Shao G, Li H, Tao Z, Yang Y, Deng Z, Liu B, Ma Z, Zhang Y, Shi G, Lam TTY, Wu JT, Gao GF, Cowling BJ, Yang B, Leung GM, Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fogarty H, Townsend L, Ni Cheallaigh C, Bergin C, Martin-Loeches I, Browne P, Bacon CL, Gaule R, Gillett A, Byrne M, Ryan K, O’Connell N, O’Sullivan JM, Conlon N, O’Donnell JS. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020 doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landi A, De Servi S. The burden of thrombotic complications in critically ill patients with COVID-19: charting the uncharted. Intern Emerg Med. 2020;15:893–895. doi: 10.1007/s11739-020-02393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J. 2020;41(19):1821–1829. doi: 10.1093/eurheartj/ehaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao L, Jiang D, Wen XS, Cheng XC, Sun M, He B, You LN, Lei P, Tan XW, Qin S, Cai GQ, Zhang DY. Prognostic value of NT-proBNP in patients with severe COVID-19. Respir Res. 2020 doi: 10.1186/s12931-020-01352-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rath D, Petersen-Uribe Á, Avdiu A, Witzel K, Jaeger P, Zdanyte M, Heinzmann D, Tavlaki E, Müller K, Gawaz MP. Impaired cardiac function is associated with mortality in patients with acute COVID-19 infection. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karbalai Saleh S, Oraii A, Soleimani A, Hadadi A, Shajari Z, Montazeri M, Moradi H, Talebpour M, Sadat Naseri A, Balali P, Akhbari M, Ashraf H. The association between cardiac injury and outcomes in hospitalized patients with COVID-19. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Libby P. The heart in COVID19: primary target or secondary bystander? JACC Basic to Transl Sci. 2020 doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with Coronavirus Disease 2019 (COVID-19): possible mechanisms. J Card Fail. 2020 doi: 10.1016/j.cardfail.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomasoni D, Italia L, Adamo M, Inciardi RM, Lombardi CM, Solomon SD, Metra M. <scp>COVID</scp> 19 and heart failure: from infection to inflammation and angiotensin <scp>II</scp> stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020 doi: 10.1002/ejhf.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skulstad H, Cosyns B, Popescu BA, Galderisi M, Di Salvo G, Donal E, Petersen S, Gimelli A, Haugaa KH, Muraru D, Almeida AG, Schulz-Menger J, Dweck MR, Pontone G, Sade LE, Gerber B, Maurovich-Horvat P, Bharucha T, Cameli M, Magne J, Westwood M, Maurer G, Edvardsen T. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21(6):592–598. doi: 10.1093/ehjci/jeaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Favaloro E, Thachil J. Reporting of D-dimer data in COVID-19: some confusion and potential for misinformation. Clin Chem Lab Med. 2020 doi: 10.1515/CCLM-2020-0573. [DOI] [PubMed] [Google Scholar]
- 22.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 23.Blackshear JL, Safford RE, Thomas CS, Bos JM, Ackerman MJ, Geske JB, Ommen SR, Shapiro BP, Johns GS. Platelet function analyzer 100 and brain natriuretic peptide as biomarkers in obstructive hypertrophic cardiomyopathy. Am J Cardiol. 2018;121:768–774. doi: 10.1016/j.amjcard.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Li L, Da XuM, Wu J, Luo D, Zhu YS, Li BX, Song XY, Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, Yan J, Ping H, Zhou Q. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int J Cardiol. 2020 doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.https://www.epicentro.iss.it/coronavirus/bollettino/Bollettino-sorveglianza-integrata-COVID-19_7-maggio-2020.pdf (last accessed on June 1st 2020).
- 27.Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 29.Christie DB, Nemec HM, Scott AM, Buchanan JT, Franklin CM, Ahmed A, Khan MS, Callender CW, James EA, Christie AB, Ashley DW. Early outcomes with utilization of tissue plasminogen activator in COVID-19 associated respiratory distress: a series of five cases. J Trauma Acute Care Surg. 2020 doi: 10.1097/TA.0000000000002787. [DOI] [PubMed] [Google Scholar]
- 30.Gattinoni L, Vassalli F, Romitti F. Benefits and risks of the P/F approach. Intensive Care Med. 2018;44:2245–2247. doi: 10.1007/s00134-018-5413-4. [DOI] [PubMed] [Google Scholar]
- 31.Leonard-Lorant I, Delabranche X, Severac F, Helms J, Pauzet C, Collange O, Schneider F, Labani A, Bilbault P, Moliere S, Leyendecker P, Roy C, Ohana M. Acute pulmonary embolism in COVID-19 patients on CT angiography and relationship to D-Dimer levels. Radiology. 2020 doi: 10.1148/radiol.2020201561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kang Y, Chen T, Mui D, Ferrari V, Jagasia D, Scherrer-Crosbie M, Chen Y, Han Y. Cardiovascular manifestations and treatment considerations in covid-19. Heart. 2020 doi: 10.1136/heartjnl-2020-317056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coccheri S. COVID-19: the crucial role of blood coagulation and fibrinolysis. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boral BM, Williams DJ, Boral LI. Disseminated intravascular coagulation. Am J Clin Pathol. 2016;146:670–680. doi: 10.1093/ajcp/aqw195. [DOI] [PubMed] [Google Scholar]
- 36.Hendren NS, Drazner MH, Bozkurt B, Cooper LT., Jr Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation. 2020;141(23):1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS, Thursz M, Manousou P, Corbett R, Goldin R, Al-Sarraj S, Abdolrasouli A, Swann OC, Baillon L, Penn R, Barclay WS, Viola P, Osborn M. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure F-X, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D’Arminio Monforte A, Ismail S, Kato H, Lapadula G, L’Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate use of Remdesivir for patients with severe Covid-19. N Engl J Med. 2020 doi: 10.1056/nejmoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panati K, Narala VR. COVID-19 outbreak: an update on therapeutic options. SN Compr Clin Med. 2020;2:379–380. doi: 10.1007/s42399-020-00264-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatment for different phenotypes ? Intensive Care Med. 2020 doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim L, Garg S, O’Halloran A, Whitaker M, Pham H, Anderson EJ, Armistead I, Bennett NM, Billing L, Como-Sabetti K, Hill M, Kim S, Monroe ML, Muse A, Reingold AL, Schaffner W, Sutton M, Talbot HK, Torres SM, Yousey-Hindes K, Holstein R, Cummings C, Brammer L, Hall AJ, Fry AM, Langley GE. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, Arbel Y, Topilsky Y. The spectrum of cardiac manifestations in coronavirus disease 2019 (COVID-19)—a systematic echocardiographic study. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request.