Highlights
-
•
Malignant peritoneal mesothelioma, particularly the sarcomatoid type, is rare and aggressive.
-
•
Accurate diagnosis by ascites cytology is difficult.
-
•
Histological examination such as laparoscopy aids in diagnosis.
-
•
There is no clear consensus treatment for MPM and an extensive research program is needed.
1. Case
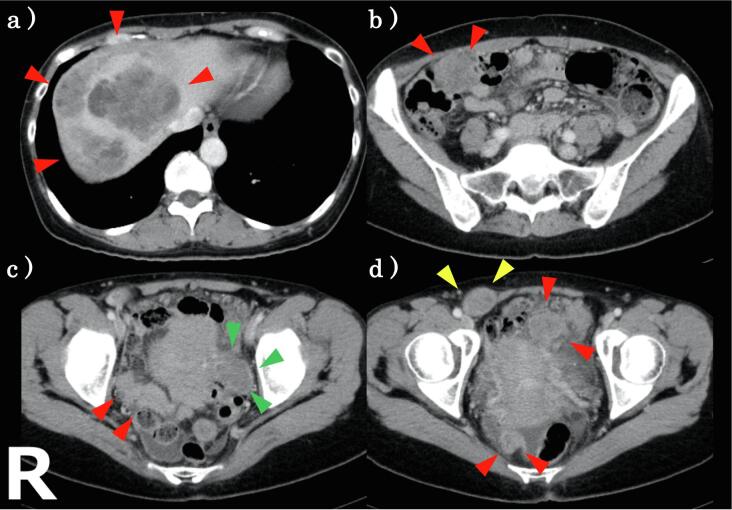
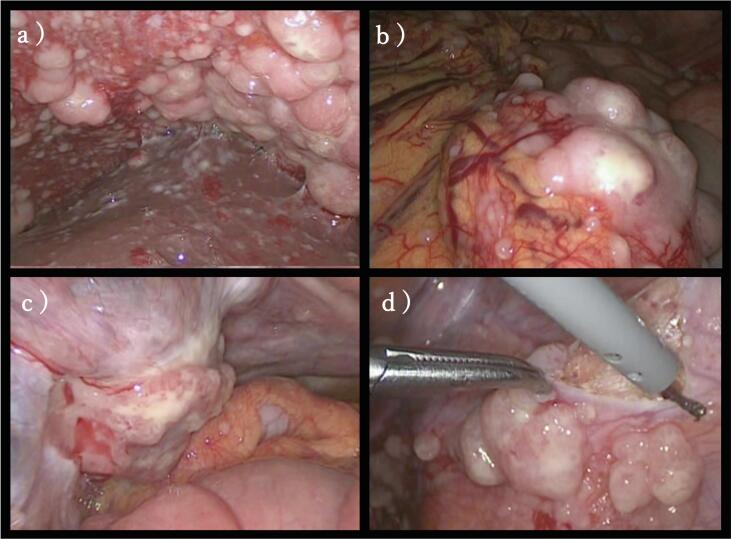
A 51-year-old woman came to our hospital for a “a small palpable mass in her lower right abdomen”. She had a right salpingo-oophorectomy for fibroma of the right ovary two years ago. She had never been exposed to asbestos, and there was no family history of malignant mesothelioma. Contrast enhanced computed tomography (CECT) showed a solid tumor in the lower right abdomen measuring about 2.7 cm with nodular enhancement (Fig. 1). We suspected that this was the palpable mass. At this time, it was not clear whether this was an intraabdominal mass or the abdominal wall mass. In addition, there was a 4 cm tumor in the left side of the uterus, and a 10 cm tumor between the liver and the right diaphragm, and many solid tumors in the abdominal cavity. Upper endoscopy and colonoscopy revealed normal findings. Tumor markers were normal (carcinoembryonic antigen 3.1 μg/L and CA 19–9 4.0U/mL) except for CA-125 that was 156.2 U/mL. We therefore suspected peritoneal cancer, ovarian cancer, peritoneal mesothelioma, etc. and performed staging laparoscopy (Fig. 2). Alternatively, we considered CT-guided core needle biopsy or incisional/excisional biopsy of the palpable mass in her lower right abdomen, which are generally less invasive than staging laparoscopy. However, we chose laparoscopy, because (1) the possibility of the mass being in the abdominal cavity could not be ruled out, (2) it allowed us to observe the entire abdominal cavity and determine the degree of the mass expansion, and (3) it provided a more adequate amount of specimen than CT-guided core needle biopsy or incisional/excisional biopsy. During surgery, diffuse and widespread masses and nodules were found in the peritoneal cavity, and the large tumor between the liver and the right diaphragm was pressed against part of the liver. In addition, we observed omental cake and a 4 cm tumor on the left side of the uterus that had invaded the left adnexa. There was only a small amount of pale yellow ascites. A mass in the lower right abdomen that was the patient’s chief complaint was located outside the abdominal cavity and we realized it was an abdominal wall mass. She underwent a left salpingo-oophorectomy and part of the peritoneum was biopsied. She recovered well after surgery with no complications.
Fig. 1.
CECT. (a) a 10 cm tumor between the liver and the right diaphragm. (b–d) a 4 cm tumor in the left side of the uterus (green) and other many solid tumors in the abdominal cavity with a small amount of ascites. (d) a solid tumor in the lower right abdomen measuring about 2.7 cm which was the patient’s chief complaint (yellow). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Operative finding. There were diffuse and widespread masses and nodules in the peritoneal cavity and the large tumor between the liver and the right diaphragm pressed against part of the liver (a). In addition, omental cake (b) and a 4 cm tumor on the left side of the uterus which invaded the left adnexa (c) was observed. There was only a small amount of pale yellow ascites. We performed biopsy on part of the peritoneum (d).
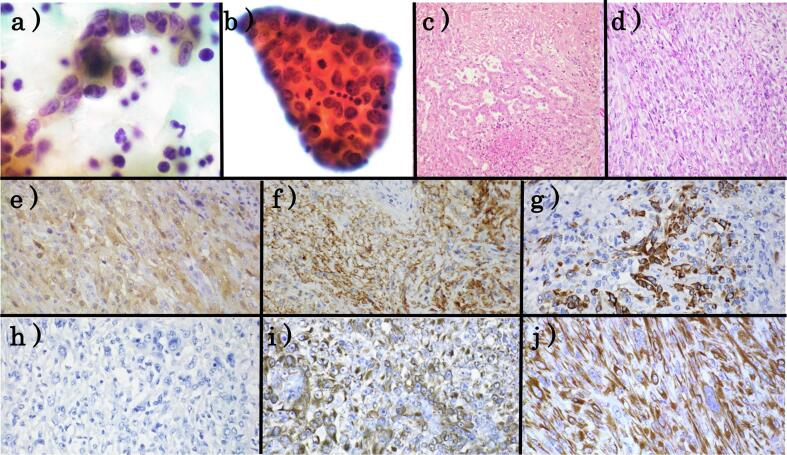
Ascites fluid cytology demonstrated many scattered single or loosely aggregated cells in an inflammatory background composed of lymphocytes (Fig. 3). These cells had short-spindle to round nuclei with irregular nuclear contours and prominent nucleoli and the cytoplasma was scant or fine and wispy. There were a few papillary clusters. Mitoses were frequent. In contrast, intercellular spaces, extracellular matrix core, membrane protrusions and microvilli characteristic of peritoneal mesothelioma were not detected. Based on these cytological features, serous carcinoma and poorly differentiated tumors were suspected.
Fig. 3.
Cytological, histological and immunohistochemical findings. (a,b) Cytological findings in the ascites fluid. (c.d ) Histological finding of the left ovary. (e–j) Immunohistochemical staining. (e) calretinin (f) D2-40 (g) CK5/6h) MOC31 (i) AE1/AE3 (j) CAM5.2.
Histologically, the area where tubule structures characteristic of epithelioid type accounted for only 1–2% of the total mass, while most were sarcoma-type areas composed of spindle to round-shaped cells (Fig. 3). Pleomorphic nuclei, mitoses and the multinucleated giant cells were also observed. Immunohistochemically, these cells were positive for mesothelioma markers except for WT1 and included calretinin, D2-40, cytokeratins5/6 and EMA, and were negative for carcinoma markers such as CEA, MOC31, Ber-EP4, Pax8 and ER (Fig. 3). Moreover, these cells were positive for AE1/AE3 and CAM5.2, which are usually positive in sarcomatoid MPM and negative for sarcoma. Therefore, we finally diagnosed sarcomatoid MPM.
A month after the biopsy, systemic chemotherapy was administered and included pemetrexed 500 mg/m2 and cisplatin 75 mg/m2 every 21 days. After four cycles, a CT scan revealed that the masses had diminished, but renal function was decreased. During the drug wash-out period for recovery of renal function, a marked increase in tumor sizes on CT scan were observed. Moreover, she also noticed increased abdominal pain with a reduced ability to carry out her activities of daily living. Therefore, after consultation with her and her family, aggressive management was discontinued and replaced with best supportive care alone. The patient died one month later (6 months after diagnosis).
2. Discussion
Malignant peritoneal mesothelioma (MPM) is a rare disease. Age-adjusted mesothelioma incidence rate across all ages from 1973 to 2002 in the United States was 9.8 per million persons per year and MPM accounts for only 11% (647/6078) of all cases of mesothelioma compared with 83% (5073/6078) pleural mesothelioma [1]. In contrast to pleural mesothelioma with predominance of males, females comprise approximately one-half of all cases of MPM. A study of 10,589 mesothelioma cases in the United States reported that females comprised a larger proportion of MPM than in the pleural mesothelioma cohort (43.8% vs. 19.1%, respectively) [2]. It was reported that the mean age at diagnosis of MPM was younger than that of pleural mesothelioma (63 years old vs. 71 years old, respectively) [2].
There is a strong relationship between asbestos exposure and mesothelioma development and it is the most recognized risk factor. However, the paper by Robert Spirtas indicated that in MPM, the link is not as strong as in pleural mesothelioma, particularly among females. Among males, the attributable risk (AR) of asbestos exposure for pleural mesothelioma and MPM was 88% and 58%, respectively. On the other hands, the risk for females was 23% for both sites combined [3]. The cause of this difference in AR by gender is unclear but could be due to the following: (1) the number of female cases is small, (2) females are less likely than male to be in occupations expected to have the highest exposure to asbestos and they are often indirectly exposed by cohabiter such as their husbands and fathers, (3) therefore, misclassification of exposure may be greater among females. In addition, the involvement of inherited susceptibility in mesothelioma has been recently actively researched and reported. This patient had no history of asbestos exposure and no family history of malignant mesothelioma, although genetic research was not conducted.
Fluid cytology has potential to provide useful information, however, its effectiveness is limited. V. de Pangher Manzini reported that, in 61 cases with fluid cytology, it was positive for mesothelioma in 31(51%), positive for non-mesothelioma malignancy in 13(21%), negative for mesothelioma with associated atypical or activated mesothelial cells in 9 (15%) and negative for mesothelioma in 8 (13%) [4]. Moreover, because the sarcomatoid component seldom into the abdominal cavity fluid, the detection of the sarcomatoid type is difficult. In our case, based on ascites fluid cytology, serous carcinoma and poorly differentiated tumors were suspected and mesothelioma could not be detected.
Therefore, the main diagnostic procedures are surgical, such as laparoscopy, laparotomy and CT-guided needle biopsy. Currently, laparoscopy is a useful diagnostic tool because of its minor invasiveness. We also chose laparoscopy for diagnosing this case. However, Paul H. Sugarbaker, et al. reported that port site recurrence is observed at nearly all trochar sites on follow-up, and extreme caution is essential [5].
The histopathologic examination by hematoxylin and eosin stain enables MPM to be classified into three histologic subtypes: epithelioid, sarcomatoid, and biphasic. The sarcomatoid type is typically composed of tightly packed spindle cells with the area of epithelioid type accounting for less than 10% of the overall histology. Sarcomatoid type, as in this case, is the rarest and epithelioid type is the most common. Yan TD reported that of 62 cases with MPM, 92%(57/62) were epithelioid type, 8% (5/62) were biphasic type, and there were no sarcomatoid type [6].
Immunohistochemical staining is quite useful for diagnosis of MPM. However, a single IHC stain cannot diagnose MPM definitively, because there is no MPM specific marker. The use of two mesothelioma markers and two carcinoma markers as an initial diagnostic screen has been recommended. The diagnosis of MPM requires positive staining for mesothelioma markers such as calretinin, WT-1, D2-40, cytokeratins5/6 and EMA, and negative staining for carcinoma markers such as CEA, BerEP4, MOC31, ER, PAX8, and claudin 4[7]. This case was negative for only WT-1 and positive for other mesothelioma markers, and negative for all carcinoma markers. Moreover, AE1/AE3 and CAM5.2 were positive in this case, which are usually positive in sarcomatoid MPM and negative for sarcoma.
There are no randomized controlled trials for MPM and no clear consensus treatment for MPM because of its rarity. Mortality of MPM is mostly due to the progression of primary disease within the peritoneal cavity, rather than distant metastases and generally has a poor prognosis without treatment. Particularly, Sarcomatoid type, as in this case, and biphasic type are considered to have poorer prognosis than the epithelioid type. For selected patients who have a good performance status, are without extra-abdominal metastasis and who could achieve complete surgical cytoreduction, cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) are proposed as the mainstay of treatment. Median overall survival with this method was reported to range from 19 to 92 months [8]. If CRS + HIPEC is not indicated, systemic chemotherapy could be a treatment option. The most common regimen is pemetrexed in combination with a platinum agent including pemetrexed plus cisplatin as in this case. When using the regimen including pemetrexed, folic acid and vitamin B12 supplementation is required to reduce toxicity. Pasi A. Jänne, et al. demonstrated that the rate of disease control (CR + PR + SD) was 71.2% with a median survival of 13.1 months among patients who received pemetrexed plus cisplatin compared with 8.7 months among those receiving pemetrexed alone [9]. Moreover, in the recent phase 3 trial conducted in only patients with malignant pleural mesothelioma, it was reported that addition of bevacizumab to pemetrexed plus cisplatin markedly improved OS compared with pemetrexed plus cisplatin without bevacizumab [10].
3. Conclusion
MPM, in particular sarcoma type, is rare. Its non-clinical features include a higher proportion of female, a younger age of diagnosis and a lower association with asbestos compared with pleural mesothelioma. The diagnostic accuracy of ascites cytology is low, therefore histological examination such as laparoscopy is useful. MPM has a poor prognosis and no consensus treatment. An extensive research program on MPM is needed.
Patient consent statement
Written informed consent was obtained.
Author contributions
M Kobayasi, K Masuda, YN, EY, AT, NK, KK, HN, HO and TY diagnosed this case. JT treated this patient clinically. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Teta M.J., Mink P.J., Lau E., Sceurman B.K., Foster E.D. US mesothelioma patterns 1973–2002: Indicators of change and insights into background rates. Eur. J. Cancer Prev. 2008;17:525–534. doi: 10.1097/CEJ.0b013e3282f0c0a2. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez D., Cheung M.C., Housri N., Koniaris L.G. Malignant abdominal mesothelioma: Defining the role of surgery. J. Surg. Oncol. 2009;99:51–57. doi: 10.1002/jso.21167. [DOI] [PubMed] [Google Scholar]
- 3.Spirtas R., Heineman E.F., Bernstein L., Beebe G.W., Keehn R.J., Stark A., Harlow B.L., Benichou J. Malignant mesothelioma: Attributable risk of asbestos exposure. Occup. Environ. Med. 1994;51:804–811. doi: 10.1136/oem.51.12.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Pangher Manzini V., Recchia L., Cafferata M., Porta C., Siena S., Giannetta L., Morelli F., Oniga F., Bearz A., Torri V., Cinquini M. Malignant peritoneal mesothelioma: A multicenter study on 81 cases. Ann. Oncol. 2010;21:348–353. doi: 10.1093/annonc/mdp307. [DOI] [PubMed] [Google Scholar]
- 5.Sugarbaker P.H., Acherman Y.I.Z., Gonzalez-Moreno S., Ortega-Perez G., Stuart O.A., Marchettini P., Yoo D. Diagnosis and treatment of peritoneal mesothelioma: The Washington Cancer Institute experience. Semin. Oncol. 2002;29:51–61. doi: 10.1053/sonc.2002.30236. [DOI] [PubMed] [Google Scholar]
- 6.Yan T.D., Popa E., Brun E.A., Cerruto C.A., Sugarbaker P.H. Sex difference in diffuse malignant peritoneal mesothelioma. Br. J. Surg. 2006;93:1536–1542. doi: 10.1002/bjs.5377. [DOI] [PubMed] [Google Scholar]
- 7.Husain A.N., Colby T.V., Ordóñez N.G., Allen T.C., Attanoos R.L., Beasley M.B., Butnor K.J., Chirieac L.R., Churg A.M., Dacic S., Galateau-Sallé F., Gibbs A., Gown A.M., Krausz T., Litzky L.A., Marchevsky A., Nicholson A.G., Roggli V.L., Sharma A.K., Travis W.D., Walts A.E., Wick M.R. Guidelines for pathologic diagnosis of Malignant Mesothelioma: 2017 Update of the consensus statement from the International Mesothelioma Interest Group. Arch. Pathol. Lab. Med. 2017;142(2018):89–108. doi: 10.5858/arpa.2017-0124-RA. [DOI] [PubMed] [Google Scholar]
- 8.Helm J.H., Miura J.T., Glenn J.A., Marcus R.K., Larrieux G., Jayakrishnan T.T., Donahue A.E., Gamblin T.C., Turaga K.K., Johnston F.M. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2015;22:1686–1693. doi: 10.1245/s10434-014-3978-x. [DOI] [PubMed] [Google Scholar]
- 9.Jänne P.A., Wozniak A.J., Belani C.P., Keohan M.L., Ross H.J., Polikoff J.A., Mintzer D.M., Taylor L., Ashland J., Ye Z., Monberg M.J., Obasaju C.K. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: Outcomes of an expanded access program. Clin. Lung Cancer. 2005;7:40–46. doi: 10.3816/CLC.2005.n.020. [DOI] [PubMed] [Google Scholar]
- 10.Zalcman G., Mazieres J., Margery J., Greillier L., Audigier-Valette C., Moro-Sibilot D., Molinier O., Corre R., Monnet I., Gounant V., Rivière F., Janicot H., Gervais R., Locher C., Milleron B., Tran Q., Lebitasy M.P., Morin F., Creveuil C., Parienti J.J., Scherpereel A. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): A randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405–1414. doi: 10.1016/S0140-6736(15)01238-6. [DOI] [PubMed] [Google Scholar]