Abstract
Background and Purpose
Airway hyperresponsiveness (AHR) is a central abnormality in asthma. IL‐5 may modulate AHR in animal models of asthma, but the available data is inconsistent on the impact of targeting IL‐5 pathway against AHR. The difference between targeting IL‐5 or the IL‐5 receptor, α subunit (IL‐5Rα) in modulating AHR remains to be investigated in human airways. The aim of this study was to compare the role of the anti‐IL‐5Rα benralizumab and the anti‐IL‐5 mepolizumab against AHR and to assess whether these agents influence the levels of cAMP.
Experimental Approach
Passively sensitized human airways were treated with benralizumab and mepolizumab. The primary endpoint was the inhibition of AHR to histamine. The secondary endpoints were the protective effect against AHR to parasympathetic activation and mechanical stress, and the tissue modulation of cAMP.
Key Results
Benralizumab and mepolizumab significantly inhibited the AHR to histamine (maximal effect −134.14 ± 14.93% and −108.29 ± 32.16%, respectively), with benralizumab being 0.73 ± 0.10 logarithm significantly more potent than mepolizumab. Benralizumab and mepolizumab significantly inhibited the AHR to transmural stimulation and mechanical stress. Benralizumab was 0.45 ± 0.16 logarithm significantly more potent than mepolizumab against AHR to parasympathetic activation. The effect of these agents was significantly correlated with increased levels of cAMP.
Conclusion and Implications
Targeting the IL‐5/IL‐5Rα axis is an effective strategy to prevent the AHR. Benralizumab was more potent than the mepolizumab and the concentration‐dependent beneficial effects of both these monoclonal antibodies were related to improved levels of cAMP in hyperresponsive airways.
Keywords: airway hyperresponsiveness, airway smooth muscle, benralizumab, head to head, IL‐5, mepolizumab, severe asthma
Abbreviations
- A kinase
cAMP‐dependent protein kinase A
- AHR
airway hyperresponsiveness
- EFS
electrical field stimulation
- Emax
maximal effect
- FEV1
forced expiratory volume in first second
- FRC
frequency–response curve
- GINA
Global Initiative for Asthma
- IL‐5Rα
IL‐5 receptor, α subunit
- Th2
T‐helper type 2
What is already known
Targeting IL‐5 pathways is an effective therapeutic strategy to treat severe asthmatic patients.
What this study adds
First‐time characterization of benralizumab and mepolizumab in a human ex vivo model of AHR.
This study provides mechanisms behind the deleterious effect of IL‐5 on isolated airways.
What is the clinical significance
Benralizumab and mepolizumab are effective in preventing AHR, with benralizumab being more potent than mepolizumab.
At effective doses, these monoclonal antibodies only partially inhibit parasympathetic hyperresponsiveness in human airways.
1. INTRODUCTION
Airflow limitation represents one of the most important and treatable characteristics of asthma. Together with other traits such as inflammation, the level of airflow limitation triggers the risk of an asthma attack (Pavord et al., 2018). Airflow limitation is mainly due to repeated contraction of airway smooth muscle, inflammatory oedema of the airway wall and intraluminal factors (Pavord et al., 2018). Airway hyperresponsiveness is a central abnormality in patients with asthma that induces enhanced sensitivity to a wide variety of stimuli, leading to an increased narrowing of the airways in vivo (Rabe, 1998).
In the recent years, passive sensitization, a validated procedure that reproduces ex vivo the airway hyperresponsiveness typical of asthmatic airways in vivo (Mitchell, Rabe, Magnussen, & Leff, 1997; Rabe, 1998; Schaafsma, Zuidhof, Nelemans, Zaagsma, & Meurs, 2006; Schmidt, Ruehlmann, Branscheid, Magnussen, & Rabe, 1999), has been extensively applied to pharmacologically characterize of the impact of several medications recommended in Step 1–4 therapy of asthma (Calzetta, Matera, Facciolo, Cazzola, & Rogliani, 2018; Cazzola, Calzetta, Rogliani, et al., 2016; Rogliani et al., 2020). Interestingly, passive sensitization has been used also to assess the effect of omalizumab, a monoclonal antibody recommended in Step 5 therapy of asthma, on the contractile tone of human hyperresponsive isolated bronchi (Berger et al., 2007). IL‐5 plays a pivotal role in modulating airway hyperresponsiveness in vivo in animal models of airway sensitization and asthma (Hamelmann et al., 1997; Leckie et al., 2000. Passive sensitization of airway smooth muscle cells elicits sequential autocrine and paracrine release of IL‐5 resulting in altered contractility (Damera, Panettieri, & Reynold, 2011; Gounni et al., 2005).
Benralizumab, a humanized anti‐L‐5 receptor, α subunit (IL‐5Rα) monoclonal antibody, blocks IL‐5 signalling and hence type‐2 inflammation, while inducing antibody‐dependent cell‐mediated cytotoxicity of eosinophils and basophils (European Medicines Agency, 2018; US Food and Drug Administration, 2017). On the other hand, mepolizumab, a humanized anti‐IL‐5 monoclonal antibody that prevents IL‐5 from binding to its receptor on the surface of eosinophils and basophils, modulates type‐2 inflammation occurring in approximately 50% of patients with asthma (European Medicines Agency, 2015; Farne, Wilson, Powell, Bax, & Milan, 2017; US Food and Drug Administration, 2015). The treatments with benralizumab and mepolizumab are recommended in Step 5 therapy of asthma and have been shown to induce clinical improvement in patients suffering from severe eosinophilic disease (Bleecker et al., 2016; FitzGerald et al., 2016; Nair et al., 2017; Ortega et al., 2014).
Indeed, some evidence generated by clinical trials indicated that benralizumab (Bleecker et al., 2016; FitzGerald et al., 2016) is effective in improving lung function expressed as forced expiratory volume in first second (FEV1). Conversely, conflicting data are available with respect to the impact of mepolizumab on lung function, with some studies reporting no effect on FEV1 (Flood‐Page et al., 2007; Haldar et al., 2009) and others showing some improvement in FEV1 (Ortega et al., 2014; Pavord et al., 2012). That improvement however was generally smaller than that induced by benralizumab (Bleecker et al., 2016; FitzGerald et al., 2016). A greater numerical effect of benralizumab compared to mepolizumab on FEV1 was also reported by a Cochrane analysis (Farne et al., 2017). Considering that generally lung function was not the primary endpoint of the clinical trials investigating monoclonal antibodies against IL‐5 and IL‐5Rα, the difference between benralizumab and mepolizumab on the improvement in FEV1 could be probably related to the different mechanisms of action of these monoclonal antibodies (Farne et al., 2017).
Beyond the effect of anti‐IL‐5 and anti‐IL‐5Rα monoclonal antibodies on lung function, although it is recognized that IL‐5 itself may modulate airway hyperresponsiveness, further inconsistent data are currently available concerning the real efficacy of targeting IL‐5 pathway in preventing airway hyperresponsiveness, at least in animal models of asthma. In this respect, while some studies indicated that acting on IL‐5 pathway may inhibit airway hyperresponsiveness (Mauser et al., 1995; Nag, Xu, Hamid, & Renzi, 2003; Shardonofsky, Venzor, Barrios, Leong, & Huston, 1999), others failed to report any effect on abnormal airway smooth muscle contractility (Eum, Maghni, Tolloczko, Eidelman, & Martin, 2005; Mathur et al., 1999; Tanaka, Nagai, & Maeda, 1998). Thus, the difference between targeting IL‐5 or IL‐5Rα in modulating airway hyperresponsiveness remains to be fully established. Therefore considering that, unlike omalizumab (Berger et al., 2007), neither benralizumab nor mepolizumab has been pharmacologically characterized in human hyperresponsive airways, the aim of this study was to compare the efficacy and potency of benralizumab and mepolizumab in passively sensitized human airways and to identify whether acting on the IL‐5/IL‐5Rα axis may protect against airway hyperresponsiveness by increasing the tissue synthesis of cAMP.
2. METHODS
2.1. Tissue collection and preparation
Regions of lungs were taken from uninvolved areas of neoplastic lesions and resected from 16 patients undergoing lobectomy surgery for lung cancer. Tissues were placed in Krebs–Henseleit buffer solution as previously described (Cazzola et al., 2011) and transported to the Laboratory of Respiratory Clinical Pharmacology at the University of Rome “Tor Vergata” (Italy) from a nearby hospital. None of the patients had been chronically treated with bronchodilators or corticosteroids, and serum IgE levels were in the normal range (<100 IU/ml−1). Preoperative lung function parameters were normal in all the patients who were not affected by chronic obstructive respiratory disorders. Detailed demographic and metric characteristics of donors are reported in e‐Table 1.
In the laboratory, the airways were cut into rings (subsegmental bronchi: thickness 1–2 mm, diameter 4–6 mm) and transferred into a 10‐ml High Tech 8 Channels Manual Compact Organ Bath system (Panlab Harvard Apparatus, Spain) containing Krebs–Henseleit buffer solution (37°C) and aerated with O2/CO2 (95:5%). Tissues were allowed to equilibrate, and the Krebs–Henseleit buffer solution was constantly changed.
2.2. Passive sensitization
Isolated airways were rotated overnight at room temperature in tubes containing Krebs–Henseleit buffer solution in the presence of 10%/vol−1 sensitizing serum (passively sensitized bronchi) or 10%/vol−1 non‐sensitizing serum (non‐sensitized bronchi). Sensitizing and non‐sensitizing sera were prepared by centrifugation from the whole blood. Sensitizing serum was obtained from a patient suffering from severe atopic asthma (total IgE 1,000 U/ml−1 specific against common aeroallergens), whereas non‐sensitizing serum was obtained from a non‐atopic donor (total IgE 45 U/ml−1). The serum from the non‐atopic donor was used as a negative control (C−) while serum from a patient with severe atopic asthma was used as a positive control (C+). The subjects provided signed consent for serum donation. Sera were frozen at −80°C in 250‐μl aliquots until required. The next morning bronchial tissues were transferred into the isolated organ bath system containing Krebs–Henseleit buffer solution (37°C) and continuously gassed with O2/CO2 (95:5%). The passive sensitization is a model that closely mimics important functional characteristics of airway hyperresponsiveness, a typical trait of asthmatic patients as previously reported (Pavord et al., 2018; Schaafsma et al., 2006; Schmidt et al., 1999; Schmidt & Rabe, 2000).
2.3. Transmural stimulation
Transmural stimulation, also called electrical field stimulation, was performed by placing tissues between two wire platinum electrodes (20 mm apart, Panlab Harvard Apparatus), connected to a 3165 multiplexing pulse booster stimulator (Ugo Basile, VA, Italy). Bronchial rings were contracted by electrical field stimulation at increasing frequencies (electrical field stimulation1–50 Hz, 10 V, 10 s, 0.5 ms, biphasic pulse) in order to activate vagus nerve terminals (the parasympathetic pathway) as observed in humans and thus producing frequency–response curves via endogenous cholinergic contractile response (Cazzola et al., 2011).
2.4. Materials
The following drugs were used:‐ benralizumab (Creative Biolabs Inc., NY, USA) diluted in distilled water, histamine (Sigma‐Aldrich, Milan, Italy) diluted in distilled water, and mepolizumab (Creative Biolabs Inc., NY, USA) diluted in distilled water. Compounds were stored in small aliquots at −80°C until their use.
2.5. Contraction measurement
Bronchial rings were connected to isometric force transducers Fort25 (WPI, UK). The signal was amplified by a PowerLab 8/36 and Octal Bridge Amp system (AD instruments, UK), recorded and analysed via the LabChart 7 interface software (AD instruments). Tissues were mounted on hooks and attached with thread to a stationary rod and the other tied with thread to an isometric force displacement transducer. Airways were allowed to equilibrate by flushing with fresh Krebs–Henseleit buffer solution. Passive tension was determined by gentle stretching of tissue (0.5–1.0 g) during equilibration. The isometric change in tension was measured by the transducer, and the tissue vitality assessed by electrical field stimulation25 Hz. These procedures allowed the bronchial rings to be correctly positioned between the hooks. When the passive contractile tone reached the plateau, rings were washed three times with fresh Krebs–Henseleit buffer solution and allowed to equilibrate for further 45 min.
2.6. Experimental design
2.6.1. Study characteristics
This study was designed as an ex vivo, prospective, randomized, negative and positive controlled, blinded, parallel groups, head‐to‐head comparison between benralizumab and mepolizumab.
2.6.2. Endpoints
The primary endpoint of this study was to assess the protective effect of benralizumab and mepolizumab against the airway hyperresponsiveness elicited by histamine in hyperresponsive human bronchi.
The secondary endpoints included the impact of benralizumab and mepolizumab against the airway hyperresponsiveness elicited by electrical field stimulation and mechanical stress induced by quick stretch and the effect of these monoclonal antibodies on the modulation of cAMP levels in hyperresponsive bronchial tissue.
2.6.3. Study 1. Effect of benralizumab and mepolizumab on the concentration–response curve to histamine
Concentration–response curves to histamine were generated in isolated airways. The concentration–response curves were produced via the cumulative administration of histamine. Airways were stimulated with histamine until a stable level of contractility was reached for each concentration, usually for 5–15 min. After that, the next concentration of agonist was administered. Experiments were performed in C−, in airways incubated overnight with sensitizing serum (positive control, C+), and in passively sensitized airways that were incubated overnight with different concentrations of either benralizumab or mepolizumab during the sensitizing procedure.
2.6.4. Study 2. Effect of concentration–response curves to benralizumab and mepolizumab on the contractile plateau to specific histamine concentrations
Histamine was administered at concentrations inducing different levels of contractile plateau to isolated airways that were previously incubated overnight with different concentrations of either benralizumab or mepolizumab during the sensitizing procedure. The concentrations of histamine used to elicit the specific contractile plateau were the half maximal effective concentration (EC50), the 70% effective concentration (EC70) and the 90% effective concentration (EC90) with respect to the maximal contractile response (Emax) detectable in C+ isolated bronchi. The response to the different concentrations of each specific histamine concentration was recorded until the contractile plateau was reached, usually for 5–15 min. These experiments were also performed in C− and C+ tissues.
2.6.5. Study 3. Effect of benralizumab and mepolizumab on the frequency–response curves to electrical field stimulation
Frequency–response curves to electrical field stimulation1–50 Hz were generated in isolated airways. Each electrical field stimulation was delivered after that the contractile response induced by the previous electrical field stimulation was terminated, usually after 3–5 min. Experiments were performed in C− and C+ tissues and in passively sensitized airways that were incubated overnight with different concentrations of either benralizumab or mepolizumab during the sensitizing procedure.
2.6.6. Study 4. Effect of concentration–response curves to benralizumab and mepolizumab on the contractile response to specific electrical field stimulation frequencies
Isolated airways were stimulated by electrical field stimulation delivered at frequencies inducing different levels of contractile response to isolated airways that were previously incubated overnight with different concentrations of either benralizumab or mepolizumab during the sensitizing procedure. The electrical field stimulation frequencies delivered to elicit the specific contractile responses were the half maximal effective frequency (EF50), the 70% effective frequency (EF70) and the 90% effective frequency (EF90) with respect to the Emax detectable in C+ isolated bronchi. Each specific electrical field stimulation frequency was delivered after that the contractile response induced by the previous electrical field stimulation was terminated, usually in 3–5 min. Experiments were performed also in C− and C+ tissues.
2.6.7. Study 5. Effect of benralizumab and mepolizumab on the contractile response to quick stretch
Quick stretch of 0.5 mm was elicited in isolated airways, as previously described (Mitchell et al., 1997). Briefly, a calibrated thumbscrew enabled the accurate measurement of the angular rotation of the threaded rod that was used to induce quick stretch. Since the pitch of the screw was 1.0 mm, a rapid (<200 ms) 180° rotation was performed to elicit a quick stretch of 0.5 mm on the bronchial rings. The resulting myogenic contractile response was recorded for 10 min. quick stretch was performed in C− and C+ tissues and in passively sensitized airways that were incubated overnight with different concentrations of either benralizumab or mepolizumab during the sensitizing procedure.
2.6.8. Quantification of IL‐5 and cAMP
The supernatant of C− and C+ tissues was collected at different time points during the overnight incubation in the presence of sensitizing and non‐sensitizing sera in order to assess the release of IL‐5 from the bronchial tissue. The concentrations of IL‐5 were quantified by using human IL‐5 elisa kit (RayBiotech, Inc., GA, USA) according to manufacturers' instructions (available at https://www.raybiotech.com/files/manual/ELISA/ELH-IL5.pdf).
At the end of experiments, isolated bronchi treated with either benralizumab or mepolizumab were collected along with C− and C+ airways in order to quantify the tissue levels of cAMP by using a cAMP elisa kit (RayBiotech, Inc.) according to manufacturers' instructions (available at https://www.raybiotech.com/files/manual/EIA/EIA-CAMP.pdf).
2.6.9. Time controls
For every seven bronchial rings mounted in the isolated organ bath system, one was used as a time control.
2.7. Data Analysis and Statistics
2.7.1. Pharmacological analysis
The airway smooth muscle contractile force in response to histamine, electrical field stimulation, and quick stretch was reported as milligrams normalized for 100 mg of bronchial tissue (mg/100 mg−1). The protective effect of benralizumab and mepolizumab against airway hyperresponsiveness induced by contractile stimuli in passively sensitized airways was expressed as the percentage of the maximal airway hyperresponsiveness inhibition that in turn was identified as the δ between the airway hyperresponsiveness induced in C+ airways and the normal contractile response elicited in C− airways. Since the effect of each single concentration of the monoclonal antibodies investigated in this research was tested after overnight incubation during the sensitizing procedure, the concentration–response curves to benralizumab and mepolizumab on the contractile plateau to specific histamine concentrations and electrical field stimulation frequencies were generated by plotting in the same graph the data originated from different bronchial rings treated with different concentrations of the monoclonal antibodies. Appropriate curve fitting to sigmoidal models were used to calculate the effect (E), Emax, EC50, EC70, EC90, EF50, EF70, EF90 and the half maximal inhibitory concentration (IC50). The equations used to describe the models used in this study were as follows: Y = Bottom + (Top − Bottom)/(1 + 10^((logEC50_or_EF50 − X))) and Y = Bottom + (Top − Bottom)/(1 + 10^((X − logIC50))). EC50, EF50, and IC50 were transformed in pEC50 (−logEC50), pEF50 (−logEF50), and pIC50 (−logIC50) to perform the statistical analysis of the potency.
Data originated from elisa kits were elaborated after normalization for the wet weight (100 mg) of isolated airways.
2.7.2. Group size, randomization, blinding, and data and statistical analysis
No published data are currently available concerning the impact of benralizumab and mepolizumab against airway hyperresponsiveness in human isolated bronchi. The only paper regarding the effect of a monoclonal antibody on the airway hyperresponsiveness induced by histamine in passively sensitized bronchi has been performed on the anti‐IgE monoclonal antibody omalizumab (Berger et al., 2007). The results of that study reported that repeating experiments on n = 4 different bronchi permitted to detect a small but significant reduction (− ≃30%, P < 0.05) of histamine‐induced airway hyperresponsiveness in passively sensitized bronchi treated with omalizumab, compared to control tissue (Berger et al., 2007).
In the present study, it is not possible the calculation of the sample size due by the fact that no studies have been conducted so far on neither benralizumab nor mepolizumab in an experimental setting by using passively sensitized human isolated bronchi. However, we can set n = 5 as the number of repetitions required to have >95% possibility (P < 0.05) to detect, with at least 80% power, a relatively small but significant alteration of bronchial contractility induced by benralizumab and mepolizumab, accordingly with the results reported by Berger et al. (2007). This sample size satisfies the guidelines of the British Journal of Pharmacology for preclinical studies, where n refers to independent values and not replicates (available at https://bpspubs.onlinelibrary.wiley.com/hub/journal/14765381/author-guidelines.html).
The total number of bronchial rings necessary to complete the study was n = 366, including C−, C+ and time control tissues. Isolated airways were collected from at least n = 5 different donors. The treatment with each specific concentration of either benralizumab or mepolizumab was performed by using specimens collected from the same patient, and experiments were repeated five times in samples originating from five different donors. In the case the amount of samples from a subject did not permit to test all the benralizumab and mepolizumab concentrations in the same experiment, the remaining concentrations were assessed in specimens collected from other patients in parallel with further C−, C+ and time controls in order to provide controlled results not affected by potential bias.
The protective effects of different concentrations of benralizumab and mepolizumab against airway hyperresponsiveness were compared each other and with C− and C+ tissues. Values were reported as mean ± SEM, the statistical significance was assessed by the Student's t‐test and ANOVA. The post hoc tests were conducted only if F in ANOVA achieved P < 0.05 and there was no significant variance inhomogeneity. Sample sizes subjected to statistical analysis was at least 5 isolated airways per group (n = 5), where n = number of independent values. The level of statistical significance was defined as P < 0.05. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Detailed dataset of sectional tissues is reported in e‐Table 2. Data were collected and managed in order to perform pre‐specified statistical analysis; any so‐called p‐hacking were avoided in order to prevent that any potential bias could affect the robustness of results, as reported by Head, Holman, Lanfear, Kahn, and Jennions (2015).
Each bronchial ring was randomly assigned to a specific treatment by using a computer‐generated sequence. All the study procedures were performed under blinded condition, in which both the operator and data analysis were blinded.
All data analysis was performed using computer software GraphPad Prism 5 (GraphPad Prism, RRID:SCR_002798; La Jolla, CA, USA), and OpenEpi (available at https://www.openepi.com) was used for the power calculation and randomization.
2.8. Ethics approval and consent to participate
Ethical approval (R.S. 37/20, 2020; Independent Ethical Committee, Fondazione PTV Policlinico Tor Vergata) and informed consent were consistent with the National Committee of Bioethics and Committee for Bio‐safety, Biotechnology and Life Sciences (available at http://old.iss.it/binary/eric/cont/Informed_consent.pdf), the recommendations on the collection of biological samples for research purposes (available at https://search.coe.int/cm/Pages/result_details.aspx?ObjectID=09000016805d84f0), the ethical and legal recommendations concerning the biobank and the research biorepository (available at https://www.oeci.eu/Documents/OECI_Biobank.pdf), and the Comitato Nazionale per la Biosicurezza, le Biotecnologie e le Scienze per la Vita (Raccolta di campioni biologici a fini di ricerca, consenso informato, 2009; available at http://bioetica.governo.it/media/3457/p2009-misto-2-raccolta-di-campioni-biologici-a-fini-di-ricerca-consenso-informato-it.pdf).
2.9. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY in http://www.guidetopharmacology.org and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander et al., 2019).
3. RESULTS
3.1. Baseline characteristics of isolated airways
No significant differences were found in the baseline characteristics of isolated airways used in this study. Passive sensitization induced significant airway hyperresponsiveness in C+ bronchi and the maximal concentration of IL‐5 was 12.23 ± 2.80‐fold greater in C+ than in C− tissues (e‐Figure 1). The smoking habit of donors did not significantly modulate the airway hyperresponsiveness and IL‐5 release induced by passive sensitization. The amount of tissues available to complete this study permitted the use of the same tissue for all conditions (C+, C−, different drug concentrations) in each experiment. Further details are reported in the supporting information.
3.2. Primary endpoint
3.2.1. Effect of benralizumab and mepolizumab on the airway hyperresponsiveness to histamine
Both benralizumab and mepolizumab inhibited the airway hyperresponsiveness mediated by the histamine induced tone in passively sensitized bronchi.
The Emax elicited by the concentration–response curve to histamine was significantly reduced when passively sensitized airways were incubated with benralizumab at concentrations ≥1 μg/ml−1, Figure 1a), whereas at least 3 μg/ml−1 of mepolizumab were necessary to significantly inhibit the histamine‐mediated airway hyperresponsiveness (Figure 1b). When either benralizumab or mepolizumab was administered at concentrations ≥10 μg/ml−1, the airway hyperresponsiveness induced by concentration–response curve to histamine in passively sensitized bronchi was completely prevented, with the contractile response of hyperresponsive airway smooth muscle reduced at the same level of that detectable in C− airways or even significantly lower for benralizumab administered at 100 μg/ml−1 ( (Figure 1a,b). Benralizumab administered at concentrations ≥10 μg/ml−1 significantly (δ pEC50 0.65 ± 0.20 ) reduced the potency of histamine in passively sensitized airways, whereas 100 μg/ml−1 of mepolizumab were necessary to elicit the same significant impact (δ pEC50 0.65 ± 0.22) on the pEC50 of histamine. Details on the effect of different concentrations of benralizumab and mepolizumab on the pharmacological characteristics of concentration–response curve to histamine (Emax and pEC50) in passively sensitized bronchi are reported in Table 1.
FIGURE 1.

Effect of overnight incubation with different concentrations of benralizumab (a) and mepolizumab (b) on the airway hyperresponsiveness (AHR) to histamine concentration–response curves (CRCs) in passively sensitized bronchi. *P < 0.05 and versus C+ (statistical analysis assessed by two‐way ANOVA); points represent the mean ± SEM of n = 5 bronchial tissue from different subjects. C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi
TABLE 1.
Effect of overnight incubation with different concentrations of benralizumab and mepolizumab on the concentration–response curves to histamine in passively sensitized bronchi
| C− | C+ | Benralizumab | Mepolizumab | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 μg·ml−1 | 3 μg·ml−1 | 10 μg·ml−1 | 30 μg·ml−1 | 100 μg·ml−1 | 1 μg·ml−1 | 3 μg·ml−1 | 10 μg·ml−1 | 30 μg·ml−1 | 100 μg·ml−1 | |||
| Histamine Emax (mg·100 mg−1 bronchial tissue) | 996.80 ± 100.00 | 1,857.00 ± 171.60 # | 1,510.00 ± 109.00* | 1,342.00 ± 86.58* | 1,244.00 ± 127.70*** | 848.30 ± 160.00*** | 524.90 ± 80.07* | 1,822.00 ± 145.80 | 1,394.00 ± 170.70* | 1,145.00 ± 125.40* | 1,006.00 ± 149.80* | 1,008.00 ± 442.40* |
| Histamine pEC50 | 5.49 ± 0.15 | 6.15 ± 0.17 # | 5.75 ± 0.18 | 5.80 ± 0.18 | 5.51 ± 0.19* | 5.55 ± 0.16* | 5.50 ± 0.20* | 6.30 ± 0.30 | 6.09 ± 0.47 | 5.82 ± 0.31 | 5.92 ± 0.36 | 5.50 ± 0.22* |
Note: Data represent the mean ± SEM of n = 5 bronchial tissue from different subjects.
Abbreviations: C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi; EC50, concentration inducing 50% Emax; Emax, maximal effect; pEC50, −logEC50.
P < 0.05 versus C− (statistical analysis assessed by the Student's t‐test).
P < 0.05 versus C+ (statistical analysis assessed by one‐way ANOVA).
The concentration–response curves to either benralizumab or mepolizumab modulated the contractile plateau to specific concentrations of histamine in hyperresponsive airways (Figure 2a–f). Specifically, both benralizumab and mepolizumab prevented the airway hyperresponsiveness in passively sensitized airways contracted by histamine administered at EC50 (Figure 2a,d), EC70 (Figure 2b,e) and EC90 (Figure 2c,f) in a potent concentration‐dependent manner. Benralizumab resulted 0.73 ± 0.10 logarithm significantly more potent than mepolizumab (Table 2).
FIGURE 2.
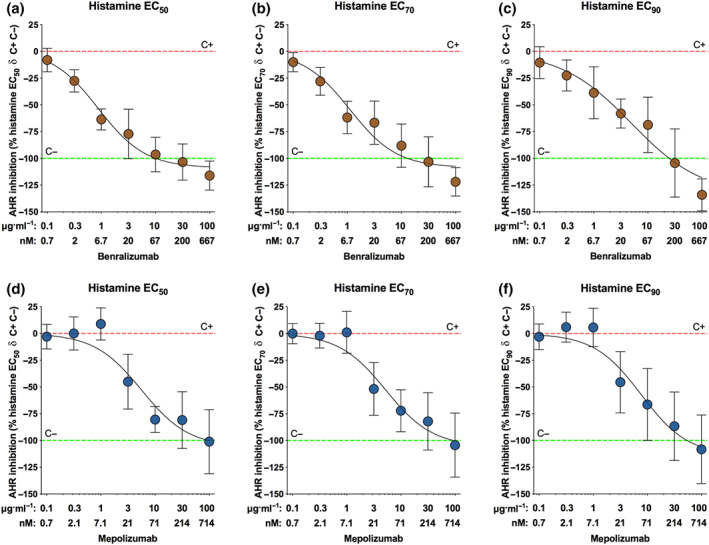
Inhibition of airway hyperresponsiveness (AHR) to different concentrations of histamine (EC50: a and d; EC70: b and e; EC90: c and f) by overnight incubation with benralizumab (a–c) and mepolizumab (d–f) in passively sensitized bronchi. Points represent the mean ± SEM of n = 5 bronchial tissue from different subjects. C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi; ECn, concentration inducing n% Emax; Emax, maximal effect
TABLE 2.
Efficacy and potency of benralizumab and mepolizumab after overnight incubation on the airway hyperresponsiveness to different concentrations of histamine (EC50–90) in passively sensitized bronchi
| Contractile tone to histamine at | Benralizumab | Mepolizumab | ||||
|---|---|---|---|---|---|---|
| EC50 | EC70 | EC90 | EC50 | EC70 | EC90 | |
| Benralizumab or mepolizumab Emax (mg·100 mg−1 bronchial tissue) | −116.05 ± 13.60 | −111.90 ± 13.28 | −134.14 ± 14.93 | −101.06 ± 29.91 | −104.33 ± 29.93 | −108.29 ± 32.16 |
| Benralizumab or mepolizumab pIC50 | 8.24 ± 0.16** | 8.13 ± 0.12* | 7.94 ± 0.19* | 7.41 ± 0.18 | 7.44 ± 0.17 | 7.28 ± 0.19 |
Note: The pharmacological analysis was performed by assessing Emax as the difference in airway contractility between C+ and C− tissues. Data represent the mean ± SEM of n = 5 bronchial tissue from different subjects.
Abbreviations: C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi; ECn, concentration inducing n% Emax; Emax, maximal effect; IC50, concentration inducing 50% inhibition airway hyperresponsiveness to histamine in passively sensitized bronchi; pIC50, −logIC50.
P < 0.05 versus mepolizumab (statistical analysis assessed by the Student's t‐test).
3.3. Secondary endpoints
3.3.1. Effect of benralizumab and mepolizumab on the airway hyperresponsiveness to electrical field stimulation
Both benralizumab and mepolizumab inhibited the airway hyperresponsiveness mediated by the parasympathetic tone elicit by electrical field stimulation in passively sensitized bronchi.
The Emax elicited by the frequency–response curves to electrical field stimulation was significantly reduced when hyperresponsive airways were incubated with benralizumab at concentrations ≥1 μg/ml−1 (Figure 3a) or mepolizumab at concentrations ≥10 μg/ml−1 (Figure 3b). Benralizumab and mepolizumab both normalized the contractile response induced by frequency–response curves to electrical field stimulation in hyperresponsive airway smooth muscle when these monoclonal antibodies were administered at concentrations ≥10 μg/ml−1, leading to non‐significantly different frequency–response curvess compared with that inducible in C− tissue (Figure 3a,b). Neither benralizumab nor mepolizumab significantly modulated the potency of frequency–response curves to electrical field stimulation in passively sensitized airways. Details on the effect of different concentrations of benralizumab and mepolizumab on the contractile response to frequency–response curves induced by electrical field stimulation (Emax and pEF50) in hyperresponsive airways are reported in e‐Table 3.
FIGURE 3.
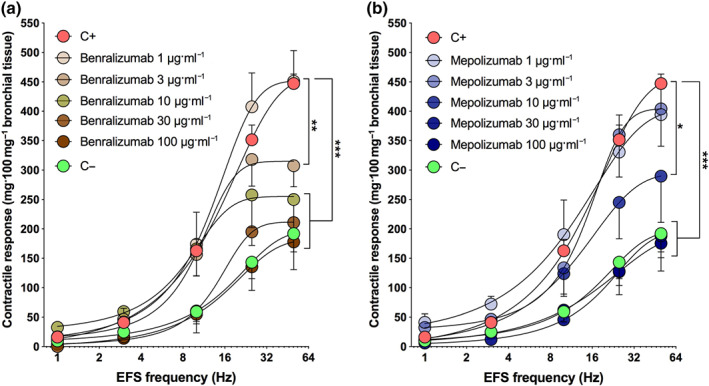
Effect of overnight incubation with different concentrations of benralizumab (a) and mepolizumab (b) on the airway hyperresponsiveness (AHR) to electrical field stimulation (EFS) frequency–response curves (FRCs) in passively sensitized bronchi. *P < 0.05 versus C+ (statistical analysis assessed by two‐way ANOVA); points represent the mean ± SEM of n = 5 bronchial tissue from different subjects. AHR, airway hyperresponsiveness; C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi
The concentration–response curves to either benralizumab or mepolizumab inhibited the contractile response to specific electrical field stimulation frequencies in passively sensitized bronchi (Figure 4a–f). Specifically, both benralizumab and mepolizumab prevented the airway hyperresponsiveness in passively hyperresponsive airways stimulated by electrical field (EF) stimulation delivered at EF50 (Figure 4a,d), EF70 (Figure 4b,e) and EF90 (Figure 4c,f) in a concentration‐dependent manner. At EF90, benralizumab was significantly more potent than mepolizumab (e‐Table 4).
FIGURE 4.
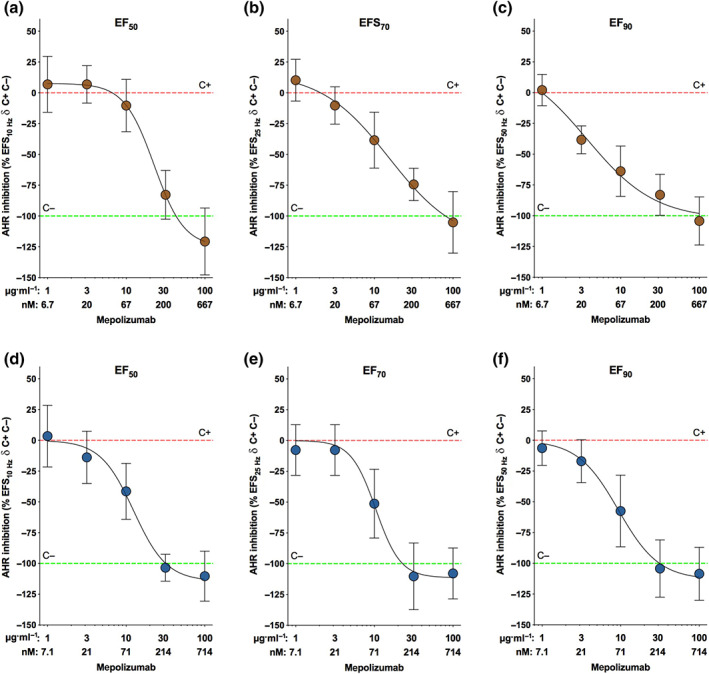
Inhibition of airway hyperresponsiveness (AHR) to different electrical field stimulation (EFS) frequencies (EF50: a and d; EF70: b and e; EF90: c and f) by overnight incubation with benralizumab (a–c) and mepolizumab (d–f) in passively sensitized bronchi. Points represent the mean ± SEM of n = 5 bronchial tissue from different subjects. C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi; EFn, frequency inducing n% Emax; Emax, maximal effect
3.3.2. Effect of benralizumab and mepolizumab on the airway hyperresponsiveness to quick stretch
Both benralizumab and mepolizumab prevented the hyperresponsive myogenic tone induced by quick stretch in passively sensitized bronchi.
Benralizumab administered at concentrations ≥10 μg/ml−1 significantly reduced the airway hyperresponsiveness when compared with C+ bronchi conversely, at least 30 μg/ml−1 of mepolizumab were necessary to induce the same significant effect in hyperresponsive airways ( Figure 5). Benralizumab at 10 μg/ml−1, but not mepolizumab, inhibited the contractile response to quick stretch at a level significantly lower than that detectable in C− tissue.
FIGURE 5.
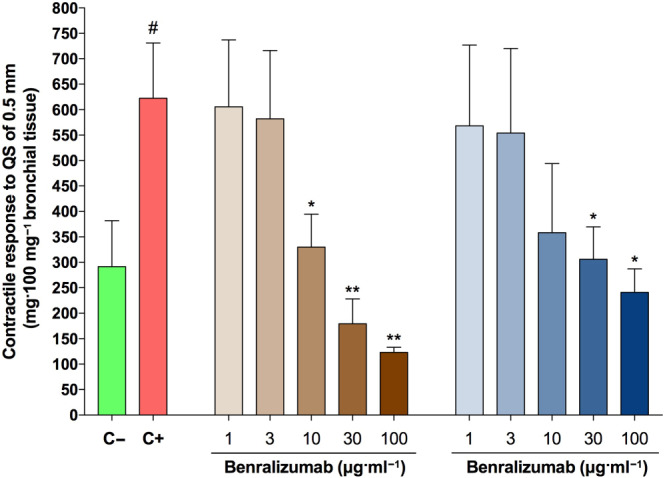
Effect of overnight incubation with different concentrations of benralizumab and mepolizumab on the airway hyperresponsiveness (AHR) to quick stretch (QS) of 0.5 mm in passively sensitized bronchi. # P < 0.05 versus C−; *P < 0.05 versus C+ (statistical analysis assessed by the Student's t test); bars represent the mean ± SEM of n = 5 bronchial tissue from different subjects. C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi
3.3.3. Effect of benralizumab and mepolizumab on the levels of cAMP in hyperresponsive airways
Both benralizumab and mepolizumab prevented the cAMP depletion elicited by passive sensitization in hyperresponsive bronchi, by normalizing the concentrations of cAMP when administered at 10 μg/ml−1. At concentrations ≥30 μg/ml−1, both the monoclonal antibodies restored the cAMP concentrations at levels significantly higher than those detected in C+ tissue (Figure 6a). Benralizumab at 100 μg/ml−1, but not mepolizumab, increased the cAMP concentrations at a level significantly greater than that found in C− tissue.
FIGURE 6.
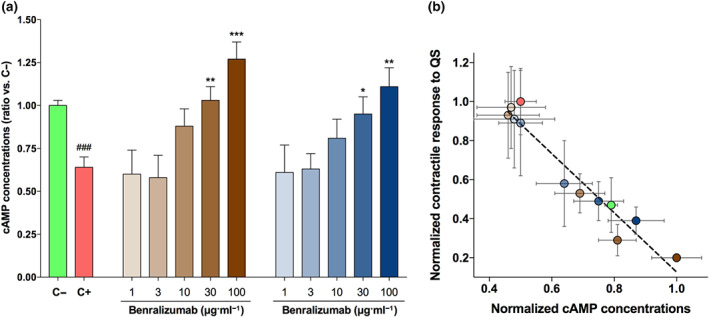
Effect of overnight incubation with different concentrations of benralizumab and mepolizumab on the cAMP concentrations in hyperresponsive bronchial tissue (a) and correlation between the cAMP concentrations and the modulation of myogenic tone to quick stretch (QS) induced by the investigated treatments (b). # P < 0.05 versus C−; *P < 0.05, versus C+ (statistical analysis assessed by the Student's t‐test); bars and points represent the mean ± SEM of n = 5 bronchial tissue from different subjects. The colours of bars in (a) and the relative treatments are consistent with the colours of points in (b). C+, positive control, passively sensitized bronchi; C−, negative control, non‐sensitized bronchi
The levels of cAMP were significantly correlated with the inhibition of airway hyperresponsiveness to quick stretch induced by benralizumab and mepolizumab (Figure 6b).
4. DISCUSSION AND CONCLUSIONS
The results of this study demonstrate that targeting the IL‐5 pathway with monoclonal antibodies is a potent and effective strategy to prevent in a concentration‐dependent manner the airway hyperresponsiveness in passively sensitized airways, a condition leading to a massive production of IL‐5 in bronchial tissue. Benralizumab and mepolizumab reached the primary endpoint since both the agents flattened and shifted rightwards the concentration–response curve to histamine, although benralizumab was more potent than mepolizumab. Concerning the secondary endpoints, benralizumab was more potent than mepolizumab when the tissues were stimulated by higher electrical field stimulation frequencies. Both the drugs also prevented the hyperresponsive myogenic tone in response to quick stretch, although only benralizumab reduced the airway smooth muscle contractility at a level lower than that detectable in non‐sensitized airways. This study further supports the evidence (Cazzola, Calzetta, Rogliani, et al., 2016) that passive sensitization may alter the concentration of cAMP in hyperresponsive airways, a condition that was reverted in a concentration‐dependent manner by both benralizumab and mepolizumab. Again, only benralizumab improved the cAMP concentrations at a level greater than that detected in non‐sensitized tissue.
Several complex indirect mechanisms (Molfino, Gossage, Kolbeck, Parker, & Geba, 2012; Mukherjee, Sehmi, & Nair, 2014) are involved in the prevention of airway hyperresponsiveness elicited by the inhibition of the IL‐5/IL‐5Rα axis in hyperresponsive airways. Whatever the pathways implicated, we have shown that benralizumab and mepolizumab can restore physiological levels of cAMP concentrations in passively sensitized airways. Increased intracellular concentrations of cAMP in airway smooth muscle promote bronchodilation via activation of cAMP‐dependent protein kinase (A kinase) (Rogliani et al., 2016). However, conflicting findings are currently available regarding the real impact of cAMP on airway inflammation, with some evidences suggesting for anti‐inflammatory effects and other reporting increased cytokine synthesis and T cell adhesion to airway smooth muscle cells induced by enhanced concentrations of cAMP (Black, Panettieri, Banerjee, & Berger, 2012). Isolated airways include several residential cells such as airway smooth muscle cells, fibroblasts, parasympathetic ganglia cells, epithelial cells and inflammatory cells such as T‐helper type 2 (Th2) lymphocytes, eosinophils and mast cells, most of them are stimulated or activated in response to passive sensitization (Schmidt & Rabe, 2000). Therefore, in the isolated airways used in our experiments, it was not possible to discern the exact origin of the increase in cAMP induced by the investigated monoclonal antibodies. However, the strong correlation between the cAMP levels and the inhibition of airway hyperresponsiveness contractility induced by benralizumab and mepolizumab suggests that the improvement in cAMP concentrations represents a key mechanism by which the inhibition of IL‐5/IL‐5Rα axis may converge and, thus, protect airway smooth muscle from hyperresponsiveness.
Unexpectedly, in passively sensitized airways, benralizumab but not mepolizumab improved airway hyperresponsiveness and cAMP at levels greater than those detected in untreated non‐sensitized tissue. Isolated airways are characterized by a certain degree of intrinsic tone mediated by the spontaneous generation of histamine and leukotrienes. Therefore, since eosinophil secretory granules contain both histaminase and leukotrienes (Bagnasco et al., 2017; Bandeira‐Melo, Bozza, & Weller, 2002), it was expected that by preventing the activation and degranulation of eosinophils by administering either benralizumab or mepolizumab would have led to the same effect. Perhaps, the difference in the efficacy between benralizumab and mepolizumab can be explained by considering that, although both these monoclonal antibodies counteract the IL‐5 pathway, only benralizumab induces antibody‐dependent cell‐mediated cytotoxicity leading to the apoptosis of eosinophils, mast cells, and basophils caused by natural killer cells (Kolbeck et al., 2010). Thus, the combination of IL5Rα blockade and antibody‐dependent cell‐mediated cytotoxicity activity of benralizumab may be responsible for the greater potency of this monoclonal antibody compared to mepolizumab against the airway hyperresponsiveness to histamine. Furthermore, while at low concentrations benralizumab can antagonize IL‐5Rα and elicit antibody‐dependent cell‐mediated cytotoxicity (Kolbeck et al., 2010), low concentrations of mepolizumab may not be sufficient to neutralize the massive release of IL‐5 in the sensitized tissue.
Such a condition can be translated to asthmatic patients before the next dose administration of a monoclonal antibody. Accordingly with pharmacokinetic studies in healthy subjects (Martin et al., 2019; Shabbir et al., 2019) that received approved doses of monoclonal antibodies (European Medicines Agency, 2015, 2018; US Food and Drug Administration, 2015, 2017), while the maximum plasma concentrations of benralizumab and mepolizumab were ≃3 and ≃12 μg/ml−1, respectively, the trough concentrations were ≃1 and ≃5 μg/ml−1, respectively. Indeed, our results indicate that at these concentrations both the agents are effective in submaximally inhibiting the airway hyperresponsiveness to histamine in isolated airways. However, while the efficacy of the circulating levels of mepolizumab at trough could be neutralized by the cytokine storm during an asthma exacerbation (Borish, 2016), the protective effect of low concentrations of benralizumab can be preserved as directed on IL‐5Rα and antibody‐dependent cell‐mediated cytotoxicity regardless of the amount of circulating IL‐5.
Taken together the data from pharmacokinetic studies with those of our study, it is also evident that the concentrations of benralizumab and mepolizumab detectable in plasma can only partially prevent the airway hyperresponsiveness induced by parasympathetic activation. This translational evidence is certainly of interest in optimizing the pharmacological treatment of severe asthmatics in which a high intrinsic parasympathetic tone has been documented (Liccardi et al., 2016). Probably, the current Global Initiative for Asthma (GINA, 2020) recommendations should be improved by considering this specific asthma phenotype in Step 5, in which combining an anti‐IL‐5/IL‐5Rα monoclonal antibody with a long‐acting muscarinic antagonist (LAMA) as preferred controller could lead to clinical and functional benefits.
Airway sensitization contributes to the adhesion of eosinophils to parasympathetic nerves, leading to their priming, activation and degranulation with consequent release of major basic protein. Major basic protein increases ACh release due to loss of function of the neuronal M2 muscarinic autoreceptor expressed on postganglionic parasympathetic nerves (Drake et al., 2018). Since such an intimate interaction between eosinophils and airway cholinergic nerves contributes to the airway hyperresponsiveness in the course of tissue sensitization, favourable synergistic interaction could result by combining a long‐acting muscarinic antagonist that inhibits the cholinergic transmission with an anti‐IL‐5/IL‐5Rα monoclonal antibody that protects vagal parasympathetic fibres from the deleterious influence of activated eosinophils.
From a strict pharmacological viewpoint, the findings of our study suggest that the profile of loss of Emax to histamine and electrical field stimulation in passively sensitized airways treated with either benralizumab or mepolizumab are due to an indirect inhibition of endogenous bronchoconstricting intermediaries release that, in turn, lead to the airway hyperresponsiveness. This evidence is supported by the fact that the airway hyperresponsiveness induced by histamine in passively sensitized bronchi is mediated not only by the direct activation of histamine receptors expressed on airway smooth muscle but also by an indirect facilitator effect on ACh release from parasympathetic nerves (Cazzola, Calzetta, Puxeddu, et al., 2016). Moreover, the airway hyperresponsiveness elicited by electrical field stimulation is mainly indirect and mediated by the sensitization of vagal parasympathetic fibres leading to increased release of endogenous ACh from these nerve terminals that in turn activate muscarinic M3 receptor expressed on airway smooth muscle (Ichinose et al., 1996; Mitchell, Ndukuw, Ikeda, Arbetter, & Leff, 1993).
This present research also provides supplementary observations mainly concerning the validity of passive sensitization of human isolated airways as a suitable model of airway hyperresponsiveness, a typical characteristic of asthmatic patients. Although this has already been demonstrated at a cellular level in human airway smooth muscle (Grunstein et al., 2002; Hakonarson, Maskeri, Carter, Chuang, & Grunstein, 1999; Hakonarson, Maskeri, Carter, & Grunstein, 1999), here we provide, for the first time, the evidence that passive sensitization of the whole human bronchial tissue induces an extensive synthesis of IL‐5 and that IL‐5 represents a key factor leading to the airway hyperresponsiveness in human airways. Certainly targeting the IL‐5 pathway counteracts the airway hyperresponsiveness in human subsegmental bronchi. Nevertheless, Manson et al. (2019) have recently demonstrated that IL‐5 does not induce hyperresponsiveness in human small airways. Perhaps, the lack of IL‐5‐mediated airway hyperresponsiveness in small airways could be due to the preponderance of airway smooth muscle in the bronchioles wall and the significantly lower, and almost absent, number of eosinophils in bronchioles compared to medium bronchi we have used in our experiments (Faul et al., 1997; Hyde, Hamid, & Irvin, 2009). Furthermore, a possible bias leading to the absence of airway hyperresponsiveness in the bronchioles used by Manson et al. (2019) is that their tissues were not passively sensitized, a procedure that induces airway hyperresponsiveness via IgE in the presence of further serum factors (Ichinose et al., 1996; Mitchell et al., 1997; Schmidt & Rabe, 2000; Schmidt et al., 1999). In fact, it is noteworthy that airway hyperresponsiveness is dependent upon still unknown serum factors that seem to be IgE related (Watson, Ruhlmann, Magnussen, & Rabe, 1998). In our study, we have also found that the passive sensitization of subsegmental bronchi (4‐ to 6‐mm inner diameter) elicits airway hyperresponsiveness in response to electrical field stimulation. Since no augmentation to electrical field stimulation was detected in previous studies in smaller airways (2‐ to 3‐mm inner diameter) regardless of the frequency delivered (Mitchell et al., 1997), it can be assumed that the distribution of functional parasympathetic innervation at the level of airways ≤3 mm is sparse and/or hyporesponsive to passive sensitization (Calzetta, Matera, & Cazzola, 2018), making these smaller airways not appropriate to reproduce ex vivo the airway hyperresponsiveness mediated by vagal activation.
Interestingly, in asthmatic patients, inhaled IL‐5 does not induce acute airway hyperresponsiveness. Conversely, 24 h post‐administration, inhaled IL‐5 significantly increased airway hyperresponsiveness in response to a provocative concentration of inhaled methacholine (20% fall in FEV1 [PC20]) (Shi et al., 1998). Thus, considering that in the ex vivo model of airway hyperresponsiveness isolated airways were incubated overnight with sensitizing serum for 18 h, the airway hyperresponsiveness detected in our experiments roughly overlapped the peak of airway hyperresponsiveness induced in asthmatic subjects by IL‐5 (Shi et al., 1998). Overall, these findings support the translational validity of the model used in our study to reproduce ex vivo the airway hyperresponsiveness that is a main treatable trait in asthma (Pavord et al., 2018) and the crucial role of IL‐5 in modulating airway hyperresponsiveness itself. Really IL‐5 represents one of those previously unknown factors beyond IgE that modulate airway hyperresponsiveness. Thus, the current knowledge provides the evidence that passive sensitization is a model of human airway hyperresponsiveness related to IL‐5 sensitivity. However, we cannot exclude that other cytokines may contribute to the airway hyperresponsiveness elicited by passive sensitization, although assessing this issue is beyond the aim of this research.
Although the treatment with IL‐5 or IL‐5Rα monoclonal antibodies elicits small but statistically significant improvement in FEV1 in asthmatic patients and prevents airway hyperresponsiveness in passively sensitized human isolated airways, it is well known that these are not the main effects of these drugs. The main limitation of this study is intrinsic to the isolated bronchial model itself, as it only permitted the characterization of the effect of benralizumab and mepolizumab against airway hyperresponsiveness in a sub‐acute ex vivo experimental setting but not after a long‐term exposure. In this regard, since airway hyperresponsiveness has been proposed to be a main treatable trait towards precision medicine in eosinophilic asthmatic patients (Agusti et al., 2016; Bel & Ten Brinke, 2017), well‐designed head‐to‐head randomized clinical trials are needed to compare the efficacy of chronic treatment with benralizumab and mepolizumab specifically against airway hyperresponsiveness in severe asthma. In fact, across the main randomized clinical trials on benralizumab, the impact of this monoclonal antibody on airway hyperresponsiveness in severe asthmatic patients has never been included into the investigated outcomes. Only few data are available for mepolizumab, reporting no beneficial effect on airway hyperresponsiveness (Flood‐Page, Menzies‐Gow, Kay, & Robinson, 2003; Haldar et al., 2009), but these clinical trials were performed in highly selected and small to very small groups of patients and thus these results should not be extrapolated to different wider asthmatic populations.
Taken together, the findings of this study demonstrate that passive sensitization induces a massive release of IL‐5 from human airways and that targeting the IL‐5/IL‐5Rα axis with monoclonal antibodies prevents, in a concentration‐dependent manner, the airway hyperresponsiveness in response to histamine, parasympathetic activation and mechanical stress. Benralizumab, by blocking the receptor IL‐5Rα, was more potent than the anti‐IL‐5 mepolizumab and the beneficial effects of both these agents were correlated with improved levels of cAMP in hyperresponsive airways. These observations also indicate that IL‐5 is a key factor in determining airway hyperresponsiveness in passively sensitized airways. Further head‐to‐head clinical trials evaluating the impact of long‐term treatment with benralizumab and mepolizumab against airway hyperresponsiveness are needed in severe asthmatic subjects, with specific focus on patients characterized by a high intrinsic parasympathetic tone.
AUTHOR CONTRIBUTIONS
L.C., B.L.R., M.G.M., F.F. and P.R. have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; L.C., B.L.R., M.G.M., F.F. and P.R. have been involved in drafting the manuscript or revising it critically for important intellectual content; and L.C., B.L.R., M.G.M., F.F. and P.R. gave the final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST
L.C. reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, non‐financial support from AstraZeneca, grants from Chiesi Farmaceutici, grants from Almirall, personal fees from ABC Farmaceutici, personal fees from Edmond Pharma, grants and personal fees from Zambon, personal fees from Verona Pharma and personal fees from Ockham Biotech. B.L.R. reports no conflict of interest. MGM reports personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, personal fees from Chiesi Farmaceutici, personal fees from Almirall, personal fees from ABC Farmaceutici and personal fees from GlaxoSmithKline. F.F. reports no conflict of interest. P.R. reports grants and personal fees from Boehringer Ingelheim, grants and personal fees from Novartis, personal fees from AstraZeneca, grants and personal fees from Chiesi Farmaceutici, grants and personal fees from Almirall, grants from Zambon, personal fees from Biofutura, personal fees from GlaxoSmithKline, personal fees from Menarini and personal fees from Mundipharma.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis and as recommended by funding agencies, publishers, and other organizations engaged with supporting research.
Supporting information
Data S1. e‐Table 1. Main characteristics of donors and normal ranges in agreement with GINA recommendations (GINA, 2020)
e‐Table 2. Dataset of sectional tissues used in this study
e‐Table 3. Effect of overnight incubation with different concentrations of benralizumab and mepolizumab on the FRCs to EFS in passively sensitized bronchi
e‐Table 4. Efficacy and potency of benralizumab and mepolizumab after overnight incubation on the AHR to different EFS frequencies (EF50–90) in passively sensitized bronchi. The pharmacological analysis was performed by assessing Emax as the difference in airway contractility between passively sensitized and non‐sensitized bronchi
e‐Figure 1. Levels of IL‐5 detectable in the supernatant of C− and C+ during the sensitizing procedure. ** P < 0.01 and *** P < 0.001 vs. C− (statistical analysis assessed by two‐way ANOVA); bars represent the mean ± SEM of n = 5 bronchial tissue from different subjects. C+: positive control, isolated bronchi incubated with sensitizing serum; C−: negative control, isolated bronchi incubated with non‐sensitizing; IL‐5: interleukin 5
Data S2: Table 1: Rigor Adherence Table
Table 2: Key Resources Table
Calzetta L, Ritondo BL, Matera MG, Facciolo F, Rogliani P. Targeting IL‐5 pathway against airway hyperresponsiveness: A comparison between benralizumab and mepolizumab. Br J Pharmacol. 2020;177:4750–4765. 10.1111/bph.15240
Luigino Calzetta and Beatrice Ludovica Ritondo contributed equally to the study.
REFERENCES
- Agusti, A. , Bel, E. , Thomas, M. , Vogelmeier, C. , Brusselle, G. , Holgate, S. , … Pavord, I. D. (2016). Treatable traits: Toward precision medicine of chronic airway diseases. The European Respiratory Journal, 47, 410–419. 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnasco, D. , Ferrando, M. , Varricchi, G. , Puggioni, F. , Passalacqua, G. , & Canonica, G. W. (2017). Anti‐interleukin 5 (IL‐5) and IL‐5Ra biological drugs: Efficacy, safety, and future perspectives in severe eosinophilic asthma. Frontiers in Medicine, 4, 135 10.3389/fmed.2017.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandeira‐Melo, C. , Bozza, P. T. , & Weller, P. F. (2002). The cellular biology of eosinophil eicosanoid formation and function. The Journal of Allergy and Clinical Immunology, 109, 393–400. 10.1067/mai.2002.121529 [DOI] [PubMed] [Google Scholar]
- Bel, E. H. , & Ten Brinke, A. (2017). New anti‐eosinophil drugs for asthma and COPD: Targeting the trait! Chest, 152, 1276–1282. 10.1016/j.chest.2017.05.019 [DOI] [PubMed] [Google Scholar]
- Berger, P. , Scotto‐Gomez, E. , Molimard, M. , Marthan, R. , Le Gros, V. , & Tunon‐de‐Lara, J. M. (2007). Omalizumab decreases nonspecific airway hyperresponsiveness in vitro. Allergy, 62, 154–161. 10.1111/j.1398-9995.2006.01243.x [DOI] [PubMed] [Google Scholar]
- Black, J. L. , Panettieri, R. A. Jr. , Banerjee, A. , & Berger, P. (2012). Airway smooth muscle in asthma: Just a target for bronchodilation? Clinics in Chest Medicine, 33, 543–558. 10.1016/j.ccm.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker, E. R. , FitzGerald, J. M. , Chanez, P. , Papi, A. , Weinstein, S. F. , Barker, P. , … SIROCCO study investigators . (2016). Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high‐dosage inhaled corticosteroids and long‐acting β2‐agonists (SIROCCO): A randomised, multicentre, placebo‐controlled phase 3 trial. Lancet, 388, 2115–2127. 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- Borish, L. (2016). The immunology of asthma: Asthma phenotypes and their implications for personalized treatment. Annals of Allergy, Asthma & Immunology, 117, 108–114. 10.1016/j.anai.2016.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzetta, L. , Matera, M. G. , & Cazzola, M. (2018). Pharmacological mechanisms leading to synergy in fixed‐dose dual bronchodilator therapy. Current Opinion in Pharmacology, 40, 95–103. 10.1016/j.coph.2018.03.011 [DOI] [PubMed] [Google Scholar]
- Calzetta, L. , Matera, M. G. , Facciolo, F. , Cazzola, M. , & Rogliani, P. (2018). Beclomethasone dipropionate and formoterol fumarate synergistically interact in hyperresponsive medium bronchi and small airways. Respiratory Research, 19, 65 10.1186/s12931-018-0770-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola, M. , Calzetta, L. , Page, C. P. , Rinaldi, B. , Capuano, A. , & Matera, M. G. (2011). Protein prenylation contributes to the effects of LPS on EFS‐induced responses in human isolated bronchi. American Journal of Respiratory Cell and Molecular Biology, 45, 704–710. 10.1165/rcmb.2010-0306OC [DOI] [PubMed] [Google Scholar]
- Cazzola, M. , Calzetta, L. , Puxeddu, E. , Ora, J. , Facciolo, F. , Rogliani, P. , & Matera, M. G. (2016). Pharmacological characterisation of the interaction between glycopyrronium bromide and indacaterol fumarate in human isolated bronchi, small airways and bronchial epithelial cells. Respiratory Research, 17, 70 10.1186/s12931-016-0386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzola, M. , Calzetta, L. , Rogliani, P. , Puxeddu, E. , Facciolo, F. , & Matera, M. G. (2016). Interaction between corticosteroids and muscarinic antagonists in human airways. Pulmonary Pharmacology & Therapeutics, 36, 1–9. 10.1016/j.pupt.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damera, G. , Panettieri, J. , & Reynold, A. (2011). Does airway smooth muscle express an inflammatory phenotype in asthma? British Journal of Pharmacology, 163, 68–80. 10.1111/j.1476-5381.2010.01165.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake, M. G. , Lebold, K. M. , Roth‐Carter, Q. R. , Pincus, A. B. , Blum, E. D. , Proskocil, B. J. , … Nie, Z. (2018). Eosinophil and airway nerve interactions in asthma. Journal of Leukocyte Biology, 104, 61–67. 10.1002/JLB.3MR1117-426R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eum, S.‐Y. , Maghni, K. , Tolloczko, B. , Eidelman, D. H. , & Martin, J. G. (2005). IL‐13 may mediate allergen‐induced hyperresponsiveness independently of IL‐5 or eotaxin by effects on airway smooth muscle. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 288, L576–L584. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency . (2015). Nucala ‐ EMEA/H/C/003860 ‐ N/0027. Available at https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf Last accessed March 21, 2020.
- European Medicines Agency . (2018). Fasenra ‐ EMEA/H/C/004433 ‐ II/0014/G. Available at https://www.ema.europa.eu/en/documents/product-information/fasenra-epar-product-information_en.pdf. Last accessed March 21, 2020.
- Farne, H. A. , Wilson, A. , Powell, C. , Bax, L. , & Milan, S. J. (2017). Anti‐IL5 therapies for asthma. Cochrane Database of Systematic Reviews, 9, CD010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, J. L. , Tormey, V. J. , Leonard, C. , Burke, C. M. , Farmer, J. , Horne, S. J. , & Poulter, L. W. (1997). Lung immunopathology in cases of sudden asthma death. The European Respiratory Journal, 10, 301–307. 10.1183/09031936.97.10020301 [DOI] [PubMed] [Google Scholar]
- FitzGerald, J. M. , Bleecker, E. R. , Nair, P. , Korn, S. , Ohta, K. , Lommatzsch, M. , … CALIMA study investigators . (2016). Benralizumab, an anti‐interleukin‐5 receptor α monoclonal antibody, as add‐on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double‐blind, placebo‐controlled phase 3 trial. Lancet, 388, 2128–2141. 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- Flood‐Page, P. , Swenson, C. , Faiferman, I. , Matthews, J. , Williams, M. , Brannick, L. , … on behalf of the International Mepolizumab Study Group . (2007). A study to evaluate safety and efficacy of mepolizumab in patients with moderate persistent asthma. American Journal of Respiratory and Critical Care Medicine, 176, 1062–1071. 10.1164/rccm.200701-085OC [DOI] [PubMed] [Google Scholar]
- Flood‐Page, P. T. , Menzies‐Gow, A. N. , Kay, A. B. , & Robinson, D. S. (2003). Eosinophil's role remains uncertain as anti‐interleukin‐5 only partially depletes numbers in asthmatic airway. American Journal of Respiratory and Critical Care Medicine, 167, 199–204. 10.1164/rccm.200208-789OC [DOI] [PubMed] [Google Scholar]
- GINA . (2020). Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. Retrieved from https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf
- Gounni, A. S. , Wellemans, V. , Yang, J. , Bellesort, F. , Kassiri, K. , Gangloff, S. , … Lamkhioued, B. (2005). Human airway smooth muscle cells express the high affinity receptor for IgE (FcεRI): A critical role of FcεRI in human airway smooth muscle cell function. The Journal of Immunology, 175, 2613–2621. 10.4049/jimmunol.175.4.2613 [DOI] [PubMed] [Google Scholar]
- Grunstein, M. M. , Hakonarson, H. , Leiter, J. , Chen, M. , Whelan, R. , Grunstein, J. S. , & Chuang, S. (2002). IL‐13‐dependent autocrine signaling mediates altered responsiveness of IgE‐sensitized airway smooth muscle. American Journal of Physiology. Lung Cellular and Molecular Physiology, 282, L520–L528. 10.1152/ajplung.00343.2001 [DOI] [PubMed] [Google Scholar]
- Hakonarson, H. , Maskeri, N. , Carter, C. , Chuang, S. , & Grunstein, M. M. (1999). Autocrine interaction between IL‐5 and IL‐1β mediates altered responsiveness of atopic asthmatic sensitized airway smooth muscle. The Journal of Clinical Investigation, 104, 657–667. 10.1172/JCI7137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakonarson, H. , Maskeri, N. , Carter, C. , & Grunstein, M. M. (1999). Regulation of TH1‐ and TH2‐type cytokine expression and action in atopic asthmatic sensitized airway smooth muscle. The Journal of Clinical Investigation, 103, 1077–1087. 10.1172/JCI5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar, P. , Brightling, C. E. , Hargadon, B. , Gupta, S. , Monteiro, W. , Sousa, A. , … Pavord, I. D. (2009). Mepolizumab and exacerbations of refractory eosinophilic asthma. The New England Journal of Medicine, 360, 973–984. 10.1056/NEJMoa0808991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelmann, E. , Oshiba, A. , Loader, J. , Larsen, G. L. , Gleich, G. , Lee, J. , & Gelfand, E. W. (1997). Antiinterleukin‐5 antibody prevents airway hyperresponsiveness in a murine model of airway sensitization. American Journal of Respiratory and Critical Care Medicine, 155, 819–825. 10.1164/ajrccm.155.3.9117011 [DOI] [PubMed] [Google Scholar]
- Head, M. L. , Holman, L. , Lanfear, R. , Kahn, A. T. , & Jennions, M. D. (2015). The extent and consequences of p‐hacking in science. PLoS Biology, 13, e1002106 10.1371/journal.pbio.1002106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde, D. M. , Hamid, Q. , & Irvin, C. G. (2009). Anatomy, pathology, and physiology of the tracheobronchial tree: Emphasis on the distal airways. The Journal of Allergy and Clinical Immunology, 124, S72–S77. 10.1016/j.jaci.2009.08.048 [DOI] [PubMed] [Google Scholar]
- Ichinose, M. , Miura, M. , Tomaki, M. , Oyake, T. , Kageyama, N. , Ikarashi, Y. , … Shirato, K. (1996). Incubation with IgE increases cholinergic neurotransmission in human airways in vitro. American Journal of Respiratory and Critical Care Medicine, 154, 1272–1276. 10.1164/ajrccm.154.5.8912735 [DOI] [PubMed] [Google Scholar]
- Kolbeck, R. , Kozhich, A. , Koike, M. , Peng, L. , Andersson, C. K. , Damschroder, M. M. , … Coyle, A. J. (2010). MEDI‐563, a humanized anti‐IL‐5 receptor α mAb with enhanced antibody‐dependent cell‐mediated cytotoxicity function. The Journal of Allergy and Clinical Immunology, 125, 1344–1353 e1342. [DOI] [PubMed] [Google Scholar]
- Leckie, M. J. , ten Brinke, A. , Khan, J. , Diamant, Z. , O'Connor, B. J. , Walls, C. M. , … Barnes, P. J. (2000). Effects of an interleukin‐5 blocking monoclonal antibody on eosinophils, airway hyper‐responsiveness, and the late asthmatic response. Lancet, 356, 2144–2148. 10.1016/S0140-6736(00)03496-6 [DOI] [PubMed] [Google Scholar]
- Liccardi, G. , Salzillo, A. , Calzetta, L. , Cazzola, M. , Matera, M. G. , & Rogliani, P. (2016). Can bronchial asthma with an highly prevalent airway (and systemic) vagal tone be considered an independent asthma phenotype? Possible role of anticholinergics. Respiratory Medicine, 117, 150–153. 10.1016/j.rmed.2016.05.026 [DOI] [PubMed] [Google Scholar]
- Manson, M. L. , Säfholm, J. , James, A. , Johnsson, A.‐K. , Bergman, P. , Al‐Ameri, M. , … Adner, M. (2019). IL‐13 and IL‐4, but not IL‐5 nor IL‐17A, induce hyperresponsiveness in isolated human small airways. Journal of Allergy and Clinical Immunology, 145(3), 808–817. [DOI] [PubMed] [Google Scholar]
- Martin, U. J. , Fuhr, R. , Forte, P. , Barker, P. , Axley, M. J. , Aurivillius, M. , et al. (2019). Comparison of autoinjector with accessorized prefilled syringe for benralizumab pharmacokinetic exposure: AMES trial results. Journal of Asthma, 1–9. 10.1080/02770903.2019.1663428 [DOI] [PubMed] [Google Scholar]
- Mathur, M. , Herrmann, K. , Li, X. , Qin, Y. , Weinstock, J. , Elliott, D. , … Padrid, P. (1999). TRFK‐5 reverses established airway eosinophilia but not established hyperresponsiveness in a murine model of chronic asthma. American Journal of Respiratory and Critical Care Medicine, 159, 580–587. 10.1164/ajrccm.159.2.9712018 [DOI] [PubMed] [Google Scholar]
- Mauser, P. J. , Pitman, A. M. , Fernandez, X. , Foran, S. K. , Adams, G. K. 3rd , Kreutner, W. , … Chapman, R. W. (1995). Effects of an antibody to interleukin‐5 in a monkey model of asthma. American Journal of Respiratory and Critical Care Medicine, 152, 467–472. 10.1164/ajrccm.152.2.7633694 [DOI] [PubMed] [Google Scholar]
- Mitchell, R. W. , Ndukuw, I. M. , Ikeda, K. , Arbetter, K. , & Leff, A. R. (1993). Effect of immune sensitization on stimulated ACh release from trachealis muscle in vitro. The American Journal of Physiology, 265, L13–L18. 10.1152/ajplung.1993.265.1.L13 [DOI] [PubMed] [Google Scholar]
- Mitchell, R. W. , Rabe, K. F. , Magnussen, H. , & Leff, A. R. (1997). Passive sensitization of human airways induces myogenic contractile responses in vitro. Journal of Applied Physiology (1985), 83, 1276–1281. [DOI] [PubMed] [Google Scholar]
- Molfino, N. A. , Gossage, D. , Kolbeck, R. , Parker, J. M. , & Geba, G. P. (2012). Molecular and clinical rationale for therapeutic targeting of interleukin‐5 and its receptor. Clinical and Experimental Allergy, 42, 712–737. 10.1111/j.1365-2222.2011.03854.x [DOI] [PubMed] [Google Scholar]
- Mukherjee, M. , Sehmi, R. , & Nair, P. (2014). Anti‐IL5 therapy for asthma and beyond. World Allergy Organization Journal, 7, 32 10.1186/1939-4551-7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag, S. S. , Xu, L. J. , Hamid, Q. , & Renzi, P. M. (2003). The effects of IL‐5 on airway physiology and inflammation in rats. Journal of Allergy and Clinical Immunology, 111, 558–566. 10.1067/mai.2003.131 [DOI] [PubMed] [Google Scholar]
- Nair, P. , Wenzel, S. , Rabe, K. F. , Bourdin, A. , Lugogo, N. L. , Kuna, P. , … ZONDA Trial Investigators . (2017). Oral glucocorticoid‐sparing effect of benralizumab in severe asthma. The New England Journal of Medicine, 376, 2448–2458. 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- Ortega, H. G. , Liu, M. C. , Pavord, I. D. , Brusselle, G. G. , FitzGerald, J. M. , Chetta, A. , … MENSA Investigators . (2014). Mepolizumab treatment in patients with severe eosinophilic asthma. The New England Journal of Medicine, 371, 1198–1207. 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- Pavord, I. D. , Beasley, R. , Agusti, A. , Anderson, G. P. , Bel, E. , Brusselle, G. , … Bush, A. (2018). After asthma: Redefining airways diseases. Lancet, 391, 350–400. 10.1016/S0140-6736(17)30879-6 [DOI] [PubMed] [Google Scholar]
- Pavord, I. D. , Korn, S. , Howarth, P. , Bleecker, E. R. , Buhl, R. , Keene, O. N. , … Chanez, P. (2012). Mepolizumab for severe eosinophilic asthma (DREAM): A multicentre, double‐blind, placebo‐controlled trial. Lancet, 380, 651–659. 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- Rabe, K. F. (1998). Mechanisms of immune sensitization of human bronchus. American Journal of Respiratory and Critical Care Medicine, 158, S161–S170. 10.1164/ajrccm.158.supplement_2.13tac130 [DOI] [PubMed] [Google Scholar]
- Rogliani, P. , Calzetta, L. , Capuani, B. , Facciolo, F. , Cazzola, M. , Lauro, D. , & Matera, M. G. (2016). Glucagon‐like peptide 1 receptor: A novel pharmacological target for treating human bronchial hyperresponsiveness. American Journal of Respiratory Cell and Molecular Biology, 55, 804–814. 10.1165/rcmb.2015-0311OC [DOI] [PubMed] [Google Scholar]
- Rogliani, P. , Matera, M. G. , Facciolo, F. , Page, C. , Cazzola, M. , & Calzetta, L. (2020). Beclomethasone dipropionate, formoterol fumarate and glycopyrronium bromide: Synergy of triple combination therapy on human airway smooth muscle ex vivo. British Journal of Pharmacology, 177, 1150–1163. 10.1111/bph.14909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma, D. , Zuidhof, A. B. , Nelemans, S. A. , Zaagsma, J. , & Meurs, H. (2006). Inhibition of Rho‐kinase normalizes nonspecific hyperresponsiveness in passively sensitized airway smooth muscle preparations. European Journal of Pharmacology, 531, 145–150. 10.1016/j.ejphar.2005.12.043 [DOI] [PubMed] [Google Scholar]
- Schmidt, D. , & Rabe, K. F. (2000). Immune mechanisms of smooth muscle hyperreactivity in asthma. The Journal of Allergy and Clinical Immunology, 105, 673–682. 10.1067/mai.2000.105705 [DOI] [PubMed] [Google Scholar]
- Schmidt, D. , Ruehlmann, E. , Branscheid, D. , Magnussen, H. , & Rabe, K. F. (1999). Passive sensitization of human airways increases responsiveness to leukotriene C4. The European Respiratory Journal, 14, 315–319. 10.1034/j.1399-3003.1999.14b13.x [DOI] [PubMed] [Google Scholar]
- Shabbir, S. , Pouliquen, I. J. , Bentley, J. H. , Bradford, E. S. , Kaisermann, M. C. , & Albayaty, M. (2019). The pharmacokinetics and relative bioavailability of mepolizumab 100mg liquid formulation administered subcutaneously to healthy participants: A randomized trial. Clinical Pharmacology in Drug Development, 9(3), 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shardonofsky, F. R. , Venzor, J. 3rd , Barrios, R. , Leong, K. P. , & Huston, D. P. (1999). Therapeutic efficacy of an anti‐IL‐5 monoclonal antibody delivered into the respiratory tract in a murine model of asthma. The Journal of Allergy and Clinical Immunology, 104, 215–221. 10.1016/S0091-6749(99)70138-7 [DOI] [PubMed] [Google Scholar]
- Shi, H. Z. , Xiao, C. Q. , Zhong, D. , Qin, S. M. , Liu, Y. , Liang, G. R. , … Xie, Z. F. (1998). Effect of inhaled interleukin‐5 on airway hyperreactivity and eosinophilia in asthmatics. American Journal of Respiratory and Critical Care Medicine, 157, 204–209. 10.1164/ajrccm.157.1.9703027 [DOI] [PubMed] [Google Scholar]
- Tanaka, H. , Nagai, H. , & Maeda, Y. (1998). Effect of anti‐IL‐4 and anti‐IL‐5 antibodies on allergic airway hyperresponsiveness in mice. Life Sciences, 62, PL169–PL174. 10.1016/S0024-3205(98)00047-2 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2017). Fasenra (benralizumab) 30 mg/mL Injection. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761070Orig1s000TOC.cfm. Last accessed March 21, 2020.
- US Food and Drug Administration (2015). NUCALA (mepolizumab) for injection, for subcutaneous use. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125526s004lbl.pdf. Last accessed March 21, 2020.
- Watson, N. , Ruhlmann, E. , Magnussen, H. , & Rabe, K. F. (1998). Histamine hypersensitivity induced by passive sensitization of human bronchus: Effect of serum IgE depletion. Clinical and Experimental Allergy, 28, 679–685. 10.1046/j.1365-2222.1998.00269.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. e‐Table 1. Main characteristics of donors and normal ranges in agreement with GINA recommendations (GINA, 2020)
e‐Table 2. Dataset of sectional tissues used in this study
e‐Table 3. Effect of overnight incubation with different concentrations of benralizumab and mepolizumab on the FRCs to EFS in passively sensitized bronchi
e‐Table 4. Efficacy and potency of benralizumab and mepolizumab after overnight incubation on the AHR to different EFS frequencies (EF50–90) in passively sensitized bronchi. The pharmacological analysis was performed by assessing Emax as the difference in airway contractility between passively sensitized and non‐sensitized bronchi
e‐Figure 1. Levels of IL‐5 detectable in the supernatant of C− and C+ during the sensitizing procedure. ** P < 0.01 and *** P < 0.001 vs. C− (statistical analysis assessed by two‐way ANOVA); bars represent the mean ± SEM of n = 5 bronchial tissue from different subjects. C+: positive control, isolated bronchi incubated with sensitizing serum; C−: negative control, isolated bronchi incubated with non‐sensitizing; IL‐5: interleukin 5
Data S2: Table 1: Rigor Adherence Table
Table 2: Key Resources Table


