Abstract
Background and Purpose
Women have a higher incidence of eating disorders than men. We investigated whether the effects of ghrelin on feeding are affected by sex and stress, and to elucidate the mechanisms that may cause sex differences in stress‐mediated anorexia, focusing on ghrelin.
Experimental Approach
Acylated ghrelin was administered to naïve and psychologically stressed male and female C57BL/6J mice, followed by measurements of food intake and plasma hormone levels. Ovariectomy was performed to determine the effects of ovary‐derived oestrogen on stress‐induced eating disorders in female mice. The numbers of Agrp or c‐Fos mRNA‐positive cells and estrogen receptor α/c‐Fos protein‐double‐positive cells were assessed.
Key Results
Ghrelin administration to naïve female mice caused a higher increase in food intake, growth hormone secretion, Agrp mRNA expression in the arcuate nucleus and c‐Fos expression in the nucleus tractus solitarius (NTS) than in male mice. In contrast, psychological stress caused a more sustained reduction in food intake in females than males. The high sensitivity of naïve females to exogenous ghrelin was attenuated by stress exposure. The stress‐induced decline in food intake was not abolished by ovariectomy. Estrogen receptor‐α but not ‐β antagonism prevented the decrease in food intake under stress. Estrogen receptor‐α/c‐Fos‐double‐positive cells in the NTS were significantly increased by stress only in females.
Conclusion and Implications
Stress‐mediated eating disorders in females may be due to blockade of ghrelin signalling via estrogen receptor‐α activation in the NTS. Targeting the ghrelin signal in the brain could be a new treatment strategy to prevent these disorders.
Keywords: anorexia, ghrelin, mice, rikkunshito, sex differences, stress
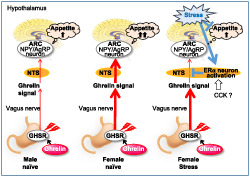
Schematic mechanism of feeding control via ghrelin reactivity in naive and stressed mice
ABBREVIATION
- AP
area postrema
- ARC
arcuate nucleus
- CCK
cholecystokinin
- CRF
corticotropin‐releasing factor
- DG6
[D‐Lys3]‐GHRP‐6
- GHSR
growth hormone secretagogue receptor (i.e., ghrelin receptor)
- HPA
hypothalamic–pituitary–adrenal
- NTS
nucleus tractus solitarius
- OVX
ovariectomized
- PVN
paraventricular nucleus
- VMH
ventromedial hypothalamus
What is already known
Women are known to have a high incidence of neuropsychiatric disorders.
Involvement of sex in feeding abnormality induced by stress has not been adequately investigated.
What this study adds
Stress‐mediated anorexia in female mice may be due to blockade of ghrelin signalling.
The data indicates that is via oestrogen receptor‐α activation in nucleus tractus solitarius.
What is the clinical significance
Our findings may contribute to the sex‐specific treatment for stress‐induced eating disorders in the future.
1. INTRODUCTION
Men and women have been reported to have different incidences of stress‐induced psychoneurotic disorders (Handa, Mani, & Uht, 2012; Handa & Weiser, 2014). In Europe and the United States, major depression or anorexia nervosa due to psychological stress is increasing year by year and differences in incidence between males and females have been reported. The prevalence of depression in women is known to be approximately twice that in men (Ehlert, Gaab, & Heinrichs, 2001; Kornstein, 1997) and anorexia nervosa is approximately three times more prevalent in women than in men (Hudson, Hiripi, Pope, & Kessler, 2007). Although such neuropsychiatric disorders are major global health issues, there is still no effective therapeutic approach or clinical evidence of the pathogenic mechanism involved (Asarian & Geary, 2013). Sex differences in the incidence of these diseases may be mediated by the different stress responses involved in the development of the disease (Handa et al., 2012; Handa & Weiser, 2014; Iwasaki‐Sekino, Mano‐Otagiri, Ohata, Yamauchi, & Shibasaki, 2009; Oyola & Handa, 2017). Sex influences the hypothalamic–pituitary–adrenal (HPA) axis. This may reflect sex differences in feedback mechanisms against stress, corticotropin‐releasing factor (CRF)‐binding protein synthesis, activation of CRF or cross‐talk with the adrenal gland (Oyola & Handa, 2017). However, the relationship between the important factors that cause stress‐induced eating abnormalities and sex differences has not been sufficiently studied and further exploration may be the key to learning its pathogenesis.
Recent reports have clarified the important role of peripheral or central appetite‐related peptides in feeding behaviour. In eating abnormalities due to stress, abnormal secretion and dysfunction of peripheral appetite‐related peptides have been observed (Misra & Klibanski, 2014; Nahata et al., 2012; Stengel, Wang, & Tache, 2011; Yamada, Saegusa, et al., 2015). In studies using rodents, it has been demonstrated that changes in feeding behaviour due to stress may depend on peripheral ghrelin concentration. After exposure to psychological stress, such as water avoidance and social defeat, appetite increased in parallel with an increase in peripheral ghrelin concentration (Stengel et al., 2011). In contrast, a decrease in appetite was observed in a stress model induced by i.c.v. urocortin (Yakabi et al., 2014) and in an immune stress model (Stengel et al., 2010), which was partially mediated by decreased ghrelin release. The majority of these studies have been conducted in male mice and only a few studies have focused on sex differences in ghrelin responsiveness and how it affects eating behaviour. One study using rodents showed that the orexigenic action of ghrelin was clearly stronger in males than in females (Clegg et al., 2007), while the other study revealed no sex differences in ghrelin‐induced feeding behaviour (Currie, Mirza, Fuld, Park, & Vasselli, 2005). Therefore, the relationship between eating abnormalities and feeding‐associated hormones has not yet been fully elucidated. Additionally, sex differences with regard to ghrelin reactivity, particularly in stressed conditions, remains controversial.
Sex hormones may be a main cause of sex differences in energy intake. The effect of estrogen on stress responses may be controlled by estrogen receptor‐α and ‐β. Estrogen receptor‐α (NR3A1) decreases food intake via cholecystokinin (CCK) signal amplification (Asarian & Geary, 2013), whereas estrogen receptor‐β (NR3A2) negatively controls the HPA axis (Handa et al., 2012). Previously, we demonstrated that stress‐induced hypophagia in aged male mice is caused by peripheral ghrelin reduction via activation of estrogen receptor‐α and consequent hypothalamic 5‐HT2C receptors activation (Yamada, Sadakane, et al., 2015). Our hypothesis is that female‐specific hypophagia depends on cross‐talk between ghrelin and estrogen receptors
In this study, our aim was to investigate whether the effects of ghrelin on feeding are essentially affected by sex and stress, and to elucidate the biological factors that may cause sex differences in stress‐mediated anorexia, focusing on the reactivity of ghrelin. First, sex differences in feeding behaviour and hormone secretion ability caused by ghrelin administration were examined. Second, the effects of psychological stress as well as sex differences on afferent ghrelin signals were investigated. Finally, we evaluated the involvement of estrogen receptor‐α neuronal activation in ghrelin signalling in the brainstem areas.
2. METHODS
2.1. Ethical statement
All animal care and experimental procedures were approved by the experimental animal ethics committees of Tsumura & Co. (Ibaraki, Japan; permit nos: 13‐012, 13‐053, 13‐0081, 14‐022, and 15‐044). The procedures conducted followed the in‐house regulations regarding laboratory animal care and use, which are in compliance with the Japanese law “Act on Welfare and Management of Animals” and the guidelines from the Ministry of Health, Labour and Welfare Japan (Act on Welfare and Management of Animals; Japan Act No. 105 of October 1, 1973, Basic Guidelines for Proper Conduct of Animal Testing and Related Activities in the Research Institutions under the Jurisdiction of the Japan Ministry of Health, Labour and Welfare; Japan Ministry of Health, Labour and Welfare Notification of June 1, 2006, Standards Relating to the Care and Management of Laboratory Animals and Relief of Pain; Japan Ministry of the Environment Notice No. 88 of April 28, 2006, Guidelines for Proper Conduct of Animal Experiments, June 1, 2006, Science Council of Japan). Animal studies are reported in compliance with the ARRIVE guidelines (Percie du Sert et al., 2020) and with the recommendations made by the British Journal of Pharmacology (Lilley et al., 2020).
2.2. Compliance with requirements for studies using animals
C57BL/6 mice are the most common inbred strain used in biomedical research (Mallien et al., 2019). They were appropriate because they have also been widely used in translational pharmacological and behavioural research on anxiety, depression and other neuropsychiatric disorders (Mallien et al., 2019; Morgan et al., 2018; Oberrauch et al., 2019). Other research on ghrelin and stress has been conducted in this strain (Hassouna et al., 2013; Liu, Yakar, Otero‐Corchon, Low, & Liu, 2002; Patterson, Khazall, Mackay, Anisman, & Abizaid, 2013). C57BL/6J mice have been used in our previous reports on novel environmental stress models and we have large amount of background data (Nahata et al., 2013; Saegusa et al., 2011). Therefore, C57BL/6J mice were used in this study.
Ten‐week‐old C57BL/6J mice (SPF grade; Charles River Co. Ltd., Japan) were used in all experiments. Mice were used at an average body weight of 25 ± 2.5 g in males and 20 ± 2.5 g in females. Mice were acclimated in 230 × 310 × 155 mm plastic cages (3 mice per cage) with sterile paper bedding in a temperature‐ (23 ± 3°C) and humidity‐controlled (30–70%) room with a 12‐h light (23:00–11:00)/dark cycle, with free access to food (certified standard diet, MF pellets; Oriental Yeast Co., Ltd., Japan) and water for at least 1 week before the experiment. All experiments were performed between 10:00 and 15:00.
2.3. Effect of acylated ghrelin on food intake
A total of 120 mice were housed in 40 cages (3 mice per cage) and randomly assigned to four groups (10 cages each group; control, ghrelin—50 nmol·kg−1, ghrelin—100 nmol·kg−1, and ghrelin—150 nmol·kg−1). The same procedure was performed for males and females. Mice were i.p. administered acylated ghrelin (Peptide Institute, Osaka, Japan) at 50, 150 or 500 nmol·kg−1, and then, total food intake per cage was measured after administration. Ghrelin doses were chosen on the basis of prior publications (Lockie, Dinan, Lawrence, Spencer, & Andrews, 2015; Nahata et al., 2014; Tsubouchi et al., 2014). Alternatively, [D‐Lys3]‐GHRP‐6, a ghrelin receptor antagonist; Bachem AG, Bubendorf, Switzerland) at 5, 10 or 20 μmol·kg−1 was administered. DG6 doses were chosen on the basis of prior reports (Asakawa et al., 2003; Endo, Hori, Ozaki, Oikawa, & Hanawa, 2014). In another experiment, a total of 72 mice were housed in 24 cages and they were randomly assigned to three groups (n = 8 cages each; control, ghrelin 3 pmol per mouse and ghrelin 30 pmol per mouse). Intracerebroventricular (i.c.v.) injections of acylated ghrelin at 3 and 30 pmol per 20 g mice were performed according to previous reports (Haley & McCormick, 1957; Mogami et al., 2016). Doses were chosen on the basis of prior reports (Asakawa et al., 2001; Currie et al., 2005). A 26‐gauge stainless‐steel needle attached to a 10‐μl microsyringe was inserted into the brain (2.6 mm below the skull surface, 1.0 mm lateral, and 0.5 mm anterior to the bregma) of held mice after light anaesthesia by isoflurane for 2 min. An injection volume of 4 μl per 20 g mouse was administered over 30 s. We observed the physical effects of i.c.v. injection on mice from the viewpoint of animal welfare and killed based on the exclusion criteria to mice exhibiting visible bleeding or abnormal behaviours (such as sedation and rotation). However, no mice met the exclusion criteria. I.c.v. administration was performed by a skilled individual who confirmed the location of the i.c.v. through dye infusions with a success rate of 97%. Saline was administered to the control group.
2.4. Novelty stress exposure
The novelty‐induced hypophagia test can evaluate the degree of anxiety or depression based on food intake suppression after exposure to a novel environment (Dulawa & Hen, 2005; Nahata et al., 2013; Saegusa et al., 2011; Yamada, Sadakane, et al., 2015). A total of 36 mice were housed in 12 cages, and they were randomly assigned to two groups: control (n = 9 cages; 27 mice) and stress (3 cages; n = 9 mice). To induce novel stress, group‐housed mice (3 mice per cage) were transferred separately to new cages (1 mouse per cage, 9 cages in total) at 0 h (starting in the dark period; see Experimental Design in Figure S1) and each parameter was evaluated during a 24‐h period. Group‐housed control mice were kept in their original cages (3 mice per cage).
2.5. Food intake and body weight
Food intake was measured at 0.5, 1, 2, 3 and/or 6 h after ghrelin administration/stress exposure. For group‐housed control mice, the total food intake per cage was divided by the number of animals to calculate the food intake per animal for each cage. Further, each mouse's body weight was measured at 24 h after administration/stress exposure. The body weight in the control group was evaluated in the same way as the food intake. The body weight increase rate was calculated by dividing the body weight at 0 h by the value at 24 h. To directly evaluate the sex difference in food intake increase induced by ghrelin administration, the relative increase rates of food intake in male and female mice were normalized by the average value in male control mice.
2.6. Plasma hormone levels
Mice were given free access to food and drinking water, and blood was collected from the abdominal vena cava between 11:00 and 15:00 under isoflurane anaesthesia after ghrelin administration/stress exposure. Animals were exsanguinated under anaesthesia by transecting the abdominal aorta and the inferior vena cava after blood collection. Collected blood was centrifuged immediately to collect the plasma. To determine blood ghrelin levels, 10% HCl (1 N) was added to the plasma. Plasma hormone concentrations were measured using the Active/Des‐acyl Ghrelin ELISA Kit (LSI Medience Corporation, Tokyo, Japan), Rat/Mouse Growth Hormone ELISA Kit (Merck Millipore, Darmstadt, Germany), and 17β‐estradiol high‐sensitivity ELISA kit (Enzo Life Sciences, PA, USA). For the measurement of growth hormone, mice were fasted before the measurement and blood was collected between 10:30 and 11:30 when the pulse had stabilized (Steyn et al., 2011). These measurements were performed according to the protocol attached to the product.
2.7. Plasma ghrelin clearance after acylated ghrelin administration
Naïve mice were administered acylated ghrelin (50 nmol·kg−1, i.p.). Food was removed after ghrelin administration. Blood was collected from the abdominal vena cava under isoflurane anaesthesia, and the plasma acylated ghrelin concentration was measured as described above.
2.8. Effect of test drugs on food intake during stress exposure
To investigate the effects of the endogenous ghrelin enhancer rikkunshito (Tsumura & Co., Tokyo, Japan), rikkunshito (1 g) was suspended in distilled water (10 ml) and p.o. administered by gavage (1000 mg per 10 ml·kg−1) to mice twice, at 18 h before and immediately after exposure to novelty stress (Figure S1c). Food intake was measured 1 h later. Distilled water was administered to the control group. The rikkunshito dose was chosen on the basis of prior reports (Fujitsuka et al., 2011; Takeda et al., 2008; Yamada, Sadakane, et al., 2015).
To clarify the effect of estrogen receptor activation on marked hypophagia in stressed female mice, the estrogen receptor‐α antagonist 1,3‐bis(4‐hydroxyphenyl)‐4‐methyl‐5‐[4‐(2‐piperidinylethoxy)phenol]‐1H‐pyrazole dihydrochloride hydrate (MPP; Sigma‐Aldrich Co. LLC., Tokyo, Japan; 150 μg·kg−1) and the estrogen receptor‐β antagonist 4‐[2‐phenyl‐5,7‐bis (trifluoromethyl)pyrazolo[1,5‐a]pyrimidin‐3‐yl]phenol (PHTPP, Sigma, 150 μg·kg−1) (Labouesse, Langhans, & Meyer, 2015; Naderi, Khaksari, Abbasi, & Maghool, 2015) were dissolved in 0.3% DMSO/saline and administered i.p. to mice 2 h before stress. The control group was administered vehicle.
2.9. Preparation of ovariectomized (OVX) mice
A total of 72 female mice were housed in 24 cages and randomly assigned to four groups: sham control (n = 9 cages; 27 mice), sham stress (3 cages; n = 9 mice), ovariectomized control (n = 9 cages; 27 mice) and ovariectomized stress (3 cages; n = 9 mice). Eight‐week‐old C57BL/6J female mice were ovariectomized under ketamine and xylazine mixed anaesthesia (Charles River Co. Ltd., Japan). Sham mice underwent blunt dissection of the right and left psoas muscles after an incision in the back. The surrounding adipose tissue was handled, and the ovary was visually checked before the incision was closed using a clip. Four days after surgery, the clip was removed after confirming the healing of incisional wounds. After 2 weeks, we confirmed that the body weight of ovariectomized mice was significantly higher than that of sham mice (sham: 19.7 ± 0.2 g, ovariectomized: 21.9 ± 0.2 g), which is a manifestation associated with ovariectomized treatment, and then, we used mice for the experiment at 10 weeks of age.
2.10. RT‐PCR analysis
The hypothalamus was rapidly removed (within 3 min) from naïve mice after exsanguination under anaesthesia at the dark period onset, and total RNA was extracted and then reverse transcribed using TaqMan Reverse Transcription Reagents kits (Applied Biosystems, CA, USA), and mRNA expression was calculated using the ΔΔCt method. We used TaqMan gene‐specific primer/probes: Rps29, Mm02342448_gH; Ghrl, Mm00445450_m1; Npy, Mm00445771_m1; Agrp, Mm00475829_g1; Hcrt, Mm01964030_s1; Pomc, Mm00435874_m1; Mc4r, Mm00457483_s1; Lepr, Mm00440174_m1; Nucb2, Mm01137144_m1; Crh, Mm01293920_s1; Esr1, Mm00433149_m1 and Esr2, Mm00599821_m1.
2.11. In situ hybridization and immunohistochemical staining
Mouse brain was perfused with fixative (G‐Fix; Genostaff Co., Ltd., Tokyo, Japan) starting at 0.5 h after ghrelin administration/stress exposure under anaesthesia. Food was removed after ghrelin administration/stress exposure (Figure S1d). Mouse brain was dissected after 1‐h perfusion and embedded in paraffin. In situ hybridization was performed with a Reagent Kit (Genostaff) according to the manufacturer's instructions. Sections were fixed, and hybridization was performed using probes Agrp (GenBank accession number: NM_001271806.1, position: 63–754) and c‐Fos (GenBank accession number: NM_010234.2, position: 798–1482). After sections were incubated with an anti‐DIG AP conjugate (Roche Molecular Biochemicals, Mannheim, Germany), sections were counterstained with a Kernechtrot staining solution. The numbers of clearly detected c‐Fos‐positive cells were manually counted in the areas related to the ghrelin‐induced orexigenic action, such as nucleus tractus solitarius (NTS), arcuate nucleus (ARC), paraventricular nucleus (PVN), area postrema (AP), ventromedial hypothalamus (VMH), or amygdala. The total (NTS and AP) and average (ARC, PVN, VMH, and amygdala) numbers of the 4 sections per mouse were calculated.
Double‐label immunohistochemistry was performed in another set of brain sections to identify c‐Fos and estrogen receptor‐α. Tissue sections (8 μm) were de‐paraffinized with G‐Nox (Genostaff) and rehydrated through an ethanol series and PBS. Antigen retrieval was performed by microwave treatment in citrate buffer pH 6.0. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 30 min, followed by incubation with G‐Block (Genostaff) and Avidin/Biotin Blocking Kit (Vector Laboratories. Inc., CA, USA). The sections were incubated with 0.1 μg·ml−1 of anti‐estrogen receptor‐α rabbit polyclonal antibody (rabbit IgG) (Cat# sc‐542, RRID:AB_631470, lot numbers F1113, dilution 1:1000; Santa Cruz Biotechnology, TX, USA) at 4°C overnight before c‐Fos staining. One per cent G‐Block/Tris buffered saline was used as the antibody diluting buffer. They were incubated with biotin‐conjugated goat anti‐rabbit Ig (Dako Products, Agilent Technologies, CA, USA) followed by the addition of peroxidase conjugated streptavidin. Peroxidase activity was visualized by ImmPACT SG (SK‐4705; Vector) and then washed with PBS. For second staining, the sections were heat‐treated with citrate buffer pH 6.0 at 90°C for 40 min in order to remove the anti‐estrogen receptor‐α antibodies. Remaining peroxidase activity was blocked with 0.3% H2O2 in methanol for 30 min, followed by incubation with G‐Block and Avidin/Biotin Blocking Kit. The sections were incubated with 0.1 μg·ml−1 of anti‐c‐Fos rabbit polyclonal antibody (rabbit IgG) (Cat# sc‐52, RRID:AB_2106783, lot numbers A2811, dilution 1:1000; Santa Cruz Biotechnology) at 4°C overnight. Peroxidase activity was visualized by diaminobenzidine (Dojindo Laboratories, Tokyo, Japan) and washed with PBS, and then, the sections were mounted with G‐Mount (Genostaff). Various solutions were not reused. The created histological slides were numbered in order to perform blinded evaluation. The numbers of clearly detected double c‐Fos‐ and estrogen receptorα‐positive cells (purple spot, Figure 5a) in the NTS were manually counted in 4 sections per mouse by a histopathologist who have no sample information. The immuno‐related procedures used comply with recommendations made by the British Journal of Pharmacology (Alexander et al., 2018).
FIGURE 5.
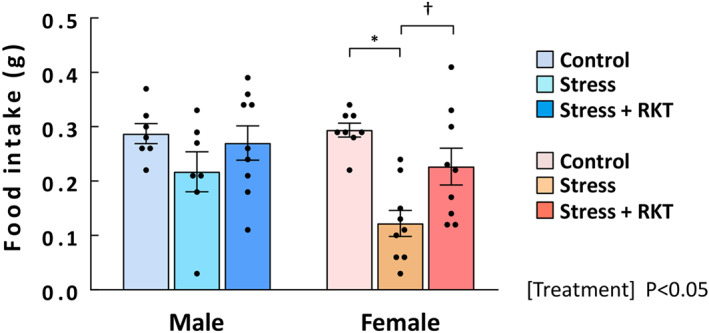
The effect of rikkunshito (RKT, ghrelin enhancer) on food intake. The effect of the endogenous ghrelin enhancer RKT at a dose of 1000 mg·kg−1 in stressed mice was evaluated, and 1‐h cumulative food intake was measured after RKT administration. Three mice were excluded due to an obvious researcher's technical mistake. Male control: n = 7 (excluded: 1); male stress: n = 7 (excluded: 2); male stress + RKT: n = 9; female control: n = 8; female‐other groups: n = 9. Results are shown as means ± SEM. *P < 0.05 vs. control; † P < 0.05 vs. stress in two‐way ANOVA followed by the Tukey–Kramer post hoc test
2.12. Data and statistical analyses
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). Statistical analysis was done only on datasets of n ≥ 5. When experiments are novel, it is difficult to perform a priori sample size calculations (Curtis et al., 2018) because the effect size and variance are unknown. Therefore, for the animal experiments, we calculated the appropriate sample sizes using the expected variance and effect size that were estimated from the previous experiments using similar methods. Animals were randomly allocated to each experimental group and matched by average body weight and food intake. Mice with wounds from fighting in the cage and mice influenced by an obvious researcher's technical mistake were excluded. These totalled eight mice. The final group sizes for each experiment are provided within the figure legends. When normalizing the increase rates in food intake to compare between male and female mice, each relative rate of increase was divided by the mean value of male control mice. According to the guidelines (Curtis et al., 2018), the y axis is labelled “fold control mean in food intake”. Statistical analyses of these data were performed using parametric tests because the data distribution is not changed by this normalization. Histological analysis was performed in a blinded manner. All other experiments were not performed in a blinded manner since the measurements could not be influenced by personal bias. Statistical analyses between two groups were performed using unpaired Student's t‐test. Differences in multiple group mean values were assessed by two‐way or three‐way ANOVA followed by the Dunnett, Tukey–Kramer, or Bonferroni post hoc test. Two‐way repeated measures ANOVA was used to compare changes over time in control and treated mice. A post hoc test was applied only when the F value reached significance, and there was no significant inhomogeneity of variance. Statistical analysis of these data was carried out using GraphPad Prism (GraphPad Software Inc., RRID:SCR_002798). Data are expressed as the mean ± SEM for each group; n indicates the number of independent experiments; P < 0.05 was considered statistically significant.
2.13. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY http://www.guidetopharmacology.org (Alexander et al., 2019).
3. RESULTS
3.1. Effects of acylated ghrelin on food intake and body weight in male and female naïve mice
To clarify the sex differences in the reactivity to ghrelin, the effect on exogenous ghrelin administration on food intake was first examined. The main effect of ghrelin was significant in female mice but not male mice. The significant interaction of ghrelin × time was observed in females. In female mice, ghrelin significantly increased food intake (Figure 1a). Two‐way ANOVA within males and females on food intake at 1 h showed significant effects of ghrelin and the interaction of ghrelin × sex (Figure 1b); i.p. injection of acylated ghrelin significantly increased 1‐h food intake in female mice at all doses (50, 150 and 500 nmol·kg−1), although the food intake in male mice did not significantly increase at 50 nmol·kg−1. The extent of the ghrelin‐induced food intake increase at 1 h was significant, with a main effect of sex and it was significantly higher in female mice at all doses than in male mice (Figure 1c). Body weight at 24 h after ghrelin administration (500 nmol·kg−1) significantly increased in female mice compared with control mice, but no interaction was observed (Figure 1d). No sex differences were observed in basal plasma ghrelin levels (males: 60.99 ± 8.02 fmol·ml−1; females: 62.91 ± 9.22 fmol·ml−1; figure not shown) before acylated ghrelin administration.
FIGURE 1.
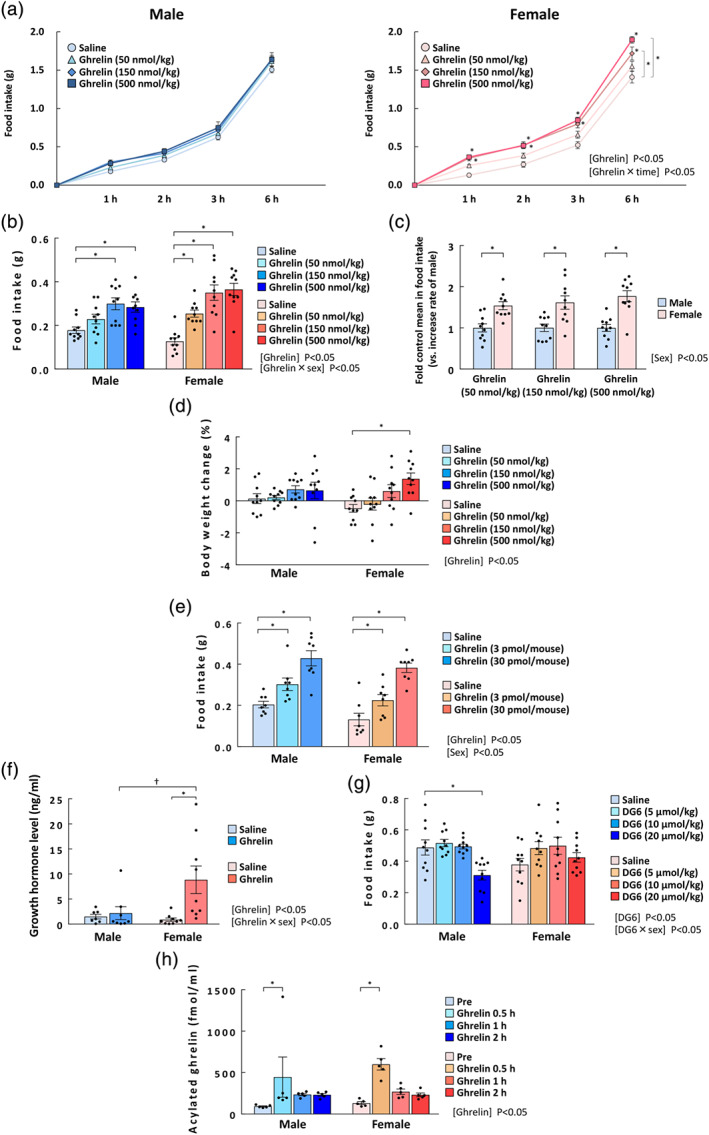
Effects of exogenous acylated ghrelin administration on food intake, body weight change, and plasma hormone levels in male and female naïve mice. (a) Sequential change in food intake, *P < 0.05 vs. saline in two‐way repeated measures ANOVA followed by the Dunnett post hoc test; (b) food intake at 1 h; (c) the relative increase in food intake at 1 h; and (d) the percentage of body weight change in male and in female naïve mice after i.p. injections of acylated ghrelin at 50, 150, or 500 nmol·kg−1. The cumulative food intake was measured at 1, 2, 3, and 6 h after ghrelin administration, and the body weight was measured before and at 24 h after ghrelin administration. n = 10 per group. (e) Effect of i.c.v. administration of ghrelin (3 and 30 pmol per mouse head) on food intake at 1 h; n = 8. (f) Plasma growth hormone levels; plasma levels were measured at 0.5 h after ghrelin administration (50 nmol·kg−1, i.p.). Mice with wounds from fighting in cages were excluded. Male saline: n = 7 (excluded: 2); male ghrelin: n = 8 (excluded: 1). Female: n = 9 per group. (g) Effect of [D‐Lys3]‐GHRP‐6 (DG6) on 3‐h food intake in naïve mice, n = 10 per group. (h) Plasma acylated ghrelin levels measured before and at 0.5, 1, and 2 h after ghrelin administration (50 nmol·kg−1, i.p.). n = 5 per group. Results are shown as means ± SEM. *, † P < 0.05 in two‐way ANOVA followed by the Dunnett or Tukey–Kramer post hoc test
Ghrelin was administered to the i.c.v. to clarify whether the different responses between females and males were peripherally dependent. Each main effect was significant, but no interaction was observed. There were no sex differences in food intake increase at 1 h between male and female mice in response to i.c.v. ghrelin administration (Figure 1e).
3.2. Effect of acylated ghrelin on plasma hormone levels in male and female naïve mice
Acylated ghrelin at the lowest dose (50 nmol·kg−1) was administered to detect plasma growth hormone levels after 0.5 h. The effects of ghrelin and the interaction of ghrelin × sex (on plasma growth hormone levels were significant. Plasma growth hormone levels in male mice did not change after we administered acylated ghrelin, however growth hormone was significantly elevated in female mice (Figure 1f).
The effects of i.p. administration of the ligand‐dependent ghrelin antagonist DG6 were significant. The interaction of DG6 × sex was significant, i.p. administration of DG6 at the highest dosage significantly reduced food intake in male mice but not in female mice (Figure 1g).
To rule out the possibility that the observed higher ghrelin responsiveness of female mice was due to sex differences in the plasma clearance of peripherally administered acylated ghrelin, we investigated the changes in peripheral ghrelin concentration after exogenous administration. The main effect of ghrelin was significant, but there were no effects of sex or interaction. Peripheral ghrelin concentrations increased after ghrelin administration (50 nmol·kg−1), peaked at 0.5 h and then decreased with no noticeable differences between male and female mice (Figure 1g). Sex differences were not observed in blood acylated ghrelin concentration at 1 or 2 h after ghrelin administration.
3.3. Effect of acylated ghrelin on ghrelin signalling in male and female naïve mice
Because we observed a higher response to exogenous ghrelin in naïve female mice independent of pharmacodynamics, we hypothesized that ghrelin signal transduction would be up‐regulated after ghrelin administration in female mice. We evaluated c‐Fos mRNA expression in the NTS, ARC, PVN, AP, VMH and amygdala of mice after acylated ghrelin administration. To see the ghrelin signals, we performed in situ hybridization for Agrp mRNA in the ARC using serial sections following administration of 50 nmol·kg−1 ghrelin (Figure 2a). We confirmed that most Agrp‐positive cells overlapped with c‐Fos‐positive cells in the ARC (double‐positive cells/total c‐Fos‐positive cells: 88.5 ± 3.2% for males and 91.8 ± 0.2% for females). No c‐Fos or Agrp mRNA was detected with the sense probe (data not shown). The effects of ghrelin and sex and the interaction of ghrelin × sex on the number of Agrp mRNA‐positive cells in the ARC were significant (Figure 2a). The number of Agrp mRNA‐positive cells in the ARC was significantly higher than in saline mice among the ghrelin‐treated female mice but not among the male mice. Ghrelin‐treated females had significantly more positive cells than ghrelin‐treated males. Ghrelin administration to male naïve mice modestly increased the number of c‐Fos‐mRNA positive cells (4.9‐fold increase vs. saline; Figure 2b). In female naïve mice, c‐Fos‐positive cells in the ARC were markedly increased by ghrelin administration (18.2‐fold vs. saline; Figure 2b). This rate of increase was significantly different between males and females. The effects of ghrelin and sex and the interaction of ghrelin × sex on the number of c‐Fos‐positive cells in the ARC were significant. Ghrelin‐treated females had significantly more positive cells than ghrelin‐treated males. In the NTS (area shown in Figure S2), the effects of ghrelin and the interaction of ghrelin × sex on the number of c‐Fos‐positive cells were significant (Figure 2c). The number of c‐Fos‐positive cells in the NTS was significantly increased by ghrelin administration in male and female naïve mice. Specifically, increases in males and females were 2.8‐ and 11.4‐fold, respectively, which were significantly different. Similarly, in the PVN, c‐Fos mRNA expression was significantly increased by ghrelin stimulation in females but not in males (Figure 2d). There was no sex difference in ghrelin responsiveness in the AP, VMH or amygdala (Figure 2e–g).
FIGURE 2.
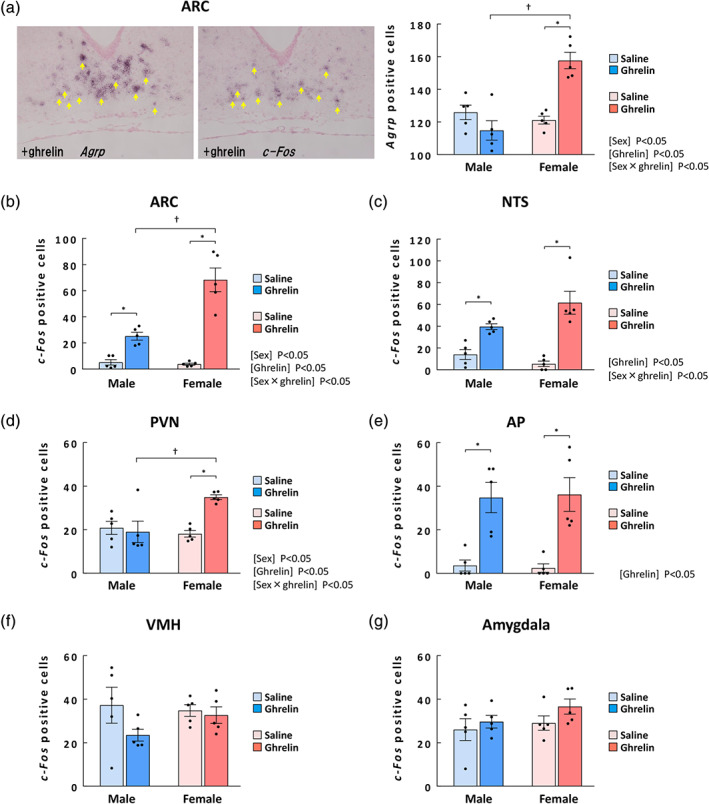
Effects of acylated ghrelin administration on ghrelin signalling in male and female naïve mice. (a) Representative images of Agrp‐positive cells (left panel) and c‐Fos‐positive cells (middle panel) indicated by yellow arrows in the ARC at 0.5 h after administration of 50 nmol·kg−1 ghrelin. Right graph: the number of Agrp‐positive cells in the ARC of males and females; (b) number of c‐Fos‐positive cells in the ARC of males and females; (c) number of c‐Fos‐positive cells in the NTS of males and females; (d) number of c‐Fos‐positive cells in the PVN of males and females; (e) number of c‐Fos‐positive cells in the AP of males and females; (f) number of c‐Fos‐positive cells in the VMH of males and females; and (g) number of c‐Fos‐positive cells in the amygdala of males and females. n = 5 per group. Results are shown as means ± SEM. *P < 0.05 vs. each saline, † P < 0.05 in two‐way ANOVA followed by the Tukey–Kramer post hoc test
3.4. Gene expression in male and female naïve mice
Gene expression in male and female hypothalami was compared by RT‐PCR. Basal Npy, Agrp, Lepr and Esr1 mRNA expression was significantly higher in female mice than in male mice (Table 1). Other appetite‐associated genes, including Crh, Preproghrelin, Hcrt, Pomc, Mc4r and Nucb2, were not different between male or female mice.
TABLE 1.
Expression levels of hypothalamic genes
| Gene name | Male | Female |
|---|---|---|
| Prepro‐ghrl | 1.00 ± 0.06 | 0.97 ± 0.04 |
| Npy | 1.00 ± 0.03 | 1.19 ± 0.02 * |
| Agrp | 1.00 ± 0.08 | 1.31 ± 0.10 * |
| Hcrt | 1.00 ± 0.06 | 0.89 ± 0.05 |
| Pomc | 1.00 ± 0.05 | 1.19 ± 0.17 |
| Mc4r | 1.00 ± 0.03 | 0.97 ± 0.03 |
| Lepr | 1.00 ± 0.03 | 1.12 ± 0.05 * |
| Nucb2 | 1.00 ± 0.03 | 0.93 ± 0.02 |
| Crh | 1.00 ± 0.02 | 1.06 ± 0.02 |
| Esr1 | 1.00 ± 0.04 | 1.34 ± 0.02 * |
| Esr2 | 1.00 ± 0.04 | 0.90 ± 0.04 |
Note: The mRNA of hypothalamus was measured in naïve mice at the dark period onset. n = 7–9 per group. Results are shown as means ± SEM.
P < 0.05 vs. male by Student's t‐test.
3.5. Effect of novelty stress on food intake and plasma hormone levels
Food intake after novelty stress exposure was not significantly different between male mice and control mice (Figure 3a, left). In contrast, the effects of stress and the interaction of stress × time on food intake in female mice were significant. Food intake in female mice exposed to novelty stress markedly decreased at 3 and 6 h compared with that in control mice (Figure 3a, right). When the food intake of males and females at 3 h was directly compared each main effect (stress and sex) was significant, but no interaction was observed. However, females had significantly reduced food intake due to stress exposure, whereas males did not (Figure 3b). The effects of sex and the interaction of stress × sex on the 0–24 h body weight change ratio were significant (Figure 3c). The body weight change of stress‐exposed females was significantly lower than that of stress‐exposed males.
FIGURE 3.
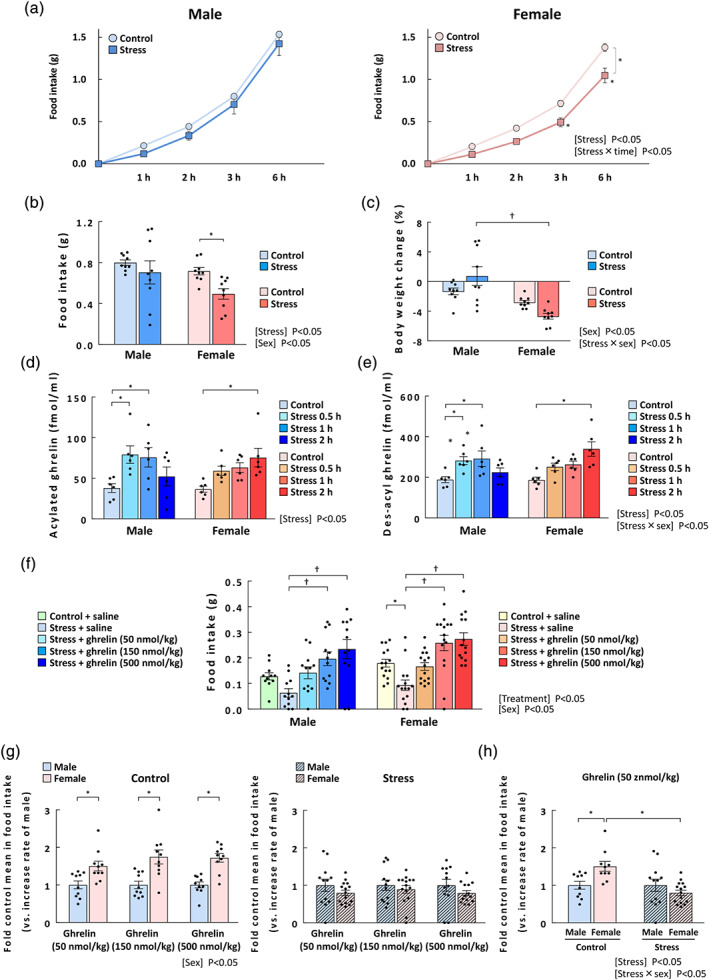
Changes in cumulative food intake, body weight, and plasma ghrelin and the effect of ghrelin on food intake in male and female stressed mice. (a) Sequential change in food intake, *P < 0.05 in two‐way repeated measures ANOVA followed by the Bonferroni post hoc test; (b) food intake at 3 h; and (c) body weight changes (%). The cumulative food intake was measured at 1, 2, 3, and 6 h after exposure to novelty stress. The body weight was measured before and at 24 h after stress exposure. n = 9 per group. (d) Plasma acylated ghrelin levels; (e) plasma des‐acyl ghrelin levels. The blood samples were collected 0.5, 1, and 2 h after exposure to novelty stress. n = 6 per group. (f) Effects of ghrelin treatments on stressed mice. The mice were i.p. administered acylated ghrelin at 50, 150, or 500 nmol·kg−1 immediately after stress exposure, and the 0.5‐h cumulative food intake was measured after ghrelin administration. Two mice were excluded due to an obvious researcher's technical mistake. Male control + saline: n = 11 (excluded: 1); male‐other groups: n = 12; female stress + ghrelin 500 nmol·kg−1: n = 14 (excluded: 1); female‐other groups: n = 15. (g) The relative increase in food intake after ghrelin administration in control mice (left) and stressed mice (right); (h) the relative increase in food intake after ghrelin administration at a dose of 50 nmol·kg−1 in male and female mice. Results are shown as means ± SEM. *P < 0.05 vs. each control or male, † P < 0.05 in two‐way ANOVA followed by the Dunnett, Bonferroni, or Tukey–Kramer post hoc test
The effect of stress on peripheral acylated ghrelin levels was significant. Ghrelin levels in male mice peaked at 0.5 h and were restored to basal levels at 2 h after stress exposure. In contrast, ghrelin levels in female mice peaked after 2 h, indicating a clear delay (Figure 3d). At 6 h after stress exposure, acylated ghrelin levels in female mice were restored to levels similar to those in control mice (control: 94.28 ± 19.55 fmol·ml−1; stress: 98.78 ± 10.21 fmol·ml−1, figure not shown). Des‐acyl ghrelin levels demonstrated the same pattern and the effects of stress and the interaction of stress × sex were significant. (Figure 3e). No further decrease in food intake was observed even when DG6 was administered under stress (Figure S3). Plasma leptin levels showed no differences between male and female mice (Figure S4). The extent and dynamics of the plasma corticosterone increase in female mice were similar to those in male mice, but a significant increase was only observed in female mice at 0.5 h (Figure S4).
3.6. Effect of acylated ghrelin on food intake in stressed mice
To clarify the sex difference in the orexigenic action of exogenous ghrelin in control or stressed mice, we evaluated the effect of acylated ghrelin on food intake in stressed mice. In stressed mice, each main effect on the 0.5‐h food intake was significant (treatment of stress + ghrelin, but no interaction was observed (Figure 3f). At 0.5 h, food intake tended to decrease in male mice and significantly decreased in female mice. Food intake under stressed conditions was increased by ghrelin administration (150 or 500 nmol·kg−1) to almost the same level in both males and females, indicating no sex differences. Additionally, the extent of the increase in 0.5‐h food intake by ghrelin administration was significantly greater in naïve female mice than in naïve male mice and the main effect of sex was significant (Figure 3g). In contrast, the stress‐loaded female mice displayed a tendency towards a decrease in relative increase in food intake due to ghrelin administration compared with male mice. To evaluate the influence of sex and stress on the ghrelin‐induced relative increase in food intake and the results are shown in Figure 3h. The effect of stress was significant, while the effect of sex was not significant, although the effect of interaction was significant. In naïve female mice administered ghrelin, the relative increase in 0.5‐h food intake was 1.50‐fold higher than in male mice that were administered ghrelin (50 nmol·kg−1). However, the relative increase in food intake in the stress‐loaded female mice showed a decrease compared with that in males and it decreased significantly compared with that in control female mice.
3.7. Changes in c‐Fos mRNA expression in the NTS, ARC, PVN, amygdala, VMH, and AP after acylated ghrelin administration in stressed mice
Since orexigenic action by exogenous ghrelin administration was obviously attenuated in stress‐loaded female mice, we evaluated ghrelin signalling after ghrelin administration in stressed mice. Representative images of c‐Fos by in situ hybridization are shown in Figure 4. The numbers of c‐Fos‐positive cells in the NTS, ARC, PVN, AP, VMH and amygdala did not show significant changes after stress exposure (Figure 4 and Table S1). The effect of the interaction of sex × stress × ghrelin on the number of c‐Fos‐positive cells in the NTS was significant. The expression of c‐Fos in the NTS was significantly increased by ghrelin administration in stressed male mice but not in female mice (Figure 4c). The effect of the interaction of sex × stress × ghrelin on the number of c‐Fos‐expressing cells in the ARC was significant. In stressed mice, c‐Fos expression in the ARC significantly increased after ghrelin administration in both males (10.4‐fold) and females (8.0‐fold; Figure 4d). In females, c‐Fos expression in ghrelin‐treated mice under stress was significantly lower than in control ghrelin‐treated mice. The effects of the interaction of sex × stress, sex × ghrelin and stress × ghrelin (on the number of c‐Fos‐expressing cells in the PVN were significant. In the PVN, the increase in the number of c‐Fos‐positive cells by ghrelin treatment in female control mice was not observed under stressed conditions (Figure 4e). Overall, the increase in the number of c‐Fos‐positive cells in the NTS, ARC and PVN by ghrelin administration was attenuated by stress exposure in female mice but not in male mice. The number of c‐Fos‐expressing cells in the AP, VMH and amygdala in the stressed groups was not changed by ghrelin administration in males or females (Table S1).
FIGURE 4.
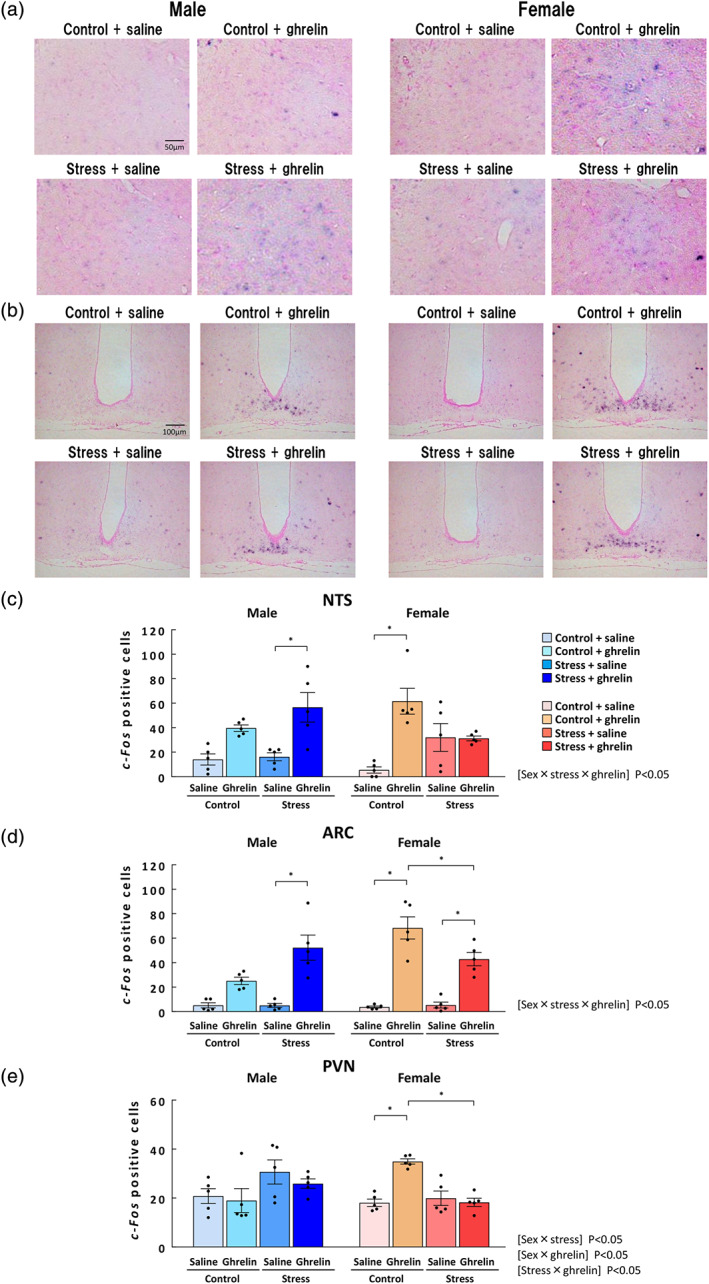
C‐Fos‐positive cells at 0.5 h after administration of ghrelin administration/stress exposure in male and female mice. (a) Representative images of c‐Fos‐positive cells (blue) in the NTS and (b) in the ARC; (c) the number of c‐Fos‐positive cells in the NTS of males/females; (d) number of c‐Fos‐positive cells in the ARC of males/females; and (e) number of c‐Fos‐positive cells in the PVN of males/females. The mice were i.p. administered acylated ghrelin at doses of 50 nmol·kg−1 immediately after stress exposure, and the brain was collected 0.5 h after the ghrelin administration. n = 5 per group. Results are shown as means ± SEM. *P < 0.05 in two‐way ANOVA followed by the Tukey–Kramer post hoc test
3.8. Effect of ghrelin enhancer rikkunshito on food intake in stressed mice
In female mice, our findings suggested that peripheral ghrelin signalling was attenuated by stress. Rikkunshito, the endogenous ghrelin signal enhancer, was administered, and food intake was measured. The main effect of stress + rikkunshito treatment on food intake was significant (F(2, 43) = 9.07), but no interaction was observed. Rikkunshito administration to stressed male mice caused no increase in food intake. In contrast, in stressed female mice, rikkunshito significantly improved the decrease in food intake (Figure 5).
3.9. Involvement of oestrogen in stressed female mice
The plasma oestrogen concentration showed no significant change after stress exposure in female mice (Figure 6a). Ovariectomized treatment of naïve female mice significantly increased food intake over that in the sham groups (Figure 6b). Meanwhile, the stress load in ovariectomized mice reduced food intake compared to in the ovariectomized control group. The decline in food intake due to stress was not suppressed in ovariectomized mice. Next, estrogen receptor antagonists were administered to stress‐loaded mice and its effect was examined. The decreased food intake in stress‐loaded female mice was significantly inhibited by administration of MPP, an estrogen receptor‐α antagonist, whereas administration of PHTPP, an estrogen receptor‐β antagonist, failed to inhibit it (Figure 6c). Food intake in stress‐loaded male mice was not affected by administration of estrogen receptor‐α antagonist MPP (Figure S5).
FIGURE 6.
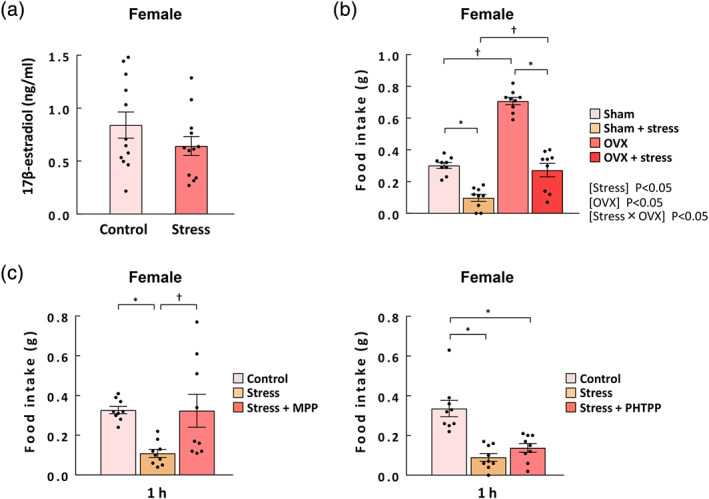
Involvement of oestrogen in stressed female mice. (a) The plasma estrogen levels in female mice under stress conditions. The blood samples were collected at 0.5 h after exposure to novelty stress. n = 12 per group. (b) The effect of ovariectomy (OVX) on 1‐h food intake in control and stressed female mice. n = 9 per group. (c) Food intake at 1 h after exposure to novel stress in female mice. MPP (estrogen receptor (ER)‐α antagonist, 150 μg·kg−1, i.p.) and PHTPP (ERβ antagonist, 150 μg·kg−1, i.p.) were administered 2 h before stress exposure. n = 9 per group. Results are shown as means ± SEM. *P < 0.05 vs. each control; † P < 0.05 by the Tukey–Kramer test
3.10. Double staining for c‐Fos and estrogen receptor‐α proteins in the NTS under stressed conditions
It was found that the load of stress is related to activation of estrogen receptorα and a decrease in feeding. estrogen receptor‐α was expressed in the NTS region, to which most abdominal vagal afferents project (Figure 7a). Significantly more estrogen receptor‐α‐positive cells were observed in females than in males (male: 187.0 ± 4.6 positive cells; female: 264.6 ± 6.5 positive cells, figure not shown). Each main effect of stress and sex and the interaction of stress × sex on the percentage of double‐positive cells (estrogen receptor‐α + c‐Fos/ estrogen receptor‐α) were significant. The percentage of double‐positive cells was significantly increased by stress exposure in female mice but not in males (Figure 7b).
FIGURE 7.
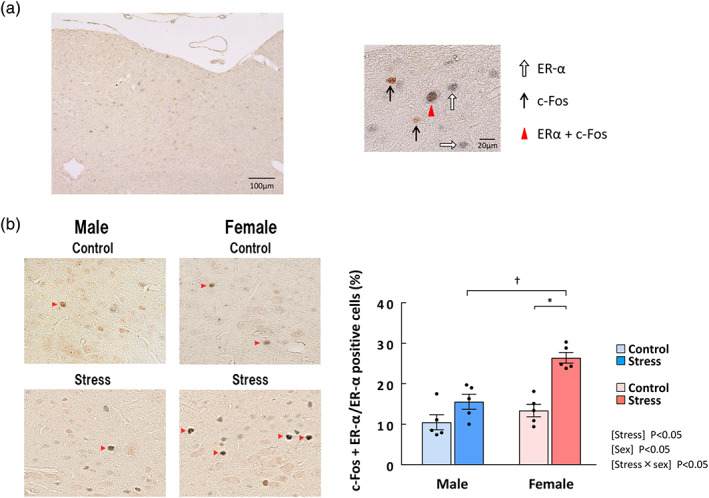
The percentage of estrogen receptor (ER)‐α/c‐Fos‐double‐positive cells among ERα‐positive cells in the NTS after stress exposure in male and female mice. (a) Left: the area containing NTS after double staining for ERα and c‐Fos. Right: Typical cells showing immunoreactivity for ERα (white arrow), c‐Fos (black arrow), and both proteins (red triangle). (b) Representative ERα/c‐Fos‐double‐positive cells in the NTS (left) and the percentage of ERα/c‐Fos‐double‐positive cells among ERα‐positive cells in the NTS of male and female mice (right). The red arrowhead indicates ERα/c‐Fos‐double‐positive cells. Mouse brains were collected 0.5 h after the onset of stress exposure. n = 5 per group. Results are shown as means ± SEM. *P < 0.05 in two‐way ANOVA followed by the Tukey–Kramer post hoc test
4. DISCUSSION AND CONCLUSIONS
There have been no other studies on the sex differences in ghrelin responsiveness under stressed conditions so far. We found that the effects of acylated ghrelin on food intake, weight gain, plasma growth hormone release, Agrp gene expression in the ARC, along with c‐Fos positivity in the NTS, ARC and PVN were noticeably higher in female mice than in male mice under naïve conditions. We also confirmed that these sex differences were not due to distinct differences in the pharmacokinetics of ghrelin. The expected strong change in neuronal activity following administration of ghrelin in female mice was abolished in the NTS when the mice were going under psychological stress. These results suggest that blockade of ghrelin signalling at the NTS occurs only in female mice during psychological stress, which may contribute to the marked and sustained reduction in feeding behaviour observed in stressed female mice.
We first demonstrated that peripheral ghrelin administration to naïve female mice clearly increased food intake over that in males. Peripherally administered ghrelin has been shown to partially accumulate in the brain and cause a physiological response (Perello et al., 2019). Our results showed no sex difference in the effect of i.c.v. administration of ghrelin (limited to the dose used). This result may rule out the possibility that ghrelin accumulation in the brain induced sex differences in the orexigenic effects of ghrelin in naïve mice. However, the site where peripherally administered ghrelin transfers to the brain does not fully coincide with the site reached by i.c.v. administration. Further, the sex difference in ghrelin transfer to the brain has not been studied, thus further research is required. These findings are in agreement with a previous study showing no sex difference were observed with regard to food intake or energy metabolism changes induced by ghrelin injection directly to the hypothalamus (Currie et al., 2005), although another study reported the that male rats were significantly more sensitive to the orexigenic effects of ghrelin (Clegg et al., 2007). There is no clear reason for this discrepancy with our results. In the Clegg et al. (2007) they used rats and the experiments were conducted in the light phase, which is the dormant period for rodents. Further, the comparison was performed between female rats subjected to the sham operation and naïve male rats, which is different from our experiments. This protocol may contain some bias in sex differences in food intake changes induced by peripheral administration of ghrelin. Our study was performed in the dark phase, the active phase of the mice, without interfering with diapause and without surgery. Additionally, we did not restrict food intake to measure feeding. Thus, in the present study the food intake measurement was performed in a more natural state for mice, which in our opinion made makes the results our study more conclusive.
Peripheral ghrelin binds to growth hormone secretagogue receptor (GHSR)‐1a, now known as the ghrelin receptor, located on vagal nerve termini to activate orexigenic neuropeptide Y/agouti‐related protein neurons in the hypothalamic ARC via the NTS (Depoortere, 2009; Wang, Saint‐Pierre, & Tache, 2002). In the present study, we found that basal Npy and Agrp mRNA expression in the hypothalamus was significantly higher in naïve female mice than in male mice. In addition, DG6, a ligand‐dependent antagonist, clearly reduced food intake in naïve male mice but did not affect food intake in female mice. Our explanation for these results is that ghrelin receptor may have a higher affinity for endogenous ghrelin in female mice and its signal may be less likely to be antagonized by DG6 in these female mice. Our findings may support the conclusion that the affinity of regular physiological ghrelin to ghrelin receptor and/or the efficacy of signal transduction is higher in female mice.
We found that most c‐Fos expression overlapped with Agrp expression in the ARC after ghrelin administration, and we measured c‐Fos‐positive cells as indicators of ghrelin signalling. When ghrelin was administered to naïve mice, the number of c‐Fos‐positive cells in the NTS clearly increased in both sexes, but the rate of increase in female mice was greater. The same result was also obtained in the ARC. In addition, the number of c‐Fos‐positive cells in the PVN, which is the destination of neuropeptide Y/agouti‐related protein neurons of the ARC, was significantly increased in naïve female but not in male mice administered ghrelin. This result may reflect the intensity and/or transmission speed of the ghrelin signal from the periphery. Interestingly, the activity of ghrelinergic neurons in areas that are not involved in orexigenic effects, such as the VMH (Merkestein et al., 2014) and amygdala (Jensen et al., 2016), was not affected by the peripheral administration of ghrelin in either male or female mice. Since AP forms a dense vascular bed and has a deficient blood–brain barrier, it senses hormonal changes in peripheral blood. After ghrelin administration, c‐Fos expression in the AP increased to the same extent in both sexes. From this, it was thought that there was no sex difference in the movement of molecules from peripheral blood to the AP. This suggests that sex differences in pharmacological action of peripheral ghrelin occur mainly in the vagal nerve pathways, NTS and ARC, and our findings are in good agreement with the results of food intake, relative increase in food intake, and growth hormone secretion following exogenous ghrelin administration.
The effect on food intake after psychological stress may also have a mild sex difference. We found in this study that there was a sex difference in changes in plasma ghrelin level after stress loading. Peripheral ghrelin has been reported to increase in stress models (Stengel et al., 2011). Increased peripheral ghrelin secretion after stress is likely to be part of the stress‐coping mechanism (Spencer, Emmerzaal, Kozicz, & Andrews, 2015), and the sharp increase in peripheral acylated ghrelin level may play a role in antagonizing stress‐induced anorexia in male mice. In female mice, the increase in plasma ghrelin level after stress was delayed compared to males. In addition, despite the increase in endogenous ghrelin level at 2 h after stress loading in female mice, no increase in food intake was observed. Moreover, we found that the high responsiveness of exogenous ghrelin in naïve female mice was abolished by stress loading. These results support our hypothesis that delayed ghrelin secretion may not be the direct cause of reduced food intake due to stress in female mice, and reduced food intake due to stress may be caused by decreased ghrelin responsiveness in female mice. Rikkunshito is a medicine that has been reported as an endogenous ghrelin enhancer (Fujitsuka et al., 2011,2016). Ghrelin signal enhancement by rikkunshito administration improved the reduction in food intake only in female mice. These results strongly suggest that stress‐loaded female mice are in a state of ghrelin resistance, in which the ghrelin signal is impaired.
It is well known that stress enhances neuron activity in the PVN (Kovacs, Schiessl, Nafz, Csernus, & Gaszner, 2018; Lin et al., 2018). Since c‐Fos expression in the PVN did not significantly increase by stress, it may indicate that novelty stress is a very mild stress and that neural activation in the PVN was minor or transient. Further assessments should be done over a more detailed and careful time course. The PVN is also a critical area that integrates the ghrelin signal with various neuropsychological factors (Dos‐Santos, Reis, Perello, Ferguson, & Mecawi, 2019). However, no in vivo studies have studied neuronal activity in the PVN when combined with peripherally administered ghrelin and stress loading. We observed that the increase in the number of c‐Fos‐positive cells in the PVN after ghrelin administration was abolished by stress exposure in female mice. This result cannot exclude the possibility that stress and ghrelin may directly interact in the second‐order neurons in the PVN. However, similar results were obtained in the ARC (the increase rate in the number of c‐Fos‐positive cells by ghrelin administration, female control: 18.2‐fold; female stress: 8.0‐fold, Figure 4d). In the NTS, the trend was even more pronounced. We considered that these results suggest that ghrelin signalling from the periphery is disturbed at the NTS or more distal sites in stressed female mice, resulting in ghrelin resistance and food intake reduction.
The relationship between the sex hormone estrogen and feeding behaviour has been demonstrated (Asarian & Geary, 2013). However, no involvement of estrogen receptors under acute psychological stress conditions has been reported. ovariectomized treatment significantly increased food intake in female mice. Thus, eating behaviour in naïve female mice is always negatively controlled by ovary‐derived oestrogen. In our results, no statistical significance was found in peripheral estrogen levels between naïve control and stressed female mice. Furthermore, the reduction in food intake due to stress was similar in ovariectomized mice compared to the sham group, indicating that ovarian oestrogen may not be involved in reducing food intake due to female mouse‐specific stress. The reversal effect of the estrogen receptor‐α antagonist MPP on the decrease in food intake in stressed female mice could be the result of blocking non‐peripheral estrogen receptors. MPP is thought to also act on receptors in the brain (Xing et al., 2018) and has an anorexigenic effect in mice depleted of peripheral oestrogen by ovariectomized (Santollo & Eckel, 2009). Thus, we believe that the stress‐induced feeding reduction in female mice is probably mediated by non‐peripheral estrogen receptor‐α activation. However, this information alone is not sufficient for us to conclude that estrogen receptor‐α in the brain is a target. To further confirm that hypothesis, more evidence will be required.
The NTS is an termination site of many afferent nerves from the periphery and the CNS (Valassi, Scacchi, & Cavagnini, 2008). Estrogen receptor‐α is highly expressed in the NTS (Asarian & Geary, 2007) and its activation has negative effects on feeding behaviour and ghrelin reactivity (Asarian & Geary, 2013). However, there is no report that anorexia due to stress is related to estrogen receptor‐α positive neuronal activation in the NTS. In our study, the ratio of estrogen receptor‐α/c‐Fos‐double‐positive cells to total estrogen receptor‐α‐positive cells in the NTS was significantly increased by stress in females but not in males. Administration of estrogen benzoate onto the surface of the hindbrain over the NTS decreased food intake in ovariectomized rats (Thammacharoen, Lutz, Geary, & Asarian, 2008). In addition, it is generally accepted that the activation of estrogen receptors‐α‐positive neurons in the NTS, which receive and processe gastrointestinal satiety signals such as CCK, reduces meal size, food intake and body weight. Estrogen receptor‐α in the NTS have been shown to synergize with CCK to enhance satiety (Asarian & Geary, 2007). Further, CCK injected i.p. causes an increase in the number of c‐Fos‐ and estrogen receptor‐α‐positive cells in the NTS (Thammacharoen et al., 2008). Thus, central estrogen receptor‐α activation plays an important role in decreasing food intake by inhibiting peripheral orexigenic signalling in the NTS via the potentiating action of CCK. Indeed, we have confirmed that an estrogen receptor‐α antagonist but not an estrogen receptors‐β antagonist prevented the decrease in food intake in female mice due to stress. This result strongly supports the involvement of estrogen receptors‐α in food intake reduction by novelty stress. It is conceivable that novelty stress may potentiate estrogen receptors‐α activation in the hindbrain and enhance the satiety signal, resulting in ghrelin resistance in female mice, although the precise mechanism is unclear and needs to be determined in future experiments. We present our hypothesis in the schema in Figure S6.
The limitations of our study are as follows:‐ (a) the sex differences in ghrelin receptor function in each part of the brain are not clearly defined; (b) there is no direct confirmation of the sex differences in ghrelin signal via the vagal afferents after vagotomy; (c) the post‐stress central nervous system activation due to ghrelin administration has not been evaluated over time and (d) no direct evidence was found to indicate the location of the estrogen receptors‐α involved i.e. whether they were in the CNS or the periphery or at both sites. The above limitations should be overcome before elucidating sex differences in stress‐mediated eating abnormalities such as anorexia nervosa.
In conclusion, peripheral ghrelin reactivity in naïve mice is higher in females than in males. Ghrelin signal suppression occurs during psychological stress only in female mice, possibly through the activation of estrogen receptors‐α‐positive neurons in the NTS, by blocking afferent ghrelin signalling at this locus. We propose a mechanism by which ghrelin signal resistance from the periphery is involved in the sex differences in stress‐mediated hypophagia.
AUTHOR CONTRIBUTIONS
C.Y. and T.H. contributed to the study concept and design; C.Y., S.I., M.N., and T.H. contributed to the acquisition of data; C.Y., T.H., and H.T. analysed and interpreted the data; C.Y., T.H., and H.T. drafted the manuscript; C.Y. performed the statistical analysis; and T.H. and H.T. supervised the study.
CONFLICT OF INTEREST
This research was funded by Tsumura & Co. H.T. received grant support from Tsumura & Co. C.Y., S.I., M.N., and T.H. are employed by Tsumura & Co. The authors have declared no other competing financial interests in relation to this work.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Natural Product Research, Design and Analysis, Immunoblotting and Immunochemistry and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Data S1 Supporting information
ACKNOWLEDGEMENTS
We would like to express deep appreciation to Genostaff Co., Ltd. for helping with the in situ hybridization and immunohistochemical techniques used in this study. We thank Y. Saegusa, S. Mogami, H. Sekine, and Y. Harada, Tsumura & Co., for their technical assistance.
Yamada C, Iizuka S, Nahata M, Hattori T, Takeda H. Vulnerability to psychological stress‐induced anorexia in female mice depends on blockade of ghrelin signal in nucleus tractus solitarius. Br J Pharmacol. 2020;177:4666–4682. 10.1111/bph.15219
REFERENCES
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Pawson, A. J. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa, A. , Inui, A. , Kaga, T. , Katsuura, G. , Fujimiya, M. , Fujino, M. A. , & Kasuga, M. (2003). Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut, 52, 947–952. 10.1136/gut.52.7.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa, A. , Inui, A. , Kaga, T. , Yuzuriha, H. , Nagata, T. , Ueno, N. , … Kasuga, M. (2001). Ghrelin is an appetite‐stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology, 120, 337–345. 10.1053/gast.2001.22158 [DOI] [PubMed] [Google Scholar]
- Asarian, L. , & Geary, N. (2007). Estradiol enhances cholecystokinin‐dependent lipid‐induced satiation and activates estrogen receptor‐alpha‐expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology, 148, 5656–5666. 10.1210/en.2007-0341 [DOI] [PubMed] [Google Scholar]
- Asarian, L. , & Geary, N. (2013). Sex differences in the physiology of eating. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 305, R1215–R1267. 10.1152/ajpregu.00446.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg, D. J. , Brown, L. M. , Zigman, J. M. , Kemp, C. J. , Strader, A. D. , Benoit, S. C. , … Geary, N. (2007). Estradiol‐dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes, 56, 1051–1058. 10.2337/db06-0015 [DOI] [PubMed] [Google Scholar]
- Currie, P. J. , Mirza, A. , Fuld, R. , Park, D. , & Vasselli, J. R. (2005). Ghrelin is an orexigenic and metabolic signaling peptide in the arcuate and paraventricular nuclei. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 289, R353–R358. 10.1152/ajpregu.00756.2004 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortere, I. (2009). Targeting the ghrelin receptor to regulate food intake. Regulatory Peptides, 156, 13–23. 10.1016/j.regpep.2009.04.002 [DOI] [PubMed] [Google Scholar]
- Dos‐Santos, R. C. , Reis, L. C. , Perello, M. , Ferguson, A. V. , & Mecawi, A. S. (2019). The actions of ghrelin in the paraventricular nucleus: Energy balance and neuroendocrine implications. Annals of the new York Academy of Sciences, 1455, 81–97. 10.1111/nyas.14087 [DOI] [PubMed] [Google Scholar]
- Dulawa, S. C. , & Hen, R. (2005). Recent advances in animal models of chronic antidepressant effects: The novelty‐induced hypophagia test. Neuroscience and Biobehavioral Reviews, 29, 771–783. 10.1016/j.neubiorev.2005.03.017 [DOI] [PubMed] [Google Scholar]
- Ehlert, U. , Gaab, J. , & Heinrichs, M. (2001). Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress‐related bodily disorders: The role of the hypothalamus–pituitary–adrenal axis. Biological Psychology, 57, 141–152. 10.1016/S0301-0511(01)00092-8 [DOI] [PubMed] [Google Scholar]
- Endo, M. , Hori, M. , Ozaki, H. , Oikawa, T. , & Hanawa, T. (2014). Rikkunshito, a Kampo medicine, ameliorates post‐operative ileus by anti‐inflammatory action. Journal of Pharmacological Sciences, 124, 374–385. 10.1254/jphs.13182FP [DOI] [PubMed] [Google Scholar]
- Fujitsuka, N. , Asakawa, A. , Morinaga, A. , Amitani, M. S. , Amitani, H. , Katsuura, G. , … Inui, A. (2016). Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Molecular Psychiatry, 21, 1613–1623. 10.1038/mp.2015.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitsuka, N. , Asakawa, A. , Uezono, Y. , Minami, K. , Yamaguchi, T. , Niijima, A. , … Inui, A. (2011). Potentiation of ghrelin signaling attenuates cancer anorexia–cachexia and prolongs survival. Translational Psychiatry, 1, 1–10, e23 10.1038/tp.2011.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley, T. J. , & McCormick, W. G. (1957). Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. British Journal of Pharmacology and Chemotherapy, 12, 12–15. 10.1111/j.1476-5381.1957.tb01354.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa, R. J. , Mani, S. K. , & Uht, R. M. (2012). Estrogen receptors and the regulation of neural stress responses. Neuroendocrinology, 96, 111–118. 10.1159/000338397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa, R. J. , & Weiser, M. J. (2014). Gonadal steroid hormones and the hypothalamo–pituitary–adrenal axis. Frontiers in Neuroendocrinology, 35, 197–220. 10.1016/j.yfrne.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassouna, R. , Labarthe, A. , Zizzari, P. , Videau, C. , Culler, M. , Epelbaum, J. , & Tolle, V. (2013). Actions of agonists and antagonists of the ghrelin/GHS‐R pathway on GH secretion, appetite, and cFos activity. Frontiers in Endocrinology (Lausanne), 4(25), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, J. I. , Hiripi, E. , Pope, H. G. Jr. , & Kessler, R. C. (2007). The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry, 61, 348–358. 10.1016/j.biopsych.2006.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki‐Sekino, A. , Mano‐Otagiri, A. , Ohata, H. , Yamauchi, N. , & Shibasaki, T. (2009). Gender differences in corticotropin and corticosterone secretion and corticotropin‐releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology, 34, 226–237. 10.1016/j.psyneuen.2008.09.003 [DOI] [PubMed] [Google Scholar]
- Jensen, M. , Ratner, C. , Rudenko, O. , Christiansen, S. H. , Skov, L. J. , Hundahl, C. , … Holst, B. (2016). Anxiolytic‐like effects of increased ghrelin receptor signaling in the amygdala. The International Journal of Neuropsychopharmacology, 19(5), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornstein, S. G. (1997). Gender differences in depression: Implications for treatment. The Journal of Clinical Psychiatry, 58(Suppl 15), 12–18. [PubMed] [Google Scholar]
- Kovacs, L. A. , Schiessl, J. A. , Nafz, A. E. , Csernus, V. , & Gaszner, B. (2018). Both basal and acute restraint stress‐induced c‐Fos expression is influenced by age in the extended amygdala and brainstem stress centers in male rats. Frontiers in Aging Neuroscience, 10(248), 1–20. 10.3389/fnagi.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labouesse, M. A. , Langhans, W. , & Meyer, U. (2015). Effects of selective estrogen receptor alpha and beta modulators on prepulse inhibition in male mice. Psychopharmacology, 232, 2981–2994. 10.1007/s00213-015-3935-9 [DOI] [PubMed] [Google Scholar]
- Lilley, E. , Stanford, S. C. , Kendall, D. E. , Alexander, S. P. H. , Cirino, G. , Docherty, J. R. , … Ahluwalia, A. (2020). ARRIVE 2.0 and the British Journal of Pharmacology: updated guidance for 2020. British Journal of Pharmacology, 177, 3611–3616. https://bpspubs.onlinelibrary.wiley.com. 10.1111/bph.15178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Itoga, C. A. , Taha, S. , Li, M. H. , Chen, R. , Sami, K. , … Xu, X. (2018). c‐Fos mapping of brain regions activated by multi‐modal and electric foot shock stress. Neurobiol Stress, 8, 92–102. 10.1016/j.ynstr.2018.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. L. , Yakar, S. , Otero‐Corchon, V. , Low, M. J. , & Liu, J. L. (2002). Ghrelin gene expression is age‐dependent and influenced by gender and the level of circulating IGF‐I. Molecular and Cellular Endocrinology, 189, 97–103. 10.1016/S0303-7207(01)00742-0 [DOI] [PubMed] [Google Scholar]
- Lockie, S. H. , Dinan, T. , Lawrence, A. J. , Spencer, S. J. , & Andrews, Z. B. (2015). Diet‐induced obesity causes ghrelin resistance in reward processing tasks. Psychoneuroendocrinology, 62, 114–120. 10.1016/j.psyneuen.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Mallien, A. S. , Soukup, S. T. , Pfeiffer, N. , Brandwein, C. , Kulling, S. E. , Chourbaji, S. , & Gass, P. (2019). Effects of soy in laboratory rodent diets on the basal, affective, and cognitive behavior of C57BL/6 mice. Journal of the American Association for Laboratory Animal Science: JAALAS, 58, 532–541. 10.30802/AALAS-JAALAS-18-000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkestein, M. , van Gestel, M. A. , van der Zwaal, E. M. , Brans, M. A. , Luijendijk, M. C. , & van Rozen, A. J. (2014). GHS‐R1a signaling in the DMH and VMH contributes to food anticipatory activity. International Journal of Obesity (Lond), 38, 610–618. https://www.nature.com/articles/ijo2013131 [DOI] [PubMed] [Google Scholar]
- Misra, M. , & Klibanski, A. (2014). Endocrine consequences of anorexia nervosa. The Lancet Diabetes & Endocrinology, 2, 581–592. 10.1016/S2213-8587(13)70180-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogami, S. , Sadakane, C. , Nahata, M. , Mizuhara, Y. , Yamada, C. , Hattori, T. , & Takeda, H. (2016). CRF receptor 1 antagonism and brain distribution of active components contribute to the ameliorative effect of rikkunshito on stress‐induced anorexia. Scientific Reports, 6(27516), 1–9. 10.1038/srep27516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, J. A. , Singhal, G. , Corrigan, F. , Jaehne, E. J. , Jawahar, M. C. , & Baune, B. T. (2018). The effects of aerobic exercise on depression‐like, anxiety‐like, and cognition‐like behaviours over the healthy adult lifespan of C57BL/6 mice. Behavioural Brain Research, 337, 193–203. 10.1016/j.bbr.2017.09.022 [DOI] [PubMed] [Google Scholar]
- Naderi, V. , Khaksari, M. , Abbasi, R. , & Maghool, F. (2015). Estrogen provides neuroprotection against brain edema and blood brain barrier disruption through both estrogen receptors α and β following traumatic brain injury. Iranian Journal of Basic Medical Sciences, 18, 138–144. [PMC free article] [PubMed] [Google Scholar]
- Nahata, M. , Muto, S. , Nakagawa, K. , Ohnishi, S. , Sadakane, C. , Saegusa, Y. , … Takeda, H. (2013). Serotonin 2C receptor antagonism ameliorates novelty‐induced hypophagia in aged mice. Psychoneuroendocrinology, 38, 2051–2064. 10.1016/j.psyneuen.2013.03.014 [DOI] [PubMed] [Google Scholar]
- Nahata, M. , Muto, S. , Oridate, N. , Ohnishi, S. , Nakagawa, K. , Sadakane, C. , … Takeda, H. (2012). Impaired ghrelin signaling is associated with gastrointestinal dysmotility in rats with gastroesophageal reflux disease. American Journal of Physiology Gastrointestinal and Liver Physiology, 303, G42–G53. 10.1152/ajpgi.00462.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahata, M. , Saegusa, Y. , Sadakane, C. , Yamada, C. , Nakagawa, K. , Okubo, N. , … Takeda, H. (2014). Administration of exogenous acylated ghrelin or rikkunshito, an endogenous ghrelin enhancer, improves the decrease in postprandial gastric motility in an acute restraint stress mouse model. Neurogastroenterology and Motility, 26, 821–831. 10.1111/nmo.12336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberrauch, S. , Sigrist, H. , Sautter, E. , Gerster, S. , Bach, D. R. , & Pryce, C. R. (2019). Establishing operant conflict tests for the translational study of anxiety in mice. Psychopharmacology, 236, 2527–2541. 10.1007/s00213-019-05315-y [DOI] [PubMed] [Google Scholar]
- Oyola, M. G. , & Handa, R. J. (2017). Hypothalamic–pituitary–adrenal and hypothalamic–pituitary–gonadal axes: Sex differences in regulation of stress responsivity. Stress, 20, 476–494. 10.1080/10253890.2017.1369523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson, Z. R. , Khazall, R. , Mackay, H. , Anisman, H. , & Abizaid, A. (2013). Central ghrelin signaling mediates the metabolic response of C57BL/6 male mice to chronic social defeat stress. Endocrinology, 154, 1080–1091. 10.1210/en.2012-1834 [DOI] [PubMed] [Google Scholar]
- Percie du Sert, N. , Hurst, V. , Ahluwalia, A. , Alam, S. , Avey, M. T. , Baker, M. , … Würbel, H. (2020). The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biology, 18(7), 1–12, e3000410 10.1371/journal.pbio.3000410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello, M. , Cabral, A. , Cornejo, M. P. , De Francesco, P. N. , Fernandez, G. , & Uriarte, M. (2019). Brain accessibility delineates the central effects of circulating ghrelin. Journal of Neuroendocrinology, 31, 1–10, e12677. [DOI] [PubMed] [Google Scholar]
- Saegusa, Y. , Takeda, H. , Muto, S. , Nakagawa, K. , Ohnishi, S. , Sadakane, C. , … Asaka, M. (2011). Decreased plasma ghrelin contributes to anorexia following novelty stress. American Journal of Physiology. Endocrinology and Metabolism, 301, E685–E696. 10.1152/ajpendo.00121.2011 [DOI] [PubMed] [Google Scholar]
- Santollo, J. , & Eckel, L. A. (2009). Effect of a putative ERα antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiology & Behavior, 97, 193–198. 10.1016/j.physbeh.2009.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer, S. J. , Emmerzaal, T. L. , Kozicz, T. , & Andrews, Z. B. (2015). Ghrelin's role in the hypothalamic–pituitary–adrenal axis stress response: Implications for mood disorders. Biological Psychiatry, 78, 19–27. 10.1016/j.biopsych.2014.10.021 [DOI] [PubMed] [Google Scholar]
- Stengel, A. , Goebel, M. , Wang, L. , Reeve, J. R. Jr. , Tache, Y. , & Lambrecht, N. W. (2010). Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: Potential role of the circulating ghrelin‐acylating enzyme GOAT. Peptides, 31, 1689–1696. 10.1016/j.peptides.2010.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel, A. , Wang, L. , & Tache, Y. (2011). Stress‐related alterations of acyl and desacyl ghrelin circulating levels: Mechanisms and functional implications. Peptides, 32, 2208–2217. 10.1016/j.peptides.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn, F. J. , Huang, L. , Ngo, S. T. , Leong, J. W. , Tan, H. Y. , Xie, T. Y. , … Chen, C. (2011). Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology, 152, 3165–3171. 10.1210/en.2011-0253 [DOI] [PubMed] [Google Scholar]
- Takeda, H. , Sadakane, C. , Hattori, T. , Katsurada, T. , Ohkawara, T. , Nagai, K. , & Asaka, M. (2008). Rikkunshito, an herbal medicine, suppresses cisplatin‐induced anorexia in rats via 5‐HT2 receptor antagonism. Gastroenterology, 134, 2004–2013. 10.1053/j.gastro.2008.02.078 [DOI] [PubMed] [Google Scholar]
- Thammacharoen, S. , Lutz, T. A. , Geary, N. , & Asarian, L. (2008). Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor‐alpha‐expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology, 149, 1609–1617. 10.1210/en.2007-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsubouchi, H. , Yanagi, S. , Miura, A. , Iizuka, S. , Mogami, S. , Yamada, C. , … Nakazato, M. (2014). Rikkunshito ameliorates bleomycin‐induced acute lung injury in a ghrelin‐independent manner. American Journal of Physiology. Lung Cellular and Molecular Physiology, 306, L233–L245. 10.1152/ajplung.00096.2013 [DOI] [PubMed] [Google Scholar]
- Valassi, E. , Scacchi, M. , & Cavagnini, F. (2008). Neuroendocrine control of food intake. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD, 18, 158–168. 10.1016/j.numecd.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Wang, L. , Saint‐Pierre, D. H. , & Tache, Y. (2002). Peripheral ghrelin selectively increases Fos expression in neuropeptide Y—Synthesizing neurons in mouse hypothalamic arcuate nucleus. Neuroscience Letters, 325, 47–51. 10.1016/S0304-3940(02)00241-0 [DOI] [PubMed] [Google Scholar]
- Xing, F. Z. , Zhao, Y. G. , Zhang, Y. Y. , He, L. , Zhao, J. K. , Liu, M. Y. , … Zhang, J. Q. (2018). Nuclear and membrane estrogen receptor antagonists induce similar mTORC2 activation‐reversible changes in synaptic protein expression and actin polymerization in the mouse hippocampus. CNS Neuroscience & Therapeutics, 24, 495–507. 10.1111/cns.12806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakabi, K. , Harada, Y. , Takayama, K. , Ro, S. , Ochiai, M. , Iizuka, S. , … Taché, Y. (2014). Peripheral α2‐β1 adrenergic interactions mediate the ghrelin response to brain urocortin 1 in rats. Psychoneuroendocrinology, 50, 300–310. 10.1016/j.psyneuen.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, C. , Sadakane, C. , Nahata, M. , Saegusa, Y. , Nakagawa, K. , Okubo, N. , … Takeda, H. (2015). Serotonin 2C receptor contributes to gender differences in stress‐induced hypophagia in aged mice. Psychoneuroendocrinology, 55C, 81–93. [DOI] [PubMed] [Google Scholar]
- Yamada, C. , Saegusa, Y. , Nahata, M. , Sadakane, C. , Hattori, T. , & Takeda, H. (2015). Influence of aging and gender differences on feeding behavior and ghrelin‐related factors during social isolation in mice. PLoS ONE, 10, 1–16, e0140094 10.1371/journal.pone.0140094 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Supporting information


