Abstract
Background and Purpose
While dipyrone is a widely used analgesic, its mechanism of action is not completely understood. Recently, we have reported that the dipyrone metabolite 4‐aminoantipyrine (4‐AA) reduces PGE2‐induced pain‐related behaviour through cannabinoid CB1 receptors. Here, we ascertained, in naive and PGE2‐induced “inflamed” conditions, both in vivo and in vitro, the molecular mechanisms involved in the 4‐AA‐induced analgesic effects.
Experimental Approach
The effect of local administration of 4‐AA (160 μg per paw) on capsaicin (0.12 μg per paw) injection‐induced pain‐related behaviour and 4‐AA's effect on 500‐nM capsaicin‐induced changes in intracellular calcium concentration ([Ca2+]i) in cultured primary sensory neurons were assessed in vivo and in vitro, respectively.
Key Results
4‐AA reduced capsaicin‐induced nociceptive behaviour in naive and inflamed conditions through CB1 receptors. 4‐AA (100 μM) reduced capsaicin‐induced increase in [Ca2+]i in a CB1 receptor‐dependent manner, when PGE2 was not present. Following PGE2 application, 4‐AA (1–50 μM) increased the [Ca2+]i. Although 4‐AA activated both TRPV1 and TRPA1 channels, increased [Ca2+]i was mediated through TRPV1 channels. Activation of TRPV1 channels resulted in their desensitisation. Blocking CB1 receptors reduced both the excitatory and desensitising effects of 4‐AA.
Conclusion and Implications
CB1 receptor‐mediated inhibition of TRPV1 channels and TRPV1‐mediated Ca2+‐influx‐ and CB1 receptor‐dependent desensitisation of TRPV1 channels contribute to the anti‐nociceptive effect of 4‐AA in naive and inflamed conditions respectively. Agonists active at both CB1 receptors and TRPV1 channels might be useful as analgesics, particularly in inflammatory conditions.
Keywords: calcium imaging, CB1, dipyrone, dorsal root ganglion, TRPA1, TRPV1
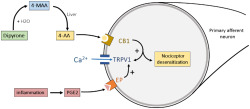
Abbreviations
- 4‐AA
4‐aminoantipyrine
- 4‐FAA
4‐formylaminoantipyrine
- 4‐MAA
4‐methylaminoantipyrine
- [Ca2+]I
intracellular calcium ion concentration
What is already known
Analgesic effects of dipyrone involves l‐arginine‐KATP channels, cannabinoids and opioid receptors activation.
A dipyrone metabolite, 4‐aminoantipyrine, is analgesic in models of inflammation, through activation of CB1 receptors
What this study add
Analgesic actions of 4‐aminoantipyrine involve desensitisation of TRPV1 channels dependent on CB1 receptors.
What is the clinical significance
4‐aminoantipyrine may be a useful lead in developing effective analgesics acting through desensitising TRPV1 channels.
1. INTRODUCTION
Dipyrone is an antipyretic and analgesic drug marketed for more than a century. The clinical use of dipyrone is based on its high analgesic efficacy, although the mechanism of action is not fully understood (Edwards et al., 2010; Dos Santos et al., 2014). In spite of the high analgesic efficacy, dipyrone has been banned in some countries (such as the United States, the United Kingdom, and Sweden) due to its association with agranulocytosis, though supporting data on this issue are still controversial (Andersohn, Konzen, & Garbe, 2007). Nevertheless, after administration, dipyrone is quickly hydrolysed to 4‐methylaminoantipyrine (4‐MAA) which is then metabolised to 4‐formylaminoantipyrine (4‐FAA), 4‐aminoantipyrine (4‐AA), and 4‐acetylaminoantipyrine (4‐AAA) (Pierre et al., 2007). As 4‐MAA and 4‐AA have the same analgesic effect as dipyrone, they are called bioactive metabolites (Pierre et al., 2007; Rogosch et al., 2012).
4‐MAA and 4‐AA are found in the plasma and CSF 30 min after dipyrone administration (Cohen, Zylber‐Katz, Caraco, Granit, & Levy, 1998). Accordingly, dipyrone produces analgesic effect both through peripheral actions and actions in the CNS (Lorenzetti & Ferreira, 1985; Vazquez, Hernandez, Escobar, & Vanegas, 2005). In a model of PGE2‐evoked pain‐related behaviour, unlike the anti‐nociceptive effect of 4‐MAA, we have shown that one of 4‐AA involves the activation of the cannabinoid CB1 receptors, expressed predominantly by neurons (Di Marzo, Stella, & Zimmer, 2014; Dos Santos et al., 2014).
Activation of TRPV1 ion channels, which is essential for the development of inflammatory heat hyperalgesia contributes to the development of PGE2‐induced pain as, through phosphorylation, PGE2 sensitises TRPV1 channels (Btesh, Fischer, Stott, & McNaughton, 2013; Caterina et al., 1997, 2000; Davis et al., 2000; Moriyama et al., 2005). Both TRPV1 channels and CB1 receptors are expressed by the overwhelming majority of nociceptive primary sensory neurons and they exhibit a huge degree of co‐localisation (Ahluwalia, Urban, Capogna, Bevan, & Nagy, 2000; Chen et al., 2016; Veress et al., 2013). In addition to the co‐localisation, CB1 receptors and TRPV1 channels exhibit a complex relationship though which CB1 receptors may inhibit or sensitise TRPV1 channels (Ahluwalia, Urban, Bevan, & Nagy, 2003; Chen et al., 2016; Fioravanti et al., 2008; Hermann et al., 2003). Therefore, the effect of 4‐AA on TRPV1‐mediated responses, especially when neurons are sensitised by inflammatory mediators, is of particular interest. Accordingly, here we have assessed, both in vivo and in vitro, the effects of 4‐AA on capsaicin‐evoked effects, both with and without sensitisation induced by prior application of PGE2.
2. METHODS
2.1. Animals
All animal care and experimental protocols were approved by the Committee on Animal Research of the State University of Campinas (protocol number: 3372‐1) and by veterinary services (Central Biological Services) at Imperial College London, UK. Experiments were conducted in accordance with the International Association for the Study of Pain (IASP) guidelines (Zimmermann, 1983), UK Animals (Scientific Procedures) Act 1986, the revised National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes. Every effort was made to minimise the number of animals used. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010), and agreeing with the editorial series updates for experiments involving animals (McGrath & Lilley, 2015) as recommended by the British Journal of Pharmacology.
A total of 84 male Wistar rats (8 weeks old, 200–250 g) were used in this study: 69 were obtained from the Multidisciplinary Center for Biological Research (CEMIB – UNICAMP, Campinas, Brazil) and 15 male Wistar rats from Charles River, UK. Animals (specific pathogen free [SPF]) were separated randomly and housed in standard plastic cages with soft bedding (four per cage) on a 12:12 light cycle, with food (commercial chow for rodents) and filtered water available ad libitum, in a temperature‐controlled room (~23°C). Testing sessions took place during the light phase (09:00 a.m.–5:00 p.m.) in a quiet room maintained at ~23°C.
2.1.1. Drugs and treatments in vivo
We used PGE2 (100 ng per paw), one of the most prominent inflammatory mediators to induce an “inflammatory” conditions (Moriyama et al., 2005; St‐Jacques & Ma, 2013), capsaicin (0.12 μg per paw) as an acute nociceptive stimuli activating TRPV1 channels, 4‐AA (160 μg per paw; Romero & Duarte, 2013; Vivancos et al., 2004), and AM251 as a selective cannabinoid CB1 receptor antagonist (50 μg per paw; Dos Santos et al., 2014). PGE2 was dissolved in ethanol to a concentration of 10 mg·ml−1 and then diluted in 0.9% NaCl. Capsaicin was initially dissolved in ethanol to a concentration of 0.2 mg·ml−1 and then diluted further with 0.9% NaCl for behaviour tests. AM251 was dissolved in propylene glycol and 10% DMSO; 4‐AA was dissolved in 0.9% NaCl. Drugs or vehicle were injected subcutaneously (50 μl) in the rat hind paw (intraplantar), using a BD Ultra‐Fine® (30 gauge) needle.
2.2. Behaviour measurements
Rats were placed separately in acrylic boxes for a habituation period of 15–30 min before testing. Following capsaicin injection, the number of flinches and licking was counted during a 5‐min period. A “flinch” was defined as a rapid jerk of the injected paw and “licking” as the time spent licking the hind paw. Assessing flinch frequency is simple to perform and one of the best scoring approaches for stereotyped behaviour in models of chemogenic nociception (Taylor, Peterson, & Basbaum, 1995; Tjølsen, Berge, Hunskaar, Rosland, & Hole, 1992). To analyse PGE2‐induced sensitisation of TRPV1 channels, PGE2 (100 ng per paw) was injected 3 h before capsaicin. Control experiments were performed in all studies using the capsaicin vehicle, which did not produce any significant nociceptive behaviour when injected into the hind paw. All behaviour tests were performed using six rats per condition and carried out without knowledge of the treatment given (blind assessment).
2.3. Primary cultures of sensory neurons
Cultures were prepared as described by Chen and co‐workers (Chen et al., 2016). Briefly, DRGs (C1 to the S1 segment) were dissected and placed into Ham's nutrient F12 culture medium (Sigma, UK) supplemented with 1‐mM l‐glutamine (Invitrogen, UK), 5,000 IU·ml−1 penicillin (Invitrogen, UK), 5,000 μg·ml−1 streptomycin (Invitrogen, UK), and 2% Ultroser G (Biospectra, France). Ganglia were incubated in type IV collagenase (Lorne Diagnostics, UK, 300 U·ml−1) for 3 h and in trypsin (0.25%) for 30 min at 37°C at 5% CO2. Following washes, ganglia were triturated, and dissociated cells plated on poly‐dl‐ornithine (Sigma, UK)‐coated glass coverslips in the supplemented medium. Cells were grown in the supplemented medium in the presence of 50 ng·ml−1 nerve growth factor (NGF, Promega, USA) for 12–24 h.
2.3.1. Drugs in vitro
PGE2 (1 μM, Mistry et al., 2014), capsaicin (500 nM, Mistry et al., 2014), 4‐AA (100, 50, 25, 10, and 1 μM; Nassini et al., 2015), AM251 (10 μM; Patil, Patwardhan, Salas, Hargreaves, & Akopian, 2011), capsazepine (10 μM; Chen et al., 2016), and the TRPA1 channel antagonist, HC‐030031 (10 μM; Boiko et al., 2017). AM251, capsaicin, and capsazepine stock solutions were prepared with DMSO. PGE2 stock solution was prepared in ethanol. Other drugs were dissolved in 0.9% NaCl.
2.4. Ca2+ imaging
For assessing changes in the [Ca2+]i, primary sensory neurons cultured for 1 day were loaded with Fura‐2 acetoxymethyl ester (Fura‐2 AM; 5 μM) in the presence of 2‐mM probenecid for 30 min at 37°C in a HEPES‐buffered saline (in mM): NaCl 122; KCl 3.3; CaCl2 1.3; MgSO4 0.4; KH2PO4 1.2; HEPES 25; glucose 10; adjusted with NaOH to pH 7.4. Coverslips were placed in a laminar flow perfusion chamber (Warner Instrument Corp., UK) and superfused with extracellular solution (in mM: NaCl 160; KCl 2.5; CaCl2 1; MgCl2 2; HEPES 10; glucose 10; pH 7.4) continuously via a six‐channel perfusion system. The following test solutions were applied to the cells: PGE2 (1 μM), AM251 (10 μM), capsazepine (10 μM), HC‐030031 (10 μM), 4‐AA (100, 50, 25, 10, and 1 μM), capsaicin (500 nM), and KCl (50 mM). The outlet of the perfusion system was positioned about 2–3 mm from the group of neurons from which the recordings were obtained. Application of drugs was controlled manually. Experiments were performed at 37°C. Only one field of view was tested on each coverslip.
Images of Fura‐2 loaded cells with the excitation wavelength alternating between 355 and 380 nM were captured with a Peltier element‐cooled slow scan charge‐coupled camera (optiMOS, QImaging, Canada) connected to a PC which ran the WinFluor software package (Dr Dempster, Strathclyde University, UK). The ratio of fluorescence intensity at the two wavelengths as a function of time (rate 0.5 Hz) was calculated automatically (R = F355/F380) by the WinFluor software package. Data were further analysed by the pClamp 10 software package (Molecular Devices, USA).
2.5. Data and statistical analysis
The significance of differences between groups was established with unpaired t‐test, one‐way ANOVA followed by Tukey's test or Fisher's exact test, as appropriate. A value of P < 0.05 was set as the threshold of statistical significance. Statistical analyses were performed in GraphPad Prism v5.00 for Windows® software packages (GraphPad Software). “n” refers to the number of animals for in vivo experiments, whereas it refers to the number of neurons for in vitro measurements. In vitro, each drug application protocol was repeated in cultures from at least three animals. The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology.
2.6. Materials
Capsazepine and AM251 were bought from Tocris (Bristol, UK); Fura‐2 AM and probenecid were supplied by Molecular Probes, Inc. (Eugene, OR). All other drugs were obtained from Sigma‐Aldrich (MO, USA).
2.7. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander, Christopoulos et al., 2019; Alexander, Mathie et al., 2019).
3. RESULTS
3.1. 4‐AA reduces capsaicin‐induced pain‐related behaviour as well as capsaicin‐induced Ca2+‐influx into cultured primary sensory neurons through activation of CB1 receptors
Subcutaneous capsaicin injection (0.12 μg per paw) into the rat hind paw induced pain‐related behaviour (Figure 1a). The capsaicin‐induced pain‐related behaviour was significantly reduced by the administration of 4‐AA (160 μg per paw, Figure 1a) prior to the capsaicin injection. To analyse the involvement of CB1 receptors in the anti‐nociceptive effect of 4‐AA, the CB1 receptor antagonist (AM251, 50 μg per paw) was administered 15 min before 4‐AA. As shown in Figure 1a, AM251 completely blocked the anti‐nociceptive effect of 4‐AA.
FIGURE 1.
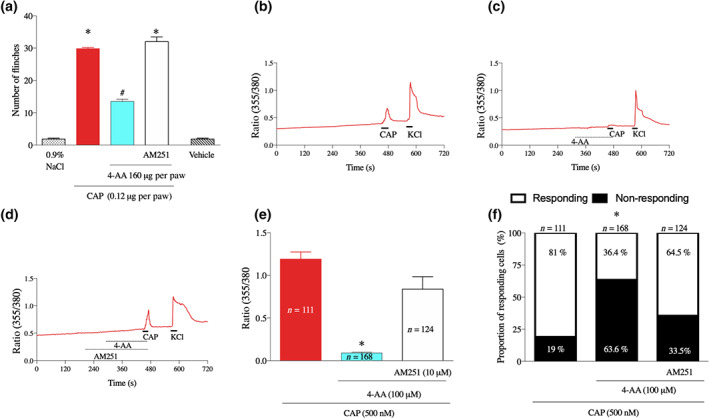
4‐aminoantipyrine (4‐AA) reduces the excitatory effect of capsaicin through CB1 receptor activation in naive (non‐inflamed) conditions. (a) Local administration of capsaicin (CAP; 0.12 μg per paw) induces flinches. The capsaicin‐induced nociceptive behaviour is reduced by the administration of 4‐AA (160 μg per paw). AM251 (50 μg per paw) reversed the anti‐nociceptive effect of 4‐AA. *P< .05, significantly different from 0.9% NaCl, vehicle and (capsaicin+4‐AA). # P< .05, significantly different from 0.9% NaCl, vehicle, capsaicin and (capsaicin+4‐AA+AM251); one‐way ANOVA, with Tukey's test. (b–d) Representative recordings of calcium imaging from cultured primary sensory neurons. Capsaicin (500 nM) induces a rise in the intracellular calcium concentration ([Ca2+]i; (b)) that is reversed by 4‐AA (100 μM; (c)). AM251 (10 μM) reversed the inhibitory effect of 4‐AA on the capsaicin‐induced calcium‐influx (ANOVA, Tukey's test, P < .05; (d)). (e) Average normalised amplitudes of capsaicin‐evoked changes in the [Ca2+]i in various conditions. (f) Proportions of neurons responding the capsaicin at various conditions; 4‐AA reduces the proportion of neurons that respond to capsaicin and AM251 reduces the inhibitory effect of 4‐AA (Fisher's exact test, P < .05). In (e–f), *P< .05, significantly different from capsaicin and (capsaicin+4‐AA+AM251); one‐way ANOVA, with Tukey's test
To confirm the CB1 receptor‐mediated inhibitory effects of 4‐AA on TRPV1, we next measured the [Ca2+]i in cultures of DRG neurons. As expected, administration of capsaicin (500 nM) induced a significant increase in the [Ca2+]I in a major sub‐population of the cells (Figure 1b,e). Application of 4‐AA alone did not produce any measurable response or affect the responsiveness of DRG neurons to KCl. However, pretreatment of the cells with 4‐AA (100 μM) significantly reduced capsaicin‐evoked responses (Figure 1c–e). Application of AM251 (10 μM) prevented the inhibitory effect of 4‐AA (Figure 1d,e). Further, 4‐AA also reduced the number of capsaicin‐responsive cells (Figure 1f) and AM251 prevented this reduction without affecting the number of cells responding to KCl (Figure 1f ).
3.2. 4‐AA produces different effects on TRPV1‐mediated responses following PGE2‐induced sensitisation in vivo and in vitro
The inhibitory effect of 4‐AA on TRPV1‐mediated responses is relevant if it is maintained in sensitised conditions which underlie the development of pathological pain experiences of various diseases including inflammation. During inflammation, inflammatory mediators sensitise TRPV1 channels. PGE2, a major component of the inflammatory soup, is one of the most powerful sensitisers of TRPV1 channels (Moriyama et al., 2005; St‐Jacques & Ma, 2013). In agreement with previous findings (Lin et al., 2006), pretreatment of the rat paw with PGE2 (100 ng, injected subcutaneously, 3 h before capsaicin injection) significantly increased the capsaicin‐induced pain‐related behaviour (Figure 2a,b).
FIGURE 2.
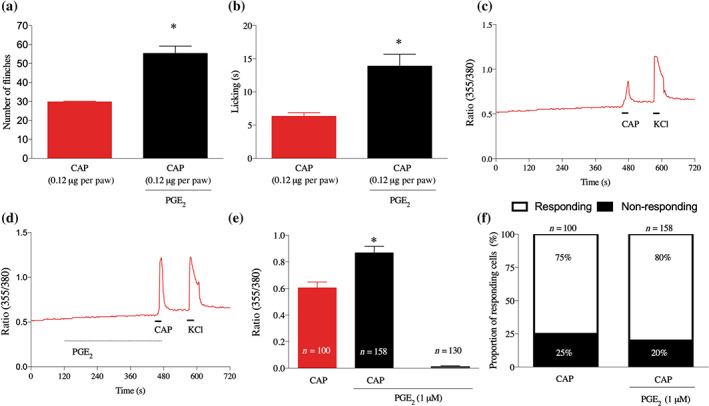
PGE2 increases capsaicin‐induced pain‐related behaviour and capsaicin‐induced increase in the [Ca2+]i in primary sensory neurons. (a) and (b) Local administration of capsaicin (0.12 μg per paw) into subcutaneous tissues of the hind paw induces pain‐related behaviour. Pretreatment with PGE2 (100 nM, 150 min) increases the capsaicin‐induced responses. *P< .05, significantly different from capsaicin alone; one‐way ANOVA with Tukey's test. (c, d) Typical recordings on changes in the [Ca2+]i in cultured primary sensory neurons. The pretreatment with PGE2 (1 μM, for 5 min) increases capsaicin‐induced increase in the [Ca2+]i (d) when compared to responses without pretreatment (c). (e) Average normalised amplitudes of capsaicin‐evoked responses in cultured primary sensory neurons without and with pretreatment with PGE2. *P< .05, significantly different from capsaicin alone; one‐way ANOVA with Tukey's test. (f) PGE2 pretreatment does not affect the number of capsaicin‐responsive neurons (Fisher's exact test, P < .05)
The sensitising effect of PGE2 was also evident in vitro, because cultured primary sensory neurons pretreated with PGE2 (1 μM, 5 min before capsaicin application) exhibited significantly greater responses to capsaicin (500 nM, for 30 s) than without PGE2 pretreatment (Figure 2c–e). However, the proportion of capsaicin‐responding neurons was not changed by PGE2 pretreatment (Figure 2f). Further, although PGE2 per se produced change in the [Ca2+]i in some cells, on average, that change did not reach the level of significance (Figure 2e).
After confirming that PGE2 could sensitise the TRPV1 channels, both in vivo and in vitro, we then assessed the effect of 4‐AA following PGE2 administration. In vivo, 4‐AA (160 μg) was injected 3 h after PGE2 injection, which was followed by capsaicin administration 30 min later. Assessing pain‐related behaviour following 4‐AA injection did not reveal any excitatory effect of 4‐AA following PGE2 injection (Figure 3a,b). However, similar to the observations in the absence of PGE2, 4‐AA did reduce the capsaicin‐induced pain‐related behaviour (Figure 3a,b). To find whether this inhibitory effect was mediated through CB1 receptors, we administered AM251 (10 μM) 2 min before 4‐AA injection. As found in the absence of PGE2, AM251 completely prevented the anti‐nociceptive effect of 4‐AA (Figure 3a,b).
FIGURE 3.
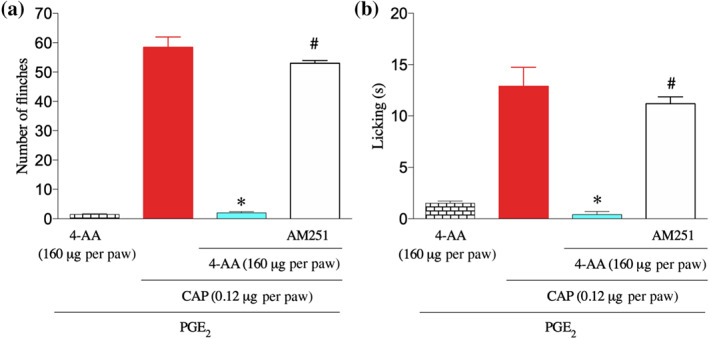
4‐AA reduces capsaicin‐induced pain‐related behaviour following PGE2 pretreatment. (a, b) 4‐AA reduces capsaicin‐induced pain‐related behavioural responses following pretreatment with PGE2. *P< .05, significantly different from (PGE2+capsaicin), #P< .05, significantly different from (PGE2+capsaicin+4‐AA); one‐way ANOVA with Tukey's test
To confirm the CB1 receptor‐mediated inhibitory effect of 4‐AA on sensitised TRPV1 channels in vitro, we pretreated cultured primary sensory neurons with PGE2 (1 μM) and then administered 4‐AA for 2 min. Unexpectedly, 4‐AA (50, 25, 10, and 1 μM), in a concentration‐dependent manner, increased the [Ca2+]i in primary sensory neurons in this condition (Figure 4a,c). The number of cells responding to 4‐AA (25 and 50 μM) was also increased (Figure 4b).
FIGURE 4.
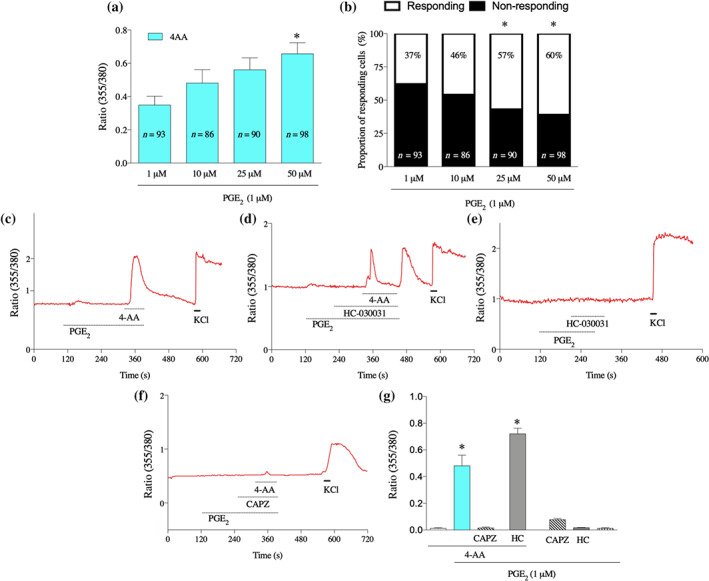
4‐AA increases [Ca2+]i through activation of TRPV1 channels in cultured primary sensory neurons following pretreatment of the cells with PGE2. (a) 4‐AA (50, 25, 10, and 1 μM) increases [Ca2+]i in primary sensory neurons, sensitised by PGE2 in a concentration‐dependent manner. (b) The proportion of 4‐AA‐responding cells is also increased following PGE2 pretreatment. *P< .05, significant effect of 4‐AA. (c–f) Representative recordings of changes in the [Ca2+]i by various conditions. Following PGE2 pretreatment, 4‐AA (50 μM) induces an increase in the [Ca2+]i in the presence of PGE2 (c). While the 4‐AA‐induced increases in the [Ca2+]i were not affected by the TRPA1 channel antagonist HC‐030031 (10 μM) in the presence of PGE2 (1 μM), at the start of washing PGE2, HC‐030031 and 4‐AA out from the recording chamber, a “buffer‐evoked” calcium transient emerged (d). Importantly, no such “buffer‐evoked” calcium transient was observed when 4‐AA was missing from the superfusate (e). The TRPV1 channel antagonist capsazepine (CAPZ; 10 μM), but not the TRPA1 channel antagonist, blocked the 4‐AA‐evoked calcium transient in the presence of PGE2 (f). (g) Average normalised amplitudes of responses of cultured primary sensory neurons in various conditions. Data shown are means SEM from a number of neurons, as follows: 4‐AA, n = 156; PGE2 +4‐AA, n = 156; PGE2+4‐AA+CAPZ, n = 142; PGE2+4‐AA+HC, n = 100; PGE2 +CAPZ, n = 115; PGE2+HC, n = 289: PGE2, n = 156. * P< .05, significant differences; one‐way ANOVA with Tukey's test
4‐AA has recently been found to be a potent activator of TRPV1 channels and another related group of cation channels, the TRPA1 channels (Schenk, Dick, Herzog, Eberhardt, & Leffler, 2019). Further, in addition to its effects on TRPV1 channels, PGE2 also contributes to the pathophysiological role of TRPA1 channels (Dall'Acqua et al., 2014; Moriyama et al., 2005; Btesh et al., 2013). Therefore, we reasoned that the co‐application of 4‐AA and PGE2 (Figure 4c) might have resulted in TRPA1‐ and/or TRPV1‐mediated increase in [Ca2+]i. Accordingly, first, we repeated the experiment in the presence of the TRPA1 channel antagonist, HC030031 (10 μM; Figure 4d) and found that HC‐030031 did not change the 4‐AA‐induced increase in the [Ca2+]i (Figure 4c,d,g). Unexpectedly, however, at the start of washing out the PGE2, 4‐AA and HC030031 out from the recording chamber, a calcium transient developed (Figure 4d). This transient did not develop when only PGE2 and HC030031 were washed out from the cells (Figure 4e). Hence, the effect must depend on the presence of 4‐AA, indicating that, in addition to another channel, the TRPA1 channel did contribute, to some extent, to the increase in [Ca2+]i induced by 4‐AA after PGE2 administration, in vitro.
To find whether or not that other channel is TRPV1, we repeated the experiment in the presence of capsazepine (10 μM), which has been used as a selective and specific TRPV1 channel antagonist (Bevan et al., 1992), though above 10‐μM capsazepine does activate and desensitise TRPA1 channels (Kistner et al., 2016). As shown in Figure 4f,g, the 4‐AA‐induced increase in the [Ca2+]i was completely blocked by capsazepine. These findings together indicate that, although TRPA1 channels are activated by 4‐AA in certain conditions, the TRPV1 channels mediate the 4‐AA‐evoked increase in [Ca2+]i in primary sensory neurons, in the presence of PGE2.
3.3. 4‐AA desensitises TRPV1 channels with the contribution of CB1 receptor
When 4‐AA was applied for 2 min to cultured primary sensory neurons, the change in [Ca2+]i after reaching a peak exhibited a rapid recovery indicating TRPV1 channel desensitisation (Figure 4d). Indeed, the increase in the [Ca2+]i by 4‐AA (10, 25, and 50 μM) significantly reduced in subsequent responses to capsaicin (Figure 5a,b).
FIGURE 5.
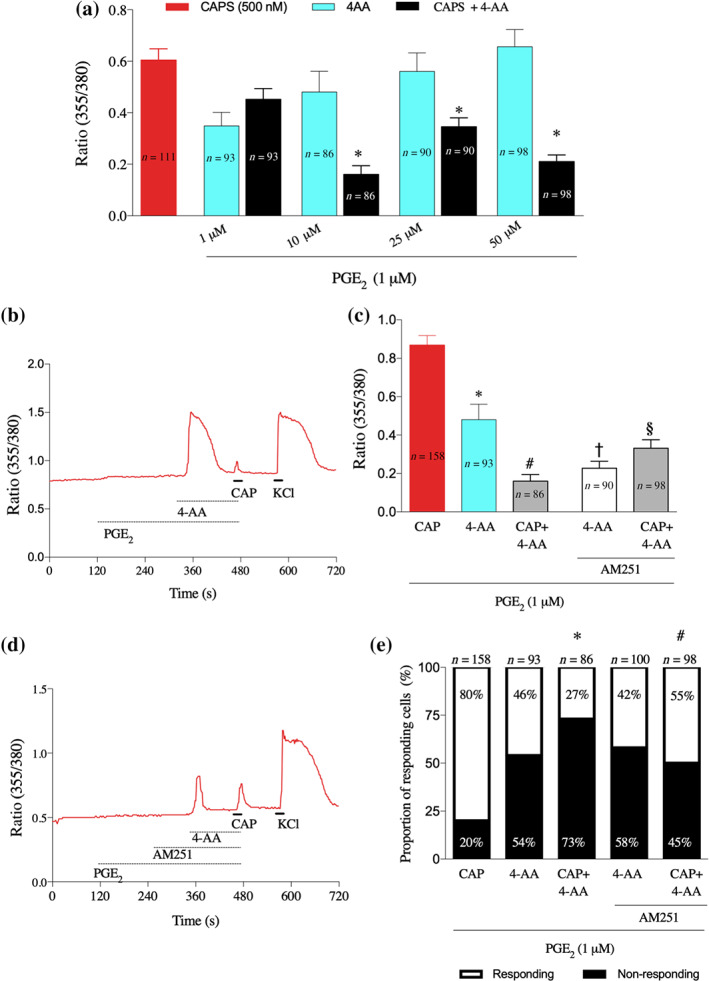
4‐AA desensitises TRPV1 channels, involving CB1 receptors. (a) Average normalised amplitudes of primary sensory neurons to 4‐AA (1–50 μM) or capsaicin (CAPS; 500 nM) with and without PGE2 (1 μM) pretreatment. Note that 4‐AA applied before capsaicin, at and above 10 μM, significantly reduces the amplitude of capsaicin‐evoked responses. *P< .05, significantly different from capsaicin alone; one‐way ANOVA with Tukey's test. (b) Representative recording of changes in the [Ca2+]i from a cultured primary sensory neuron by the application of 4‐AA (10 μM), capsaicin (CAP; 500 nM) in the presence of PGE2 (1 μM). Note that capsaicin induces only a small response. (c) Average normalised amplitudes of primary sensory neurons to 4‐AA (10 μM) and capsaicin (500 nM) in the presence of PGE2 (1 μM) and without or with AM251 (10 μM) pretreatment. Note the opposing changes in the 4‐AA‐ and 4‐AA + capsaicin‐evoked increase in the [Ca2+]i by AM251; while the 4‐AA‐induced responses are decreased, the 4‐AA + capsaicin‐evoked responses are increased. *P< .05, significantly different from capsaicin, #P< .05, significantly different from capsaicin, †P< .05, significantly different from 4‐AA, §P< .05, significantly different from (capsaicin+4‐AA); one‐way ANOVA with Tukey's test. (d) A typical recording of changes in the [Ca2+]i by 4‐AA (10 μM) and capsaicin (500 nM) in the presence of PGE2 (1 μM) and AM251 (10 μM) from a cultured primary sensory neuron. (e) AM251 (10 μM) attenuates the effect of 4‐AA on the number of capsaicin‐responding cells whereas it has no effect on the number of cells responsive to 4‐AA (Fisher's exact test, P < .05). *P< .05, significantly different from capsaicin, #P< .05, significantly different from capsaicin; one‐way ANOVA with Tukey's test
In order to find out whether the CB1 receptors played any part in the 4‐AA‐increased [Ca2+]I , we pretreated the cells with AM251 (10 μM) before applying 4‐AA. The increase in the [Ca2+]i induced by 4‐AA (10 μM) was significantly reduced by this pretreatment (Figure 5c,d). In addition, desensitisation of TRPV1 channels was significantly reduced (Figure 5c,d). Further, 4‐AA reduced the number of capsaicin‐responsive neurons to 27% when compared to capsaicin application alone, and the pretreatment with the CB1 receptor antagonist increased the number of responsive neurons to capsaicin to 55% (Figure 5e). AM251 did not change the number of cells responding to 4‐AA (Figure 5e).
4. DISCUSSION
Recently, we have shown that CB1 receptors are involved in 4‐AA's analgesic effect after PGE2 administration (Dos Santos et al., 2014). Here, we report that 4‐AA also reduces capsaicin‐induced nociceptive behaviour as well capsaicin‐evoked responses assessed by measuring [Ca2+]i in cultured primary sensory neurons. However, while in vivo, the 4‐AA‐produced inhibition of capsaicin‐evoked nocifensive behaviour was blocked by the CB1 receptor antagonist AM251, in vitro, 4‐AA through the desensitisation of TRPV1 channels reduced the TRPV1‐mediated increase in the [Ca2+]i, though this latter effect also depended on CB1 receptors. Although both CB1 receptors and TRPV1 channels might be expressed on cells other than peripheral terminals of primary sensory neurons (Ahluwalia et al., 2000; Basu & Srivastava, 2005; Bodó et al., 2005; Caterina et al., 1997; Chen et al., 2016; Sacerdote, Massi, Panerai, & Parolaro, 2000; Ständer, Schmelz, Metze, Luger, & Rukwied, 2005; Veress et al., 2013), our findings together indicate that the 4‐AA‐induced behavioural effects following local applications were mediated, at least predominantly, directly via primary sensory neurons.
Although there is an apparent difference in the molecular pathway in vitro and that used in vivo, we propose that the mechanisms of actions are the same in vitro and in vivo, under conditions of inflammation. The molecular events in these pathways include the PGE2‐induced sensitisation of several Ca2+‐permeable ion channels (Bonet, Fischer, Parada, & Tambeli, 2013; Dall'Acqua et al., 2014; Lopshire & Nicol, 1998; Mistry et al., 2014; Patil et al., 2020). We have found evidence that 4‐AA activated at least two of those sensitised channels, TRPV1 and TRPA1. Recently, Schenk et al. (Schenk et al., 2019) have reported that heterologously expressed human TRPV1 channels are indeed activated by as low as 100‐nM 4‐AA. However, 100‐μM 4‐AA did not increase the [Ca2+]i when cells were not pretreated with PGE2, indicating that either human and rat TRPV1 channels, or the heterologously expressed and native channels, are significantly different regarding 4‐AA responsiveness. Nevertheless, our findings indicate that PGE2 pretreatment of primary sensory neurons results in a significant increase both in the efficacy and potency of 4‐AA on TRPV1 channels; 100 μM had no excitatory effect under naive condition, whereas 1 μM produced a significant increase in the [Ca2+]i under inflamed conditions.
We also found that at the start of PGE2, HC030031, and 4‐AA wash out, a 4‐AA‐dependent increase in the [Ca2+]i was induced in the great majority of the cells. The most plausible explanation for that response is that during the co‐application of PGE2, HC030031, and 4‐AA, the TRPA1 channel/channel gating blocker HC030031 and 4‐AA both occupy their respective binding sites. At the start of the wash out, HC030031, which has an IC50 of ~5 (McNamara et al., 2007) is removed significantly faster than 4‐AA from TRPA1; hence, 4‐AA is still able to open the channel. Although the 4‐AA‐evoked, TRPA1‐mediated, increase in the [Ca2+]i was expected, its appearance only after PGE2 pretreatment was surprising, because similarly to heterologously expressed human TRPV1 channels, heterologously expressed human TRPA1 channels also respond to 4‐AA at 100 nM (Schenk et al., 2019). Hence, similarly to TRPV1, TRPA1 channels may have differing responses to 4‐AA in human and rat or/and at heterologous or native conditions. Interestingly, after PGE2 pretreatment, TRPA1 channels responded to 4‐AA even after the presence of this agent for 2 min and a TRPV1‐mediated Ca2+ influx.
Although 4‐AA activated TRPA1 channels, the findings that HC030031 did not affect 4‐AA‐evoked responses, together with the TRPV1 antagonist/TRPA1 activator/desensitiser capsazepine completely blocking the 4‐AA‐evoked responses, indicate that the increase in the [Ca2+]i by 4‐AA is mediated primarily by TRPV1 channels in primary sensory neurons. The apparent lack of TRPA1 channels to contribute to the 4‐AA‐evoked responses is difficult to explain. However, TRPV1 and TRPA1 channels can form molecular complexes, where TRPV1 channels have been shown to play a predominant, controlling role over TRPA1 channel responses (Patil et al., 2020). Although HC030031 acts only on TRPA1 channels, inhibiting the channel gating might disrupt the control exerted by TRPV1 channels, resulting in a transient activation of TRPA1 channels, immediately after removing the TRPA1 channel antagonist.
TRPV1‐mediated Ca2+ influx induces desensitisation of TRPV1 channels (Koplas, Rosenberg, & Oxford, 1997). Our present in vitro data indicate that, in the presence of PGE2, TRPV1 channels are desensitised after a brief, 4‐AA‐induced, activation. Hence, it is feasible to propose that similar TRPV1 desensitisation by 4‐AA also occurs in vivo. However, while desensitisation of TRPV1 channels is most often preceded by excitatory responses such as pain‐related behaviour in vivo (LaMotte, Shain, Simone, & Tsai, 1991), we did not observe any nociceptive responses following the injection of 4‐AA in the inflamed condition. The development of nociceptive responses is initiated by action potential generation in nociceptive primary sensory neurons. Therefore, the most plausible explanation for the apparent lack of any nociceptive response following 4‐AA injection is that although 4‐AA activated TRPV1 channels, that activation did not result in action potential generation in nociceptive primary sensory neurons. Such lack of action potential generation could occur if the rate of depolarisation is too slow for the activation of voltage‐gated sodium channels (Hille, 2001). Previously, the ultrapotent TRPV1 activator resiniferatoxin has been shown to induce slow depolarisation, which then results in the generation of very few action potentials in primary sensory neurons and, hence, no significant pain‐related behaviour by resiniferatoxin (Raisinghani, Pabbidi, & Premkumar, 2005). While we have not specifically studied this point, 4‐AA, similarly to resiniferatoxin may produce slow depolarisation per se. Alternatively, by acting also on the CB1 receptors, 4‐AA may reduce the rate of depolarisation, for example, by increasing K+ currents (Vásquez et al., 2003; Di Marzo et al., 2014).
Following the administration of the CB1 receptor antagonist AM251, no inhibitory effects by 4‐AA on capsaicin‐induced nociceptive responses were observed either in naive condition or after PGE2 administration. AM251 also blocked the 4‐AA‐evoked inhibitory effect on capsaicin‐evoked increase in the [Ca2+]i when cells were not pretreated with PGE2 as well as the 4‐AA‐induced increase in the [Ca2+]i and the subsequent TRPV1 channel desensitisation after PGE2 pretreatment. As a result, the capsaicin‐evoked increase in the [Ca2+]i was significantly greater than without AM251 pretreatment after PGE2 pretreatment. Importantly, in our in vitro experiment, AM251, which had been shown to activate TRPA1 channels in trigeminal ganglion neurons (Patil et al., 2020), did not produce any change in the [Ca2+]i either without or with PGE2 pretreatment. Further, AM251 administration per se did not produce any pain‐related behaviour in either condition. These findings together suggest that the AM251‐induced effects were mediated by CB1 receptors both in vivo and in vitro.
The CB1 receptor‐mediated inhibitory effect on TRPV1‐mediated responses in naive conditions is in agreement with previous data (Mahmud, Santha, Paule, & Nagy, 2009; Patwardhan et al., 2006; Sántha, Jenes, Somogyi, & Nagy, 2010). Previous findings also show that the CB1 receptors, in addition to inhibiting TRPV1 channel activity, also contribute to TRPV1 channel sensitisation (Chen et al., 2016; Fioravanti et al., 2008; Hermann et al., 2003). Notably, while such CB1 receptor‐mediated increases in TRPV1‐mediated responsiveness is evident in naive conditions (Chen et al., 2016; Fioravanti et al., 2008), our present findings suggest that it may become significantly more pronounced in inflammatory conditions.
Taken together, our in vivo and in vitro findings strongly suggest that 4‐AA induced the following events both in vitro and in vivo under inflammatory conditions. First, inflammatory mediators and CB1 receptors, activated by 4‐AA, sensitise TRPV1 channels. That sensitisation results in significantly increased excitatory efficacy and potency of 4‐AA on TRPV1 and TRPA1 channels. Activation of TRPV1 channels by 4‐AA induces Ca2+ influx, which, while desensitising TRPV1 channels, does not result in action potential generation. As a result, capsaicin‐evoked responses are reduced.
Importantly, inflammation‐associated “switching” from an exclusively CB1 receptor‐mediated, to a mixed CB1 receptor‐ and TRPV1‐mediated, inhibitory effect, as we found in the present study, has been reported at the central terminals of nociceptive primary sensory neurons (Nerandzic et al., 2018). This effect could be due to inflammation‐induced change in the CB1 receptor–TRPV1 channel protein–protein interaction in a major sub‐population of primary sensory neurons (Chen et al., 2016). As a result, while in the naive condition the CB1 receptor is able to reduce activity of TRPV1 channels (Mahmud et al., 2009; Sántha et al., 2010), under inflammatory conditions, its predominant effect appears to be sensitisation of TRPV1 channels.
In agreement with previous suggestions, our findings also indicate that dual CB1 receptor/TRPV1 channel agonists, particularly when restricted to the periphery, could produce significant analgesic effects in inflammation. Some capsaicinoids including olvanil, arvanil, and palvinil, though with various potency and efficacy, do exhibit such activity, which is thought to be a major mechanism for their anti‐nociceptive effects (Alsalem et al., 2016; Brand et al., 1987; De Petrocellis et al., 2011; Hoffmann et al., 2012; Melck et al., 1999). However, the majority of the already known CB1 receptor / TRPV1 channel agonists also have significant effects on other components of the endocannabinoid/endovanilloid system (Melck et al., 1999). While it is not known whether 4‐AA has any effects on other parts of the endocannabinoid/endovanilloid system, our data do extend the list of CB1 receptor / TRPV1 channel agonists. As a consequence, 4‐AA should be included into the list of compounds, which serve as a starting point to develop highly potent and effective analgesics to control inflammatory pain.
AUTHOR CONTRIBUTIONS
G.G.S. designed the project, performed in vitro and in vivo experiments and data analysis, and wrote the manuscript. R.L., M.P.E.N. performed calcium‐imaging. W.F.V. performed calcium‐imaging and drafted the manuscript. J.B.P.M. performed behavioral pharmacology tests. I.N. designed in vitro studies, finalized the manuscript. C.H.T. designed the behavior experiments and data analysis. C.A.P. designed the project, supervised the experiments, and contributed to writing the manuscript. All authors reviewed the final text version.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for Design & Analysis and Animal Experimentation, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENT
This study was supported by Sao Paulo Research Foundation ‐ Fapesp (2017/2345‐0), and G.G.S. by Coordination of Improvement of Higher Education Personnel (CAPES), Brazil. The authors thank Dr. Catarine Massucato Nishijima and Cesar Bissoto for their technical support.
Goncalves dos Santos G, Li R, Ng MPE, et al. CB1 receptor‐dependent desensitisation of TRPV1 channels contributes to the analgesic effect of dipyrone in sensitised primary sensory neurons. Br J Pharmacol. 2020;177:4615–4626. 10.1111/bph.15170
Contributor Information
Cláudia Herrera Tambeli, Email: chtambeli@unicamp.br.
Carlos Amilcar Parada, Email: caparada@unicamp.br.
REFERENCES
- Ahluwalia, J. , Urban, L. , Bevan, S. , & Nagy, I. (2003). Anandamide regulates neuropeptide release from capsaicin‐sensitive primary sensory neurons by activating both the cannabinoid 1 receptor and the vanilloid receptor 1 in vitro. The European Journal of Neuroscience, 17, 2611–2618. 10.1046/j.1460-9568.2003.02703.x [DOI] [PubMed] [Google Scholar]
- Ahluwalia, J. , Urban, L. , Capogna, M. , Bevan, S. , and Nagy, I. (2000). Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. [DOI] [PubMed]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176, S142–S228. 10.1111/bph.14749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsalem, M. , Millns, P. , Altarifi, A. , El‐Salem, K. , Chapman, V. , & Kendall, D. A. (2016). Anti‐nociceptive and desensitizing effects of olvanil on capsaicin‐induced thermal hyperalgesia in the rat. BMC Pharmacology and Toxicology, 17(1). 10.1186/s40360-016-0074-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersohn, F. , Konzen, C. , & Garbe, E. (2007). Systematic Review: Agranulocytosis Induced by Nonchemotherapy Drugs. Annals of Internal Medicine, 146(9), 657 10.7326/0003-4819-146-9-200705010-00009 [DOI] [PubMed] [Google Scholar]
- Basu, S. , & Srivastava, P. (2005). Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proceedings of the National Academy of Sciences of the United States of America, 102, 5120–5125. 10.1073/pnas.0407780102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan, S. , Hothi, S. , Hughes, G. , James, I. F. , Rang, H. P. , Shah, K. , … Yeats, J. C. (1992). Capsazepine: A competitive antagonist of the sensory neurone excitant capsaicin. British Journal of Pharmacology, 107, 544–552. 10.1111/j.1476-5381.1992.tb12781.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodó, E. , Bíró, T. , Telek, A. , Czifra, G. , Griger, Z. , Tóth, B. I. , … Paus, R. (2005). A hot new twist to hair biology: Involvement of vanilloid receptor‐1 (VR1/TRPV1) signaling in human hair growth control. The American Journal of Pathology, 166, 985–998. 10.1016/S0002-9440(10)62320-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boiko, N. , Medrano, G. , Montano, E. , Jiang, N. , Williams, C. R. , Madungwe, N. B. , … Eaton, B. A. (2017). TrpA1 activation in peripheral sensory neurons underlies the ionic basis of pain hypersensitivity in response to vinca alkaloids. PLOS ONE, 12(10), e0186888 10.1371/journal.pone.0186888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonet, I. J. M. , Fischer, L. , Parada, C. A. , & Tambeli, C. H. (2013). The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan‐induced hyperalgesia. Neuropharmacology, 65, 206–212. 10.1016/j.neuropharm.2012.09.020 [DOI] [PubMed] [Google Scholar]
- Brand, L. , Berman, E. , Schwen, R. , Loomans, M. , Janusz, J. , Bohne, R. , … Farmer, R. (1987). NE‐19550: A novel, orally active anti‐inflammatory analgesic. Drugs under Experimental and Clinical Research, 13, 259–265. [PubMed] [Google Scholar]
- Btesh, J. , Fischer, M. J. M. , Stott, K. , & McNaughton, P. (2013). Mapping the binding site of TRPV1 on AKAP79: Implications for inflammatory hyperalgesia. The Journal of Neuroscience, 33, 9184–9193. 10.1523/JNEUROSCI.4991-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina, M. J. , Leffler, A. , Malmberg, A. B. , Martin, W. J. , Trafton, J. , Petersen‐Zeitz, K. R. , … Julius, D. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science, 288(80), 306–313. 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- Caterina, M. J. , Schumacher, M. A. , Tominaga, M. , Rosen, T. A. , Levine, J. D. , & Julius, D. (1997). The capsaicin receptor: A heat‐activated ion channel in the pain pathway. Nature, 389, 816–824. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Varga, A. , Selvarajah, S. , Jenes, A. , Dienes, B. , Sousa‐Valente, J. , … Nagy, I. (2016). Spatial Distribution of the Cannabinoid Type 1 and Capsaicin Receptors May Contribute to the Complexity of Their Crosstalk. Scientific Reports, 6(1). 10.1038/srep33307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, O. , Zylber‐Katz, E. , Caraco, Y. , Granit, L. , & Levy, M. (1998). Cerebrospinal fluid and plasma concentrations of dipyrone metabolites after a single oral dose of dipyrone. European Journal of Clinical Pharmacology, 54, 549–553. 10.1007/s002280050511 [DOI] [PubMed] [Google Scholar]
- Dall'Acqua, M. C. , Bonet, I. J. M. , Zampronio, A. R. , Tambeli, C. H. , Parada, C. A. , & Fischer, L. (2014). The contribution of transient receptor potential ankyrin 1 (TRPA1) to the in vivo nociceptive effects of prostaglandin E2. Life Sciences, 105(1‐2), 7–13. 10.1016/j.lfs.2014.02.031 [DOI] [PubMed] [Google Scholar]
- Davis, J. B. , Gray, J. , Gunthorpe, M. J. , Hatcher, J. P. , Davey, P. T. , Overend, P. , … Sheardown, S. A. (2000). Vanilloid receptor‐1 is essential for inflammatory thermal hyperalgesia. Nature, 405, 183–187. 10.1038/35012076 [DOI] [PubMed] [Google Scholar]
- De Petrocellis, L. , Ligresti, A. , Moriello, A. S. , Allarà, M. , Bisogno, T. , Petrosino, S. , et al. (2011). Effects of cannabinoids and cannabinoid‐enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British Journal of Pharmacology, 163, 1479–1494. 10.1111/j.1476-5381.2010.01166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo, V. , Stella, N. , & Zimmer, A. (2014). Endocannabinoid signalling and the deteriorating brain. Nature Reviews. Neuroscience, 16, 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, J. , Meseguer, F. , Faura, C. , Moore, R. A. , McQuay, H. J. , & Derry, S. (2010). Single dose dipyrone for acute postoperative pain. Cochrane Database of Systematic Reviews, 1(1), 1–41, CD003227 10.1002/14651858.CD003227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravanti, B. , De Felice, M. , Stucky, C. L. , Medler, K. A. , Luo, M.‐C. , Gardell, L. R. , et al. (2008). Constitutive activity at the cannabinoid CB1 receptor is required for behavioral response to noxious chemical stimulation of TRPV1: Antinociceptive actions of CB1 inverse agonists. The Journal of Neuroscience, 28, 11593–11602. 10.1523/JNEUROSCI.3322-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, H. , De Petrocellis, L. , Bisogno, T. , Moriello, A. S. , Lutz, B. , & Di Marzo, V. (2003). Dual effect of cannabinoid CB1 receptor stimulation on a vanilloid VR1 receptor‐mediated response. Cellular and Molecular Life Sciences, 60, 607–616. 10.1007/s000180300052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. (2001). Ion channels of excitable membranes (3rd ed.). [Google Scholar]
- Hoffmann, J. , Supronsinchai, W. , Andreou, A. P. , Summ, O. , Akerman, S. , & Goadsby, P. J. (2012). Olvanil acts on transient receptor potential vanilloid channel 1 and cannabinoid receptors to modulate neuronal transmission in the trigeminovascular system. Pain, 153, 2226–2232. 10.1016/j.pain.2012.07.006 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner, K. , Siklosi, N. , Babes, A. , Khalil, M. , Selescu, T. , Zimmermann, K. , … Engel, M. A. (2016). Systemic desensitization through TRPA1 channels by capsazepine and mustard oil ‐ a novel strategy against inflammation and pain. Scientific Reports, 6(1), 28611–28622. 10.1038/srep28621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplas, P. A. , Rosenberg, R. L. , & Oxford, G. S. (1997). The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. The Journal of Neuroscience, 17, 3525–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte, R. H. , Shain, C. N. , Simone, D. A. , & Tsai, E. F. (1991). Neurogenic hyperalgesia: Psychophysical studies of underlying mechanisms. Journal of Neurophysiology, 66, 190–211. 10.1152/jn.1991.66.1.190 [DOI] [PubMed] [Google Scholar]
- Lin, C. R. , Amaya, F. , Barrett, L. , Wang, H. , Takada, J. , Samad, T. A. , & Woolf, C. J. (2006). Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. The Journal of Pharmacology and Experimental Therapeutics, 319, 1096–1103. 10.1124/jpet.106.105569 [DOI] [PubMed] [Google Scholar]
- Lopshire, J. C. , & Nicol, G. D. (1998). The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin‐elicited current in rat sensory neurons: Whole‐cell and single‐channel studies. The Journal of Neuroscience, 18, 6081–6092. 10.1523/JNEUROSCI.18-16-06081.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti, B. B. , & Ferreira, S. H. (1985). Mode of analgesic action of dipyrone: Direct antagonism of inflammatory hyperalgesia. European Journal of Pharmacology, 114, 375–381. 10.1016/0014-2999(85)90383-8 [DOI] [PubMed] [Google Scholar]
- Mahmud, A. , Santha, P. , Paule, C. C. , & Nagy, I. (2009). Cannabinoid 1 receptor activation inhibits transient receptor potential vanilloid type 1 receptor‐mediated cationic influx into rat cultured primary sensory neurons. Neuroscience, 162, 1202–1211. 10.1016/j.neuroscience.2009.05.024 [DOI] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP. British Journal of Pharmacology, 172(13), 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara, C. R. , Mandel‐Brehm, J. , Bautista, D. M. , Siemens, J. , Deranian, K. L. , Zhao, M. , … Fanger, C. M. (2007). TRPA1 mediates formalin‐induced pain. Proceedings of the National Academy of Sciences of the United States of America, 104, 13525–13530. 10.1073/pnas.0705924104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melck, D. , Bisogno, T. , De Petrocellis, L. , Chuang, H. H. , Julius, D. , Bifulco, M. , et al. (1999). Unsaturated long‐chain N‐acyl‐vanillyl‐amides (N‐AVAMs): Vanilloid receptor ligands that inhibit anandamide‐facilitated transport and bind to CB1 cannabinoid receptors. Biochemical and Biophysical Research Communications, 262, 275–284. 10.1006/bbrc.1999.1105 [DOI] [PubMed] [Google Scholar]
- Mistry, S. , Paule, C. C. , Varga, A. , Photiou, A. , Jenes, A. , Avelino, A. , … Nagy, I. (2014). Prolonged exposure to bradykinin and prostaglandin E2 increases TRPV1 mRNA but does not alter TRPV1 and TRPV1b protein expression in cultured rat primary sensory neurons. Neuroscience Letters, 564, 89–93. 10.1016/j.neulet.2014.02.006 [DOI] [PubMed] [Google Scholar]
- Moriyama, T. , Higashi, T. , Togashi, K. , Iida, T. , Segi, E. , Sugimoto, Y. , … Tominaga, M. (2005). Sensitization of TRPV1 by EP1and IP Reveals Peripheral Nociceptive Mechanism of Prostaglandins. Molecular Pain, 1(1), 1744–8069. 10.1186/1744-8069-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassini, R. , Fusi, C. , Materazzi, S. , Coppi, E. , Tuccinardi, T. , Marone, I. M. , … Benemei, S. (2015). The TRPA1 channel mediates the analgesic action of dipyrone and pyrazolone derivatives. British Journal of Pharmacology, 172, 3397–3411. 10.1111/bph.13129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerandzic, V. , Mrozkova, P. , Adamek, P. , Spicarova, D. , Nagy, I. , & Palecek, J. (2018). Peripheral inflammation affects modulation of nociceptive synaptic transmission in the spinal cord induced by N‐arachidonoylphosphatidylethanolamine. British Journal of Pharmacology, 175(12), 2322–2336. 10.1111/bph.13849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, M. , Patwardhan, A. , Salas, M. M. , Hargreaves, K. M. , & Akopian, A. N. (2011). Cannabinoid receptor antagonists AM251 and AM630 activate TRPA1 in sensory neurons. Neuropharmacology, 61, 778–788. 10.1016/j.neuropharm.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, M. J. , Salas, M. , Bialuhin, S. , Boyd, J. T. , Jeske, N. A. , & Akopian, A. N. (2020). Sensitization of small‐diameter sensory neurons is controlled by TRPV1 and TRPA1 association. The FASEB Journal, 34, 287–302. 10.1096/fj.201902026R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan, A. M. , Jeske, N. A. , Price, T. J. , Gamper, N. , Akopian, A. N. , & Hargreaves, K. M. (2006). The cannabinoid WIN 55,212‐2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proceedings of the National Academy of Sciences, 103, 11393–11398. 10.1073/pnas.0603861103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre, S. C. , Schmidt, R. , Brenneis, C. , Michaelis, M. , Geisslinger, G. , & Scholich, K. (2007). Inhibition of cyclooxygenases by dipyrone. British Journal of Pharmacology, 151, 494–503. 10.1038/sj.bjp.0707239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisinghani, M. , Pabbidi, R. M. , & Premkumar, L. S. (2005). Activation of transient receptor potential vanilloid 1 (TRPV1) by resiniferatoxin. The Journal of Physiology, 567, 771–786. 10.1113/jphysiol.2005.087874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogosch, T. , Sinning, C. , Podlewski, A. , Watzer, B. , Schlosburg, J. , Lichtman, A. H. , … Imming, P. (2012). Novel bioactive metabolites of dipyrone (metamizol). Bioorganic & Medicinal Chemistry, 20, 101–107. 10.1016/j.bmc.2011.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, T. R. L. , & Duarte, I. D. G. (2013). Involvement of ATP‐sensitive K+ channels in the peripheral antinociceptive effect induced by ketamine. Veterinary Anaesthesia and Analgesia, 40, 419–424. 10.1111/vaa.12024 [DOI] [PubMed] [Google Scholar]
- Sacerdote, P. , Massi, P. , Panerai, A. E. , & Parolaro, D. (2000). In vivo and in vitro treatment with the synthetic cannabinoid CP55,940 decreases the in vitro migration of macrophages in the rat: Involvement of both CB1 and CB2 receptors. Journal of Neuroimmunology, 109, 155–163. 10.1016/s0165-5728(00)00307-6 [DOI] [PubMed] [Google Scholar]
- Sántha, P. , Jenes, A. , Somogyi, C. , & Nagy, I. (2010). The endogenous cannabinoid anandamide inhibits transient receptor potential vanilloid type 1 receptor‐mediated currents in rat cultured primary sensory neurons. Acta Physiologica Hungarica, 97, 149–158. 10.1556/APhysiol.97.2010.2.1 [DOI] [PubMed] [Google Scholar]
- Santos, G. D. , Vieira Dias, E. , Teixeira, J. M. , Carolina, M. , Athie, M. C. P. , Magayewski Bonet, I. J. , … Parada, C. A. (2014). The analgesic effect of dipyrone in peripheral tissue involves two different mechanisms: Neuronal K ATP channel opening and CB 1 receptor activation. European Journal of Pharmacology, 741, 124–131. 10.1016/j.ejphar.2014.07.019 [DOI] [PubMed] [Google Scholar]
- Schenk, S. A. , Dick, F. , Herzog, C. , Eberhardt, M. J. , & Leffler, A. (2019). Active metabolites of dipyrone induce a redox‐dependent activation of the ion channels TRPA1 and TRPV1. PAIN Reports, 4(3), e720–730 10.1097/pr9.0000000000000720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ständer, S. , Schmelz, M. , Metze, D. , Luger, T. , & Rukwied, R. (2005). Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. Journal of Dermatological Science, 38, 177–188. 10.1016/j.jdermsci.2005.01.007 [DOI] [PubMed] [Google Scholar]
- St‐Jacques, B. , & Ma, W. (2013). Prostaglandin E2/EP4 signalling facilitates EP4 receptor externalization in primary sensory neurons in vitro and in vivo. Pain, 154, 313–323. 10.1016/j.pain.2012.11.005 [DOI] [PubMed] [Google Scholar]
- Taylor, B. K. , Peterson, M. A. , & Basbaum, A. I. (1995). Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. The Journal of Neuroscience, 15(11), 7575–7584. 10.1523/jneurosci.15-11-07575.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjølsen, A. , Berge, O. G. , Hunskaar, S. , Rosland, J. H. , & Hole, K. (1992). The formalin test: An evaluation of the method. Pain, 51, 5–17. 10.1016/0304-3959(92)90003-T [DOI] [PubMed] [Google Scholar]
- Vásquez, C. , Navarro‐Polanco, R. A. , Huerta, M. , Trujillo, X. , Andrade, F. , Trujillo‐Hernández, B. , & Hernández, L. (2003). Effects of cannabinoids on endogenous K+ and Ca2+ currents in HEK293 cells. Canadian Journal of Physiology and Pharmacology, 81, 436–442. 10.1139/y03-055 [DOI] [PubMed] [Google Scholar]
- Vazquez, E. , Hernandez, N. , Escobar, W. , & Vanegas, H. (2005). Antinociception induced by intravenous dipyrone (metamizol) upon dorsal horn neurons: Involvement of endogenous opioids at the periaqueductal gray matter, the nucleus raphe magnus, and the spinal cord in rats. Brain Research, 1048, 211–217. 10.1016/j.brainres.2005.04.083 [DOI] [PubMed] [Google Scholar]
- Veress, G. , Meszar, Z. , Muszil, D. , Avelino, A. , Matesz, K. , Mackie, K. , & Nagy, I. (2013). Characterisation of cannabinoid 1 receptor expression in the perikarya, and peripheral and spinal processes of primary sensory neurons. Brain Structure & Function, 218, 733–750. 10.1007/s00429-012-0425-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos, G. G. , Verri, W. A. Jr. , Cunha, T. M. , Schivo, I. R. S. , Parada, C. A. , Cunha, F. Q. , & Ferreira, S. H. (2004). An electronic pressure‐meter nociception paw test for rats. Brazilian Journal of Medical and Biological Research, 37, 391–399. [DOI] [PubMed] [Google Scholar]
- Zimmermann, M. (1983). Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16, 109–110. 10.1016/0304-3959(83)90201-4 [DOI] [PubMed] [Google Scholar]


