Abstract
Recent advances in human induced pluripotent stem cell (iPSC) technology may provide unprecedented opportunities to study patient‐specific responses to anaesthetics and opioids. In this review, we will (1) examine the advantages and limitations of iPSC technology, (2) summarize studies using iPSCs that have contributed to our current understanding of anaesthetics and opioid action on the cardiovascular system and central nervous system (CNS), and (3) describe how iPSC technology can be used to further develop personalized analgesic and sedative pharmacotherapies with reduced or minimal detrimental cardiovascular effects.
Keywords: anaesthetics, brain, cardiomyocyte, iPSC, ketamine, opioids, propofol
Abbreviations
- DARPA
Defense Advanced Research Projects Agency
- EHT
engineered heart tissue
- FDA
US Food and Drug Administration
- HTS
high‐throughput screening
- iPSC
induced pluripotent stem cell
- iPSC‐CM
iPSC‐derived cardiomyocyte
- iPSC‐NC
iPSC‐derived neuronal cell
- MEA
multi‐electrode array
- NCATS
US National Center for Advancing Translational Science
- OSKM
Oct3/4, Sox2, Klf4, and c‐Myc
- PDCD4
programmed cell death protein 4
- scRNA‐seq
single‐cell RNA sequencing
1. INTRODUCTION
Drug development efforts frequently fail because cell culture or small‐animal models do not adequately replicate human physiology (Cook et al., 2014). The discovery by Nobel laureate Dr. Shinya Yamanaka that activation of only four different transcription factors (Oct3/4, Sox2, Klf4, and c‐Myc; also known as “OSKM”) can transform terminally differentiated somatic cells into induced pluripotent stem cells (iPSCs) (Takahashi & Yamanaka, 2006) has enabled the unlimited generation of human iPSC‐derived cardiomyocytes (iPSC‐CMs) and neuronal cells (iPSC‐NCs), among other cells of interest. Historically, these two cell types are difficult to obtain from humans and have limited accessibility for genetic, functional, and drug testing. To date, iPSC technologies have already led to new drug discoveries, but how would it accelerate the progress towards precision medicine in anaesthesia, perioperative and pain medicine? To address this question, we will first briefly describe the main receptor systems relevant to anaesthesia. In the next section, we will elucidate the current methods available for anaesthetic drug development and testing using iPSC‐CMs and iPSC‐NCs. In the concluding section, we will use the previously described receptor systems as examples of how iPSC‐CMs and iPSC‐NCs can be utilized to evaluate anaesthetic drugs and to discover individualized drug reactions. We will focus on cardiomyocytes and neuronal cells, as they are among the most relevant cells in determining immediate and long‐term outcomes of patients after surgery.
2. BACKGROUND
For the past several decades, development of new anaesthetics has stagnated for at least two reasons: (1) Finding new small molecules or compounds with the potential to induce anaesthesia has been challenging, and the development of new drugs is both time consuming and costly. (2) Anaesthesia has been considered safe and served its designated purpose well (i.e., inducing unconsciousness during medical procedures). However, the analysis of improved electronic medical records (EMRs) revealed that anaesthetic and pain medications might have unforeseen side effects as the following examples illustrate:
Anaesthetics have been shown to negatively affect postoperative brain function in elderly patients (Hardcastle et al., 2019) and increase postoperative delirium (Shaw et al., 1987; Shi et al., 2019; Sprung et al., 2017) (for review, see Mahanna‐Gabrielli et al., 2019). In addition, these drugs are suspected to be associated with congenital heart disease and brain defects as well as autism spectrum disorders (Brambrink et al., 2010; Davidson et al., 2016).
Individual responses to anaesthetics and pain medications may depend on genetic differences. For instance, while the number and type of Na+ channels expressed within the dorsal ganglion may explain the diverse response to analgesics in chronic pain patients (Chang et al., 2018; Meents et al., 2019), other patients suffer from a condition labelled as “triple low” (i.e., patients presenting with low BP and low cerebral electrical activity despite being exposed to a low concentration of anaesthetics) that increases perioperative morbidity and mortality (Sessler et al., 2012, 2019). These associations suggest a strong link between pharmacogenetics and perioperative cardiovascular and cerebral outcome.
Patients frequently take more than 10 different medications at the time when anaesthetics are administered (Reynolds, Pierce, Weitendorf, & Linder, 2017), which raises concerns about drug–drug and/or gene–drug interactions. It appears that perioperative outcome worsens in patients receiving a multitude of home medications (McIsaac, Wong, Bryson, & van Walraven, 2018). Therefore, personalizing medicine may reduce adverse side effects by avoiding unwanted drug–drug interactions.
By collecting somatic cells, for instance from peripheral blood or skin biopsy, directly from a patient and reprogramming those cells into iPSCs, any desired somatic cells with the patient's genetic information can then be differentiated. While genetic testing will provide some information about the genetic variant leading to a patient‐specific phenotype, these results are often confounded by a variant of unknown significance for certain pathogenicity. For example, investigators have used iPSC and genome editing technologies to help classify variants of unknown significance as either pathogenic in long QT syndrome (Garg et al., 2018) or benign in hypertrophic cardiomyopathy (Ma et al., 2018). These examples illustrate how iPSC can be used to identify patient‐specific variance resulting in clinical phenotypes. In addition, patient‐specific iPSC derivatives can be exposed to a range of drugs to detect drug–drug and/or drug–gene interactions (Lau, Paik, & Wu, 2019), test new anaesthetic compounds (Chang et al., 2020), complement gene‐editing strategies (Christidi, Huang, & Brunham, 2018), and discover new potential drug targets (Paik, Chandy, & Wu, 2020) (Figure 1). Multiple commercially produced human iPSC‐CM and iPSC‐NC lines are currently available (Table 1). Nevertheless, individualized drugs and treatment plans should be based on studies of iPSCs from a specific patient rather than a uniform iPSC stock, to achieve personalized patient care.
FIGURE 1.
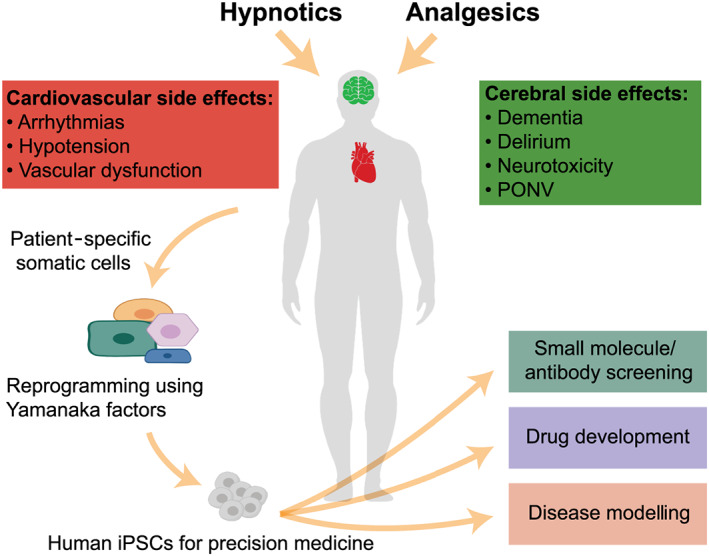
Currently used anaesthetics cause a multitude of cerebral and cardiovascular side effects. Reprogramming of patient‐specific somatic cells into induced pluripotent stem cells (iPSCs) allows the generation of iPSC‐derived cardiomyocytes and iPSC‐derived neuronal cells for later small molecule/antibody screening, drug development, and disease modelling. PONV, postoperative nausea and vomiting
TABLE 1.
Commercially available ESC and iPSC lines for in vitro cell‐based anaesthesia studies
| Cell type | Drug | Main question | Receptor | Ref. |
|---|---|---|---|---|
| NSC/NPC | Dynorphin, U50,488 | Function | OPRK1 | (Sheng et al., 2007) |
| ESC‐derived NC (H1 cell line, WiCell Res. Institute) | Isoflurane | Neurogenesis | GABAA | (Zhao et al., 2013) |
| Propofol | Cytotoxicity | GABAA | (Twaroski et al., 2014) | |
| Propofol | Cytotoxicity | GABAA | (Twaroski et al., 2015) | |
| Ketamine | Cytotoxicity | NMDA | (Bosnjak et al., 2012) | |
| iPSC‐derived dopaminergic neurons, ReNcell® | Ketamine | Cytotoxicity | NMDA | (Ito et al., 2015) |
| iPSC‐NC (iCell®) | Diazepam | Receptor expression | GABAA | (Yuan et al., 2016) |
| iPSC‐CM (ReproCardio2®) | Propofol | Toxicity | GABAA | (Kido et al., 2018) |
| iPSC‐CM (iCell®) | Milrinone, citalopram, nifedipine, lidocaine | EHT function | Multiple | (Mannhardt et al., 2017) |
Abbreviations: EHT, engineered heart tissue; NPC, neuronal progenitor cell; NSC, neuronal stem cell; OPRK1, κ‐opioid receptor.
Using iPSCs to understand how anaesthetic drugs affect cardiac and neuronal cells is extremely valuable because anaesthetic receptor–ligand interactions are overly complex. Typical allosteric modulators can either enhance or diminish receptor signalling in response to an anaesthetic, but new drugs may act on both allosteric and orthosteric binding sites (Wisler, Rockman, & Lefkowitz, 2018). Receptor homodimers may also react differently to drugs than heterodimeric receptors (Cechova, Lan, Barthes, Jung, & Ulbrich, 2020). Finally, many of these receptors exhibit tissue‐specific expression and multiple splice variants (Barker et al., 1993; Knierim et al., 2019; Michel et al., 2014) (Figure 2).
FIGURE 2.
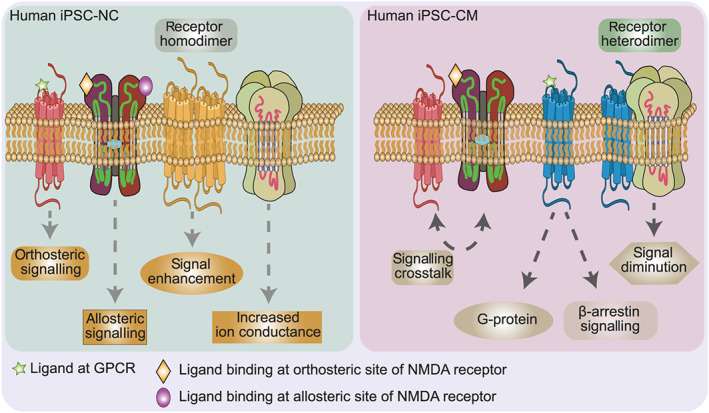
iPSC technology allows the study of anaesthesia‐relevant receptors on human iPSC‐derived cardiomyocytes (iPSC‐CMs) and iPSC‐derived neuronal cells (iPSC‐NCs). These cells reflect the cell type‐specific receptor expression profile of individual patients. The iPSC technology can also be used to determine cell‐type‐specific isoforms of receptors, as well as receptor co‐localization and clustering (i.e., homodimerization or heterodimerization), allosteric modulation, and cell‐type‐specific receptor densities. This Figure shows a schematic expression of GABA receptors, NMDA receptors, and/or GPCR opioid receptors in iPSC‐CMs and iPSC‐NCs to illustrate potential patient‐ and tissue‐specific signalling and receptor differences
A comprehensive review of all receptors and ion channels within the cardiomyocytes and neuronal cells is beyond the scope of this review. Instead, we will use three major receptor systems ‐ Gamma‐aminobutyric acid (GABA) receptors, N‐Methyl‐d‐aspartate (NMDA) receptors, and G‐protein coupled receptors (GPCRs) ‐ (Figure 3) to illustrate how iPSC‐CMs and iPSC‐NCs have been used to evaluate the toxicity and function of anaesthetic drugs.
FIGURE 3.
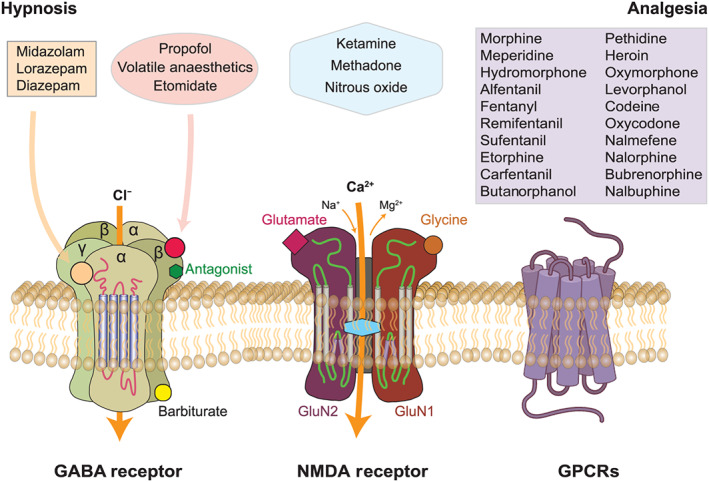
Hypnotic and analgesic properties of common anaesthetics are mediated through three receptor systems: GABA receptors, NMDA receptors, and/or GPCR opioid receptors
2.1. GABAA receptors
Most of the commonly used hypnotics such as propofol, or volatile anaesthetics, such as isoflurane, mediate their effect via GABAA receptors, a pentameric ligand‐gated ion channel assembled from different subunits encoded in 19 different genes (Olsen & Sieghart, 2009). The response to ligands depends on subunit composition (i.e., α2βγ2 subunits: anxiolytic effect of diazepam; α5βγ2 subunits: learning and memory, Olsen & Sieghart, 2009) and cell or tissue type. After binding of the anaesthetic, a central pore within the receptor opens, which increases chloride conductance and hyperpolarizes the post‐synaptic membrane (Garcia, Kolesky, & Jenkins, 2010; Nourmahnad et al., 2016). Functional GABAA receptors are considered a hallmark of mature iPSC‐NCs (Badja et al., 2014).
2.2. NMDA receptors
Blockade of NMDA receptors by certain anaesthetics (e.g., ketamine, nitrous oxide, and methadone) plays a crucial role in preventing postoperative chronic pain. The NMDA receptor is a Ca2+‐conducting glutamate receptor mainly located within the central nervous sytem (CNS), although some forms of it are abundant within the cardiovascular system (Leung et al., 2002). NMDA receptors assemble as obligate heteromers that may draw from GluN1, GluN2A, GluN2B, GluN2C, GluN2D, GluN3A, and GluN3B subunits (Alexander, Mathie, et al., 2019). Depending on where they are expressed, NMDA receptors can promote cell survival, cause cell death, contribute to neurodegeneration, or play a role in vascular remodelling that is associated with pulmonary hypertension (Dumas et al., 2018). While synaptic NMDA receptors are believed to promote cell survival, recent data suggest that the activation of extrasynaptic NMDA receptors might cause cell death and could be involved in neurodegenerative diseases such as Alzheimer's disease (Hardingham & Bading, 2010). NMDA receptors have recently gained attention with reports that blockade by the anaesthetic ketamine improved treatment‐resistant depression (Y. Yang, Cui, et al., 2018).
2.3. GPCRs
Most perioperative prescribed medications (i.e., opioids, antiemetics, and vasopressors) mediate their effects through GPCRs. This highly diverse group of more than 1,000 different receptors regulates a wide array of intracellular signalling pathways. iPSC‐CMs express GPCRs similar to those of native cardiomyocytes, and GPCR signalling has been associated with dilated cardiomyopathy (Wu et al., 2015), hypertrophic (Han et al., 2014; Lan et al., 2013), arrhythmogenic right ventricular (Kim et al., 2013), and diabetic cardiomyopathies (Drawnel et al., 2014). iPSC‐CMs might be an excellent model to study anaesthetic‐induced cardiomyopathies. Similarly, GPCRs, particularly opioid receptors, have been well described within different brain regions (Bachmutsky, Wei, Kish, & Yackle, 2020; Kieffer, 1999; Peng, Sarkar, & Chang, 2012), and in situ hybridization and microarray data revealed the expression of neuropeptides and their corresponding GPCR within different human brain regions (i.e., Allen Brain Atlas, Hawrylycz et al., 2012). Since iPSCs express a variety of GPCRs, they constitute an ideal platform to study GPCR function using multiple biosensors (for in‐depth reviews, see Kauk & Hoffmann, 2018; Lohse, Nuber, & Hoffmann, 2012) or to develop tissue‐specific drugs with fewer unwanted side effects.
In summary, human iPSC‐CMs and iPSC‐NCs have been used in a wide range of applications, from monolayers or co‐culture systems to complex organoids. The different testing modalities are discussed in the next paragraph.
3. PHARMACOLOGICAL TESTING IN iPSC‐CMs AND iPSC‐NCs
The pharmacological profile of a compound differs depending on whether a 2D monolayer or a 3D tissue/organoid platform will be used as microenvironmental signals can boost somatic plasticity, metabolism, function, and cell survival (Caiazzo et al., 2016; Luca et al., 2013). The following sections will review techniques to evaluate iPSC‐CMs and iPSC‐NCs for pharmacological testing. While 3D tissues describe iPSC‐derived simple cell structures aligned in certain porous 3D scaffolds fabricated with hydrogels (i.e., engineered heart tissue [EHT], Zimmermann & Cesnjevar, 2009), 3D organoids are defined as tiny, self‐organized, three‐dimensional tissues reflecting the cell composition and function of an organ such as a heart or brain (Liu, Oikonomopoulos, Sayed, & Wu, 2018; Xia & Izpisua Belmonte, 2019) in which the interactions between multiple different cell types can be evaluated. The advantage of 3D tissues consists of its scalability for high‐throughput screening (HTS) in contrast to 3D organoids which better reflect organ‐specific cell organization and cell–cell interaction.
3.1. iPSC‐derived cardiovascular cells/tissues/organoids
Using gene‐editing strategies, iPSC‐CMs have been generated that mimic diverse genetic cardiovascular disorders (Chang et al., 2016; Davis et al., 2016; Liang et al., 2016; Paci, Passini, Severi, Hyttinen, & Rodriguez, 2017; Sun et al., 2012; Wu et al., 2015; K.‐C. Yang, Breitbart, et al., 2018). Depending on the differentiation protocol, functional iPSC‐CMs (i.e., contracting cells) can be detected within 7–9 days of differentiation (Burridge, Holmström, & Wu, 2015). Unfortunately, these cells are relatively immature (see Sayed, Liu, & Wu, 2016) and require an additional 90–120 days to express a more adult‐like phenotype (Ebert et al., 2019). The maturation process has been successfully expedited either by exposure to mechanical stress (Abilez et al., 2018; Leonard et al., 2018) or by adjusting culture conditions (i.e., using conductive biomimetic surface materials, Wang et al., 2017). The resulting changes in myofibril ultrastructure, Ca2+ handling, and electrophysiological properties need to be considered in pharmacological or toxicological studies. A variety of different assays and tests have been developed to describe iPSC‐CM function, as described below.
Perioperative medications often affect a multitude of cardiovascular ion channels, thereby directly influencing electrophysiological properties of a variety of cardiac cells. Preclinical assessment of drug–drug or drug–gene interactions altering QTc duration is essential as QTc prolongation constitutes a critical benchmark during drug development (Blinova et al., 2019). Many methods, including the use of patch‐clamp techniques (Neher & Sakmann, 1976) and different fluorescence dyes for HTS (Bedut et al., 2016; Lee et al., 2012; Shinnawi et al., 2015), have allowed investigators to record electrophysiological changes of iPSC‐CMs. For example, ACEA Bioscience recently introduced a CardioECR platform that combines a multi‐electrode array (MEA) and contractility measurements for comprehensive excitation–contraction coupling analysis (Guo, Eldridge, Furniss, Mussio, & Davis, 2015). While this system was limited to short‐term recordings, newer approaches, based on nanomeshes, now allow investigators to record electrical properties of iPSC‐CMs over 96 h (Lee et al., 2019).
While measuring the contractility of iPSC‐CMs in 2D culture is well described (Burridge, Sharma, & Wu, 2015; Zhang et al., 2019), EHT platforms require different measurement methods. Hydrogel‐based traction force microscopy (Xie, Hawkins, & Sun, 2017) and the deflection of microposts of a polydimethyl‐siloxane micropost array are currently used to calculate contractile force (Beussman et al., 2016). Recent advances in microscopy and visual data analysis allow investigators to monitor fluorescently tagged sarcomere movements of pharmacologically challenged or genetically altered iPSC‐CMs (Toepfer et al., 2019). The introduction of piezo‐bending actuators will make EHTs more suitable for drug HTS (Mannhardt, Warncke, Trieu, Müller, & Eschenhagen, 2019).
More recently, microphysiological (“heart‐on‐a‐chip”) systems have been developed, allowing the study of structure and function of native tissues in vitro. With the evolvement of multi‐material 3D printing, Lind et al. (2017) assembled microchips to study contractile development and electrophysiology in iPSC‐CMs. These microchips can be produced in a programmable microfabrication process, which will allow high‐throughput tissue engineering for toxicology and drug screening purposes in the future (see Liu et al., 2018). The importance of this technology becomes evident by the fact that the US National Center for Advancing Translational Science (NCATS) developed partnerships with the US Food and Drug Administration (FDA) and the Defense Advanced Research Projects Agency (DARPA), providing $76 million over 5 years for developing and validating compounds, assays, and biomarkers in microphysiological systems (Sutherland, Fabre, & Tagle, 2013).
As the quality of bioprinting improves (see Moroni et al., 2018), the generation of 3D electro‐mechanically coupled, fluid‐ejecting miniature ventricle‐like cardiac organoid chambers (Li et al., 2018) and vascularized cardiac and brain organoids are becoming available (Bordoni et al., 2018; Cakir et al., 2019; Mansour et al., 2018).
These new techniques may soon allow us to study anaesthetics in progressively more sophisticated human heart tissues that more closely reflect natural cellular and mechanical properties.
3.2. iPSC‐derived neuronal cells/tissues/organoids
Although most tests conducted in iPSC‐CMs are also applicable with slight modifications in iPSC‐NCs, electrophysiological studies in iPSC‐CMs and iPSC‐NCs differ significantly. Human iPSC‐CMs build a homogenous syncytium of similar cells and mainly transfer electrical signals via gap junctions and voltage‐gated ion channels (Stoppel, Kaplan, & Black, 2016). By contrast, mature neuronal cells send their projections to a variety of different cell types and mainly communicate through synapses within complex neuronal networks. Functional 3D neuronal networks comprising astrocytes, oligodendroglia, and neurons have been described (Izsak et al., 2019). The cell heterogeneity makes it harder to assess genetic and electrophysiological properties, but newer techniques (i.e., “Patch‐seq,” combined patch‐clamp electrophysiological measurement and single‐cell RNA sequencing [scRNA‐seq; Fuzik et al., 2016], HTS [McKeithan et al., 2017; Sharma et al., 2017], Ca2+ screens [Jones & Bunnage, 2017; Stacey et al., 2018], and 3D tissue chips [Spira, Shmoel, Huang, & Erez, 2018]) may help to solve some of these challenges.
Through modifying culture conditions, iPSC‐derived neurospheres (Lancaster et al., 2013; Pacitti, Privolizzi, & Bax, 2019) can be differentiated into a variety of different brain regions (Jo et al., 2016; Qian et al., 2016). Neurospheres cultured in cell suspension have been directly differentiated into cortical cells (Paşca et al., 2015), cerebellar cells (Muguruma, Nishiyama, Kawakami, Hashimoto, & Sasai, 2015), hippocampal‐choroid plexus organoids (Sakaguchi et al., 2015), and human forebrain structures with reproducible numbers and diversity of neuronal cells types (Velasco et al., 2019). Although these organoids are already being used successfully to develop pharmacological tests and drugs to counteract neurodegenerative diseases (Choi et al., 2014; Park et al., 2018) and cerebral malignancies (Plummer et al., 2019), the technology remains underutilized for testing new anaesthetics and analgesic drugs (Table 1). Recently, an optical screening tool was able to test 5,215 bioactive compounds for their specific effects on biological pathways and molecular targets by automatically determining nuclear and neurite characteristics (Sherman & Bang, 2018). In addition to expected hits (i.e., kinase inhibitors), these investigators discovered that the Na+‐channel blocker dibucaine activated and the δ‐opioid receptor blocker BNTX inhibited neurite growth.
A unique subtype of neuronal organoids reflects the blood–brain barrier, which is a cellular structure built of pericytes, astrocytes, and neurons that protects the brain from circulating toxins or pathogens, while allowing vital nutrients to reach cerebral structures. As the blood–brain barrier communicates directly with specified microvascular endothelial cells (Campisi et al., 2018), it is a substantial barrier to overcome for potential new anaesthetics. Therefore, iPSC‐derived blood–brain barrier‐forming experimental models are of particular interest (Jamieson, Linville, Ding, Gerecht, & Searson, 2019) because they allow the study of transporter proteins responsible for transfer of molecules and drugs such as opioids (Chaves, Remiao, Cisternino, & Decleves, 2017). With the rapid advancement of organ chip technology (Vatine et al., 2019), iPSC‐derived neuronal‐vascular units (Campisi et al., 2018; Park et al., 2019) are becoming readily available to study these mechanisms.
4. USING iPSC TECHNOLOGY TO EVALUATE ANAESTHETIC AGENTS IN CARDIOVASCULAR AND NEURONAL TISSUES
As discussed above, iPSC technology has facilitated the development and validation of new agents in different medical specialities but has been limited in use for perioperative medicine. By focusing on the receptor systems described above, the following section highlights the use of iPSC technology for evaluating anaesthetic effects on cardiovascular and neuronal tissues (Figure 4 and Table 2).
FIGURE 4.
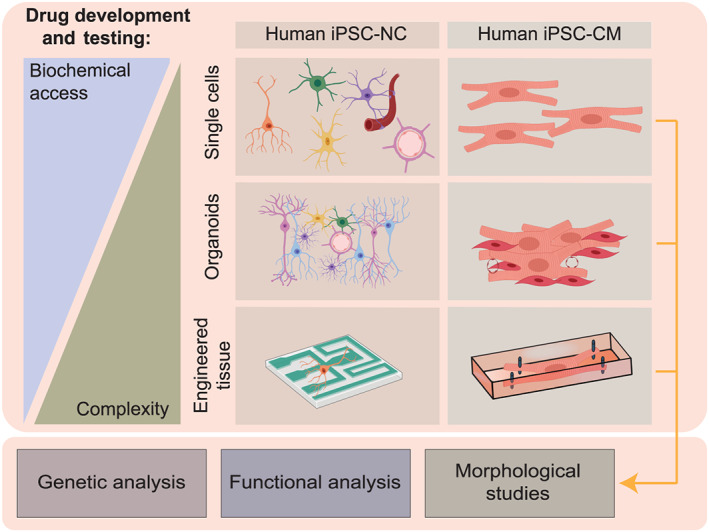
While human cardiac and neuronal cells are usually not directly accessible for new drug testing, reprogramming patient‐specific somatic cells to iPSC‐CMs and iPSC‐NCs can facilitate genetic, functional, and/or morphological studies. While assays using single‐cell preparations are simple in design and may provide maximum insight into biochemical processes, experimental models using engineered tissues increase in complexity and allow more physiological assessments
TABLE 2.
Patient‐derived iPSC lines for testing anaesthesia‐relevant medication
| Cell type | Drug | Study type | Tests | Gene targets | Drug function | Ref. |
|---|---|---|---|---|---|---|
| iPSC‐NC | Levetiracetam | Treatment of SMA | Cell viability, function | CACNA1B, ABCB1 | Inh | (Ando et al., 2019) |
| iPSC‐NC | Lacosamide | Treatment of SFN | Electrophysiology | SCN3A, SCN9A, SCN10A | B | (Namer et al., 2019) |
| iPSC‐NC | VPA | Function gene expression | Dopamine transporter function | DRD2, OPRK1, OPRD1 | PA | (Sheng et al., 2016a) |
| iPSC‐NC | VPA | Opioid dependency | DRD2 | PA | (Sheng et al., 2016b) | |
| iPSC‐NC | Ketamine | Neuroplasticity | Dendritic growth | GluR1, GluR2 | Off‐target | (Collo et al., 2019) |
| iPSC‐NC | Ketamine, diazepam | Neuronal network communication | Receptor function and signal transmission | GRIN1, GRIN2A, GRIN2B, GRIN2C, GrIN2D, CHRNA7, GABRA1 | AG, PAM | (Larsen, Hansen, Mikkelsen, Hyttel, & Stummann, 2019) |
| iPSC‐NC | (2R,6R)‐Hydroxynorketamine | Neuroplasticity | Dendrite growth | AMPA | Off‐target | (Collo, Cavalleri, Chiamulera, & Merlo Pich, 2018) |
| iPSC‐CM | VPA, diazepam, acetaminophen, phenobarbital | Cardiotoxicity screening | Functional assessment, cell viability, mitochondrial toxicity | N/A | On‐target/off‐target | (Sirenko et al., 2017) |
| iPSC‐CM | KSEB01‐S2, propofol, etomidate | Cardiotoxicity screening | Functional assessment | N/A | Off‐target | Chang et al., 2020) |
Note: Gene targets, according to the Concise Guide to PHARMACOLOGY (Alexander, Christopoulos, et al., 2019).
Abbreviations: AG, antagonist; B, blocker; DA, dopaminergic; N/A, not applicable; Inh, inhibitor; PA, partial agonist; PAM, positive allosteric modulator; SFN, small fibre neuropathy; SMA, spinal muscular atrophy; VPA, valproic acid.
4.1. GABAA receptors
Propofol is by far the most often used intravenous anaesthetic acting on GABAA receptors. Despite its favourable safety profile, administration of high doses of propofol for prolonged period of time has been associated with a life‐threatening condition called “propofol infusion syndrome” (Hemphill, McMenamin, Bellamy, & Hopkins, 2019). Propofol‐induced mitochondrial dysfunction has been proposed as a key mechanism. Kido, Ito, Yamamoto, Makita, and Uchida (2018) treated iPSC‐CMs with different propofol concentrations (0–50 μg·ml−1) for 48 hours and measured mitochondrial function. At concentrations above 10 μg·ml−1, propofol caused mitochondrial dysfunction while higher concentrations induced apoptosis.
Effects of different intravenous anaesthetics on cell survival and proliferation have been studied using human embryonic stem cell (ESC)‐derived neurons (Twaroski, Yan, Olson, Bosnjak, & Bai, 2014). In this case, propofol induced cell death via down‐regulation of microRNA‐21 and up‐regulation of sprouty 2. This observation was followed by experiments demonstrating propofol‐induced up‐regulation of programmed cell death protein 4 (PDCD4), which constitutes a direct target of microRNA‐21 (Twaroski et al., 2015). These studies point to the importance of microRNAs in preventing anaesthetic‐induced neurotoxicity (Jiang et al., 2014; Xu et al., 2015). However, the BP reducing and cardiodepressive effects of propofol are concerning, especially in elderly and critically ill patients. Therefore, other, new, hypnotics are needed. To test one such new compound KSEB01‐S2 (a potent GABAA receptor ligand), investigators used patient‐derived iPSC‐CMs and demonstrated that KSEB01‐S2 reduced cardiomyocyte function less than propofol (Chang et al., 2020). These data acquired in human tissue are important to pave the way towards future clinical testing.
Volatile anaesthetics (i.e., isoflurane, sevoflurane, and desflurane) also exert their hypnotic ability at least to some degree through GABAA receptors. Increasing concerns of potential neurotoxicity (Diaz et al., 2016) led to studies using commercially available human neural progenitor cells to study the effects of prolonged exposure to volatile anaesthetics on neuronal survival and neurogenesis (Zhao et al., 2013). Using Ca2+‐sensing fluorescent probes, these investigators demonstrated that isoflurane induced moderate cytosolic Ca2+ concentrations and protected neuronal progenitor cells while higher concentrations resulted in cell death (Zhao et al., 2013). Another study used neuronal progenitor cells and determined that inhibition of the JNK signalling pathway protects against sevoflurane‐mediated cell death (Z. Yang et al., 2017). These studies illustrate the value of human iPSC‐CMs and iPSC‐NCs for determining the impact of anaesthetics on both cell viability and function, especially when screening protocols include multiple test conditions.
4.2. NMDA receptors
Multiple anaesthetics (e.g., ketamine, nitrous oxide, and methadone) mediate their hypnotic effect through NMDA receptors. Among those, ketamine has been studied most often in iPSC‐NCs. Its unexpected emotion‐stabilizing effect and frequent clinical usage as potential anti‐depressant raised questions about long‐term safety. Recently, iPSCs were differentiated into floor plate‐derived midbrain dopaminergic neurons to evaluate whether the ion channel AMPA receptor and its subunits, GluR1 and GluR2, play a role in modulating the anti‐depressant effect of ketamine (Collo, Cavalleri, Chiamulera, & Merlo Pich, 2019). Importantly, ketamine increased the structural plasticity and expression of both receptor subunits, suggesting that a new NMDA receptor‐independent mechanism may be responsible for the positive “side effect” of ketamine. This example demonstrates the utility of using iPSC technology to discover new drug mechanisms, which might lead to new indications. Despite its positive effect, ketamine may also increase the production of ROS, accompanied by increased release of mitochondrial cytochrome C and nuclear DNA fragmentation (Bai et al., 2013). This effect might be dose‐dependent, as ketamine administration reduced ATP levels and increased the NADH/NAD+ ratio at lower concentrations but resulted in mitochondrial dysfunction, caspase activation, and subsequent cell death at higher concentrations and long‐term exposure (Bai et al., 2013; Ito, Uchida, & Makita, 2015). Collectively, these data expand our knowledge about a relatively “old” drug by using human iPSC‐NCs.
4.3. GPCRs
Opioids are by far the most prominent GPCR ligands in the perioperative setting. While the effect of opioids on rodent ESCs has been shown multiple times, human stem cells have been rarely tested. The endogenous κ‐opioid receptor ligand dynorphin was shown to increase neuronal progenitor migration and proliferation (Sheng et al., 2007). The iPSC technology can also be used as a tool to understand individualized response to opioids. The μ‐opioid receptor gene (OPRM1) A118G single nucleotide polymorphism (SNP rs1799971) encoding receptor variant has been associated with opioid addiction. By replicating the corresponding gene defect in iPSC‐NCs, investigators detected a robust decrease of synaptic function in response to different opioids (Halikere et al., 2019). These results were confirmed in iPSC‐NCs generated from different individuals, suggesting that the SNP rs1799971 alone is responsible for a decreased opioid‐mediated neurotransmitter release and may be a key component of opioid addiction.
In a similar approach, investigators generated dopaminergic iPSC‐NCs from opioid‐dependent patients (Sheng et al., 2016a, 2016b). Surprisingly, these iPSC‐NCs expressed fewer D2 receptors compared to control patients, which is critical as the dopaminergic system is a major substrate of reward and reinforcement for addictive drugs. These examples show how the iPSC technology can reveal genetic variances affecting individual responses to anaesthetic medications. By finding these critical genetic targets, future drugs may be better able to target only beneficial receptor variations while avoiding mutated receptors (Table 2).
5. LIMITATIONS OF iPSC TECHNOLOGY
Even though introduced over a decade ago, iPSC technology still has some roadblocks to overcome before its full perioperative application. iPSCs are generally less mature than their natural counterparts: iPSC‐CMs start to beat after 7–9 days (Burridge, Holmström, & Wu, 2015) but might require another 90–120 days to express an more mature‐like phenotype (Ebert et al., 2019). Similarly, although the development of complex neuronal networks (Quadrato et al., 2017) in organoids has been reported to replicate remarkably well early in vivo brain development, these tissues reflect brain structures at the mostly late mid‐fetal period (19–24 gestational weeks) (Paşca et al., 2015; Qian et al., 2016). Although the study in human‐derived tissue provides clear advantages over animal studies, behaviour studies essential for the interpretation of medications acting on the CNS are not feasible with iPSCs. Interestingly, when studying electroencephalograms from cortical organoids and human fetal brains, organoids displayed a more variable trajectory than human brains, whereas the developmental pattern over time was similar between both (Trujillo et al., 2019). These data suggest that neuronal networks derived from iPSC‐NCs do not precisely reflect human brain development in vivo, but the trends appear to be similar. In addition to new methods for increasing maturation and vascularization of brain organoids, several groups have started to study the interaction between different cell types within cerebral organoids (Bagley, Reumann, Bian, Lévi‐Strauss, & Knoblich, 2017; Quadrato et al., 2017) and neuro‐skeletal junction (Marton et al., 2019; Swartz et al., 2020). Integrating human iPSC‐derived cerebral organoids into rodents will further facilitate our understanding of how vasculature and circulating cells interact with brain tissue (Cakir et al., 2019; Mansour et al., 2018). Therefore, with the advancements in biotechnology and bioengineering, we may overcome these limitations, and the clinical translation may occur sooner rather than later.
6. POSSIBLE CLINICAL APPLICATION
Despite the current limitations, iPSC technology might help soon to address conditions such as postoperative vomiting or delirium or uncontrolled hypotension based on patient‐specific genetic conditions. Several pathways would allow the use of patient‐specific iPSCs as an optimization tool for perioperative care (Figure 5). One scenario could include collecting the blood of healthy patients well before any surgical or medical procedure by their general physician (i.e., patient reaching a certain age or suffering from a positive family history of inherited disease susceptibility). Reprogramming of collected somatic cells and differentiation into iPSC‐CMs or iPSC‐NCs would then allow for testing of multiple medications and combination of medications to identify the most advantageous drug profile with the fewest side effects. In a second scenario, patients would be seen in the perioperative assessment clinic where their blood cells would be reprogrammed and differentiated into iPSC‐derived somatic cells for testing of the most advantageous drug combination usable in planned upcoming surgical and perioperative care period. In both scenarios, generated iPSCs or derived somatic cells could be cryopreserved for later testing.
FIGURE 5.
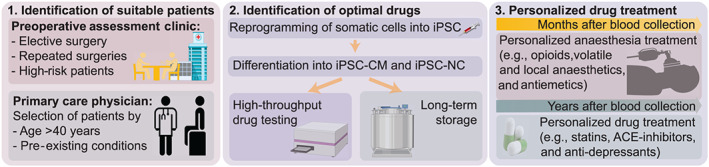
Possible clinical scenarios for the use of iPSC technology in perioperative care: (1) Patients might either be identified directly before a planned surgical procedure in the preoperative assessment clinic or (without planned procedure) at a regular visit at the primary care physician if the patient presents with specific selection criteria. (2) In both cases, blood will be drawn, iPSC‐CM and iPSC‐NC generated and tested for the best possible drug combination for each individual. Cells might also be cryopreserved for future testing. (3) Patients will receive a specialized cocktail of anaesthesia/intensive care medications with the most beneficial cardiac and neuronal risk profile. Later drug therapy can be optimized by selecting medications (e.g., statins, ACE‐inhibitors, or anti‐depressants) with a minimal risk profile based on the prior testing on iPSCs. iPSC‐CM, iPSC‐derived cardiomyocyte; iPSC‐NC, iPSC‐derived neuronal cell
7. CONCLUSIONS
Anaesthesia‐related drugs have largely remained the same for the past 50 years. Although iPSCs are a valuable tool in the arsenal of pharmacologists and clinicians to test drugs in an HTS and personalized manner, the generation and maintenance of iPSC‐CMs and iPSC‐NCs is still challenging. Nevertheless, recent paradigm‐shifting developments in iPSC and tissue engineering technologies can be used to shorten the time of drug development and to create the next generation of improved anaesthetic agents.
7.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in the IUPHAR/BPS Guide to PHARMACOLOGY (http://www.guidetopharmacology.org) and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019; Alexander, Mathie, et al., 2019).
CONFLICT OF INTEREST
J.C.W. is a co‐founder of Khloris Biosciences but has no competing interests because the work presented here is completely independent. D.O. declares no competing interests.
ACKNOWLEDGEMENTS
The authors would like to acknowledge funding support from the National Institutes of Health (NIH) UH3 TR002588, R01 HL146690, and R01 HL141371 (J.C.W.), Burroughs Wellcome Foundation IRSA 1015009 (J.C.W.), Tabacco‐related Disease Research Program TRDRP 27IR‐0012 (J.C.W.), Stanford Cardiovascular Institute Seed grant, and Transdisciplinary Initiatives Program (TIP) Award, Stanford Maternal & Child Health Research Institute (D.O.). Due to space constraints, the authors apologize in advance for not including all relevant citations on the subject matter.
Obal D, Wu JC. Induced pluripotent stem cells as a platform to understand patient‐specific responses to opioids and anaesthetics. Br J Pharmacol. 2020;177:4581–4594. 10.1111/bph.15228
Contributor Information
Detlef Obal, Email: obal@stanford.edu.
Joseph C. Wu, Email: joewu@stanford.edu.
REFERENCES
- Abilez, O. J. , Tzatzalos, E. , Yang, H. , Zhao, M.‐T. , Jung, G. , Zöllner, A. M. , … Wu, J. C. (2018). Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells, 36, 265–277. 10.1002/stem.2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … Pawson, A. J. (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176(Suppl 1), S21–S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Mathie, A. , Peters, J. A. , Veale, E. L. , Striessnig, J. , Kelly, E. , … Sharman, J. L. (2019). The Concise Guide to PHARMACOLOGY 2019/20: Ion channels. British Journal of Pharmacology, 176(Suppl 1), S142–S228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, S. , Funato, M. , Ohuchi, K. , Inagaki, S. , Sato, A. , Seki, J. , … Hara, H. (2019). The protective effects of levetiracetam on a human iPSCs‐derived spinal muscular atrophy model. Neurochemical Research, 44, 1773–1779. 10.1007/s11064-019-02814-4 [DOI] [PubMed] [Google Scholar]
- Bachmutsky, I. , Wei, X. P. , Kish, E. , & Yackle, K. (2020). Opioids depress breathing through two small brainstem sites. eLife, 9, e52694 10.7554/eLife.52694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badja, C. , Maleeva, G. , El‐Yazidi, C. , Barruet, E. , Lasserre, M. , Tropel, P. , … Magdinier, F. (2014). Efficient and cost‐effective generation of mature neurons from human induced pluripotent stem cells. Stem Cells Translational Medicine, 3, 1467–1472. 10.5966/sctm.2014-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley, J. A. , Reumann, D. , Bian, S. , Lévi‐Strauss, J. , & Knoblich, J. A. (2017). Fused cerebral organoids model interactions between brain regions. Nature Methods, 14, 743–751. 10.1038/nmeth.4304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, X. , Yan, Y. , Canfield, S. , Muravyeva, M. Y. , Kikuchi, C. , Zaja, I. , … Bosnjak, Z. J. (2013). Ketamine enhances human neural stem cell proliferation and induces neuronal apoptosis via reactive oxygen species‐mediated mitochondrial pathway. Anesthesia and Analgesia, 116, 869–880. 10.1213/ANE.0b013e3182860fc9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, P. A. , Lomen‐Hoerth, C. , Gensch, E. M. , Meakin, S. O. , Glass, D. J. , & Shooter, E. M. (1993). Tissue‐specific alternative splicing generates two isoforms of the trkA receptor. The Journal of Biological Chemistry, 268, 15150–15157. [PubMed] [Google Scholar]
- Bedut, S. , Seminatore‐Nole, C. , Lamamy, V. , Caignard, S. , Boutin, J. A. , Nosjean, O. , … Coge, F. (2016). High‐throughput drug profiling with voltage‐ and calcium‐sensitive fluorescent probes in human iPSC‐derived cardiomyocytes. American Journal of Physiology. Heart and Circulatory Physiology, 311, H44–H53. 10.1152/ajpheart.00793.2015 [DOI] [PubMed] [Google Scholar]
- Beussman, K. M. , Rodriguez, M. L. , Leonard, A. , Taparia, N. , Thompson, C. R. , & Sniadecki, N. J. (2016). Micropost arrays for measuring stem cell‐derived cardiomyocyte contractility. Methods, 94, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinova, K. , Schocken, D. , Patel, D. , Daluwatte, C. , Vicente, J. , Wu, J. C. , & Strauss, D. G. (2019). Clinical trial in a dish: Personalized stem cell‐derived cardiomyocyte assay compared with clinical trial results for two QT‐prolonging drugs. Clinical and Translational Science, 12, 687–697. 10.1111/cts.12674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordoni, M. , Rey, F. , Fantini, V. , Pansarasa, O. , Di Giulio, A. M. , Carelli, S. , & Cereda, C. (2018). From neuronal differentiation of iPSCs to 3D neuro‐organoids: Modelling and therapy of neurodegenerative diseases. International Journal of Molecular Sciences, 19 10.3390/ijms19123972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosnjak, Z. J. , Yan, Y. , Canfield, S. , Muravyeva, M. Y. , Kikuchi, C. , Wells, C. W. , … Bai, X. (2012). Ketamine induces toxicity in human neurons differentiated from embryonic stem cells via mitochondrial apoptosis pathway. Current Drug Safety, 7, 106–119. 10.2174/157488612802715663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink, A. M. , Evers, A. S. , Avidan, M. S. , Farber, N. B. , Smith, D. J. , Zhang, X. , … Olney, J. W. (2010). Isoflurane‐induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology, 112, 834–841. 10.1097/ALN.0b013e3181d049cd [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge, P. W. , Holmström, A. , & Wu, J. C. (2015). Chemically defined culture and cardiomyocyte differentiation of human pluripotent stem cells. Current Protocols in Human Genetics, 87, 21.3.1–21.3.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge, P. W. , Sharma, A. , & Wu, J. C. (2015). Genetic and epigenetic regulation of human cardiac reprogramming and differentiation in regenerative medicine. Annual Review of Genetics, 49, 461–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiazzo, M. , Okawa, Y. , Ranga, A. , Piersigilli, A. , Tabata, Y. , & Lutolf, M. P. (2016). Defined three‐dimensional microenvironments boost induction of pluripotency. Nature Materials, 15, 344–352. 10.1038/nmat4536 [DOI] [PubMed] [Google Scholar]
- Cakir, B. , Xiang, Y. , Tanaka, Y. , Kural, M. H. , Parent, M. , Kang, Y.‐J. , … Park, I. H. (2019). Engineering of human brain organoids with a functional vascular‐like system. Nature Methods, 16, 1169–1175. 10.1038/s41592-019-0586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi, M. , Shin, Y. , Osaki, T. , Hajal, C. , Chiono, V. , & Kamm, R. D. (2018). 3D self‐organized microvascular model of the human blood‐brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials, 180, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechova, K. , Lan, C. , Barthes, N. P. F. , Jung, M. , & Ulbrich, M. H. (2020). Kappa but not delta or mu opioid receptors form homodimers at low membrane densities. BioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, A. C. Y. , Chang, A. C. H. , Nicin, L. , Weber, G. J. , Holbrook, C. , Davies, M. F. , … Bertaccini, E. J. (2020). An in vitro model for identifying cardiac side effects of anesthetics. Anesthesia and Analgesia, 130(1), e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, A. C. Y. , Ong, S.‐G. , LaGory, E. L. , Kraft, P. E. , Giaccia, A. J. , Wu, J. C. , & Blau, H. M. (2016). Telomere shortening and metabolic compromise underlie dystrophic cardiomyopathy. Proc. Natl. Acad. Sci. USA, 113, 13120–13125. 10.1073/pnas.1615340113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, W. , Berta, T. , Kim, Y. H. , Lee, S. , Lee, S.‐Y. , & Ji, R.‐R. (2018). Expression and role of voltage‐gated sodium channels in human dorsal root ganglion neurons with special focus on Nav1.7, species differences, and regulation by paclitaxel. Neuroscience Bulletin, 34, 4–12. 10.1007/s12264-017-0132-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves, C. , Remiao, F. , Cisternino, S. , & Decleves, X. (2017). Opioids and the blood‐brain barrier: A dynamic interaction with consequences on drug disposition in brain. Current Neuropharmacology, 15, 1156–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S. H. , Kim, Y. H. , Hebisch, M. , Sliwinski, C. , Lee, S. , D'Avanzo, C. , … Klee, J. B. (2014). A three‐dimensional human neural cell culture model of Alzheimer's disease. Nature, 515, 274–278. 10.1038/nature13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christidi, E. , Huang, H. M. , & Brunham, L. R. (2018). CRISPR/Cas9‐mediated genome editing in human stem cell‐derived cardiomyocytes: Applications for cardiovascular disease modelling and cardiotoxicity screening. Drug Discovery Today: Technologies, 28, 13–21. 10.1016/j.ddtec.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Collo, G. , Cavalleri, L. , Chiamulera, C. , & Merlo Pich, E. (2018). (2R,6R)‐Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell‐derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport, 29, 1425–1430. 10.1097/WNR.0000000000001131 [DOI] [PubMed] [Google Scholar]
- Collo, G. , Cavalleri, L. , Chiamulera, C. , & Merlo Pich, E. (2019). Ketamine increases the expression of GluR1 and GluR2 α‐amino‐3‐hydroxy‐5‐methy‐4‐isoxazole propionate receptor subunits in human dopaminergic neurons differentiated from induced pluripotent stem cells. Neuroreport, 30, 207–212. 10.1097/WNR.0000000000001185 [DOI] [PubMed] [Google Scholar]
- Cook, D. , Brown, D. , Alexander, R. , March, R. , Morgan, P. , Satterthwaite, G. , & Pangalos, M. N. (2014). Lessons learned from the fate of AstraZeneca's drug pipeline: A five‐dimensional framework. Nature Reviews. Drug Discovery, 13, 419–431. 10.1038/nrd4309 [DOI] [PubMed] [Google Scholar]
- Davidson, A. J. , Disma, N. , de Graaff, J. C. , Withington, D. E. , Dorris, L. , Bell, G. , … GAS consortium . (2016). Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake‐regional anaesthesia in infancy (GAS): An international multicentre, randomised controlled trial. Lancet, 387, 239–250. 10.1016/S0140-6736(15)00608-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. , Davis, L. C. , Correll, R. N. , Makarewich, C. A. , Schwanekamp, J. A. , Moussavi‐Harami, F. , … Molkentin, J. D. (2016). A tension‐based model distinguishes hypertrophic versus dilated cardiomyopathy. Cell, 165, 1147–1159. 10.1016/j.cell.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, L. K. , Gaynor, J. W. , Koh, S. J. , Ittenbach, R. F. , Gerdes, M. , Bernbaum, J. C. , … Nicolson, S. C. (2016). Increasing cumulative exposure to volatile anesthetic agents is associated with poorer neurodevelopmental outcomes in children with hypoplastic left heart syndrome. The Journal of Thoracic and Cardiovascular Surgery, 152, 482–489. 10.1016/j.jtcvs.2016.03.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drawnel, F. M. , Boccardo, S. , Prummer, M. , Delobel, F. , Graff, A. , Weber, M. , … Iacone, R. (2014). Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Reports, 9, 810–821. 10.1016/j.celrep.2014.09.055 [DOI] [PubMed] [Google Scholar]
- Dumas, S. J. , Bru‐Mercier, G. , Courboulin, A. , Quatredeniers, M. , Rücker‐Martin, C. , Antigny, F. , … Cohen‐Kaminsky, S. (2018). NMDA‐type glutamate receptor activation promotes vascular remodeling and pulmonary arterial hypertension. Circulation, 137, 2371–2389. 10.1161/CIRCULATIONAHA.117.029930 [DOI] [PubMed] [Google Scholar]
- Ebert, A. , Joshi, A. U. , Andorf, S. , Dai, Y. , Sampathkumar, S. , Chen, H. , … Wu, J. C. (2019). Proteasome‐dependent regulation of distinct metabolic states during long‐term culture of human iPSC‐derived cardiomyocytes. Circulation Research, 125, 90–103. 10.1161/CIRCRESAHA.118.313973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuzik, J. , Zeisel, A. , Máté, Z. , Calvigioni, D. , Yanagawa, Y. , Szabó, G. , … Harkany, T. (2016). Integration of electrophysiological recordings with single‐cell RNA‐seq data identifies neuronal subtypes. Nature Biotechnology, 34, 175–183. 10.1038/nbt.3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, P. S. , Kolesky, S. E. , & Jenkins, A. (2010). General anesthetic actions on GABAA receptors. Current Neuropharmacology, 8, 2–9. 10.2174/157015910790909502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg, P. , Oikonomopoulos, A. , Chen, H. , Li, Y. , Lam, C. K. , Sallam, K. , … Wu, J. C. (2018). Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. Journal of the American College of Cardiology, 72, 62–75. 10.1016/j.jacc.2018.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L. , Eldridge, S. , Furniss, M. , Mussio, J. , & Davis, M. (2015). Use of human induced pluripotent stem cell‐derived cardiomyocytes (hiPSC‐CMs) to monitor compound effects on cardiac myocyte signaling pathways. Curr Protoc Chem Biol, 7, 141–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halikere, A. , Popova, D. , Scarnati, M. S. , Hamod, A. , Swerdel, M. R. , Moore, J. C. , … Pang, Z. P. (2019). Addiction associated N40D mu‐opioid receptor variant modulates synaptic function in human neurons. Molecular Psychiatry, 25, 1406–1419. 10.1038/s41380-019-0507-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, L. , Li, Y. , Tchao, J. , Kaplan, A. D. , Lin, B. , Li, Y. , … Yang, L. (2014). Study familial hypertrophic cardiomyopathy using patient‐specific induced pluripotent stem cells. Cardiovascular Research, 104, 258–269. 10.1093/cvr/cvu205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle, C. , Huang, H. , Crowley, S. , Tanner, J. , Hernaiz, C. , Rice, M. , … Price, C. C. (2019). Mild cognitive impairment and decline in resting state functional connectivity after total knee arthroplasty with general anesthesia. Journal of Alzheimer's Disease, 69, 1003–1018. 10.3233/JAD-180932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham, G. E. , & Bading, H. (2010). Synaptic versus extrasynaptic NMDA receptor signalling: Implications for neurodegenerative disorders. Nature Reviews. Neuroscience, 11, 682–696. 10.1038/nrn2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz, M. J. , Lein, E. S. , Guillozet‐Bongaarts, A. L. , Shen, E. H. , Ng, L. , Miller, J. A. , … Jones, A. R. (2012). An anatomically comprehensive atlas of the adult human brain transcriptome. Nature, 489, 391–399. 10.1038/nature11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemphill, S. , McMenamin, L. , Bellamy, M. C. , & Hopkins, P. M. (2019). Propofol infusion syndrome: A structured literature review and analysis of published case reports. British Journal of Anaesthesia, 122, 448–459. 10.1016/j.bja.2018.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H. , Uchida, T. , & Makita, K. (2015). Ketamine causes mitochondrial dysfunction in human induced pluripotent stem cell‐derived neurons. PLoS ONE, 10, e0128445 10.1371/journal.pone.0128445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izsak, J. , Seth, H. , Andersson, M. , Vizlin‐Hodzic, D. , Theiss, S. , Hanse, E. , … Illes, S. (2019). Robust generation of person‐specific, synchronously active neuronal networks using purely isogenic human iPSC‐3D neural aggregate cultures. Frontiers in Neuroscience, 13, 351 10.3389/fnins.2019.00351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson, J. J. , Linville, R. M. , Ding, Y. Y. , Gerecht, S. , & Searson, P. C. (2019). Role of iPSC‐derived pericytes on barrier function of iPSC‐derived brain microvascular endothelial cells in 2D and 3D. Fluids Barriers CNS, 16, 15 10.1186/s12987-019-0136-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X.‐L. , Du, B.‐X. , Chen, J. , Liu, L. , Shao, W.‐B. , & Song, J. (2014). MicroRNA‐34a negatively regulates anesthesia‐induced hippocampal apoptosis and memory impairment through FGFR1. International Journal of Clinical and Experimental Pathology, 7, 6760–6767. [PMC free article] [PubMed] [Google Scholar]
- Jo, J. , Xiao, Y. , Sun, A. X. , Cukuroglu, E. , Tran, H.‐D. , Göke, J. , … Ng, H. H. (2016). Midbrain‐like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin‐producing neurons. Cell Stem Cell, 19, 248–257. 10.1016/j.stem.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, L. H. , & Bunnage, M. E. (2017). Applications of chemogenomic library screening in drug discovery. Nature Reviews. Drug Discovery, 16, 285–296. 10.1038/nrd.2016.244 [DOI] [PubMed] [Google Scholar]
- Kauk, M. , & Hoffmann, C. (2018). Intramolecular and intermolecular FRET sensors for GPCRs—Monitoring conformational changes and beyond. Trends in Pharmacological Sciences, 39, 123–135. 10.1016/j.tips.2017.10.011 [DOI] [PubMed] [Google Scholar]
- Kido, K. , Ito, H. , Yamamoto, Y. , Makita, K. , & Uchida, T. (2018). Cytotoxicity of propofol in human induced pluripotent stem cell‐derived cardiomyocytes. Journal of Anesthesia, 32, 120–131. 10.1007/s00540-017-2441-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer, B. L. (1999). Opioids: First lessons from knockout mice. Trends in Pharmacological Sciences, 20, 19–26. [DOI] [PubMed] [Google Scholar]
- Kim, C. , Wong, J. , Wen, J. , Wang, S. , Wang, C. , Spiering, S. , … Chen, H. S. V. (2013). Studying arrhythmogenic right ventricular dysplasia with patient‐specific iPSCs. Nature, 494, 105–110. 10.1038/nature11799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim, A. B. , Röthe, J. , Çakir, M. V. , Lede, V. , Wilde, C. , Liebscher, I. , … Schöneberg, T. (2019). Genetic basis of functional variability in adhesion G protein‐coupled receptors. Scientific Reports, 9, 11036 10.1038/s41598-019-46265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, F. , Lee, A. S. , Liang, P. , Sanchez‐Freire, V. , Nguyen, P. K. , Wang, L. , … Wu, J. C. (2013). Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient‐specific induced pluripotent stem cells. Cell Stem Cell, 12, 101–113. 10.1016/j.stem.2012.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, M. A. , Renner, M. , Martin, C.‐A. , Wenzel, D. , Bicknell, L. S. , Hurles, M. E. , … Knoblich, J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501, 373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, H. M. , Hansen, S. K. , Mikkelsen, J. D. , Hyttel, P. , & Stummann, T. C. (2019). Alpha7 nicotinic acetylcholine receptors and neural network synaptic transmission in human induced pluripotent stem cell‐derived neurons. Stem Cell Research, 41, 101642 10.1016/j.scr.2019.101642 [DOI] [PubMed] [Google Scholar]
- Lau, E. , Paik, D. T. , & Wu, J. C. (2019). Systems‐wide approaches in induced pluripotent stem cell models. Annual Review of Pathology, 14, 395–419. 10.1146/annurev-pathmechdis-012418-013046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, P. , Klos, M. , Bollensdorff, C. , Hou, L. , Ewart, P. , Kamp, T. J. , … Herron, T. J. (2012). Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell‐derived cardiac myocyte monolayers. Circulation Research, 110, 1556–1563. 10.1161/CIRCRESAHA.111.262535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. , Sasaki, D. , Kim, D. , Mori, M. , Yokota, T. , Lee, H. , … Someya, T. (2019). Ultrasoft electronics to monitor dynamically pulsing cardiomyocytes. Nature Nanotechnology, 14, 156–160. 10.1038/s41565-018-0331-8 [DOI] [PubMed] [Google Scholar]
- Leonard, A. , Bertero, A. , Powers, J. D. , Beussman, K. M. , Bhandari, S. , Regnier, M. , … Sniadecki, N. J. (2018). Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. Journal of Molecular and Cellular Cardiology, 118, 147–158. 10.1016/j.yjmcc.2018.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J. C. , Travis, B. R. , Verlander, J. W. , Sandhu, S. K. , Yang, S.‐G. , Zea, A. H. , … Silverstein, D. M. (2002). Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 283, R964–R971. 10.1152/ajpregu.00629.2001 [DOI] [PubMed] [Google Scholar]
- Li, R. A. , Keung, W. , Cashman, T. J. , Backeris, P. C. , Johnson, B. V. , Bardot, E. S. , … Costa, K. D. (2018). Bioengineering an electro‐mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials, 163, 116–127. 10.1016/j.biomaterials.2018.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, P. , Sallam, K. , Wu, H. , Li, Y. , Itzhaki, I. , Garg, P. , … Wu, J. C. (2016). Patient‐specific and genome‐edited induced pluripotent stem cell‐derived cardiomyocytes elucidate single‐cell phenotype of Brugada syndrome. Journal of the American College of Cardiology, 68, 2086–2096. 10.1016/j.jacc.2016.07.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind, J. U. , Busbee, T. A. , Valentine, A. D. , Pasqualini, F. S. , Yuan, H. , Yadid, M. , … Parker, K. K. (2017). Instrumented cardiac microphysiological devices via multimaterial three‐dimensional printing. Nature Materials, 16, 303–308. 10.1038/nmat4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. , Oikonomopoulos, A. , Sayed, N. , & Wu, J. C. (2018). Modeling human diseases with induced pluripotent stem cells: From 2D to 3D and beyond. Development, 145, dev156166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohse, M. J. , Nuber, S. , & Hoffmann, C. (2012). Fluorescence/bioluminescence resonance energy transfer techniques to study G‐protein‐coupled receptor activation and signaling. Pharmacological Reviews, 64, 299–336. [DOI] [PubMed] [Google Scholar]
- Luca, A. C. , Mersch, S. , Deenen, R. , Schmidt, S. , Messner, I. , Schäfer, K.‐L. , … Stoecklein, N. H. (2013). Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS ONE, 8, e59689 10.1371/journal.pone.0059689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, N. , Zhang, J. Z. , Itzhaki, I. , Zhang, S. L. , Chen, H. , Haddad, F. , … Wu, J. C. (2018). Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human‐induced pluripotent stem cells. Circulation, 138, 2666–2681. 10.1161/CIRCULATIONAHA.117.032273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanna‐Gabrielli, E. , Schenning, K. J. , Eriksson, L. I. , Browndyke, J. N. , Wright, C. B. , Culley, D. J. , … Deiner, S. (2019). State of the clinical science of perioperative brain health: Report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. British Journal of Anaesthesia, 123, 464–478. 10.1016/j.bja.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhardt, I. , Eder, A. , Dumotier, B. , Prondzynski, M. , Krämer, E. , Traebert, M. , … Hansen, A. (2017). Blinded contractility analysis in hiPSC‐cardiomyocytes in engineered heart tissue format: Comparison with human atrial trabeculae. Toxicological Sciences, 158, 164–175. 10.1093/toxsci/kfx081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhardt, I. , Warncke, C. , Trieu, H. K. , Müller, J. , & Eschenhagen, T. (2019). Piezo‐bending actuators for isometric or auxotonic contraction analysis of engineered heart tissue. Journal of Tissue Engineering and Regenerative Medicine, 13, 3–11. 10.1002/term.2755 [DOI] [PubMed] [Google Scholar]
- Mansour, A. A. , Gonçalves, J. T. , Bloyd, C. W. , Li, H. , Fernandes, S. , Quang, D. , … Gage, F. H. (2018). An in vivo model of functional and vascularized human brain organoids. Nature Biotechnology, 36, 432–441. 10.1038/nbt.4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton, R. M. , Miura, Y. , Sloan, S. A. , Li, Q. , Revah, O. , Levy, R. J. , … Pașca, S. P. (2019). Differentiation and maturation of oligodendrocytes in human three‐dimensional neural cultures. Nature Neuroscience, 22, 484–491. 10.1038/s41593-018-0316-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIsaac, D. I. , Wong, C. A. , Bryson, G. L. , & van Walraven, C. (2018). Association of polypharmacy with survival, complications, and healthcare resource use after elective noncardiac surgery: A population‐based cohort study. Anesthesiology, 128, 1140–1150. [DOI] [PubMed] [Google Scholar]
- McKeithan, W. L. , Savchenko, A. , Yu, M. S. , Cerignoli, F. , Bruyneel, A. A. N. , Price, J. H. , … Mercola, M. (2017). An automated platform for assessment of congenital and drug‐induced arrhythmia with hiPSC‐derived cardiomyocytes. Frontiers in Physiology, 8, 766 10.3389/fphys.2017.00766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meents, J. E. , Bressan, E. , Sontag, S. , Foerster, A. , Hautvast, P. , Rösseler, C. , … Lampert, A. (2019). The role of Nav1.7 in human nociceptors: Insights from human induced pluripotent stem cell‐derived sensory neurons of erythromelalgia patients. Pain, 160, 1327–1341. 10.1097/j.pain.0000000000001511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, L. Y. M. , Verkaart, S. , Koopman, W. J. H. , Willems, P. H. G. M. , Hoenderop, J. G. J. , & Bindels, R. J. M. (2014). Function and regulation of the Na+‐Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle. The Journal of Biological Chemistry, 289, 11293–11303. 10.1074/jbc.M113.529388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroni, L. , Burdick, J. A. , Highley, C. , Lee, S. J. , Morimoto, Y. , Takeuchi, S. , & Yoo, J. J. (2018). Biofabrication strategies for 3D in vitro models and regenerative medicine. Nature Reviews Materials, 3, 21–37. 10.1038/s41578-018-0006-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muguruma, K. , Nishiyama, A. , Kawakami, H. , Hashimoto, K. , & Sasai, Y. (2015). Self‐organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Reports, 10, 537–550. [DOI] [PubMed] [Google Scholar]
- Namer, B. , Schmidt, D. , Eberhardt, E. , Maroni, M. , Dorfmeister, E. , Kleggetveit, I. P. , … Lampert, A. (2019). Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient‐specific iPS cell‐derived nociceptors. eBioMedicine, 39, 401–408. 10.1016/j.ebiom.2018.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher, E. , & Sakmann, B. (1976). Single‐channel currents recorded from membrane of denervated frog muscle fibres. Nature, 260, 799–802. 10.1038/260799a0 [DOI] [PubMed] [Google Scholar]
- Nourmahnad, A. , Stern, A. T. , Hotta, M. , Stewart, D. S. , Ziemba, A. M. , Szabo, A. , & Forman, S. A. (2016). Tryptophan and cysteine mutations in M1 helices of α1β3γ2L γ‐aminobutyric acid type A receptors indicate distinct intersubunit sites for four intravenous anesthetics and one orphan site. Anesthesiology, 125, 1144–1158. 10.1097/ALN.0000000000001390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen, R. W. , & Sieghart, W. (2009). GABAA receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology, 56, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paci, M. , Passini, E. , Severi, S. , Hyttinen, J. , & Rodriguez, B. (2017). Phenotypic variability in LQT3 human induced pluripotent stem cell‐derived cardiomyocytes and their response to antiarrhythmic pharmacologic therapy: An in silico approach. Heart Rhythm, 14, 1704–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacitti, D. , Privolizzi, R. , & Bax, B. E. (2019). Organs to cells and cells to organoids: The evolution of in vitro central nervous system modelling. Frontiers in Cellular Neuroscience, 13, 129 10.3389/fncel.2019.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik, D. T. , Chandy, M. , & Wu, J. C. (2020). Patient and disease‐specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacological Reviews, 72, 320–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. , Wetzel, I. , Marriott, I. , Dréau, D. , D'Avanzo, C. , Kim, D. Y. , … Cho, H. (2018). A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer's disease. Nature Neuroscience, 21, 941–951. 10.1038/s41593-018-0175-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, T.‐E. , Mustafaoglu, N. , Herland, A. , Hasselkus, R. , Mannix, R. , FitzGerald, E. A. , … Ingber, D. E. (2019). Hypoxia‐enhanced Blood‐Brain Barrier Chip recapitulates human barrier function and shuttling of drugs and antibodies. Nature Communications, 10, 2621 10.1038/s41467-019-10588-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca, A. M. , Sloan, S. A. , Clarke, L. E. , Tian, Y. , Makinson, C. D. , Huber, N. , … Paşca, S. P. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nature Methods, 12, 671–678. 10.1038/nmeth.3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J. , Sarkar, S. , & Chang, S. L. (2012). Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real‐time RT‐PCR. Drug and Alcohol Dependence, 124, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer, S. , Wallace, S. , Ball, G. , Lloyd, R. , Schiapparelli, P. , Quiñones‐Hinojosa, A. , … Pamies, D. (2019). A human iPSC‐derived 3D platform using primary brain cancer cells to study drug development and personalized medicine. Scientific Reports, 9, 1407 10.1038/s41598-018-38130-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, X. , Nguyen, H. N. , Song, M. M. , Hadiono, C. , Ogden, S. C. , Hammack, C. , … Ming, G. L. (2016). Brain‐region‐specific organoids using mini‐bioreactors for modeling ZIKV exposure. Cell, 165, 1238–1254. 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato, G. , Nguyen, T. , Macosko, E. Z. , Sherwood, J. L. , Min Yang, S. , Berger, D. R. , … Arlotta, P. (2017). Cell diversity and network dynamics in photosensitive human brain organoids. Nature, 545, 48–53. 10.1038/nature22047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, K. K. , Pierce, D. L. , Weitendorf, F. , & Linder, M. W. (2017). Avoidable drug–gene conflicts and polypharmacy interactions in patients participating in a personalized medicine program. Personalized Medicine, 14, 221–233. [DOI] [PubMed] [Google Scholar]
- Sakaguchi, H. , Kadoshima, T. , Soen, M. , Narii, N. , Ishida, Y. , Ohgushi, M. , … Sasai, Y. (2015). Generation of functional hippocampal neurons from self‐organizing human embryonic stem cell‐derived dorsomedial telencephalic tissue. Nature Communications, 6, 8896 10.1038/ncomms9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed, N. , Liu, C. , & Wu, J. C. (2016). Translation of human‐induced pluripotent stem cells: From clinical trial in a dish to precision medicine. Journal of the American College of Cardiology, 67, 2161–2176. 10.1016/j.jacc.2016.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessler, D. I. , Sigl, J. C. , Kelley, S. D. , Chamoun, N. G. , Manberg, P. J. , Saager, L. , … Greenwald, S. (2012). Hospital stay and mortality are increased in patients having a “triple low” of low blood pressure, low bispectral index, and low minimum alveolar concentration of volatile anesthesia. Anesthesiology, 116, 1195–1203. 10.1097/ALN.0b013e31825683dc [DOI] [PubMed] [Google Scholar]
- Sessler, D. I. , Turan, A. , Stapelfeldt, W. H. , Mascha, E. J. , Yang, D. , Farag, E. , … Kurz, A. (2019). Triple‐low alerts do not reduce mortality: A real‐time randomized trial. Anesthesiology, 130, 72–82. 10.1097/ALN.0000000000002480 [DOI] [PubMed] [Google Scholar]
- Sharma, A. , Burridge, P. W. , McKeithan, W. L. , Serrano, R. , Shukla, P. , Sayed, N. , … Wu, J. C. (2017). High‐throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Science Translational Medicine, 9, eaaf2584 10.1126/scitranslmed.aaf2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw, P. J. , Bates, D. , Cartlidge, N. E. , French, J. M. , Heaviside, D. , Julian, D. G. , & Shaw, D. A. (1987). Long‐term intellectual dysfunction following coronary artery bypass graft surgery: A six month follow‐up study. The Quarterly Journal of Medicine, 62, 259–268. [PubMed] [Google Scholar]
- Sheng, W. S. , Hu, S. , Herr, G. , Ni, H. T. , Rock, R. B. , Gekker, G. , … Peterson, P. K. (2007). Human neural precursor cells express functional κ‐opioid receptors. The Journal of Pharmacology and Experimental Therapeutics, 322, 957–963. 10.1124/jpet.107.121988 [DOI] [PubMed] [Google Scholar]
- Sheng, Y. , Filichia, E. , Shick, E. , Preston, K. L. , Phillips, K. A. , Cooperman, L. , … Luo, Y. (2016a). Lower dopamine D2 receptor expression levels in human dopaminergic neurons derived from opioid‐dependent iPSCs. The American Journal of Psychiatry, 173, 429–431. 10.1176/appi.ajp.2015.15121545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng, Y. , Filichia, E. , Shick, E. , Preston, K. L. , Phillips, K. A. , Cooperman, L. , … Luo, Y. (2016b). Using iPSC‐derived human DA neurons from opioid‐dependent subjects to study dopamine dynamics. Brain and Behavior: A Cognitive Neuroscience Perspective, 6, e00491 10.1002/brb3.491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, S. P. , & Bang, A. G. (2018). High‐throughput screen for compounds that modulate neurite growth of human induced pluripotent stem cell‐derived neurons. Disease Models & Mechanisms, 11, dmm031906 10.1242/dmm.031906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, Z. , Mei, X. , Li, C. , Chen, Y. , Zheng, H. , Wu, Y. , … Shen, Y. (2019). Postoperative delirium is associated with long‐term decline in activities of daily living. Anesthesiology, 131, 492–500. 10.1097/ALN.0000000000002849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnawi, R. , Huber, I. , Maizels, L. , Shaheen, N. , Gepstein, A. , Arbel, G. , … Gepstein, L. (2015). Monitoring human‐induced pluripotent stem cell‐derived cardiomyocytes with genetically encoded calcium and voltage fluorescent reporters. Stem Cell Reports, 5, 582–596. 10.1016/j.stemcr.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirenko, O. , Grimm, F. A. , Ryan, K. R. , Iwata, Y. , Chiu, W. A. , Parham, F. , … Tice, R. R. (2017). In vitro cardiotoxicity assessment of environmental chemicals using an organotypic human induced pluripotent stem cell‐derived model. Toxicology and Applied Pharmacology, 322, 60–74. 10.1016/j.taap.2017.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira, M. E. , Shmoel, N. , Huang, S.‐H. M. , & Erez, H. (2018). Multisite attenuated intracellular recordings by extracellular multielectrode arrays, a perspective. Frontiers in Neuroscience, 12, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprung, J. , Roberts, R. O. , Weingarten, T. N. , Nunes Cavalcante, A. , Knopman, D. S. , Petersen, R. C. , … Warner, D. O. (2017). Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. British Journal of Anaesthesia, 119, 316–323. 10.1093/bja/aex130 [DOI] [PubMed] [Google Scholar]
- Stacey, P. , Wassermann, A. M. , Kammonen, L. , Impey, E. , Wilbrey, A. , & Cawkill, D. (2018). Plate‐based phenotypic screening for pain using human iPSC‐derived sensory neurons. SLAS Discov., 23, 585–596. [DOI] [PubMed] [Google Scholar]
- Stoppel, W. L. , Kaplan, D. L. , & Black, L. D. (2016). Electrical and mechanical stimulation of cardiac cells and tissue constructs. Advanced Drug Delivery Reviews, 96, 135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, N. , Yazawa, M. , Liu, J. , Han, L. , Sanchez‐Freire, V. , Abilez, O. J. , … Pavlovic, A. (2012). Patient‐specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med., 4, 130ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland, M. L. , Fabre, K. M. , & Tagle, D. A. (2013). The National Institutes of Health Microphysiological Systems Program focuses on a critical challenge in the drug discovery pipeline. Stem Cell Res Ther, 4(Suppl 1), I1 10.1186/scrt361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, E. W. , Shintani, G. , Wan, J. , Maffei, J. S. , Wang, S. H. , Miller, B. L. , … Coppola, G. (2020). Establishment of a human induced pluripotent stem cell‐derived neuromuscular co‐culture under optogenetic control. BioRxiv. [Google Scholar]
- Takahashi, K. , & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676. [DOI] [PubMed] [Google Scholar]
- Toepfer, C. N. , Sharma, A. , Cicconet, M. , Garfinkel, A. C. , Mücke, M. , Neyazi, M. , … Seidman, C. E. (2019). SarcTrack: An adaptable software tool for efficient large‐scale analysis of sarcomere function in hiPSC‐cardiomyocytes. Circ Res, 124, 1172–1183. 10.1161/CIRCRESAHA.118.314505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo, C. A. , Gao, R. , Negraes, P. D. , Gu, J. , Buchanan, J. , Preissl, S. , … Muotri, A. R. (2019). Complex oscillatory waves emerging from cortical organoids model early human brain network development. Cell Stem Cell, 25, 558–569.e7. 10.1016/j.stem.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroski, D. M. , Yan, Y. , Olson, J. M. , Bosnjak, Z. J. , & Bai, X. (2014). Down‐regulation of microRNA‐21 is involved in the propofol‐induced neurotoxicity observed in human stem cell‐derived neurons. Anesthesiology, 121, 786–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twaroski, D. M. , Yan, Y. , Zaja, I. , Clark, E. , Bosnjak, Z. J. , & Bai, X. (2015). Altered mitochondrial dynamics contributes to propofol‐induced cell death in human stem cell‐derived neurons. Anesthesiology, 123, 1067–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatine, G. D. , Barrile, R. , Workman, M. J. , Sances, S. , Barriga, B. K. , Rahnama, M. , … Svendsen, C. N. (2019). Human iPSC‐derived blood‐brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell, 24, 995–1005.e6. 10.1016/j.stem.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Velasco, S. , Kedaigle, A. J. , Simmons, S. K. , Nash, A. , Rocha, M. , Quadrato, G. , … Arlotta, P. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature, 570, 523–527. 10.1038/s41586-019-1289-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Cui, C. , Nan, H. , Yu, Y. , Xiao, Y. , Poon, E. , … Chen, M. (2017). Graphene sheet‐induced global maturation of cardiomyocytes derived from human induced pluripotent stem cells. ACS Applied Materials & Interfaces, 9, 25929–25940. 10.1021/acsami.7b08777 [DOI] [PubMed] [Google Scholar]
- Wisler, J. W. , Rockman, H. A. , & Lefkowitz, R. J. (2018). Biased G protein‐coupled receptor signaling: Changing the paradigm of drug discovery. Circulation, 137, 2315–2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H. , Lee, J. , Vincent, L. G. , Wang, Q. , Gu, M. , Lan, F. , … Wu, J. C. (2015). Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β‐adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell, 17, 89–100. 10.1016/j.stem.2015.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia, Y. , & Izpisua Belmonte, J. C. (2019). Design approaches for generating organ constructs. Cell Stem Cell, 24, 877–894. [DOI] [PubMed] [Google Scholar]
- Xie, T. , Hawkins, J. , & Sun, Y. (2017). Traction force measurement using deformable microposts. Methods in Molecular Biology, 1627, 235–244. [DOI] [PubMed] [Google Scholar]
- Xu, H. , Zhang, J. , Zhou, W. , Feng, Y. , Teng, S. , & Song, X. (2015). The role of miR‐124 in modulating hippocampal neurotoxicity induced by ketamine anesthesia. The International Journal of Neuroscience, 125, 213–220. [DOI] [PubMed] [Google Scholar]
- Yang, K.‐C. , Breitbart, A. , De Lange, W. J. , Hofsteen, P. , Futakuchi‐Tsuchida, A. , Xu, J. , … Pabon, L. (2018). Novel adult‐onset systolic cardiomyopathy due to MYH7 E848G mutation in patient‐derived induced pluripotent stem cells. JACC Basic Transl. Sci., 3, 728–740. 10.1016/j.jacbts.2018.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Cui, Y. , Sang, K. , Dong, Y. , Ni, Z. , Ma, S. , & Hu, H. (2018). Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature, 554, 317–322. 10.1038/nature25509 [DOI] [PubMed] [Google Scholar]
- Yang, Z. , Lv, J. , Li, X. , Meng, Q. , Yang, Q. , Ma, W. , … Ke, Z. J. (2017). Sevoflurane decreases self‐renewal capacity and causes c‐Jun N‐terminal kinase‐mediated damage of rat fetal neural stem cells. Scientific Reports, 7, 46304 10.1038/srep46304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, N. Y. , Poe, M. M. , Witzigmann, C. , Cook, J. M. , Stafford, D. , & Arnold, L. A. (2016). Characterization of GABAA receptor ligands with automated patch‐clamp using human neurons derived from pluripotent stem cells. Journal of Pharmacological and Toxicological Methods, 82, 109–114. 10.1016/j.vascn.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J. Z. , Termglinchan, V. , Shao, N.‐Y. , Itzhaki, I. , Liu, C. , Ma, N. , … Wu, J. C. (2019). A human iPSC double‐reporter system enables purification of cardiac lineage subpopulations with distinct function and drug response profiles. Cell Stem Cell, 24, 802–811.e5. 10.1016/j.stem.2019.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, X. , Yang, Z. , Liang, G. , Wu, Z. , Peng, Y. , Joseph, D. J. , … Wei, H. (2013). Dual effects of isoflurane on proliferation, differentiation, and survival in human neuroprogenitor cells. Anesthesiology, 118, 537–549. 10.1097/ALN.0b013e3182833fae [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, W.‐H. , & Cesnjevar, R. (2009). Cardiac tissue engineering: Implications for pediatric heart surgery. Pediatric Cardiology, 30, 716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]


