Abstract
Crotalaria longirostrata (chipilin) leaves contain phenolic compounds with antioxidant activity. These phenolic compounds, however, could easily degrade after extraction. Microencapsulation is a possible solution for avoiding this degradation. Frequently, microencapsulation is carried out using conventional encapsulating agents. The aim of this work was to evaluate the effect of several non-conventional encapsulating agents on microencapsulation by spray drying of phenolic compounds from chipilin, stability and release of phenolic compounds were also studied. Maltodextrin (MD), gum Arabic (GA), soy protein (SP), cocoa shell pectin (CSP), and protein (PC), as well as the gum (GC) of Cajanus cajan seeds were used. Different blends of these matrixes containing phenolic compounds from chipilin leaves were spray dried at 120 °C. After drying, the yield and microencapsulation efficiency were determined. All results were analyzed by an ANOVA test (p < 0.05). The release kinetics of phenolic compounds were modeled using zero, first-order, Higuchi and Korsmeyer-Peppas models. The R2 was calculated for each model. The blends of encapsulating agents allowed the formation of an efficient polymer matrix with yields between 46 and 64% and microencapsulation efficiency between 65 and 92%. Results show that maltodextrin with soy protein allowed the highest (92%) microencapsulation efficiency, although maltodextrin and cocoa shell pectin were more effective protective agents, showing greater stability. The Korsmeyer-Peppas model was the best in predicting the phenolic compounds release with R2 values higher than 98%. The stability time for microcapsules with MD–CSP was 8.88 years and 1.43 years at 4 °C and 30 °C, respectively.
Keywords: Crotalaria longirostrata extract, Cajanus cajan, Spray drying, Cocoa shell pectin, Gum of Cajanus cajan
Introduction
Phenolic compounds are secondary metabolites that are synthesized in plants and possess biological properties such as antioxidant activity and the elimination of free radicals (Cai et al. 2004). These free radicals are responsible for premature aging and diseases like cancer; therefore, antioxidants are a powerful tool in reducing oxidative stress in the human body (Yashin et al. 2013). Polyphenols, however, show low water solubility and low stability in environmental conditions, such as exposure to light, oxygen, temperature and enzymatic activities (Paini et al. 2015). The polyphenols are then degraded/oxidized principally during storage and shelf life (Wang et al. 2009). For example, a rich in polyphenols is unstable and has a short shelf life (Robert et al. 2010). Therefore, the microencapsulation of polyphenols could be an alternative for stability and could increase in shelf life (Desai and Park 2005).
Chipilin (Crotalaria longirostrata) leaves are an important source of phenolic compounds with antioxidant activity (Jiménez-Aguilar and Grusak 2015). Cruz-Rodríguez et al. (2017) reported the content of phenolic compounds in methanolic extracts of chipilin branches: total phenols (1332.79 μg gallic acid mL−1, saponins (3228.97 μg diosgenin mL−1); flavones and flavonols (309.56 μg quercetin mL−1) and flavonoids (1012.34 μg rutin mL−1). On the other hand, Jiménez-Aguilar and Grusak (2015) obtained the phenolic concentration and antioxidant activity of C. longirostrata leaves that had a maximum concentration of 3.38 mg gallic acid g−1 fresh leaf and 60.47 μmol Trolox g−1 fresh leaf, as well as antioxidant activity, respectively.
Spray drying is one of the best drying methods for converting, in a single step, fluid materials into solid particles (Murugesan and Orsat 2012), which could be applied to food. Spray drying of food bioactive ingredients has advanced considerably in recent years, with the main goal of scientists being the optimization of the process for specific bioactives. Another main trend has been the evaluation of the stability of encapsulated ingredients during storage and the release profile of materials (Assadpour and Jafari 2019). Among the advantages of spray drying is that it is suitable for heat-sensitive materials because of the short exposure times at high temperatures. In addition, spray drying has a low operating cost, a high microcapsules quality, fast solubility, a small size and a high microcapsules stability (Madene et al. 2006). Among its weaknesses, spray drying produces microcapsules with several surface structures depending on the encapsulating agent used (Ribeiro et al. 2019), and there is a limitation in the choice of encapsulating material (Madene et al. 2006) depending on the microcapsule application. The encapsulating agents frequently used for the spray drying process include carbohydrates, gums, pectin and proteins or mixtures (Desai and Park 2005). The diversity of the materials that are used in the microencapsulation is due to the fact that not all meet the desired characteristics. Good rheological properties, easy handling, good dispersal or emulsifying abilities and maintaining their stability are conditions desired for encapsulating agents. The encapsulating agents must protect compounds during storage from the action of external factors (oxygen, temperature, humidity, and light), among others (Robert et al. 2010; Paini et al. 2015). The evaluation of new encapsulating materials would overcome difficulties in terms of availability and flexibility in choosing materials for specific purposes (Murugesan and Orsat 2012). In this way, Kalusevic et al. (2017) reported the use of maltodextrin, gum Arabic and skim milk powder to encapsulate grape skin extract. Sun-Waterhouse et al. (2013), using four different fiber polymers as encapsulating agents (sodium alginate, methyl β-cyclodextrin (MβCD), hydroxypropylmethyl cellulose (HPMC) and inulin) found that inulin showed higher encapsulation efficiency than quercetin and vanillin (18.5% and 53.3% respectively). Belščak-Cvitanović et al. (2015) used alginate (5%, w/v), pectin (5%, w/v), carrageenan (2%, w/v), modified corn starch (5%, w/v), acacia gum (2%, w/v), guar gum (1%, w/v), xanthan (0.5%, w/v), locust bean gum (0.5%, w/v), whey proteins (10%, w/v), pea flour (7%, w/v), oligofructose (10%, w/v) or inulin (10%, w/v) to encapsulate green tea bioactive compounds. They found that inulin accompanied with pectin had the highest total polyphenols (67.5–82.2%) for green tea encapsulation. Therefore, the search for new materials and their concentration has become very important because product yield and encapsulation efficiencies could be improved.
The Cajanus cajan seed has a low fat concentration, a moderate amount of fiber and a good amount of protein and starches, as well as an adequate balance of minerals. These seeds are used as both animal feed and human food (Tiwari et al. 2011), but in Mexico few people consume it. Flour from Cajanus cajan has gained importance as a protein energy source of native origin, so its protein and galactomannan fraction have been studied. Robles-Flores et al. (2018) extracted protein isolates and gum from Cajanus cajan seeds that were used as ingredients in the formulation of edible coatings for fresh strawberries fruits. Thus, these proteins and gum could then be used in the microencapsulation process as an unconventional encapsulating agent. Moreover, an interesting residue to be evaluated is the cocoa husks generated from cocoa processing. These cocoa by-products contain pectin that can be obtained and used as an encapsulating agent, as well as alternatively giving an added value. The pectin has been used as a coating material together with other encapsulating agents such as saffron extracts (Esfanjani et al. 2015) and nissin (Wang et al. 2017).
Because no work has reported the spray drying of Crotalaria longirostrata phenolic compounds, in this work, several encapsulating agents (gum Arabic, Cajanus cajanus gum, cocoa shell pectin, Cajanus cajan protein and soy protein) were mixed with maltodextrin and the methanolic extract of Crotalaria longirostrata leaves, then dried by spray drying to evaluate new materials for microencapsulation. The effect of these mixtures of encapsulating agents was evaluated on the yield and microencapsulation efficiency, as well as the antioxidant activity, the phenolic compounds’ release and the stability of microencapsulated phenolic compounds.
Materials and methods
Materials
Maltodextrin 10DE (MD) (INAMALT, IMSA Guadalajara, Mexico), gum Arabic (GA) (Hycel, Mexico), soy protein 90% (SP) (Soyatein, Mexico), protein (PC), the gum (GC) of Cajanus cajan seeds and cocoa shell pectin (CSP) were used as encapsulating agents. After obtaining PC, GC and CSP, these were characterized in terms of protein content (factor 6.25), which were for PC, GC and CSP 67%, 6% and 1.6%, respectively. The carbohydrates’ content for PC, GC and CSP were 21.5%, 80% and 77%, respectively.
CSP was extracted from cocoa husks according to Vriesmann et al. (2012) with some modifications. Flour of cocoa husks was mixed in citric acid 0.1 N (pH 2.2) at 90 °C for 90 min in a 1:50 solid: solution (w/v) ratio. The suspension was cooled at room temperature for 6 h and centrifuged at 4500 rpm for 15 min at 4 °C. The supernatant was separated and suspended in 96% ethanol (1:1 v/v), then left for 16 h at 4 °C. Afterward, the solution was centrifuged at 4500 rpm for 20 min, and the precipitate was separated with a filter cloth and washed with 96% ethanol until the ethanol was colorless. The pectin obtained was dried at 50 °C for 4 h.
The protein (PC) and gum (GC) of Cajanus cajan seeds were obtained as reported by Robles-Flores et al. (2018). Chipilin (Crotalaria longirostrata) methanolic extract was obtained as reported by Cruz-Rodríguez et al. (2017) using chipilin leaves obtained from a local supermarket. Previous to extraction, the leaves were washed with water and dried in the shade at 40 °C to constant weight. The dried leaves were ground to a particle size of 100. The extraction yield for methanolic extract was 5.37 ± 0.31% (5.37 g of extract lyophilized from 100 g of dried leaves).
Preparation of feed blends for spray drying
Six different blends were spray dried (Table 1). Before microencapsulation, each encapsulating agent was dispersed in distilled water and kept refrigerated for 12 h for complete hydration. Subsequently, the blends were added and mixed with an IKA brand Turrax homogenizer (IKA, Delaware, USA) at 10,000 rpm for 5 min. For all treatments, the total solids’ concentration was 18 g 100 g−1 of which 17 g 100 g−1 were of encapsulating agents and 1 g 100 g−1 of methanolic extract of chipilin. Once the mixtures were made, they were fed into the spray dryer. Kinematic viscosities of the blend were determined at 25 °C using a SVM 3000 Stabinger viscometer (Anton Paar, Graz, Austria).
Table 1.
Encapsulating agents used for spray drying encapsulation of chipilin (Crotalaria longirostrata) methanolic extracts
| Treatment | Composition (g 100 g−1) | ||||||
|---|---|---|---|---|---|---|---|
| MD | GA | GC | CSP | PC | SP | CME | |
| T1 | 15 | 2 | – | – | – | – | 1 |
| T2 | 15 | – | 2 | – | – | – | 1 |
| T3 | 15 | – | – | 2 | – | – | 1 |
| T4 | 15 | – | – | – | 2 | – | 1 |
| T5 | 15 | – | – | – | – | 2 | 1 |
| T6 | 17 | – | – | – | – | – | 1 |
MD maltodextrin, GA gum Arabic, GC gum Cajanus cajan seeds, CSP cocoa shell pectin, PC protein Cajanus cajan seeds, SP soy protein, CME chipilin methanolic extract, – not added
Microencapsulation by spray drying
A BUCHI mini B-290 laboratory-type spray dryer (BUCHI, Flawil, Switzerland) with a standard 0.5-mm nozzle was used. The inlet and outlet air temperatures were 120 °C and 60 °C. A feed flow of the solution to be dried of 3 mL min−1, a spray air flow of 742 L h−1 and a pressure drop of 1.35 bar were used. The aspirator had a gas flow rate of 35 m3 h−1. To maintain the homogeneity of the solution, suspensions were gently shaken using magnetic agitation. After spray drying, powders were recovered, weighed and stored in vacuum-closed metal bags, and, finally, they were stored in refrigeration until evaluation.
Spray drying yields was determined with the mass of the final product obtained compared with the total amount of solids fed into the dryer. The yield was calculated with Eq. 1:
| 1 |
Powder properties
Moisture content and water activity
Moisture content was determined according to the AOAC method (AOAC 2000). The measurement of water activity was carried out by using a HygroPalm AW1 hygrometer (Rotronic, Zurich, Switzerland).
Bulk density
Bulk density was determined according to Fazaeli et al. (2012) with modifications. One g of microcapsules was deposited in an empty 10-mL graduated cylinder and vortexed for 1 min. The ratio between mass and volume occupied by the powder in the cylinder determined the bulk density value.
Water solubility index (WSI), water absorption rate (WAR) and swelling capacity (SC)
The WSI was determined according to Paini et al. (2015). For that, 1 g of microcapsules was added to 12 mL of distilled water, mixed vigorously and incubated at 30 °C for 30 min. The mixture was then centrifuged at 3500 rpm for 10 min. The supernatant was collected in a Petri dish and was finally dried at 105 °C to constant weight. The WSI, WAR and SC were calculated by Eqs. 2, 3 and 4, respectively:
| 2 |
| 3 |
| 4 |
Microcapsule morphology
The microcapsules morphology was examined by scanning electron microscopy (SEM) using a high-resolution, high-vacuum JEOL SM-71480 microscope (JEOL, Massachusetts, USA). Microcapsules were attached to the specimen holder with double-sided adhesive tape, and SEM images were taken at room temperature and examined by using an acceleration voltage of 2 kV.
Microencapsulation efficiency
Microencapsulation efficiency was calculated by using the surface and total phenolic compounds in the microcapsules as described by Robert et al. (2010). Briefly, 200 mg of microcapsules were mixed with 2 mL of methanol:acetic acid:water solution (50:8:42 v/v/v). The mixture was vortexed for 1 min, sonicated twice in a Cole-Parmer ultrasonic model 08855-00 bath (Vernon Hills, Illinois, USA) at 25 °C for 20 min, and finally centrifuged at 4000 rpm for 5 min. The total phenolic content was determined with Folin-Ciocalteu reagent, using the method described by Singleton et al. (1999) with gallic acid as the standard. For the surface phenolic compounds’ content, 200 mg of the microcapsules were mixed with 2 mL of ethanol:methanol (1:1) and vortexed for 1 min, then centrifuged at 4000 rpm for 5 min. The surface phenolic compounds content was determined as the total phenolic compounds’ content. The microencapsulation efficiency was determined by Eq. 5 (Mahdavi et al. 2016):
| 5 |
where PCtotal is the total phenolic compound (mg gallic acid g−1) and PCsup is the superficial phenolic compound (mg gallic acid g−1).
Determination of antioxidant activity
The antioxidant capacity was determined by scavenging the radical 2,2-diphenyl-1-picrylhydrazyl (DPPH), according to Shekhar and Anju (2014). The method is based on the reduction of the DPPH radical to 517 nm in the presence of an antioxidant that donates an electron or hydrogen atom to a radical (Hosseini et al. 2013). The effect of antioxidants on DPPH radical quenching was expressed as the absorbance. Briefly, several solutions of microcapsules using distilled water were prepared (of 1, 10, 15, 20, and 25 mg mL−1). Three mL of these solutions were mixed with 1 mL of DPPH 0.1 mM at room temperature. After 30 min of incubation in darkness, the absorbance of the solution was determined to 517 nm. The EC50 value (the average effective concentration) of the sample, which is the concentration of the sample required to inhibit 50% of the free DPPH radical, was calculated using the inhibition curve.
Release analysis and modeling of phenolic compounds
In vitro release test
The in vitro release of chipilin’ methanolic extract microencapsulated was determined according to Cheraghali et al. (2018) with modifications. Two grams of microcapsules were dispersed in 25 mL of distilled water at room temperature with constant agitation. At 5-min intervals, 5 mL of dispersion were taken and replaced with an equivalent volume of distilled water. Aliquots were centrifuged at 9000 rpm for 5 min, and the phenolic compounds released were quantified as previously described in terms of the mg of gallic acid g−1 of powder.
The release kinetics of phenolic compounds were calculated using models reported by Assadpour et al. (2017):
| 6 |
| 7 |
| 8 |
where k is the model constant, t is time (min), Qt is the released concentration of phenolic compounds at time t (mg g−1), Q0 is the initial concentration of phenolic compound within solutions (usually Q0= 0), Ct is the remaining concentration of phenolic compounds within capsules after time t (mg g−1), and C0 is the initial concentration of phenolic compounds within capsules (mg g−1).
The cumulative phenolic compound released (CPCR) was also calculated. The CPCR was fitted to the Korsmeyer-Peppas semi-empirical model (Hosseini et al. 2013; Cheraghali et al. 2018):
| 9 |
where is the cumulative phenolic compound release (%), K is the release rate constant, n is the release exponent that could be used to indicate the mechanism of release and t is the time in s. The R2 was calculated for each model. Constants k, K and n were determined by estimation using Statgraphics Centurion XV software (StatPoint Technologies, USA).
Accelerated storage stability test
The accelerated storage stability test was determined by the accelerated test with Rancimat equipment (Metrohm AG, Herisau, Switzerland) by evaluating the oxidation of microencapsulated phenolic compounds in accelerated conditions. Two temperatures (160 °C and 180 °C) and an airflow of 10 L h−1 were evaluated. For that, 1.5 g of microcapsules (powder) were placed in each reaction vessel, and air at 160 °C and 180 °C was applied. The Rancimat equipment detected the oxidation product released during the test. During this test, the phenolic compounds are oxidized, and volatile secondary products of the reaction are formed, which are transported to the measuring vessel by the airflow and the absorption in the measuring solution (triple distilled water). The electrical conductivity is recorded in the measurement solution; in this way, the change can be detected. The time that elapses until the appearance of the secondary products of the reaction is called the induction time, characterizing the oxidation stability of the phenolic compounds. The induction time values were automatically deducted and recorded by the Rancimat equipment. The tests were performed in triplicate. The stability time (t) of the microencapsulation at 4 °C was obtained by extrapolating using Eq. 10 at the usual storage temperatures:
| 10 |
where ts is the stability time (years), a and b are the model constants, and T is the temperature (°C).
The stability time is calculated from the slope of the line obtained by representing the natural logarithm of the induction times as a function of the temperature.
Experimental design
For microencapsulation, a completely random experimental design of six treatments with two repetitions was used. The results were analyzed by ANOVA (p < 0.05), and the Tukey test was used for the mean comparison using the Statgraphics Centurion XVI software. Response variables were spray drying yield, microencapsulation efficiency, phenolic compound encapsulated, phenolic compound release, the microcapsules’ morphology and stability. Some graphs were drawn by Excel (Microsoft Office 2019).
Results and discussion
Yield of spray drying
Spray drying yields ranged from 46 to 64%, and the highest yield was obtained with T1 and the lowest with T2. Drying yield is directly related to the adhesion of the microcapsules to the drying chamber and the losses of the product because of the stickiness of the powder. Bhandari et al. (1997) showed that because of the synergy between the components of the mixture to be dried the glass transition temperature (Tg) is likely to be altered, causing the dust still in the drying chamber to pass from the vitreous state to the gummy state, which creates adhesions in the drying chamber.
The kinematic viscosity of mixtures to be dried was analyzed (Table 2). The kinematic viscosity of the solutions was affected by the composition of the blends; the results suggest than when a high kinematic viscosity was used, the spray drying yield decreased. Treatments T2 and T3 were statistically higher than other treatments. Treatments with higher and lower yields (T1 and T2) have gum in their composition: GA in T1 and GC for T2. Differences could be explained by the composition of each gum. Authors like Cui (2005) have reported that the functional properties and physicochemical characteristics of gums may vary depending on molecular weight, chemical composition, monosaccharide sequence and the position of glycosidic bonds, which cause differences between viscosities and perhaps in the drying yield. GA is obtained from the Acacia tree and contains rhamnose, arabinose and galactose (Mirhosseini and Amid 2012), whereas Cajanus cajan gum contains arabinose (54%) as the main sugar, with small amounts of galactose, glucose, xylose, rhamnose and/or fucose. Thus, these differences in composition could contribute to the ability to form hydrogen bonds with the polyphenols increasing or decreasing the yield.
Table 2.
Spray drying yield process, and kinematic viscosity, moisture content, water activity, bulk density, water solubility index, water absorption rate and swelling capacity properties of microcapsules obtained by spray drying using different encapsulating agents
| Treatment | Encapsulating agents | Spray drying yield (%) |
Kinematic viscosity (mPa s) |
Moisture content (%, w.b.) |
Water activity | Bulk density (g cm−3) |
Water solubility index (%) |
Water absorption rate (g g−1) |
Swelling capacity (g g−1) |
|---|---|---|---|---|---|---|---|---|---|
| T1 | MD–GA | 64.39 ± 1.07a | 3.90 ± 0.23b | 3.72 ± 0.22ab | 0.26 ± 0.01b | 0.23 ± 0.02a | 86.11 ± 6.24ab | 0.26 ± 0.05b | 0.009 ± 0.007ab |
| T2 | MD–GC | 46.71 ± 5.70c | 42.99 ± 2.49a | 3.01 ± 0.68b | 0.39 ± 0.01a | 0.26 ± 0.2a | 82.15 ± 0.78b | 0.90 ± 0.05a | 0.004 ± 0.0008b |
| T3 | MD–CSP | 51.13 ± 3.03bc | 38.71 ± 2.90a | 4.31 ± 0.41a | 0.31 ± 0.04ab | 0.24 ± 0.02a | 84.25 ± 3.05ab | 0.66 ± 0.16a | 0.005 ± 0.001ab |
| T4 | MD–PC | 61.74 ± 4.10ab | 3.06 ± 0.03b | 3.71 ± 0.44ab | 0.25 ± 0.02b | 0.21 ± 0.02a | 84.70 ± 2.33ab | 0.58 ± 0.08ab | 0.005 ± 0.001ab |
| T5 | MD–SP | 60.67 ± 2.96ab | 4.73 ± 1.36b | 3.94 ± 0.35ab | 0.29 ± 0.004b | 0.22 ± 0.01a | 86.54 ± 4.14ab | 0.70 ± 0.32a | 0.006 ± 0.002ab |
| T6 | MD | 62.43 ± 1.53ab | 3.08 ± 0.03b | 3.15 ± 0.14b | 0.25 ± 0.004b | 0.23 ± 0.01a | 91.98 ± 1.24a | 0.25 ± 0.04b | 0.01 ± 0.001a |
| LSD | 12.85 | 6.61 | 0.93 | 0.088 | 0.049 | 7.84 | 0.35 | 0.007 |
MD maltodextrin, GA gum Arabic, SP soy protein, CSP cocoa shell pectin, PC protein Cajanus cajan seeds, GC gum Cajanus cajan seeds
Powder properties
Moisture content and water activity
Table 2 shows the moisture content and water activity of microcapsules after spray drying. The moisture content varied between 3 and 4%, and visually no agglomerate formation was observed in any treatment. This low moisture content in the microcapsules could be due to the drying conditions. Several authors mention that in a spray drying system lower moisture content can be reached with the increase of the inlet air temperature (Fazaeli et al. 2012; Paini et al. 2015), despite the use of different encapsulating agents. In addition, this is because at a higher input temperature the rate of heat transfer to the particle is higher, which provides a greater driving force for moisture evaporation (Fazaeli et al. 2012).
Water activity varied between 0.25 and 0.39. Tonon et al. (2009) showed that the Aw of 0.3 or lower is better for the stability of the powder because less free water is available for the growth of microorganisms and biochemical reactions and therefore increasing the shelf life.
Bulk density determination of the powders
The bulk density of powders in the different treatments was statistically equal (p > 0.05), varying from 0.210 to 0.260 g cm−3. These values are lower compared with the bulk density of microencapsulated obtained by Pieczykolan and Kurek (2019), who reported bulk densities of up to 0.95 g cm−3 using maltodextrin and inulin as encapsulating agents of anthocyanins. They explained that differences could be attributed to the difference of the molecular weight of the coating material. The reduction in bulk density is probably due to an increase in particle size and a greater tendency for particles to be hollow. The drying rate is also important for this parameter, as the surface of the particle can preserve the original shape by hardening the capsule when drying occurs quickly or can contract when the water contained inside evaporates as a result of the process (Pieczykolan and Kurek 2019).
Morphology of microcapsules
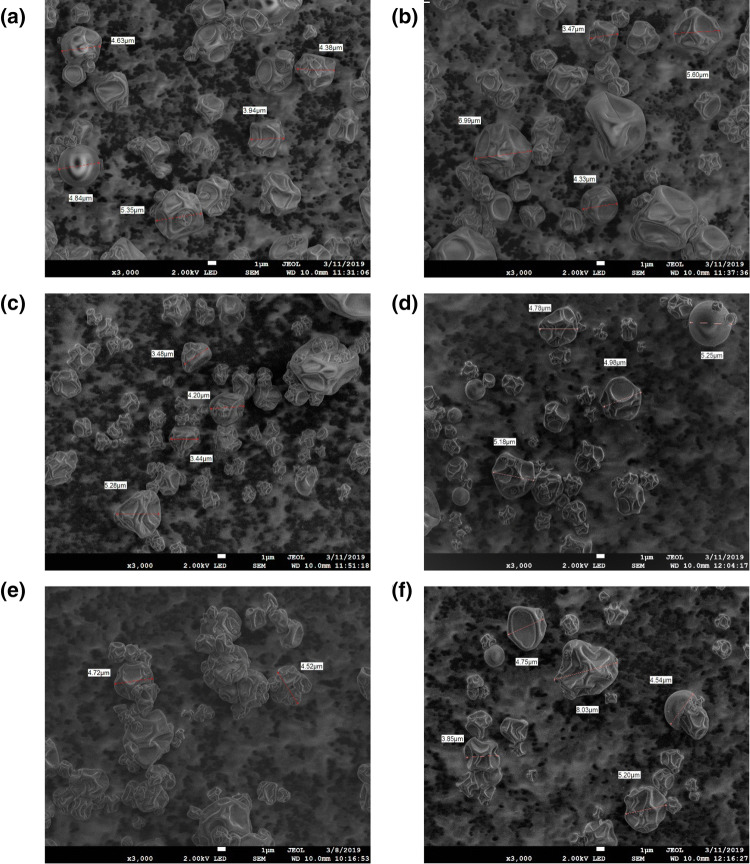
Micrographs (Fig. 1) show that these microcapsules possessed a continuous wall, with mostly irregular amorphous structures, and smooth and depression surfaces were observed. No cracks were observed, which proves how good the phenolic compounds’ encapsulation is. During atomization in the chamber of the spray dryer, the drops are rapidly expanded, and hollow particles with a matrix-type structure are produced as reported Akbarbaglu et al. (2019).
Fig. 1.
SEM micrographs of spray-dried powder particle containing Crotalaria longirostrata methanolic extract (CME) microencapsulated with different encapsulating agents: a MD–GA, b MD–GC, c MD–CSP, d MD–PC, e MD–SP, f MD. MD maltodextrin, GA gum Arabic, SP soy protein, CSP cocoa shell pectin, PC protein Cajanus cajan seeds, GC gum Cajanus cajan seeds
The size of microcapsules varied between 3 and 8 μm for all treatments, but no statistically significant difference was observed in the average particle size between treatments (Fig. 2a). The particle size distribution is similar for blends of MD with other encapsulating agents with close 65% for a size particle of 4 μm. When MD was used, the particle size distribution of this was lower (56%) for a size particle of 4 μm (Fig. 2b). These characteristics of microcapsules have been reported by other authors such as Akbarbaglu et al. (2019), who reported an average particle size of 5 μm for microcapsules of flaxseed protein hydrolysates obtained by spray drying. Sarabandi et al. (2019), however, reported a particle size higher than 50 μm for the microencapsulation of eggplant peel extract using gum Arabic and maltodextrin. The difference could be due to inlet air temperature (> 140 °C) and the encapsulating agents used. Paini et al. (2015) mentioned that the inlet air temperature also influences the microstructure of the microcapsules. A temperature of 160 °C caused a greater degradation of the spherical structure of the particles, leading to a greater porosity and a lower apparent density of the microparticles. Fazaeli et al. (2012) mentioned that the differences between the particle size or shape of microcapsules can be explained by the molecular structure of the carrier agents. In this work, however, no morphological variations were observed, even though different encapsulating agents were used. This may be because maltodextrin was used in all treatments in the same proportion (15%) and was greater in concentration compared with other encapsulating agents (2%).
Fig. 2.
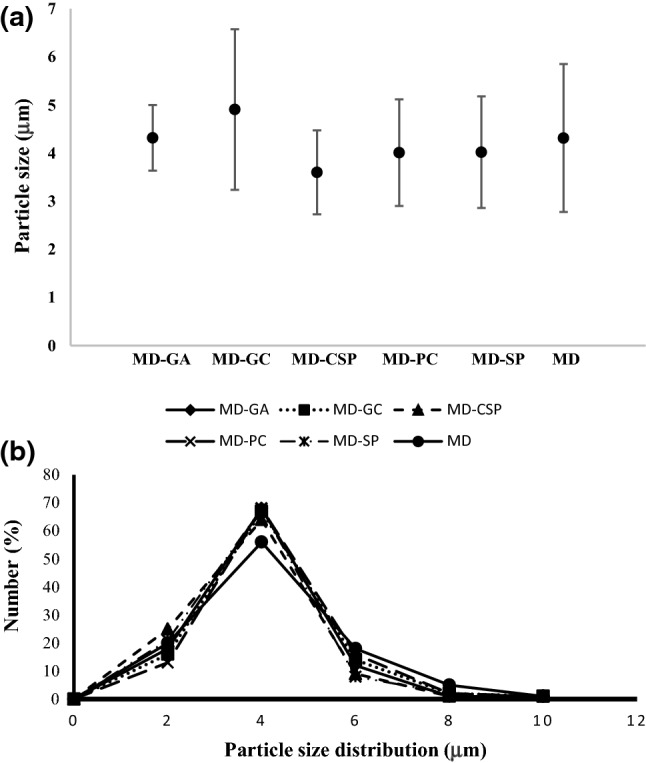
a Average particle size and b particle size distribution of microcapsules containing Crotalaria longirostrata methanolic extract (CME) microencapsulated with different encapsulating agents: MD–GA, MD–GC, MD–CSP, MD–PC, MD–SP, MD. MD maltodextrin, GA gum Arabic, SP soy protein, CSP cocoa shell pectin, PC protein Cajanus cajan seeds, GC gum Cajanus cajan seeds
Water solubility index (WSI), water absorption rate (WAR) and swelling capacity (SC)
The rehydration properties of the microparticles were evaluated in terms of water solubility index (WSI), water absorption index (WAI) and swelling capacity (SC). WSI values were between ranges of 82–91% (Table 2). These values are similar to those obtained by Paini et al. (2015) for the microencapsulation of olive pomace and higher than those obtained by Kha et al. (2010) and Ahmed et al. (2010) for the encapsulation of Momordica cochinchinensis extract (38%) and the purple potato (40–57%), respectively.
The high solubility of microcapsules could be because all encapsulating agents had high solubility, and of the 18% of the solids in the mixtures, 15% was maltodextrin, which has high solubility in water and is why it is mainly used in the drying process (Fazaeli et al. 2012), favoring the solubility of the microencapsulated extract.
WAR values ranged from 0.2 to 0.9 g g−1 (Table 2), similar to those reported by Paini et al. (2015) (0.28–0.58 g g−1) and lower than those obtained by Ahmed et al. (2010), who reported a WAR of 0.86–1.20 g g−1. The variation in WAR may be related to the availability of hydroxyl groups present in the structure of encapsulating agents for forming hydrogen bonds between encapsulating agents and water (Ahmed et al. 2010).
On the other hand, the swelling capacity (Table 2) values of microencapsulated powders were very low (0.004–0.01 g g−1) compared with the results shown by Paini et al. (2015), who reported values between 2 and 3 g g−1. Ahmed et al. (2010) mentioned that in treatments where the concentration of maltodextrin was increased granular swelling was reduced; the authors mentioned that the low swelling capacity is caused by the granular stability of the microcapsules, thus reducing the ability to swell.
Microencapsulation efficiency (ME)
Phenolic compounds’ content in microcapsules varied significantly between treatments (Table 3). The blend of MD–GA had a higher phenolic compound (1.38 mg g−1), whereas the blend of MD–PC had the lowest retention (0.89 mg g−1). For IC50 values varied between 13.35 and 16.95 mg mL−1, and significant differences (p < 0.05) were obtained between treatments (Table 3). Treatments 1, 2, 5 and 6 needed about 13 mg of extract to inhibit the 50% of DPPH (IC50 value), but treatments 3 and 4 needed about 16 mg. The IC50 results are proportional to the phenolic compounds content in the powders, which suggests that the phenolic compounds have an important role in determining the antioxidant capacity in the methanolic extract of chipilin. For a higher phenolic compound content, fewer milligrams of powder are required to inhibit 50% of the free radicals so that the phenolic compounds’ initial content in the mixture to be encapsulated will have a direct relationship to the antioxidant activity of the microcapsule obtained after drying.
Table 3.
Phenolic compounds, IC50, microencapsulation efficiency and stability time properties of microcapsules obtained by spray drying using different encapsulating agents
| Treatment | Encapsulating agents | Phenolic compound (mg GA g−1 powder) |
IC50 (mg mL−1) |
Microencapsulation efficiency (%) |
Stability time at 4 °C (year) |
Stability time at 30 °C (year) |
|---|---|---|---|---|---|---|
| T1 | MD–GA | 1.38 ± 0.0a | 13.34 ± 0.42a | 85.95 ± 0.43b | 4.42 ± 0.49bc | 0.77 ± 0.07bc |
| T2 | MD–GC | 1.23 ± 0.04ab | 13.35 ± 0.47a | 89.83 ± 0.89ab | 3.44 ± 0.57cd | 0.63 ± 0.08c |
| T3 | MD–CSP | 1.06 ± 0.12bc | 16.35 ± 0.79b | 78.28 ± 0.98c | 8.88 ± 0.64a | 1.43 ± 0.08a |
| T4 | MD-PC | 0.89 ± 0.10c | 16.95 ± 0.28b | 75.10 ± 1.16c | 1.31 ± 0.36ed | 0.27 ± 0.06d |
| T5 | MD–SP | 1.16 ± 0.23abc | 13.96 ± 0.81a | 92.77 ± 2.03a | 0.7 ± 0.03e | 0.16 ± 0.006d |
| T6 | MD | 1.17 ± 0.04abc | 13.99 ± 0.53a | 65.40 ± 0.41d | 5.95 ± 0.86b | 0.99 ± 0.12b |
| LSD | 0.298 | 1.72 | 4.49 | 2.22 | 0.32 |
MD maltodextrin, GA gum Arabic, SP soy protein, CSP cocoa shell pectin, PC protein Cajanus cajan seeds, GC gum Cajanus cajan seeds
The treatment that had the highest retention of phenolic compounds was treatment 5 (Table 3) with an ME of 92%, and the lowest efficiency (65%) was obtained in treatment 6 (control) with a significant statistical difference (p < 0.05). Treatment T6 contains only maltodextrin as an encapsulating agent. This treatment shows that the use of a single encapsulating agent does not always guarantee the highest efficiency, so using it in combination with other materials makes it possible to improve the microencapsulation efficiency. Using maltodextrin alone as the wall material gave the lowest efficiency probably because of its lack of emulsification and low film forming capacity as reported by Mahdavi et al. (2016). The results of the present study also revealed that the gums and CSP provide lower phenolic compounds’ entrapment, while higher concentrations of protein in the blend were more efficient for protecting and entrapping the phenolic compounds. This could also be associated with the difference between the chemical structure of the gum, polysaccharides and proteins, as well as specific interactions with the phenolic compounds. In our results, when a blend was used, suggested interactions occurring between MD, GA, SP, CSP, PC, GC and phenolic compounds are the hydrogen bonds that are formed between the hydrogens of the hydroxyl groups of phenolic compounds and the oxygen atoms of the glycosidic bonds present in polysaccharides. Moreover, the microencapsulation efficiency also depends on the affinity of phenolic compounds and encapsulating agents, as well as properties such as water solubility, molecular size, conformational mobility and the shape of polyphenol (Mahdavi et al. 2016).
The high microencapsulation efficiency observed in treatment T5 may be due to the ability of SP to interact with MD and CME, with hydrophobic interactions and hydrogen bonds between proteins and phenolic compounds. Moreover, the combination of soy proteins with carbohydrates (MD) as encapsulating agents could promote better protection, oxidative stability and drying properties. The protection generated by the mixture of encapsulating agents is mainly due to the hydrogen bonds that form polysaccharides with proteins when water is removed in the drying process (Rokka and Rantamäki 2010).
The characteristics of proteins, however, play a very important role in the microencapsulation process and its efficiency in the retention of phenolic compounds. Treatments T4 and T5 show that the efficiencies between the two treatments when using Cajanus cajan and soy protein, respectively, differed significantly. It is probably due to the difference of the purity of both proteins. According to the proximate analysis reported in the “Materials and methods” section, the Cajanus cajan protein is a concentrate that contains 67% of protein, but the commercial soy protein “Soyatein” contains 90% (per information from the manufacturer). The high carbohydrate content (21.5%) in the Cajanus cajan protein suggests that proteins and carbohydrates can probably interact with phenolic compounds, modifying some interactions and the stability of microcapsule structure and decreasing the microencapsulation efficiency. In addition, the type of proteins could be playing a very important role in encapsulation efficiency. Nesterenko et al. (2013) mentioned that soybeans contain a significant fraction (35–40%) of mainly glycinin and conglycinin proteins (50–90% of total proteins). The glycinin fraction (11S globulin) has a molecular weight of approximately 350 kDa, while conglycinin (globulin fraction 7S) is approximately 70 kD, which shows particular physicochemical and functional attributes in terms of the properties of gel formation, the emulsifier and the surfactant. On the other hand, Akinhanmi et al. (2008) found globulin, albumin and alkaline glutelin to be more abundant proteins in the protein fraction of Cajanus cajan.
Release analysis and stability of phenolic compounds’ microcapsules
Stability of microencapsulated phenolic compounds in an accelerated test
Table 3 show significant statistical differences (p < 0.05) for the stability time at 4 and 30 °C. The treatment with the longest stability of the microcapsules was treatment T3 (8.8 y at 4 °C and 1.43 y at 30 °C). This could be due to the high stability of pectin and maltodextrin as a polymer because together they can protect phenolic compounds. On the other hand, the treatments where proteins were used had the lowest stability times; this may be due to the protein being more susceptible to denaturing and although they have interacted with maltodextrin. The stability time shows, however, that all encapsulating agents can provide a good protection to the phenolic compounds in the microcapsules. That is because the non-encapsulated chipilin’ methanolic extract was degraded between 3 and 5 s at 160 and 180 °C, respectively. The stability of the microencapsulated phenolic compounds could be due to the high molecular weight and to the higher glass transition temperature (Cai and Corke 2000) of encapsulating agents, giving them stability at high temperatures. The degree of protection provided by encapsulating agents, however, could not only depend on physical condition but also on other factors such as the structure and the characteristics of each agent (Mahdavi et al. 2016). It is also possible that the porous structure of the vitreous matrices will allow oxygen to penetrate the structure of the microcapsule, oxidizing the phenolic compounds.
Phenolic compounds’ release controlled
The release of phenolic compounds was carried out to observe the capacity of encapsulating agents in retaining the phenolic compounds (Fig. 3). A rapid release of phenolic compounds was observed from the beginning of the test for 5 min; after 10 min, the release was constant, indicating the complete phenol release of the microcapsules, which was close to 97% for all treatments. After 2 min, a statistical difference was found between the CPCR using MD–GC and MD–CSP. The reason for this might be the fact that MD–GC provides a stronger interaction with phenolic compounds than MD–CSP. After 5 min, however, no significant differences can be observed. These results could be explained because the microcapsules were dispersed in water where the encapsulating agents are highly soluble. These results are according to Ribeiro et al. (2019) who reported values for about 20 min for complete release (100%) of microencapsulated elderberry extract using modified chitosan, sodium alginate and gum Arabic as encapsulating agents.
Fig. 3.
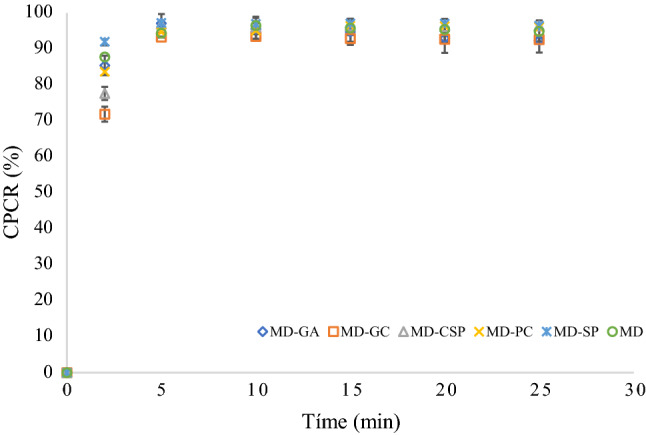
Cumulative phenolic compound released (CPCR) of microencapsulated phenolic compounds of Crotalaria longirostrata (chipilin) using several mixtures of encapsulating agents: MD maltodextrin, GA gum Arabic, SP soy protein, CSP cocoa shell pectin, PC protein Cajanus cajan seeds, GC gum Cajanus cajan seeds
The high solubility and release of phenols are related to the amorphous structure of microcapsules as well as their dimensions. Sansone et al. (2011) mentioned that amorphous structures and the small diameter of microcapsules improve the total surface area exposed to the solvent facilitating the release, increasing the microcapsule-water interaction and facilitating the solubility (Ahmed et al. 2010; Paini et al. 2015).
Table 4 shows the coefficients (k, K and n) for the zero, the first-order, the Higuchi model and the Korsmeyer-Peppas model found for all treatments. In relation to k values for zero, first-order and the Higuchi models, these varied between 0.0064 and 0.021 for the zero model, between 0.0387 and 0.146 for the first-order model and between 0.0167 and 0.064 for the Higuchi model. These values are similar to those reported by Assadpour et al. (2017) for folic acid release from spray dried powder particles of pectin-whey protein nanocapsules. R2 values varied between 43 and 92% for the zero, first-order and Higuchi models, where the first-order model had the highest values (of 66–92%). For the Korsmeyer-Peppas model, however, all R2 values were higher than 98%. Values for K varied between 54.54 and 86.60 s−1, whereas n values changed to 0.016 and 0.077 depending on the encapsulating agents used. The microcapsules composed of MD–GC and MD–CSP permitted a low K value, compared with the MD–GA and MD–SP (Table 4). The presence of CSP, GC and PC in the blend matrix reduced the K value (60.85, 54.54 and 68.99 s−1, respectively) of the phenolic compounds’ release. Among the samples, the MD–GC microcapsules showed the lowest value of release rate constant (K), and microcapsules prepared with MD–SP showed the highest release rate. For all treatments, values of the release rate constant were higher than reported by Cheraghali et al. (2018) and Hosseini et al. (2013), who reported values lower than our values (0.818 s−1 and 16.71–27.99). In relation to the n value for the Korsmeyer-Peppas equation, our values changed between 0.016 and 0.077, but these values were lower than reported by Cheraghali et al. (2018). They reported values of the diffusion release index between 0.43 and 0.85, which implied the samples followed an anomalous non-Fickian diffusion release mechanism, with a swelling effect. The difference could be because Cheraghali et al. (2018) used 21% of encapsulating agents, whereas we used 17%. The GC better controlled the release compared with other compounds added to the MD.
Table 4.
Kinetic model parameters for the release of phenolic compounds from microcapsules formulated with several encapsulating agents
| Treatment | Encapsulating agents | Zero | First-order | Higuchi | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| k | R2 | k | R2 | k | R2 | K value (s−1) |
n | R2 | ||
| T1 | MD-GA | 0.0101 | 52 | − 0.0396 | 75 | 0.0265 | 65 | 80.81 | 0.0221 | 99 |
| T2 | MD–GC | 0.021 | 52 | − 0.146 | 68 | 0.064 | 67 | 54.54 | 0.077 | 98 |
| T3 | MD–CSP | 0.0152 | 43 | − 0.0449 | 66 | 0.049 | 56 | 60.85 | 0.067 | 98 |
| T4 | MD-PC | 0.0107 | 53 | − 0.0453 | 74 | 0.0478 | 67 | 68.99 | 0.0481 | 99 |
| T5 | MD–SP | 0.0064 | 76 | − 0.0319 | 85 | 0.0167 | 88 | 86.60 | 0.016 | 99 |
| T6 | MD | 0.0094 | 75 | − 0.0387 | 92 | 0.0287 | 86 | 78.42 | 0.0287 | 99 |
MD maltodextrin, GA gum Arabic, SP soy protein, CSP cocoa shell pectin, PC protein Cajanus cajan seeds, GC gum Cajanus cajan seeds
Conclusion
The spray drying process allowed us to obtain microcapsules with moisture content and low water activity, which benefited the stability of the microcapsules. The concentration of maltodextrin (18%) and other encapsulating agents (GA, GC, CSP, PC and SP) used benefited the bulk density of the powders since a low bulk density was achieved. The amorphous structure and size of the microcapsules for all treatments also benefited their final solubility. The interactions between maltodextrin, the phenolic compounds of the chipilin extract and other encapsulating agents (GA, GC, CSP, PC or SP) allowed the formation of an efficient polymer matrix for encapsulation by spray drying, where the soy protein (SP) contributed to a better retention of phenolic compounds with 92% encapsulation efficiency. The encapsulated phenolic compounds, however, were released quickly (between 2 and 5 min), so their use would mainly be applied to cold or hot drinks. The MD–CSP and MD–GC mixtures permitted improving the retention of phenolic compounds during the release process with respect to other agents, so increasing the concentrations of CSP as well as the total solids’ content used in the mixture is suggested. The Korsmeyer-Peppas model was the best in predicting the release of phenolic compounds with R2 values higher than 98%. Therefore, GC and CSP mixed with MD could be an alternative to common biopolymers used as carriers to protect labile materials that will be encapsulated by spray drying.
Acknowledgements
Navarro-Flores thanks the Consejo Nacional de Ciencía y Tecnologia (CONACyT, Mexico) for the scholarship granted to her. The authors thank Tecnologico Nacional de México for funding this work (Project: 6840.18-P). The authors also thank Edith Ponce-Recinos, from the Universidad Politecnica de Chiapas (Mexico), for support with the scanning electron microscopy technique.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed M, Akter MS, Lee JC, Eun JB. Encapsulation by spray drying of bioactive components, physicochemical and morphological properties from purple sweet potato. LWT Food Sci Technol. 2010;43(9):1307–1312. doi: 10.1016/j.lwt.2010.05.014. [DOI] [Google Scholar]
- Akbarbaglu Z, Jafari SM, Sarabandi K, Mohammadi M, Heshmati MK, Pezeshki A. Influence of spray drying encapsulation on the retention of antioxidant properties and microstructure of flaxseed protein hydrolysates. Colloids Surf B. 2019;178:421–429. doi: 10.1016/j.colsurfb.2019.03.038. [DOI] [PubMed] [Google Scholar]
- Akinhanmi TF, Arogundade LA, Tiamiyu MO, Oloruntoba E, Osiname BJ. Protein fractions of legumes and cereals consumed in Nigeria. ASSET Int J. 2008;7:54–62. [Google Scholar]
- AOAC . Official methods of analysis of AOAC International. 17. MD: Gaithersburg; 2000. [Google Scholar]
- Assadpour E, Jafari SM. Advances in spray-drying encapsulation of food bioactive ingredients: from microcapsules to nanocapsules. Annu Rev Food Sci Technol. 2019;10:103–131. doi: 10.1146/annurev-food-032818-121641. [DOI] [PubMed] [Google Scholar]
- Assadpour E, Jarafi SM, Maghsoudlou Y. Evaluation of folic acid release from spray dried powder particles of pectin-whey protein nano-capsules. Int J Biol Macromol. 2017;95:238–247. doi: 10.1016/j.ijbiomac.2016.11.023. [DOI] [PubMed] [Google Scholar]
- Belščak-Cvitanović A, Lević S, Kalusevic A, Špoljarić I, Đorđević V, Komes D, et al. Efficiency assessment of natural biopolymers as encapsulants of green tea (Camellia sinensis L.) bioactive compounds by spray drying. Food Bioprocess Technol. 2015;8:2444–2460. doi: 10.1007/s11947-015-1592-y. [DOI] [Google Scholar]
- Bhandari BR, Datta N, Howes T. Problems associated with spray drying of sugar-rice foods. Dry Technol. 1997;15(2):671–684. doi: 10.1080/07373939708917253. [DOI] [Google Scholar]
- Cai YZ, Corke H. Production and properties of spray-dried Amaranthus Betacyanin pigments. J Food Sci. 2000;65(7):1248–1252. doi: 10.1111/j.1365-2621.2000.tb10273.x. [DOI] [Google Scholar]
- Cai Y, Luo Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–2184. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheraghali F, Shojaee-Aliabadi S, Hosseini SM, MirmoghTadaie L, Mortazavian AM, Ghanati K, et al. Characterization of microcapsule containing walnut (Juglans regia L.) green husk extract as preventive antioxidant and antimicrobial agent. Int J Prev Med. 2018;8:1–6. doi: 10.4103/ijpvm.IJPVM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Rodríguez R, Meza-Gordillo R, Rodríguez-Mendiola M, Arias-Castro C, Mancilla-Margalli N, Ávila-Miranda M, Ruiz-Valdiviezo VM, Ayora-Talavera T. Antifungal activity of Crotalaria longirostrata Hook. & Arn. extracts against phytopathogen fungi from maize. Gayana Bot. 2017;74(1):167–175. doi: 10.4067/s0717-66432017005000102. [DOI] [Google Scholar]
- Cui SW (2005) Food carbohydrates: chemistry, physical properties and applications. Ed. Taylor and Francis Group. https://ttngmai.files.wordpress.com/2012/09/foodcarbohydrates.pdf
- Desai KGH, Park HJ. Recent developments in microencapsulation of food ingredients. Dry Technol. 2005;23:1361–1394. doi: 10.1081/DRT-200063478. [DOI] [Google Scholar]
- Esfanjani AF, Jafari SM, Assadpoor E, Mohammadi A. Nano-encapsulation of saffron extract through double-layered multiple emulsions of pectin and whey protein concentrate. J Food Eng. 2015;165:149–155. doi: 10.1016/j.jfoodeng.2015.06.022. [DOI] [Google Scholar]
- Fazaeli M, Emam-Djomeh Z, Kalbasi Ashtari A, Omid M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod Process. 2012;90(4):667–675. doi: 10.1016/j.fbp.2012.04.006. [DOI] [Google Scholar]
- Hosseini SM, Hosseinia H, Mohammadifara MA, Mortazaviana AM, Mohammadia A, Khosravi-Daranib K, Shojaee-Aliabadia S, Dehghanc S, Khaksar R. Incorporation of essential oil in alginate microparticles by multiple emulsion/ionic gelation process. Int J Biol Macromol. 2013;62:582–588. doi: 10.1016/j.ijbiomac.2013.09.054. [DOI] [PubMed] [Google Scholar]
- Jiménez-Aguilar DM, Grusak MA. Evaluation of minerals, phytochemical compounds and antioxidant activity of Mexican, Central American, and African green leafy vegetables. Plant Food Hum Nutr. 2015;70(4):357–364. doi: 10.1007/s11130-015-0512-7. [DOI] [PubMed] [Google Scholar]
- Kalusevic AM, Levic SM, Calija BR, Milic JR, Pavlović VB, Bugarski BM, et al. Effects of different carrier materials on physicochemical properties of microencapsulated grape skin extract. J Food Sci Technol. 2017;54(11):3411–3420. doi: 10.1007/s13197-017-2790-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kha TC, Nguyen MH, Roach PD. Effects of spray drying conditions on the physicochemical and antioxidant properties of the gac (Momordica cochinchinensis) fruit aril powder. J Food Eng. 2010;98(3):385–392. doi: 10.1016/j.jfoodeng.2010.01.016. [DOI] [Google Scholar]
- Madene A, Jacquot M, Scher J, Desobry S. Flavour encapsulation and controlled release—a review. Int J Food Sci Technol. 2006;41(1):1–21. doi: 10.1111/j.1365-2621.2005.00980.x. [DOI] [Google Scholar]
- Mahdavi SA, Jarafi SM, Assadpoor E, Dehnad D. Microencapsulation optimization of natural anthocyanins with maltodextrin, gum Arabic and gelatin. Int J Biol Macromol. 2016;85:379–385. doi: 10.1016/j.ijbiomac.2016.01.011. [DOI] [PubMed] [Google Scholar]
- Mirhosseini H, Amid BT. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Res Int. 2012;46(1):387–398. doi: 10.1016/j.foodres.2011.11.017. [DOI] [Google Scholar]
- Murugesan R, Orsat V. Spray drying for the production of nutraceutical ingredients—a review. Food Bioprocess Technol. 2012;5(1):3–14. doi: 10.1007/s11947-011-0638-z. [DOI] [Google Scholar]
- Nesterenko A, Alric I, Durrieu V. Vegetable proteins in microencapsulation: a review of recent interventions and their effectiveness. Ind Crops Prod. 2013;42:469–479. doi: 10.1016/j.indcrop.2012.06.035. [DOI] [Google Scholar]
- Paini M, Aliakbarian B, Casazza AA, Lagazzo A, Botter R, Perego P. Microencapsulation of phenolic compounds from olive pomace using spray drying: a study of operative parameters. LWT Food Sci Technol. 2015;62(1):177–186. doi: 10.1016/j.lwt.2015.01.022. [DOI] [Google Scholar]
- Pieczykolan E, Kurek MA. Use of guar gum, gum Arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. Int J Biol Macromol. 2019;129:665–671. doi: 10.1016/j.ijbiomac.2019.02.073. [DOI] [PubMed] [Google Scholar]
- Ribeiro AM, Estevinho BN, Rocha F. Microencapsulation of polyphenols—the specific case of the microencapsulation of Sambucus nigra L. extracts—a review. Trends in Food Sci Technol. 2019 doi: 10.1016/j.tifs.2019.03.011. [DOI] [Google Scholar]
- Robert P, Gorena T, Romero N, Sepulveda E, Chavez J, Saenz C. Encapsulation of polyphenols and anthocyanins from pomegranate (Punica granatum) by spray drying. Int J Food Sci Technol. 2010;45(7):1386–1394. doi: 10.1111/j.1365-2621.2010.02270.x. [DOI] [Google Scholar]
- Robles-Flores GC, Abud-Archila M, Ventura-Canseco LMC, Meza-Gordillo R, Grajales-Lagunes A, Ruiz-Cabrera MA, Gutiérrez-Miceli FA. Development and evaluation of a film and edible coating obtained from the Cajanus cajan seed applied to fresh strawberry fruit. Food Bioprocess Technol. 2018;11:2172–2181. doi: 10.1007/s11947-018-2175-5. [DOI] [Google Scholar]
- Rokka S, Rantamäki P. Protecting probiotic bacteria by microencapsulation: challenges for industrial applications. Eur Food Res Technol. 2010;231(1):1–12. doi: 10.1007/s00217-010-1246-2. [DOI] [Google Scholar]
- Sansone F, Mencherini T, Picerno P, D’Amore M, Aquino RP, Lauro MR. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J Food Eng. 2011;105(3):468–476. doi: 10.1016/j.jfoodeng.2011.03.004. [DOI] [Google Scholar]
- Sarabandi K, Jafari SM, As Mahoonak, Mohammadi A. Application of gum Arabic and maltodextrin for encapsulation of eggplant peel extract as a natural antioxidant and color source. Int J Biol Macromol. 2019;140:59–68. doi: 10.1016/j.ijbiomac.2019.08.133. [DOI] [PubMed] [Google Scholar]
- Shekhar TC, Anju G. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. leaves. Am J Ethnomed. 2014;1(4):244–249. doi: 10.1016/S0029-5493(01)00385-5. [DOI] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sun-Waterhouse D, Wadhwa SS, Waterhouse GIN. Spray-drying microencapsulation of polyphenol bioactives: a comparative study using different natural fibre polymers as encapsulants. Food Bioprocess Technol. 2013;6:2376–2388. doi: 10.1007/s11947-012-0946-y. [DOI] [Google Scholar]
- Tiwari B, Brennan C, Jaganmohan R, Surabi A, Alagusundaram K. Utilisation of pigeon pea (Cajanus cajan L) by products in biscuit manufacture. LWT Food Sci Technol. 2011;44(6):1533–1537. doi: 10.1016/j.lwt.2011.01.018. [DOI] [Google Scholar]
- Tonon RV, Brabet C, Pallet D, Brat P, Hubinger MD. Physicochemical and morphological characterisation of açai (Euterpe oleraceae Mart.) powder produced with different carrier agents. Int J Food Sci Technol. 2009;44(10):1950–1958. doi: 10.1111/j.1365-2621.2009.02012.x. [DOI] [Google Scholar]
- Vriesmann LC, Teófilo RF, de Oliveira L, Petkowicz C. Extraction and characterization of pectin from cacao pod husks (Theobroma cacao L.) with citric acid. LWT Food Sci Technol. 2012;49(1):108–116. doi: 10.1016/j.lwt.2012.04.018. [DOI] [Google Scholar]
- Wang Y, Lu Z, Lv F, Bie X. Study on microencapsulation of curcumin pigments by spray drying. Eur Food Res Technol. 2009;229(3):391–396. doi: 10.1007/s00217-009-1064-6. [DOI] [Google Scholar]
- Wang H, Yang B, Sun H. Pectin-chitosan polyelectrolyte complex nanoparticles for encapsulation and controlled release of nisin. Am J Polym Sci Technol. 2017;3(5):82–88. doi: 10.11648/j.ajpst.20170305.11. [DOI] [Google Scholar]
- Yashin A, Yashin Y, Wang J, Nemzer B. Antioxidant and antiradical activity of coffee. Antioxidants. 2013;2(4):230–245. doi: 10.3390/antiox2040230. [DOI] [PMC free article] [PubMed] [Google Scholar]