Abstract
Anthocyanins make up the largest group of water-soluble pigments of the vegetable kingdom. These bio-compounds with antioxidant properties are attracting great interest in the food, pharmaceutical and cosmetic industry mainly because of their presence in many fruits and vegetables. The extraction of the pigment is still in need of further studies, especially concerning the extraction yield and the use of friendly solvents. The use of acidulants has shown an alternative to improve the extraction. This work presents the capability of pure solvents and binary mixtures associated with organic acids in the extraction of anthocyanins present in jabuticaba fruit skins and red cabbage leaves. The results suggest that the application of organic acids dissolved in binary mixtures formed by water and ethanol can provide an increase in the extraction of anthocyanins.
Keywords: Cabbage, Jabuticaba, Anthocyanins, Organic acids
Introduction
The quality of food products and their visual appeal are directly related to color (Veberic et al. 2015). It is therefore assumed that the quality and quantity of pigments in a food product are essential for its commercial acceptance (Shen et al. 2014). Although synthetic dyes have a lower cost of production, greater stability and greater dyeing capacity, the safety of synthetic dyes has been questioned in recent years, leading to a reduction in the number of coloring agents allowed. The interest in natural dyes, on the other hand, has increased significantly as a consequence of both legislation and consumer awareness regarding the use of synthetic additives (Cortez et al. 2017). Of the natural dyes available for use in food, anthocyanins comprise a diverse group of intensely colored pigments, responsible for the purple, red and blue colors found in fruits, flowers and vegetables. These pigments, which have been consumed for centuries without adverse effects (Smeriglio et al. 2016), are soluble in water, facilitating their incorporation in food matrices. In addition to their color attributes, anthocyanins have been met with intensified interest because of their potential health benefits. Yousuf et al. (2016) cites several studies that associate the consumption of anthocyanin extracts with increased vision intensity, antioxidant capacity, the treatment of various blood circulation disorders, anti-inflammatory effects and diabetes control, among others, due to their diversified action in various enzymes and metabolic processes. The anthocyanins belong to the family of flavonoids, phenolic compounds characterized by a basic flavilium nucleus (2-phenylbenzopyril cation) which consists of two aromatic rings joined by a unit of three carbon atoms and condensed by one oxygen atom. The molecule consists of two or three portions, one aglycone (anthocyanidin), a group of sugars and, often, a group of organic acids (Welch et al. 2008). These pigments can have different structural forms, which can take on different colors depending on several factors, especially temperature, pH value and possible bonds with other chemicals. The pH is the factor with the most influence on color, since anthocyanins may present different structures depending on the acidity or alkalinity (Lee et al. 2005). Four major chemical structures may occur in equilibrium in the medium, the basic structure of anthocyanins in the form of a flavilium cation with a red color, which is predominant in a pH lower than 3. With the increase in pH, the cation loses a proton and suffers a hydration, forming a colorless pseudobase (carbinol) in pH lower than 6. For a pH above 6, the flavilium cation loses protons, forming the quinoidal base with a blue color. With a pH between 12 and 13, the carbinol is transformed through tautomerism into a pale-yellow chalcone (Levi et al. 2004).
Among the fruits and vegetables that are sources of anthocyanins, the red cabbage (Brassica oleracea) has attracted attention because of the quantity of anthocyanins (Mizgier et al. 2016) and because its consumption has been associated with the prevention of diseases (Sarkar and Rakshit 2017). In addition, the red cabbage is characterized by its long shelf life, and it can therefore be easily stored and be available in its fresh form throughout the year (Wiczkowski et al. 2013). However, there is a lack of studies evaluating the extraction capacity and the stability of the red cabbage extract in different organic solvents (Hosseini et al. 2016). The jabuticaba (Myrciaria cauliflora), commonly sold and consumed in Brazil, is known as one of the richest sources of anthocyanins in Brazil (Rodrigues et al. 2015). The fruits grow directly on the trunk and main branches, has a diameter between 3 and 4 cm, has 1–4 seeds, and a thick skin that covers a gelatinous white pulp (Leite-Legatti et al. 2012). The fruit has a sweet and sub-acidic flavor, probably due to its sugar, organic acid and terpene content (Plagemann et al. 2012).
Organic solvents are the most commonly-used extraction method to obtain anthocyanins from fruits and vegetables. The choice of a method to extract anthocyanins will depend not only on the purpose of the extraction, but also on the nature of the constituents of the anthocyanin molecules (Ahmadiani et al. 2019), and it is equally important that such methods are not complex, time-consuming or costly. The polar characteristic of the anthocyanins make it easy to extract them with polar solvents such as water, methanol and ethanol. Concomitantly with the alcoholic solvents, the use of acids (Chung et al. 2016; Hosseini et al. 2016) favors the penetration of the solvent in fruit and vegetable tissues, in addition to increasing the stability of the extracts by hindering the emergence of fungi that degrade the anthocyanins. The acidic medium also makes it so the anthocyanins are predominantly found in the flavilium cation form. The stability of the flavilium cation in an acid medium make the use of solvents containing organic acids desirable in the extraction of anthocyanins from fruits and vegetables.
In light of the above, the importance of anthocyanins is clear, just as the importance of developing and applying mixtures that allow for the maximization of their extraction. However, the use of common acidulants in the food industry has not been studied. In this sense, the present study evaluates the capacity of solutions involving water, alcohols and organic acids (citric, adipic and nicotinic acids) in the extraction of the anthocyanins present in jabuticaba fruit skins and red cabbage leaves.
Methodology
Study material
The jabuticaba fruits (M. cauliflora) and red cabbage (B. oleracea) were acquired from rural properties in the city of Pinhalzinho, state of Santa Catarina, Brazil, during September (Winter). After their acquisition, the raw materials were cleaned and the leaves of the red cabbage were separated while the jabuticabas were depulped. The material was then dried, separately, in an oven with air circulation for a period of no less than 48 h at a temperature of 45 °C.
The dried material was ground in a food processor, separated into particles smaller than 1.19 mm with a 16 mesh sieve, packaged in 2.5 g portions in vacuum-sealed packages and maintained at a temperature of − 6 °C until the extraction was carried out.
Chemicals and reagents
For the experimental procedure, the alcohols and doubly distilled water were used without any further purification. Prior to the study, the organic acids were dried in an electrical furnace for at least 2 h under the temperature of 353.15 K and kept in a desiccator until the beginning of the experiment. The source and purity of the materials used are presented in Table 1.
Table 1.
Source and purity of the chemicals used in this work
| Component | Source | Puritya | Analysis | Purification |
|---|---|---|---|---|
| Water | Our lab | − | Refractive index | Doubly distilled |
| Methanol | Vetec-Brazil | ≥ 0.995 | Refractive index | None |
| Ethanol | Vetec-Brazil | ≥ 0.995 | Refractive index | None |
| 2-Propanol | Vetec-Brazil | ≥ 0.995 | Refractive index | None |
| Adipic acid | Sigma-Aldrich-Germany | ≥ 0.995 | None | Drying |
| Citric acid | Sigma-Aldrich-Japan | ≥ 0.995 | None | Drying |
| Nicotinic acid | Sigma-Aldrich-India | ≥ 0.995 | None | Drying |
aInformed by supplier
A digital refractometer (Atago, model RX-5000i, accuracy ± 0.00004) was used to analyze the refractive index of the solvents at 298.15 K and the results were compared with values published. The results confirm the quality of the liquid reagents used in this work.
The binary liquid water + ethanol, water + methanol and water + isopropanol solutions in the molar fractions of 50% were prepared with a semi-analytical scale. All pure solvents and binary solutions were studied in the absence and in the presence of the dissolved organic acids. The citric and adipic acids were evaluated in the concentrations of 0.1, 0.5 and 1.0 mol kg−1 and the nicotinic acid in the concentrations of 0.01, 0.03 and 0.06 mol kg−1. For each pure component, binary and ternary solution, a pH reading was made before the beginning of the extraction.
Extraction of anthocyanin
In the solid–liquid extraction (Silva et al. 2017) procedure, both liquids and solids were transferred to jacketed vessels previously connected to a thermostatic bath. A liquid to solid mass ratio of 60:1 was used for each extraction in order to get a higher mass transfer. The extraction was carried out in a magnetically stirred batch and with a controlled temperature of 25 °C for a period of 3 h. With the aid of a vacuum pump, the anthocyanic extract was then filtered with filter paper with a grammage of 250 g m−2.
Calculation of anthocyanin concentration
The total amount of monomeric anthocyanins (TMA) was determined through a spectrophotometric procedure using the pH differential method (Gordillo et al. 2018). This method is based on the structural transformation of the anthocyanin as a function of pH in two buffer solutions: potassium chloride with pH 1.0 and sodium acetate with pH 4.5. According to this method, the difference in absorbance of the solutions of pH 1.0 and 4.5 is directly proportional to the TMA concentration. The absorbance of the samples buffered in two different pHs are determined at the wavelengths of 510 nm and 700 nm.
The total amount of monomeric anthocyanins is expressed as mg L−1 of cyanidin-3-glucoside, which is the most common anthocyanin (Khoo et al. 2017), as in Eq. (1), where MW represents the molar mass of cyanidin-3-glucoside (449.2 g mol−1), DF is the dilution factor of the sample, ε is the molar extinction coefficient of cyanidin-3-glucoside (26,900 L mol−1 cm−1), 1000 represents the conversion of g to mg and 1 is the wave path in the cuvette in cm. The determinations were performed using a GENESYS™ 30 visible spectrophotometer of the brand Thermo Scientific.
| 1 |
Statistical analysis
The experimental uncertainty in the determination of TMA was established by the uncertainties propagation method. Assuming that the absorbance readings are the only experimental quantities associated with Eq. (1) propagating an uncertainty to the final result, and that the absorbance reading in the spectrophotometer has an uncertainty of ± 0.002, the amount of TMA was calculated with an experimental uncertainty of ± 0.67 mg L−1.
Results and discussion
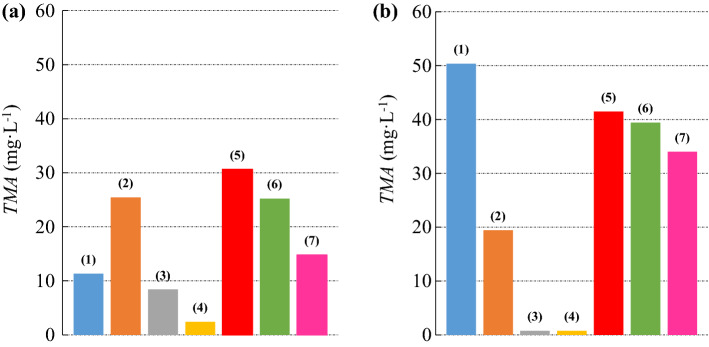
Figure 1 shows a comparison between the TMA quantities extracted from both the jabuticaba skins and the red cabbage leaves using pure solvents and liquid binary solutions.
Fig. 1.
Concentration of TMA extracted with pure solvents and with binary liquid mixtures: (a) jabuticaba; (b) red cabbage; (filled blue circle) (1) water; (filled orange circle) (2) methanol; (filled grey circle) (3) ethanol; (filled yellow cicle) (4) isopropanol; (filled red circle) (5) water + methanol; (filled green circle) (6) water + ethanol; (filled pink circle) (7) water + isopropanol; standard relative deviation 0.08 (color figure online)
Effect of solvent on extraction of TMA
In the case of the jabuticaba skins, the pure solvent with the best extraction capacity was methanol (25.42 mg L−1), followed by water (11.36 mg L−1), ethanol (8.40 mg L−1) and isopropanol (2.43 mg L−1). A comparison between the solvent with the greatest capacity to extract and the solvent with the lowest capacity to extract shows an increase of approximately 946%.
For the red cabbage leaves, the pure solvent with the highest extraction capacity was water (50.37 mg L−1), followed by methanol (19.44 mg L−1), and then by isopropanol (0.77 mg L−1) and ethanol (0.76 mg L−1) with almost identical values. A comparison shows a much higher extraction capacity of water compared to the alcohols of two and three carbon atoms.
It should be noted that with the pure solvents under study, it was possible to extract a larger amount of TMA from the cabbage leaves with an increase of approximately 98% in relation to the jabuticaba skins. For the red cabbage leaves, the results indicate a degree of dependence of the extraction capacity on the dielectric constant of the pure solvent, since it has a higher value for water followed by methanol, ethanol and isopropanol, respectively.
A possible reason for the better efficiency of methanol when compared to water in the case of jabuticaba skins is the action mechanism of the organic solvents in the extraction of bioactive compounds, in which the denaturation of cell membranes occurs, allowing for the solubilization and extraction of pigments and the formation of a concentration gradient from the inside of the material to the medium (Cai et al. 2016; Cacace and Mazza 2002).
Just as in this work, Metivier et al. (1980) also observed that methanol was 20% more effective than ethanol and 73% more effective than water in the extraction of anthocyanins from grape skins. However, many authors prefer to use ethanol (Cacace and Mazza 2003; Fan et al. 2008; Dai et al. 2009; Karabey and Mazza 2010) rather than methanol, mainly because of its low toxicity and low cost when compared to methanol (Zeng et al. 2018; Lapornik et al. 2005).
One explanation for the low value of anthocyanins extracted from red cabbage with pure organic solvents may be the non-extraction of hydrophilic anthocyanins (Chandrasekhar et al. 2012) as well as the dehydration and collapse of plant cells and denaturation of the proteins of the cell wall, hindering the dissemination of the plant's polyphenols through the extraction fluid (Garcia-Castello et al. 2015). The presence of small quantities of water is necessary for the extraction of hydrophilic anthocyanins (Willemse et al. 2013).
Effect of binary solvent on extraction of TMA
In the application of binary liquid solutions for the extraction TMA from jabuticaba skins, the highest extraction capacity was achieved with water + methanol (30.64 mg L−1), followed by water + ethanol (25.23 mg L−1) and water + isopropanol (14.79 mg L−1), respectively. The same behavior was also observed in the extraction from the red cabbage leaves, with values of 41.49 mg L−1, 39.43 mg L−1 and 34.02 mg L−1 for water + methanol, water + ethanol and water + isopropanol, respectively.
In comparison to pure alcohol, the addition of water is observed to have a striking effect on the extraction of anthocyanins. For the jabuticaba skins, the extraction capacity increases by 21%, 200% and 509%, while for the red cabbage leaves these increases are of 113%, 5088% and 4318% when compared to pure methanol, ethanol and isopropanol, respectively.
The results suggest that the binary liquid mixture formed by water and methanol is appropriate for the extraction of anthocyanins. It seems that the binary mixture incorporate the capability of water related to its high dielectric constant and the capability of methanol related to the denaturation of cell membranes.
Effect of solvent + organic acid on extraction of TMA
As mentioned earlier, three organic acids were dissolved in the pure solvents and binary liquid solutions in order to evaluate their acidulant function in the extractive capacity of the solution. The behavior as a function of organic acid concentration indicated a decrease in the extractive capacity with the increase in their concentration. This decrease in the levels of anthocyanins may be attributed to a rupture of the cell membranes, resulting in the simultaneous degradation of phenolic compounds and of the anthocyanins (Todaro et al. 2009; Mosier et al. 2002).
In general, the lowest concentration studied for each organic acid showed an increase in the extractive capacity, both in the pure solvents and in the binary solutions, resulting in the following results for the presence of citric and adipic acid at concentrations of 0.1 mol kg−1 and nicotinic acid at concentrations of 0.01 mol kg−1.
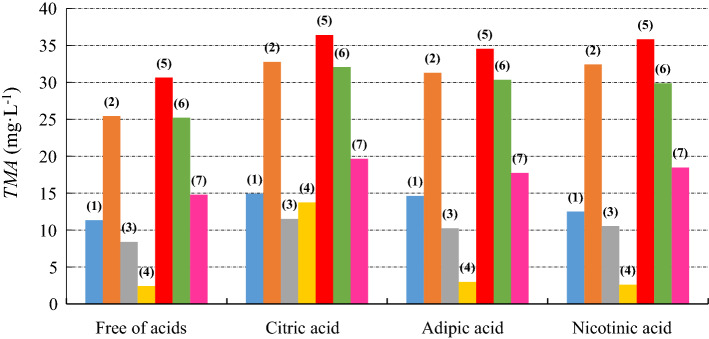
Figure 2 shows the influence of the addition of organic acids in the extraction of the anthocyanins present in the skins of the jabuticaba fruits.
Fig. 2.
Concentration of TMA extracted from jabuticaba skins with pure solvents and binary liquid mixtures, free of organic acids and with organic acids: (filled blue circle) (1) water; (filled orange circle) (2) methanol; (filled grey circle) (3) ethanol; (filled yellow cicle) (4) isopropanol; (filled red circle) (5) water + methanol; (filled green circle) (6) water + ethanol; (filled pink circle) (7) water + isopropanol; standard relative deviation 0.08 (color figure online)
Using water as a solvent, one can see that the presence of organic acids resulted in an increase in the amount of TMA extracted from the jabuticaba skin, with an increase of approximately 31%, 29% and 10% for the citric, adipic and nicotinic acid, respectively, when compared to the extraction without the acids. In relation to methanol, there was an increase in the amount of TMA of 29%, 23% and 28%. Using ethanol, the observed increase was 37%, 22% and 26%, and for isopropanol the increase observed was 465%, 23% and 8%.
When using the binary liquid solutions, the largest quantities of TMA extracted from the jabuticaba skins were observed using water + methanol, generating an increase of 19%, 13% and 17% for the citric, adipic and nicotinic acid, respectively, when compared to the solutions without the acids. For water + ethanol, there was an increase of 27%, 20% and 18%. For water + isopropanol, there was an increase of 33%, 20% and 25%.
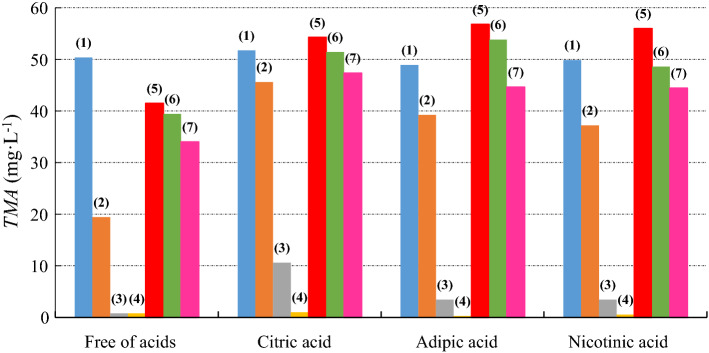
The total quantity of monomeric anthocyanins extracted from the red cabbage for each condition under study is shown in Fig. 3. Using water as a solvent, one can see an increase of 3% in the amount of TMA extracted with the presence of citric acid when compared to water without acids. In the presence of adipic and nicotinic acid, a decline in the amount of extracted TMA could be observed.
Fig. 3.
Concentration of TMA extracted from red cabbage with pure solvents and binary liquid mixtures, free of organic acids and with organic acids: (filled blue circle) (1) water; (filled orange circle) (2) methanol; (filled grey circle) (3) ethanol; (filled yellow cicle) (4) isopropanol; (filled red circle) (5) water + methanol; (filled green circle) (6) water + ethanol; (filled pink circle) (7) water + isopropanol; standard relative deviation 0.08 (color figure online)
For methanol, there was an increase in the amount of TMA extracted from the cabbage in the presence of acids when compared to the pure component in the absence of acids, i.e. and increase of 135%, 102% and 91% for citric, adipic and nicotinic acid, respectively. When using ethanol, an increase of 1292%, 352% and 353% could be observed. When using isopropanol as the solvent and in the presence of citric acid, it was possible to extract 30% more compared to the pure component in the absence of acids. In the presence of adipic and nicotinic acid, however, a decline in the amount of extracted TMA could be observed.
When using the binary liquid solutions, the largest quantities of TMA extracted from the red cabbage leaves were observed using water + methanol, generating an increase of 31%, 37% and 35% for the citric, adipic and nicotinic acid, respectively, when compared to the solutions without the acids. For water + ethanol, there was an increase of 30%, 37% and 23%. When using water + isopropanol, an increase of 39%, 31% and 31% could be observed.
The determining factor in the levels of anthocyanins was the pH condition. The lower the pH, the greater the concentration of anthocyanins extracted, confirming the theory presented by Francis (1982) that establishes that one must keep the pigment in the H+ form of the flavillium cation. The solubilization of organic acids in both the pure components and the binary liquid solutions provided a significant decrease in pH values between 71 and 30%. The lowest pH values were obtained in the presence of citric acid, followed by the adipic and nicotinic acids. The pH values for the extraction of the TMA in the jabuticaba skins and red cabbage leaves are shown in Table 2.
Table 2.
Values of pH for extraction of anthocyanins
| Solvent | FA | Jabuticaba skins | Red cabbage leaves | ||||
|---|---|---|---|---|---|---|---|
| CA | AA | NA | CA | AA | NA | ||
| Water | 7.24 | 2.15 | 2.98 | 3.40 | 2.20 | 2.65 | 3.61 |
| Methanol | 7.79 | 2.29 | 3.24 | 3.63 | 2.25 | 3.34 | 3.90 |
| Ethanol | 7.83 | 2.98 | 4.41 | 4.29 | 2.94 | 4.56 | 4.06 |
| Isopropanol | 6.90 | 2.88 | 4.81 | 4.71 | 3.43 | 4.38 | 4.30 |
| Water + methanol | 7.53 | 2.71 | 3.42 | 3.58 | 2.97 | 3.61 | 3.61 |
| Water + ethanol | 7.66 | 3.01 | 3.98 | 4.18 | 3.02 | 4.00 | 4.12 |
| Water + isopropanol | 7.44 | 3.13 | 4.44 | 4.26 | 3.32 | 4.50 | 4.13 |
FA free of acid; CA citric acid (0.1 mol kg−1); AA adipic acid (0.1 mol kg−1); NA nicotinic acid (0.01 mol kg−1); standard relative deviation 0.03
A general assessment suggests that the application of organic acids dissolved in solutions can provide an increase in the extraction of anthocyanins. In the extraction of TMA from jabuticaba skins, the largest extractive capacity (36.40 mg L−1) was observed using a solution formed by water + methanol + citric acid. For the extraction from red cabbage, the largest extractive capacity (56.80 mg L−1) was observed using a solution formed by water + methanol + adipic acid. Despite having the best results, using a solution with methanol is not recommended. An alternative would be to use a solution with ethanol.
In the extraction of the anthocyanins present in the jabuticaba skins, the solution formed by water + ethanol + citric acid had a result of 32.06 mg L−1 of TMA, and for the cabbage leaves, the water + ethanol + adipic acid solution had a value of 53.82 mg L−1 of TMA. When compared to the extractions performed only with pure water, the increases in the extractive capacity were approximately 182% for the jabuticaba skins and 7% for the red cabbage leaves.
Conclusion
The use of solutions involving methanol had the best extractive capacities, but the use of methanol is not recommended and replacing methanol by ethanol is an alternative. An optimization of the extraction of the anthocyanins in the jabuticaba skins and red cabbage leaves is achieved with the use of acidified solvents. The best extractive conditions are observed in a pH range between 2.0 and 4.0. The application of organic acids (dissolved solid) as acidulant agents showed viability. Ternary solutions formed by water + ethanol + citric acid or adipic acid showed promising extraction capabilities, creating opportunities for future investigations.
Acknowledgement
The authors wish to thank FAPESC (Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina) and UNIEDU (Programa de Bolsas Universitárias de Santa Catarina) for the financial support.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmadiani N, Sigurdson GT, Robbins RJ, Collins TM, Giusti MM. Solid phase fractionation techniques for segregation of red cabbage anthocyanins with different colorimetric and stability properties. Food Res Int. 2019;120:688–696. doi: 10.1016/j.foodres.2018.11.026. [DOI] [PubMed] [Google Scholar]
- Cacace JE, Mazza G. Extration of anthocyanins and other phenolics from black currants with sulfured water. J Agr Food Chem. 2002;50:5939–5946. doi: 10.1021/jf025614x. [DOI] [PubMed] [Google Scholar]
- Cacace JE, Mazza G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J Food Sci. 2003;68:240–248. doi: 10.1111/j.1365-2621.2003.tb14146.x. [DOI] [Google Scholar]
- Cai Z, Qu Z, Lan Y, Zhao S, Ma X, Wan Q, Jing P, Li P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016;197:266–272. doi: 10.1016/j.foodchem.2015.10.110. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar J, Madhusudhan MC, Raghavarao KSMS. Extraction of anthocyanins from red cabbage and purification using adsorption. Food Bioprod Process. 2012;9:615–623. doi: 10.1016/j.fbp.2012.07.004. [DOI] [Google Scholar]
- Chung C, Rojanasasithara T, Mutilangi W, McClements DJ. Stabilization of natural colors and nutraceuticals: inhibition of anthocyanin degradation in model beverages using polyphenols. Food Chem. 2016;212:596–603. doi: 10.1016/j.foodchem.2016.06.025. [DOI] [PubMed] [Google Scholar]
- Cortez R, Luna-Vital DA, Margulis D, Mejia EG. Natural pigments: stabilization methods of anthocyanins for food applications. Compr Rev Food Sci F. 2017;16:180–198. doi: 10.1111/1541-4337.12244. [DOI] [PubMed] [Google Scholar]
- Dai J, Gupte A, Gates L, Mumper RJ. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: Extraction methods, stability, anticancer properties and mechanisms. Food Chem Toxicol. 2009;47:837–847. doi: 10.1016/j.fct.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Fan G, Han Y, Gu Z, Chen D. Optimizing conditions for anthocyanins extraction from purple sweet potato using response surface methodology (RSM) Food Sci Tech-Brazil. 2008;41:155–160. doi: 10.1016/j.lwt.2007.01.019. [DOI] [Google Scholar]
- Francis FJ. Analysis of anthocyanins. In: Markakis P, editor. Anthocyanins as food colors. New York: Academic Press; 1982. pp. 182–205. [Google Scholar]
- Garcia-Castello EM, Rodriguez-Lopez AD, Mayor L, Ballesteros R, Conidi C, Cassano A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT Food Sci Technol. 2015;64:1114–1122. doi: 10.1016/j.lwt.2015.07.024. [DOI] [Google Scholar]
- Gordillo B, Sigurdson GT, Lao F, González-Miret ML, Heredia FJ, Giusti MM. Assessment of the color modulation and stability of naturally copigmented anthocyanin-grape colorants with different levels of purification. Food Res Int. 2018;106:791–799. doi: 10.1016/j.foodres.2018.01.057. [DOI] [PubMed] [Google Scholar]
- Hosseini S, Gharachorloo M, Ghiassi-Tarzi B, Ghavami M. Evaluation of the organic acids ability for extraction of anthocyanins and phenolic compounds from different sources and their degradation kinetics during cold storage. Pol J Food Nutr Sci. 2016;66:261–269. doi: 10.1515/pjfns-2015-0057. [DOI] [Google Scholar]
- Karabey E, Mazza G. Optimisation of antioxidant activity of grape cane extracts using response surface methodology. Food Chem. 2010;119:343–348. doi: 10.1016/j.foodchem.2009.06.029. [DOI] [Google Scholar]
- Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapornik B, Prosek M, Wondra AG. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J Food Eng. 2005;71:214–222. doi: 10.1016/j.jfoodeng.2004.10.036. [DOI] [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88:1269–1278. doi: 10.1093/jaoac/88.5.1269. [DOI] [PubMed] [Google Scholar]
- Leite-Legatti AV, Batista ÂG, Dragano NRV, Marques AC, Malta LG, Riccio MF, Eberlin MN, Machado ART, Carvalho-Silva LB, Ruiz ALTG, Carvalho JE, Pastore GM, Júnior MRM. Jaboticaba peel: antioxidant compounds, antiproliferative and antimutagenic activities. Food Res Int. 2012;49:596–603. doi: 10.1016/j.foodres.2012.07.044. [DOI] [Google Scholar]
- Levi MAB, Scarminio LS, Poppi RJ, Trevisan MG. Three-way chemometric method study and uv-vis absorbance for the study of simultaneous degradation of anthocyanins in flowers of the Hibiscus rosa-sinensys species. Talanta. 2004;62:299–305. doi: 10.1016/j.talanta.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Metivier RP, Francis FJ, Clydesdale FM. Solvent extraction of anthocyanins from wine pomace. J Food Sci. 1980;45:1099–1100. doi: 10.1111/j.1365-2621.1980.tb07534.x. [DOI] [Google Scholar]
- Mizgier P, Kucharska AZ, Sokot-Letowska A, Kolniak-Ostek J, Kidon M, Fecka I. Characterization of phenolic compounds and antioxidant and anti-inflammatory properties of red cabbage and purple carrot extracts. J Funct Foods. 2016;21:133–146. doi: 10.1016/j.jff.2015.12.004. [DOI] [Google Scholar]
- Mosier NS, Ladisch CM, Ladisch MR. Characterization of acid catalytic domains for cellulose hydrolysis and glucose degradation. Biotechnol Bioeng. 2002;79:610–618. doi: 10.1002/bit.10316. [DOI] [PubMed] [Google Scholar]
- Plagemann I, Krings U, Berger RG, Marostica MR. Volatile constituents of jabuticaba (Myrciaria jaboticaba (Vell.) O. Berg) fruits. J Essent Oil Res. 2012;24:45–51. doi: 10.1080/10412905.2012.645651. [DOI] [Google Scholar]
- Rodrigues S, Fernandes FAN, Brito ES, Sousa AD, Narain N. Ultrasound extraction of phenolics and anthocyanins from jabuticaba peel. Ind Crop Prod. 2015;69:400–407. doi: 10.1016/j.indcrop.2015.02.059. [DOI] [Google Scholar]
- Sarkar D, Rakshit A. Red cabbage as potential functional food in the present perspective. Int J Bioresour Sci. 2017;4:7–8. doi: 10.5958/2454-9541.2017.00002.0. [DOI] [Google Scholar]
- Shen Y, Zhang X, Prinyawiwatkul W, Xu Z. Simultaneous determination of red and yellow artificial food colourants and carotenoid pigments in food products. Food Chem. 2014;157:553–558. doi: 10.1016/j.foodchem.2014.02.039. [DOI] [PubMed] [Google Scholar]
- Silva S, Costa EM, Calhau C, Morais RM, Pintado ME. Anthocyanin extraction from plant tissues: a review. Crit Rev Food Sci. 2017;57:3072–3083. doi: 10.1080/10408398.2015.1087963. [DOI] [PubMed] [Google Scholar]
- Smeriglio A, Barreca D, Bellocco E, Trombetta D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother Res. 2016;30:1265–1286. doi: 10.1002/ptr.5642. [DOI] [PubMed] [Google Scholar]
- Todaro A, Cimino F, Rapisarda P, Catalano AE, Barbagallo RN, Spagna G. Recovery of anthocyanins from eggplant peel. Food Chem. 2009;114:434–439. doi: 10.1016/j.foodchem.2008.09.102. [DOI] [Google Scholar]
- Veberic R, Slatnar A, Bizjak J, Stampar F, Mikulic-Petkovsek M. Anthocyanin composition of different wild and cultivated berry species. LWT Food Sci Technol. 2015;60:509–517. doi: 10.1016/j.lwt.2014.08.033. [DOI] [Google Scholar]
- Welch CR, Wu Q, Simon JE. Recent advances in anthocyanin analysis and characterization. Curr Anal Chem. 2008;4:75–101. doi: 10.2174/157341108784587795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiczkowski W, Szawara-Nowak D, Topolska J. Red cabbage anthocyanins: profile, isolation, identification, and antioxidant activity. Food Res Int. 2013;51:303–309. doi: 10.1016/j.foodres.2012.12.015. [DOI] [Google Scholar]
- Willemse CM, Stander MA, Villiers A. Hydrophilic interaction chromatographic analysis of anthocyanins. J Chromatogr A. 2013;1319:127–140. doi: 10.1016/j.chroma.2013.10.045. [DOI] [PubMed] [Google Scholar]
- Yousuf B, Gul K, Wani AA, Singh P. Health benefits of anthocyanins and their encapsulation for potential use in food systems: a review. Crit Rev Food Sci. 2016;56:2223–2230. doi: 10.1080/10408398.2013.805316. [DOI] [PubMed] [Google Scholar]
- Zeng Y-J, Xu P, Yang H-R, Zong M-H, Lou W-Y. Purification of anthocyanins from saskatoon berries and their microencapsulation in deep eutectic solvents. LWT Food Sci Technol. 2018;95:316–325. doi: 10.1016/j.lwt.2018.04.087. [DOI] [Google Scholar]