Abstract
Adlay, as a traditional Chinese medicine, has been used in nourishing foods, which are rich in a variety of nutrients (special biological compounds). The study was designed to optimize the fermentation parameters of dehulled, polished and broken adlay fermented by Bacillus subtilis BJ3-2 with regard to tetramethylpyrazine (TMP) yield and fibrinolytic enzyme activity. Then the proximate and bioactive components of B. subtilis-fermented adlay were evaluated. Box–Behnken design results showed that the TMP yield was 6.93 mg/g DW (dried weight) of B. subtilis-fermented polished adlay, which was about 136 times higher than that of B. subtilis-fermented soybean (BSB). The fibrinolytic enzyme activity was 2236.17 U/g in B. subtilis-fermented dehulled adlay, and slightly less than in BSB. B. subtilis-fermented adlay contained higher fat, free amino acids and fatty acids contents but lower protein and starch contents than raw adlay. Except for coixol and coixan, the levels of γ-aminobutyric acid, triterpenes, phenolics, flavonoids and coixenolide in B. subtilis-fermented adlay increased by 14.05, 2.02, 2.31 and 1.36 times, respectively. The contents of phenolic acids including caffeic, gallic, catechinic and chlonogenic acids in the free phenolic extracts significantly increased (p < 0.05). The results demonstrated that the biotransformation of high-yield TMP, fibrinolytic enzyme and other bioactive components of B. subtilis-fermented adlay products was realized. B. subtilis-fermented adlay could be a promising value-added food, and that is more suitable for human consumption.
Electronic supplementary material
The online version of this article (10.1007/s13197-020-04443-0) contains supplementary material, which is available to authorized users.
Keywords: Adlay, B. subtilis BJ3-2, Tetramethylpyrazine, Fibrinolytic enzyme activity, Bioactive components
Introduction
Cereals and its ingredients are accepted as functional food and nutraceuticals because of providing proteins, dietary fibre, vitamins, minerals, polyphenols and phytosterols required for human health (Luithui et al. 2019). Cereals are beneficial in the prevention of metabolic syndrome, obesity, and associated chronic diseases such as cardiovascular disease and type 2 diabetes (Das et al. 2012). Among the broadly used herbal medicines, cereal-based adlay has long been used as a medicinal food material due to its high nutritional and bioactive components. Coix (Coix lachryma-jobi L. var. ma-yuen Stapf) is a grass crop that is widely cultivated in China, Laos and Japan (Manosroi et al. 2014). The ripe seed kernel of coix is adlay, also known as coix seed, job’s tear, or Chinese pearl barley. Many health-beneficial components have been found in adlay, including polysaccharide, coixenolide, coixol, γ-aminobutyric acid, phenolics and triterpenoid (Xu and Chen 2017). Adlay was used as a medicinal material for treating the symptoms of warts, chapped skin, neuralgia, rheumatism, female endocrine disorder and inflammatory diseases (Li 1596). In recent decades, numerous studies have shown that adlay and its extracts have beneficial effects, including antioxidant activity, osteoporosis prevention, anti-inflammatory activity, anti-hypertensive activity, antitumor, and anti-obesity effects (Zhu 2017). Adlay seeds consist of three parts from the exterior to the interior, including the hull, testa, and dehulled adlay. After polishing, dehulled adlay can be further divided into bran, polished adlay and broken adlay. Dehulled and polished adlay are the major edible parts, but broken adlay is used to produce feed (Wan et al. 2017). In general, the value-added disposal of adlay is a growing problem that needs effective solution.
Recently, as part of a general trend, the adlay-processing industry has been challenged to produce new ingredients and foods with added values for consumer health. Yeast, fungi and bacteria were used to ferment adlay to develop healthy and functional food ingredients (Tien et al 2016; Wu et al. 2013; Wang et al. 2013). Adlay fermented by Bacillus subtilis resulted in higher antioxidant activity and greatly modulated intestinal microflora compared with unfermented samples (Wang et al. 2011, 2014). B. subtilis has been used extensively to ferment natto, a well-known traditional fermented food that is prepared with cooked soybeans (Singh et al. 2015). Nattokinase is secreted from B. subtilis cell and reduces blood clotting to prevent cardiovascular diseases (Wang et al. 2011). Pyrazine is an important aroma compound with a high flavour dilution value in natto, and the tetramethylpyrazine (TMP) content was 511.72 ηg/g (Liu et al. 2018). During the fermentation of natto, B. subtilis generates various metabolites, such as peptone, peptides, amino acids, sugars, and organic acids, which enhance the organoleptic and biological properties of the final products. Therefore, the selection of promising microorganisms for fermentation is necessary for the production of high and consistent quality.
Inspired by those research ideas, adlay was fermented by B. subtilis to develop a promising value-added adlay product in current study. It is especially interesting that the TMP content of B. subtilis-fermented adlay was far higher than that of B. subtilis-fermented soybean. Therefore, it is of great significance to maximize the TMP yield and evaluate the quality properties of B. subtilis-fermented adlay. The current study was planned with the optimization of fermentation parameters to obtain high TMP yield and fibrinolytic enzyme activity from dehulled, polished and broken adlay fermented by Bacillus subtilis BJ3-2. Furthermore, the levels of proximate and bioactive components after fermentation were investigated.
Materials and methods
Materials
Polished adlay (PA), dehulled adlay (DA) and broken adlay (BA) were obtained from Guizhou Renxin Agricultural Development Co., Ltd. (Guizhou, China). Soybean (Qianzhou No. 9) was purchased from the local supermarket. Chromatographic-grade methanol, tetramethylpyrazine, glucose, γ-aminobutyric acid, coixol, gallic acid, rutin, protocatechuic acid, chlorogenic acid, catechinic acid, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid and ferulic acid were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All other chemicals and reagents were purchased from Sinopharm Chemical Reagent Co. Ltd. (Suzhou, China) and were of analytical grade.
Fermentation optimization of B. subtilis-fermented adlay
B. subtilis BJ3-2 was gathered from Dr. Wu, College of Life Sciences, Guizhou University (Jia et al. 2010). Cultures of B. subtilis BJ3-2 was maintained on peptone beef extract medium. The medium consisted of 5 g/L NaCl, 10 g/L peptone and 3 g/L beef extract. The seed culture was inoculated at 37 °C for 20 h at 180 rpm. The cells were harvested in sterile distilled water, and after adjusting to a concentration of 106 total cells mL−1.
Approximately 30 g of dehulled, polished and broken adlay were washed and soaked overnight in distilled water, respectively. After decanting the water, the soaked adlay (dehulled, polished and broken adlay) was mixed with distilled water and steam-sterilized. After cooling to room temperature, the steamed adlay (dehulled, polished and breoken adlay) was inoculated with B. subtilis BJ3-2 and then incubated in an incubator. After fermentation, B. subtilis BJ3-2 fermented dehulled adlay (BDA), B. subtilis BJ3-2 fermented polished adlay (BPA) and B. subtilis BJ3-2 fermented broken adlay (BBA) were obtained.
In primary experiments, the main variables including the ratio of water to material, initial pH, steaming time (min), loading capacity (%, v/v), inoculation amount (%, v/w), fermentation temperature (°C) and fermentation time (h) were investigated using single factor test. The results indicated that the TMP yield and fibrinolytic enzyme activity reached the maximum values when the ratio of water to material was 1.4, initial pH was 7.0, steaming time was 30 min, loading capacity was 12%, inoculation amount was 7%, fermentation temperature was 37 °C and fermentation time was 72 h. Based on the experiments of each single factor, the TMP yield and fibrinolytic enzyme activity were mainly influenced by the 3 factors, including inoculation amount (mL, X1), fermentation temperature (°C, X2) and fermentation time (h, X3). The experiments were performed on the Box–Behnken Design (BBD). The coded values of the experimental factors and their levels for the BBD are shown in Table 1. The complete design was carried out in a random order and consisted of 17 combinations including 5 replicates at central point (Table 1).
Table 1.
Box–Behnken design matrix of 3 variables with experimental values of TMP yield (mg/g DW) and fibrinolytic enzyme activity (U/g)
| Run no | X1 Inoculation amount (%) |
X2 Fermentation temperature (°C) |
X3 Fermentation time (h) |
BDA | BPA | BBA | |||
|---|---|---|---|---|---|---|---|---|---|
| TMP | Fibrinolytic enzyme activity | TMP | Fibrinolytic enzyme activity | TMP | Fibrinolytic enzyme activity | ||||
| 1 | 0 (7) | 0 (37) | 0 (84) | 6.13 | 2128.54 | 6.15 | 1938.01 | 5.15 | 2064.88 |
| 2 | − 1 (5) | 0 | 1 (96) | 4.55 | 832.24 | 4.02 | 648.59 | 2.48 | 1567.29 |
| 3 | 0 | 0 | 0 | 5.36 | 2236.19 | 6.56 | 1755.88 | 4.34 | 2021.75 |
| 4 | 0 | 0 | 0 | 5.38 | 1805.77 | 5.84 | 1843.93 | 5.11 | 1952.74 |
| 5 | 1(9) | 0 | 1 | 5.79 | 1902.77 | 4.88 | 1940.24 | 4.04 | 1806.03 |
| 6 | 0 | 0 | 0 | 5.96 | 1654.91 | 5.78 | 1987.26 | 4.59 | 1850.84 |
| 7 | 0 | 1(40) | 1 | 4.76 | 2014.86 | 6.94 | 2142.18 | 3.08 | 2074.62 |
| 8 | 0 | 1 | − 1 (72) | 2.94 | 692.37 | 5.96 | 1985.21 | 5.08 | 1490.25 |
| 9 | 1 | 1 | 0 | 5.93 | 1904.42 | 5.78 | 2016.56 | 4.33 | 1610.62 |
| 10 | 0 | − 1 (34) | − 1 | 3.24 | 744.86 | 3.89 | 980.22 | 2.13 | 975.26 |
| 11 | 0 | − 1 | 1 | 4.25 | 593.1 | 3.55 | 444.88 | 2.49 | 1114.37 |
| 12 | − 1 | − 1 | 0 | 4.37 | 487.51 | 3.23 | 366.52 | 1.49 | 1413.62 |
| 13 | − 1 | 1 | 0 | 3.12 | 1522.91 | 4.97 | 1833.81 | 3.47 | 1768.34 |
| 14 | 1 | 0 | − 1 | 4.56 | 657.93 | 3.14 | 642.21 | 3.77 | 1125.77 |
| 15 | − 1 | 0 | − 1 | 3.19 | 808.96 | 3.58 | 1062.55 | 3.56 | 2085.85 |
| 16 | 0 | 0 | 0 | 5.49 | 1950.92 | 5.93 | 2047.027 | 4.47 | 1929.42 |
| 17 | 1 | − 1 | 0 | 3.82 | 589.5 | 2.6 | 636.48 | 1.76 | 457.92 |
BDA B. subtilis-fermented dehulled adlay, BPA B. subtilis-fermented polished adlay, BBA B. subtilis-fermented broken adlay
Preparation of B. subtilis-fermented soybean (BSB)
Approximately 30 g of soybean were washed and soaked overnight in distilled water. After decanting the water, the soaked soybean were steam-sterilized at 121 °C for 30 min. After cooling to room temperature, the steamed soybean was incubated at an inoculation amount of 5% for 48 h with 40 °C. B. subtilis-fermented BJ3-2 soybean (BSB) was obtained. All fermented samples were vacuum freeze-dried, then milled through a 60-meshsieve, and stored at 4 °C for further analysis.
Determination of TMP and fibrinolytic enzyme activity
TMP content was determined by the method of Chen et al. (2010) with some modifications. TMP was ultrasonically extracted by blending the B. subtilis-fermented adlay/soybean flour (3 g) with 15 mL of 80% ethanol for 30 min. The obtained mixture was then centrifuged at 4000 g for 15 min. The supernatant was collected, and the residual pellet was re-extracted twice. All the supernatants were collected and diluted to 50 mL with 80% ethanol, and the solution was filtered through a 0.45-μm filter. The chromatography was performed on a Phecda-C18 (250 mm × 4.6 mm, 5 μm) column at 45 °C, with an aqueous mobile phase (1% acetic acid and 0.05% trifluoroacetic acid in water, pH 2.5)–(methanol) (70:30, v/v). The flow rate was 0.8 mL/min and the UV detection wavelength was 297 nm. The injection volume of each sample was 20 μL.
The fibrinolytic enzyme activity was determined by the modified fibrin plate method (Zhang et al. 2016). A standard curve was made with urokinase activity unit as ordinate and bacteriolytic circle area as abscissa, and the enzyme activity was calculated on the basis of the standard curve.
Determination of proximate compositions
The proximate compositions of B. subtilis-fermented adlay, including starch, crude fat and crude protein, were determined according to the AOAC Official Method (1990). The nitrogen factor used for crude protein calculation was 5.83 (Litchfield 1967).
Free amino acids content was measured according to the method described by Liang et al. (2018) with minor modifications. Amino acids was extracted from 0.5 g dried B. subtilis-fermented adlay flour tissue with 50 mL of 0.01 mol/L hydrochloric acid solution in an ultrasonic bath for 30 min, centrifuged at 4000 g for 15 min and filtered through Whatman No.4 filter paper. The filtrate was filtered using a 0.45 µm CA nonsterile filter, mixed with o-phthalaldehyde reagent in an Eppendorf tube, and immediately injected onto HPLC.
Fat from the samples was extracted as described in the determination of fat, but the solvent was evaporated only by the rotary vacuum dryer, without the final drying at 103 °C to avoid undesirable fatty acid oxidation. Approximately 30 mg of extracted adlay oil was placed into a sealed bottle with 2 mL of methanol sulfate solution, and was esterified at 80 °C for 0.5 h. Then, 2 mL of n-hexane was added to the sample, followed by sharking by ultrasonic method, and the supernatant was obtained. The supernatant was water-washed until neutral, and concentrated to 1 mL by 1 g of anhydrous sodium sulfate. Fatty acid methyl esters were prepared and determined using gas chromatography coupled with mass spectrometry (GC–MS). The analysis was performed using an Agilent 7890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with a HP-88 capillary column (100 m × 0.25 mm × 20 μm, Agilent Technologies, Palo Alto, CA, USA). The column temperature program started at 100 °C for 10 min, then heated at a rate of 4 °C/min to 232 °C with a delay of 15 min, and finally the run time was 58 min. The injector temperature was set at 270 °C. The injected volume was 1 µL in a split mode (100:1). Helium was used as the carrier gas at a flow rate of 1 mL/min. The concentrations of individual fatty acids were expressed as g·100 g−1 of the total fatty acid methyl esters identified.
Determination of active compounds
γ-Aminobutyric acid (GABA) was determined by the method of Park et al. (2016). Total triterpene, phenolics and flavonoids contents of each extract were analyzed using the colorimetric method described by Xu et al. (2017). The total phenolics content was expressed as milligram of gallic acid equivalent per gram dried B. subtilis-fermented adlay weight (mg GAE/g DW, dry weight). The flavonoids content was expressed as milligram of rutin equivalent per gram dry B. subtilis-fermented adlay weight (mg RE/g DW). Free and bound phenolic compounds were extracted and detected according to the method of Xu et al. (2018). Analysis phenolic compounds of the extracts were performed by Agilent 1260 HPLC system. The coixan content was determined by employing the method of Rajesh et al. (2011). The coixol and coixenolide contents were determined by employing the method of Xu et al. (2017).
Statistical analysis
The results were expressed as the mean ± standard deviation, and statistical analysis was conducted using SPSS version 18.0 (SPSS Inc., Chicago, USA). One-way ANOVA was analysed by Duncan’s multiple range test for the determination of significant differences and the significance level was p < 0.05. The curve fitting and kinetics calculations were performed using Origin Pro 8.0 software (Origin Lab Co., Northampton, MA, USA).
Results and discussion
Process model and its significance for B. subtilis-fermented adlay
The values of the response variables of B. subtilis-fermented adlay measured in this study are shown in Table 1. ANOVA was conducted to fit the model and to examine the statistical significance of the model terms (Table S1–S3).
The regression model p < 0.01 showed that the models in BDA, BPA and BBA were highly significant. However, the lack of fit of P values in BDA (0.8472 for TMP yield and 0.5770 for fibrinolytic enzyme activity), BSB (0.4637 for TMP yield and 0.1285 for fibrinolytic enzyme activity), and CDA (0.6328 for TMP yield and 0.6084 for fibrinolytic enzyme activity) indicated that the model was not significant. According to the canonical analysis, the model predicted that the maximum TMP yield and fibrinolytic enzyme activity of BDA would be achieved when inoculation amount, fermentation temperature, and fermentation time were 9%, 40°C, and 3.9 d, respectively. The predicted value of the maximum TMP yield and fibrinolytic enzyme activity were 6.13 mg/g DW and 2128.54 U/g, respectively. To confirm this prediction, a verification experiment was conducted in triplicate under the optimized conditions. After verification (Fig. 1a), the experimental TMP yield and fibrinolytic enzyme activity of BDA were 6.15 mg/g DW and 2236.47 U/g, respectively. The model predicted that the maximum TMP yield and fibrinolytic enzyme activity in BPA when the inoculation amount, fermentation temperature, and fermentation time were 8.1%, 38°C, and 4.0 d, respectively. The predicted value of the maximum TMP yield and fibrinolytic enzyme activity in BPA were 6.94 mg/g DW and 2142.18 U/g, respectively. After verification, the experimental TMP yield and fibrinolytic enzyme activity were 6.93 mg/g DW and 2097.26 U/g (Fig. 1a), which agreed with the predicted value. The model predicted that the maximum TMP yield and fibrinolytic enzyme activity in BBA would be achieved when the inoculation amount, fermentation temperature, and fermentation time are 7%, 38°C, and 3.4 d, respectively. The predicted values of the maximum TMP yield and fibrinolytic enzyme activity were 5.15 mg/g DW and 1956.88 U/g, respectively. The experimental TMP yield and fibrinolytic enzyme activity in BBA were 5.09 mg/g DW and 2011.56 U/g (Fig. 1a), respectively.
Fig.1.
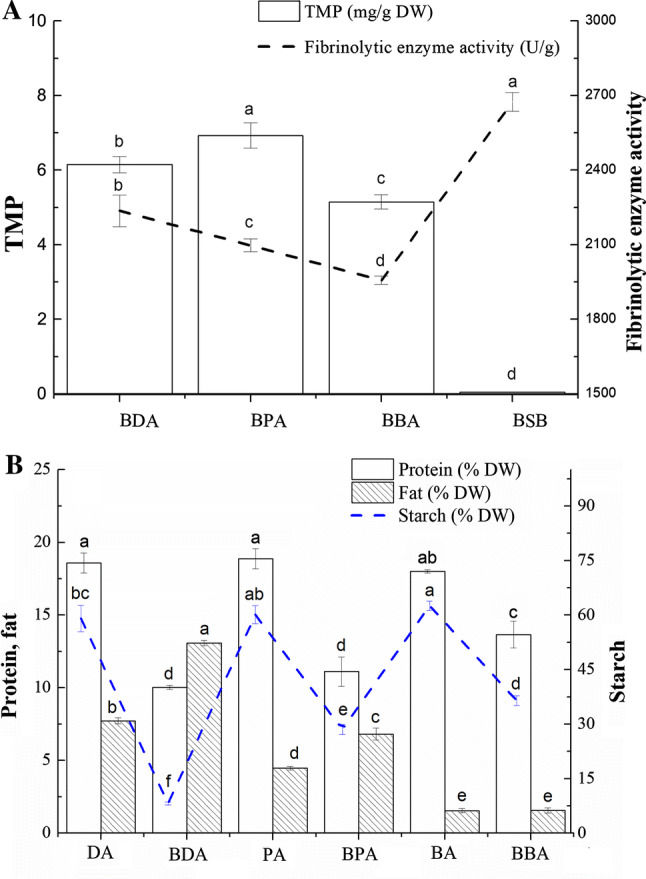
TMP yield, fibrinolytic enzyme and proximate compositions of raw and B. subtilis-fermented adlay. DA dehulled adlay, BDA B. subtilis-fermented dehulled adlay, PA polished adlay, BPA B. subtilis-fermented polished adlay, BA broken adlay, BBA B. subtilis-fermented broken adlay
Meanwhile, for analyzing the difference of the TMP yield and fibrinolytic enzyme activity between B. subtilis-fermented adlay and B. subtilis-fermented soybean (BSB), the TMP yield and fibrinolytic enzyme activity of BSB were detected. The TMP content and fibrinolytic enzyme activity in BSB were 0.05 mg/g DW and 2674.68 U/g (Fig. 1a), respectively. Based on the results, the TMP content of BPA was about 136 times higher than that of BSB, but the fibrinolytic enzyme activity of all B. subtilis-fermented adlay samples was less than that of BSB. TMP is the key biologically active component of the Chinese herb chuanxiong, Ligusticum chuanxiong, but the TMP content is low (the average content of 2.19 μg/g). The TMP content in vinegar samples and light aroma type Chinese liquors ranged from 0.001 mg/g to 0.131 mg/g and 88.70 to 1417.59 μg/L, respectively (Chen et al. 2010; Niu et al. 2017). There are many studies on producing a high level of TMP via gene modification, or adding precursors of action, 3-hydroxy-2-butanone, threonine and ammonium ions (Meng et al. 2015). Supplemented with 30.1 g/L of acetoin and 67.7 g/L of diammonium phosphate, 8.34 g/L of TMP was obtained, which was the highest yield reported (Xiao et al. 2018). In current study, 6.93 mg/g DW of TMP was obtained without adding precursors, indicating that a new method would be used to produce high-yield TMP.
The TMP yield of BBA was significantly lower (p < 0.05) than that of BPA and BDA. The main reason was the difference in the protein and starch content, and grain size of adlay. The interaction between protein and starch molecules restricts the water swelling of starch granules (Ong and Blanshard 1995). The water absorbability and starch gelatinization were directly affected by the protein content. The higher the protein content, the smaller the gap between the starch granules. When the cooking time of cereal was longer, the starch cannot be fully gelatinized, the viscosity of the rice was lower and looser (Liu et al. 2012). The high protein content and small grain size were observed in broken adlay. Therefore, broken adlay after cooking for a long time showed such high viscosity, and nutrients and growth factor were not used by B. subtilis BJ3-2 for reproduction. Dehulled and polished adlay were so loose that nutrients and growth factors were better used by B. subtilis BJ3-2 for reproduction, metabolism and biosynthesis of precursors for TMP.
Effect of fermentation on proximate compositions
The proximate compositions of raw and B. subtilis-fermented adlay are shown in Fig. 1b. During fermentation, protein and starch would be degraded to provide nutrients and energy for B. subtilis growth, which led to their lower contents. The starch and protein contents gradually decreased from 62.56 to 8.17% DW, and 18.59 to 8.09% DW, respectively. During fermentation, amylase and protease produced by B. subtilis, which hydrolyzed macromolecules of carbohydrate and protein into the more dispersible or soluble compounds of amino acids, dextrin, simple sugars, and acids (Koh et al. 2014). Those small substances were used to the growth and reproduction of B. subtilis, or biosynthesis of secondary metabolites. Therefore, the contents of protein and starch were significantly reduced after fermentation. In contrast, the fat content was considerably higher after fermentation. The fat content ranged from 1.55 to 13.07% DW, indicating that B. subtilis BJ3-2 produced less lipase to promote the decomposition of fat. Similarly, Phellinus-fermented adlay contained higher fat content but lower protein and starch contents than polished adlay (Hu et al. 2011).
Amino acids play an important role in several biological activities and represent among the most important building blocks of body tissue, thereby making them indispensable for vital bodily functions (Liang et al. 2018). The composition and amount of free amino acids before and after fermentation are shown in Table 2. Seventeen amino acids were identified, and the total content of free amino acids significantly increased from 0.11 g/100 g DW to 4.77 g/100 g DW, in the descending order of BDA > BPA > BBA > PA > DA > BA. After fermentation, the total free amino acid content in BPA, BDA and BBA reached 4.77, 4.55 and 1.86 g/100 g DW, which increased by 31 times, 41 times and 17 times compared to DA, PA, and BA, respectively. Glutamic acid, tyrosine, and phenylalanine were higher in raw adlay. However, after fermentation, the major free amino acids were leucine (0.57–1.53 g/100 g DW), alanine (0.12–0.86 g/100 g DW) and phenylalanine (0.28–0.53 g/100 g DW). Enzymes, such as proteases, contribute to the modification of adlay composition (Das et al. 2012), which could explain the higher levels of free amino acids in B. subtilis-fermented adlay. Similarly, the total free amino acid contents in monascal adlay products ranged from 0.86 to 14.11 g/100 g DW and in the descending order of MDA > MPA > DA > PA (Hao et al. 2013). Hu et al. (2007) also found that the total free amino acid content in Phellinus-fermented polished adlay (123.34 g/100 g) was much higher than in polished adlay (48.59 g/100 g).
Table 2.
Contents of free amino acids of raw and B. subtilis-fermented adlay (g/100 g DW)
| Amino acid | ASP | THR | SER | GLU | GLY | ALA | CYS | VAL | MET | ILE | LEU | TYR | PHE | HIS | LYS | AGR | PRO | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DA | ND | ND | ND | 0.06d | < 0.01 | < 0.01 | ND | < 0.01 | ND | ND | 0.06c | < 0.01 | < 0.01 | ND | < 0.01 | ND | ND | 0.15 |
| BDA | 0.02b | 0.07a | 0.09a | 0.11c | 0.03a | 0.86a | 0.01b | 0.38a | 0.05c | 0.16a | 1.53a | 0.47a | 0.53a | 0.08a | 0.06a | 0.03a | 0.35a | 4.77 |
| PA | ND | ND | ND | 0.05e | < 0.01 | < 0.01 | ND | 0.01d | ND | ND | 0.03d | < 0.01 | < 0.01 | ND | < 0.01 | ND | ND | 0.11 |
| BPA | 0.02a | 0.027a | 0.04b | 0.18a | 0.27b | 0.65b | 0.01b | 0.36b | 0.08a | 0.15b | 1.54a | 0.47a | 0.53a | 0.07b | 0.05b | 0.02b | 0.34a | 4.55 |
| BA | ND | ND | ND | 0.05f | < 0.01 | < 0.01 | ND | 0.01d | ND | ND | 0.03d | < 0.01 | < 0.01 | ND | < 0.01 | ND | ND | 0.11 |
| BBA | 0.09c | 0.02a | ND | 0.13b | 0.01c | 0.12c | 0.02a | 0.19c | 0.06b | 0.08c | 0.57b | 0.18b | 0.28b | 0.05b | 0.04c | 0.02b | 0.08b | 1.86 |
Contents are presented as mean, and the standard deviation (SD) was less than 0.01. Different lower-case letters in the same line indicate significant differences at p < 0.05.
ND not detected, DA dehulled adlay, BDA B. subtilis-fermented dehulled adlay, PA polished adlay, BPA B. subtilis-fermented polished adlay, BA broken adlay, BBA B. subtilis-fermented broken adlay
Adlay contains a high content of unsaturated fatty acids and is known as a typical “green food” (Yin et al. 2018). As shown in Table 3, the fatty acid content of B. subtilis-fermented adlay was significantly higher than that of raw adlay. The total fatty acid content significantly increased from 44.88 g/100 g DW to 60.75 g/100 g DW, in the descending order of BDA > BPA > BBA > PA > BA > DA. Oleic (23.01–30.10 g/100 g DW), linoleic (12.12–15.31 g/100 g DW) and palmitic acids (6.61–10.81 g/100 g DW) were the major fatty acids in all adlay samples. The monounsaturated fatty acids (MUFA) were the most abundant, followed by polyunsaturated fatty acids (PUFA) and saturated fatty acids (SFA). The PUFA/SFA ratio was higher than the minimum recommended value for a human diet of 0.45 (Niu et al. 2017). The PUFA/SFA ratios of dehulled, polished and broken adlay were 1.18, 1.71 and 1.67. After fermentation, the PUFA/SFA ratios of BDA, BPA and BBA were reduced to 1.09, 1.27, and 1.53, respectively. Furthermore, ω-3 PUFAs prevent the growth of atherosclerotic plaque in blood vessels, reducing blood pressure, and improving the immune function, and ω-6 PUFAs are responsible to maintain a healthy ratio between high- and low-density cholesterol. In the present study, the ω6/ω3 ratio of BDA, BPA and BBA ranged from 25.93 to 31.89, indicating its potential as a functional food.
Table 3.
Contents of fatty acids of raw and B. subtilis-fermented adlay (g/100 g DW)
| Fatty acid | DA | BDA | PA | BPA | BA | BBA |
|---|---|---|---|---|---|---|
| Hexanoic (C6:0) | 0.02 ± 0.00a | 0.01 ± 0.00b | 0.02 ± 0.00a | 0.01 ± 0.00b | 0.01 ± 0.00b | 0.01 ± 0.00b |
| Octanoic (C8:0) | 0.01 ± 0.00a | 0.01 ± 0.01a | 0.00 ± 0.00a | 0.01 ± 0.00a | 0.01 ± 0.00a | 0.01 ± 0.01a |
| Lauric (12:0) | 0.01 ± 0.00 b | 0.03 ± 0.01a | 0.01 ± 0.00b | 0.01 ± 0.00b | 0.02 ± 0.01ab | 0.02 ± 0.00ab |
| Myristic (C14:0) | 0.03 ± 0.01 b | 0.06 ± 0.02a | 0.03 ± 0.01b | 0.05 ± 0.02ab | 0.03 ± 0.01b | 0.03 ± 0.01b |
| Pentadecanoic (C15:0) | 0.02 ± 0.00c | 0.05 ± 0.01a | 0.02 ± 0.01bc | 0.03 ± 0.00b | 0.02 ± 0.00c | 0.02 ± 0.00c |
| Palmitic (C16:0) | 6.89 ± 0.59b | 10.81 ± 0.98a | 6.67 ± 0.84bc | 9.59 ± 0.38a | 6.62 ± 0.54c | 7.25 ± 0.70bc |
| Heptadecanoic (C17:0) | 0.08 ± 0.02ab | 0.12 ± 0.04a | 0.06 ± 0.02b | 0.09 ± 0.04ab | 0.07 ± 0.03ab | 0.07 ± 0.02ab |
| Stearic (C18:0) | 1.12 ± 0.37b | 2.41 ± 0.16a | 1.22 ± 0.25b | 2.12 ± 0.47a | 1.17 ± 0.20c | 1.35 ± 0.31c |
| Arachidic (C20:0) | 0.43 ± 0.05a | 0.56 ± 0.09a | 0.27 ± 0.09b | 0.50 ± 0.11a | 0.29 ± 0.08b | 0.29 ± 0.09b |
| Heneicosanoic (C21:0) | 0.03 ± 0.01ab | 0.04 ± 00a | 0.01 ± 0.00c | 0.04 ± 0.01 a | 0.02 ± 0.01bc | 0.02 ± 0.00b |
| Behenic (C24:0) | 0.17 ± 0.08ab | 0.21 ± 0.04a | 0.09 ± 0.02b | 0.18 ± 0.08ab | 0.09 ± 0.03b | 0.10 ± 0.04b |
| Lignoceric (C24:0) | 0.17 ± 0.07a | 0.17 ± 0.02a | 0.06 ± 0.03bc | 0.16 ± 0.07ab | 0.05 ± 0.02c | 0.06 ± 0.02c |
| Total SFA | 8.97 | 14.47 | 8.43 | 12.76 | 8.37 | 9.23 |
| Palmitoleic (C16:1) | 0.08 ± 0.02a | 0.11 ± 0.01a | 0.10 ± 0.05a | 0.10 ± 0.02a | 0.12 ± 0.07a | 0.12 ± 0.07a |
| Oleic (C18:1) | 23.01 ± 2.24c | 30.10 ± 1.57a | 23.76 ± 2.56c | 29.20 ± 1.08a | 23.44 ± 1.45c | 26.67 ± 1.87b |
| Gondoic (C20:1) | 0.15 ± 0.06a | 0.18 ± 0.09a | 0.11 ± 0.05a | 0.19 ± 0.05a | 0.14 ± 0.05 | 0.12 ± 0.07a |
| Total MUFA | 23.24 | 30.38 | 23.98 | 29.49 | 23.70 | 26.92 |
| α-Linolenic (C18:3) | 0.46 ± 0.10a | 0.49 ± 0.07a | 0.54 ± 0.02a | 0.53 ± 0.07a | 0.50 ± 0.12a | 0.43 ± 0.09a |
| Arachidonate (C20:4) | 0.10 ± 0.03a | 0.10 ± 0.05a | 0.03 ± 0.00b | 0.08 ± 0.02a | 0.03 ± 0.00b | 0.03 ± 0.01b |
| Linoleic (C18:2) | 12.12 ± 0.88b | 15.31 ± 0.95a | 13.89 ± 0.62a | 14.83 ± 0.75a | 13.47 ± 0.91ab | 13.70 ± 1.01ab |
| Total PUFA | 12.67 | 15.90 | 14.45 | 15.44 | 14.00 | 14.16 |
| Total FA | 44.88 | 60.75 | 46.86 | 57.69 | 46.07 | 50.31 |
| PUFA/SFA | 1.19 | 1.10 | 1.71 | 1.21 | 1.67 | 1.53 |
| ω-6/ω-3 ratio | 26.27 | 31.26 | 25.93 | 27.86 | 26.91 | 31.89 |
Results are expressed as mean ± standard error. Lower-case letters in the same line indicate significant differences at p < 0.05
SFA saturated fatty acid, PUFA polyunsaturated fatty acid, MUFA monounsaturated fatty acid, FA free fatty acids, BDA B. subtilis-fermented dehulled adlay, DA dehulled adlay, BPA B. subtilis-fermented polished adlay, PA polished adlay, BBA B. subtilis-fermented broken adlay, BA broken adslay
Effect of fermentation on GABA, triterpene, flavonoids, phenolics, coixol, coixenolide and coixan
GABA, a four-carbon nonprotein amino acid, is widely found in many cereals and used as a monomer for the production of the biodegradable plastic polyamide (Liao et al. 2013). Fermentation triggered the bio-synthesis of GABA, and a gradual increase of GABA was found in our experiment (Fig. 2a). The GABA content in raw adlay ranged from 2.00 to 2.51 mg/g DW. After fermentation, the highest GABA content was observed in BDA (30.14 mg/g DW), representing an almost 14-fold increase compared to DA. Similarly, adzuki beans were used with mixed cultures of Lactococcus lactis and Lactobacillus rhamnosus, and the highest GABA content was 201.2 mg/100 g, a 150-fold increased as compared to the non-treated adzuki beans (Liao et al. 2013). Moreover, Park et al. (2017) found that the GABA content of Rhizopus oligosporus fermented buckwheat was 34 times higher than that of raw buckwheat.
Fig. 2.
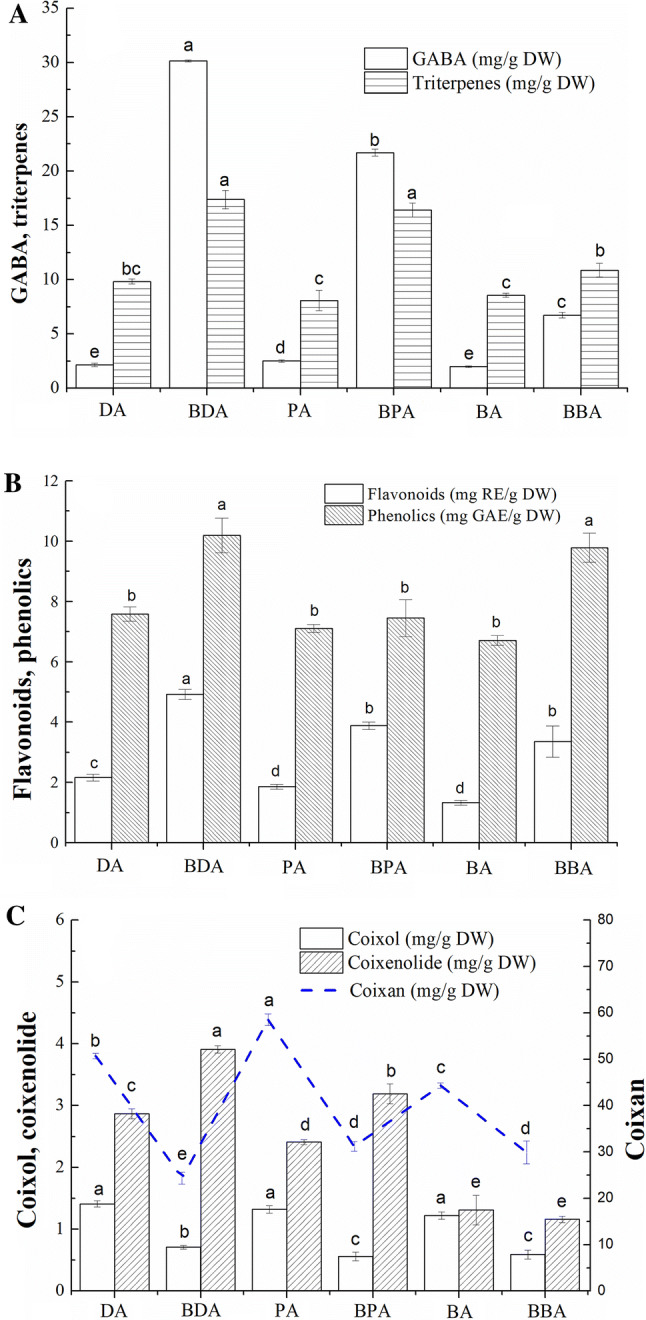
Bioactive components of raw and B. subtilis-fermented adlay. DA dehulled adlay, BDA B. subtilis-fermented dehulled adlay, PA polished adlay, BPA B. subtilis-fermented polished adlay, BA broken adlay, BBA B. subtilis-fermented broken adlay
Triterpenes have good pharmacological effects, including immune modulation, hypoglycaemic and hypolipidaemic effects, protection of liver function, anti-HIV and antitumor effects (He et al. 2014). The triterpene content in raw adlay ranged from 8.09 to 9.83 mg/g DW. The triterpene content of BDA increased significantly to 17.38 mg/g DW, and showed a significant increase (2.02 times) after fermentation.
Phenolics and flavonoids are secondary metabolites of plants, and exhibit a broad variety of biological effects, such as antioxidant, anti-inflammatory, and anti-allergic activity (Xu et al. 2017). A significantly (p < 0.05) higher contents of phenolics and flavonoids of B. subtilis-fermented adlay was found than that of the raw adlay (Fig. 2b). The highest phenolics and flavonoids were 10.19 mg GAE/g DW and 4.92 mg RE/g DW in BDA, which were approximately 1.34-fold and 1.08-fold higher than that of DA, respectively. The contents of flavonoids and phenolics in Phellinus linteus fermented adlay were higher than in raw adlay (Liang et al., 2009). Similarly, the phenolic content of fermented defatted rice bran was increased by about threefold compared to unfermented samples (Webber et al. 2014).
Coixan, coixol and coixenolide have attracted increasing attention due to their potential biological functions, especially antioxidant, anti-tumor, anti-diabetic, anti-melanin, and anti-inflammatory activities (Yin et al. 2018). The contents of coixan, coixol and coixenolide are shown in Fig. 2c. In raw materials, the coixan content ranged from 44.34 to 58.53% DW. After fermentation, the coixan contents of BDA, BPA and BBA decreased significantly to 24.37% DW, 31.17% DW and 29.88% DW. This decrease might be partly attributed to the increased amylase activity, which resulted in the hydrolysis of coixan into monosaccharides such as glucose and galactose during fermentation, and supplied the carbon and energy source to B. sutillus BJ3-2. The coixol contents of DA, PA, and BA were 1.32, 1.41 and 1.22 mg/g DW, respectively. After fermentation, the contents in BPA, BDA, and BBA decreased significantly to 0.39 mg/g DW, 0.56 mg/g DW, and 0.32 mg/g DW, respectively. The coixenolide contents increased significantly to 3.91% DW of BDA, 3.19% DW of BPA, and 1.16% DW of BBA, almost 0.76 times higher than that of the raw adlay. The main reason was that the B. subtilis produced less lipase to promote the decomposition of coixenolide, and the total mass of fermented adlay decreased.
Effect of fermentation on phenolic composition
Phenolic compounds were the important active compounds from the plant materials. The HPLC allowed the identification and quantitation of eight phenolic compounds (gallic acid, protocatechuic acid, chlorogenic acid, catechinic acid, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid and ferulic acid) in both free and bound extractions of B. sutillus-fermented adlay. As shown in Table 4, the contents of phenolic compounds of fermented samples were significantly higher than those of raw adlay, and varieties of phenolic compounds in the free extracts were significantly superior to those in the bound extracts.
Table 4.
Contents of free and bound phenolics in raw and B. subtilis-fermented adlay (μg/g)
| Phenolic acids | Gallic acid | protocatec-huic acid | Chlorogenic acid | Catechinic acid | p-hydroxybenzoic acid | Caffeic acid | p-coumaric acid | Ferulic acid |
|---|---|---|---|---|---|---|---|---|
| DA | ||||||||
| F | 23.28 ± 0.36b | 1.57 ± 0.01b | 20.59 ± 0.11a | 20.44 ± 0.95a | 6.34 ± 031a | 56.06 ± 0.14b | 3.44 ± 0.18d | 2.56 ± 0.28e |
| B | 0.28 ± 0.02h | ND | ND | ND | ND | ND | ND | 4.89 ± 0.10b |
| BDA | ||||||||
| F | 38.31 ± 0.60a | 1.71 ± 0.01a | 15.14 ± 0.99b | 21.78 ± 1.16a | 4.73 ± 0.10b | 59.98 ± 2.19a | 4.53 ± 0.22c | 4.59 ± 0.46b |
| B | 0.44 ± 0.03 g | ND | ND | ND | ND | ND | ND | 0.96 ± 0.13 g |
| PA | ||||||||
| F | 12.72 ± 0.12d | 0.97 ± 0.03d | 2.56 ± 0.08d | 7.99 ± 0.16c | 3.89 ± 0.10c | 5.93 ± 0.15e | 5.39 ± 0.25ab | 3.00 ± 0.12d |
| B | 0.52 ± 0.09 g | ND | ND | ND | ND | ND | ND | 5.22 ± 0.09a |
| BPA | ||||||||
| F | 20.36 ± 0.94c | 1.18 ± 0.05c | 8.45 ± 0.51c | 15.16 ± 1.37b | 5.06 ± 0.49b | 33.89 ± 0.82c | 3.56 ± 0.19d | 4.89 ± 0.08b |
| B | 0.35 ± 0.03 g | ND | ND | ND | ND | ND | ND | 0.74 ± 0.21g |
| BA | ||||||||
| F | 9.65 ± 0.17f | 0.69 ± 0.02f | 1.98 ± 0.11f | 5.80 ± 0.11e | 2.98 ± 0.15e | 4.83 ± 0.12f | 5.10 ± 0.08b | 2.80 ± 0.12e |
| B | 0.43 ± 0.04 g | ND | ND | ND | ND | ND | ND | 5.00 ± 0.12b |
| BBA | ||||||||
| F | 11.66 ± 0.18e | 0.90 ± 0.03e | 2.36 ± 0.03e | 7.39 ± 0.16d | 3.49 ± 0.13d | 8.30 ± 0.27d | 5.34 ± 0.11a | 3.96 ± 0.05c |
| B | 0.20 ± 0.16 h | ND | ND | ND | ND | ND | ND | 1.34 ± 0.11f |
Results are expressed as mean ± standard error. Different lower-case letters in the same line indicate significant differences at p < 0.05
ND not detected, F free form, B bound form, BDA B. subtilis-fermented dehulled adlay, DA dehulled adlay, BPA B. subtilis-fermented polished adlay, PA polished adlay, BBA B. subtilis-fermented broken adlay, BA broken adlay
After fermentation, the levels of gallic, protocatechuic, chlorogenic, catechinic and caffiec acids have a significant increase compared to raw adlay (p < 0.05). Caffeic, gallic, catechinic and chlorogrnic acids were the major phenolic compounds in all fermented adlay samples. Free caffeic acid existed in free from and showed a significant increase (1011.47%) during fermentation and reached 59.98 μg/g DW in BDA. Gallic acid existed in both free and bound forms with the contents ranging from 9.65 to 38.31 μg/g DW and 0.08 to 0.52 μg/g DW, respectively, and free gallic acid increased significantly after fermnentation (p < 0.05). Ferulic acid was also detected in both free and bound forms. The bound ferulic acid contents in fermented adlay ranged from 0.74 to 1.34 μg/g DW, and were far lower than those of raw adlay. The contents of protocatechuic acid, p-hydroxybenzoic acid and p-coumaric acid were no statistic difference between raw and fermented adlay. Bound phenolic contents of protocatechuic acid, chlorogenic acid, catechinic acid, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid were not detected in all samples. High levels of the phenolic compounds existed in conjugated and insolubly bound forms combined with polysaccharide, lipidmoieties, hemicelluloses and cellulose (Wang et al. 2018). B.subtilis produced aprotease and phytase, which accelerated the degradation of plant material, providing greater access for feruloyl esterase to hydrolyze bound phenolics (Webber et al. 2014). Therefore, the high phenolic contents of B. subtilis fermented adlay was likely to be the result of phenolic release facilitated by a high level of enzymatic activity and/or an adjuvant action among proteases, carbohydrase and phenolic esterase.
Conclusion
Adlay can be used as a substrate for TMP and fibrinolytic enzyme production from B. subtilis BJ3-2. After optimization by Box–Behnken Design, the TMP yield of BPA (6.93 mg/g DW) was 136 times higher than that o f BSB, but the fibrinolytic enzyme activity was slightly lower than that of BSB. Moreover, the levels of free amino acid, fatty acid, GABA, triterpenes, phenolics, flavonoids, coixenolide and phenolic compounds significantly increased (p < 0.05) in B. subtilis-fermented adlay compared with the raw adlay. Thus, the natural bioactivity of adlay can be further increased using fermentation bioprocesses, which can be used to improve quality of adlay-based foods to produce nutritionally superior healthy meals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The research was supported by the Natural Science Foundation of Guizhou Province [Grant No. (2019) 1111, (2014) 6023 & (2012) 4001], and Agriculture Committee of Guizhou Province [Grant No. (2018) 81].
Compliance with ethical standards
Conflict of interest
The authors declare no financial or commercial conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AOAC . Official methods of analysis. 15. Washington, DC: Association of Official Analytical Chemists; 1990. [Google Scholar]
- Chen JC, Chen QH, Guo Q, Ruan S, Ruan H, He GQ, Gu Q. Simultaneous determination of acetoin and tetramethylpyrazine in traditional vinegars by HPLC method. Food Chem. 2010;122(4):1247–1252. doi: 10.1016/j.foodchem.2010.03.072. [DOI] [Google Scholar]
- Das A, Raychaudhuri U, Chakraborty R. Cereal based functional food of Indian subcontinent: a review. J Food Sci Technol. 2012;49(6):665–672. doi: 10.1007/s13197-011-0474-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao F, Wu Q, Xu Y. Precursor supply strategy for tetramethylpyrazine production by Bacillus Subtilis on solid-state fermentation of wheat bran. Appl Biochem Biotechnol. 2013;169:1346–1352. doi: 10.1007/s12010-012-0083-0. [DOI] [PubMed] [Google Scholar]
- He QQ, Yang L, Zhang JY, Ma JN, Ma CM. Chemical constituents of gold-red apple and their α-glucosidase inhibitory activities. J Food Sci. 2014;79(10):1970–1983. doi: 10.1111/1750-3841.12599. [DOI] [PubMed] [Google Scholar]
- Hu AJ, Zhao SN, Liang HH, Qiu TQ, Chen GH. Ultrasound assisted supercritical fluid extraction of oil and coixenolide from adlay seed. Ultrason Sonochem. 2007;14(2):219–224. doi: 10.1016/j.ultsonch.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Li SZ (1596) Bencao Gangmu (systematic pharmacopoeia), Part of cereal 24, China
- Liang XN, Han HJ, Zhao X, Cao XY, Yang M, Tao DB, Wu RN, Yue XQ. Quantitative analysis of amino acids in human and bovine colostrum milk samples through iTRAQ labeling. J Sci Food Agric. 2018;98:5157–5163. doi: 10.1002/jsfa.9032. [DOI] [PubMed] [Google Scholar]
- Liang CH, Syu JL, Mau JL. Antioxidant properties of solid-state fermented adlay and rice by Phellinus linteus. Food Chem. 2009;116:841–845. doi: 10.1016/j.foodchem.2009.03.032. [DOI] [Google Scholar]
- Liao WC, Wang CY, Shyu YT. Influence of preprocessing methods and fermentation of adzuki beans on γ-aminobutyric acid (GABA) accumulation by lactic acid bacteria. J Funct Foods. 2013;5(3):1108–1115. doi: 10.1016/j.jff.2013.03.006. [DOI] [Google Scholar]
- Litchfield JH. Morel mushroom mycelium as a food flavoring material. Biotechnol Bioeng. 1967;9:289–304. doi: 10.1002/bit.260090303. [DOI] [Google Scholar]
- Liu XJ, Yang L, Mao X, Zhao LC, Zhou AM, Liu X. Study on mechanism of adlay. J Chin Inst Food Sci Technol. 2012;12(7):55–60. [Google Scholar]
- Liu Y, Su H, Song HL. Comparison of four extraction methods, SPME, DHS, SAFE, versus SDE, for the analysis of flavor compounds in Natto. Food Anal Methods. 2018;11:343–354. doi: 10.1007/s12161-017-1005-0. [DOI] [Google Scholar]
- Luithui Y, Nisha RB, Meera MS. Cereal by-products as an important functional ingredient: effect of processing. J Food Sci Technol. 2019;56(1):1–11. doi: 10.1007/s13197-018-3461-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manosroi J, Khositsuntiwong N, Manosroi A. Biological activities of fructooligosaccharide (FOS)-containing Coix lachryma-jobi Linn. extract. J Food Sci Technol. 2014;51(2):341–346. doi: 10.1007/s13197-011-0498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W, Wang RM, Xiao DG. Metabolic engineering of Bacillus subtilis to enhance the production of tetramethylpyrazine. Biotechnol Lett. 2015;37:2475–2480. doi: 10.1007/s10529-015-1950-x. [DOI] [PubMed] [Google Scholar]
- Niu YW, Yao ZM, Xiao Q, Xiao ZB, Ma N, Zhua JC. Characterization of the key aroma compounds in different light aroma type Chinese liquors by GC-olfactometry, GC-FPD, quantitative measurements, and aroma recombination. Food Chem. 2017;233:204–215. doi: 10.1016/j.foodchem.2017.04.103. [DOI] [PubMed] [Google Scholar]
- Ong MH, Blanshard JMV. Texture determinants in cooked, parboiled rice I: Rice starch amvlose and the fine structrure of amylopectin. J Cereal Sci. 1995;21:251–260. doi: 10.1006/jcrs.1995.0028. [DOI] [Google Scholar]
- Park N, Lee TK, Nguyen TH, An EB, Kim NM, You YH, Park TS, Kim D. The effect of fermented buckwheat on producing l-carnitine and γ-aminobutyric acid (GABA) enriched designer eggs. J Sci Food Agric. 2017;97:2891–2897. doi: 10.1002/jsfa.8123. [DOI] [PubMed] [Google Scholar]
- Singh R, Puri A, Panda BP. Development of menaquinone-7 enriched nutraceutical: inside into medium engineering and process modeling. J Food Sci Technol. 2015;52(8):5212–5219. doi: 10.1007/s13197-014-1600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repizo GD, Mortera P, Magni C. Disruption of the alsSD operon of Enterococcus faecalis impairs growth on pyruvate at low pH. Microbiol-Sgm. 2011;157:2708–2719. doi: 10.1099/mic.0.047662-0. [DOI] [PubMed] [Google Scholar]
- Tien AJ, Chueh TH, Hsia CP. Monascus Adlay and Monacolin K Attenuates Arterial Thrombosis in rats through the inhibition of ICAM-1 and oxidative stress. Kidney Blood Press Res. 2016;41:815–827. doi: 10.1159/000452584. [DOI] [PubMed] [Google Scholar]
- Wan LY, Wang ML, Luo GL, Ran JP. Effect of processing of grading shelling on quality and antioxidation of barley. Sci Technol Food Ind. 2017;38(17):33–37. doi: 10.13386/j.issn1002-0306.2017.17.007. [DOI] [Google Scholar]
- Wang CY, Lin HT, Wu SC. Influence of dietary supplementation with Bacillus-fermented adlay on lipid metabolism, antioxidant status and intestinal microflora in hamsters. J Sci Food Agric. 2011;91:2271–2276. doi: 10.1002/jsfa.4450. [DOI] [PubMed] [Google Scholar]
- Wang CY, Wu SJ, Shyu YT. Antioxidant properties of certain cereals as affected by food-grade bacteria Fermentation. J Biosci Bioeng. 2014;117(4):449–456. doi: 10.1016/j.jbiosc.2013.10.002. [DOI] [PubMed] [Google Scholar]
- Wang L, Luo Y, Wu YN, Wu ZQ. Impact of fermentati on degree on phenolic compositions and bioactivities during the fermentation of guava leaves with Monascus anka and Bacillus sp. J Funct Food. 2018;41:183–191. doi: 10.1016/j.jff.2017.12.044. [DOI] [Google Scholar]
- Webber DM, Hettiarachchy NS, RonnyHorax RL, Theivendran S. Phenolic profile and antioxidant activity of extracts prepared from fermented heat-stabilized defatted rice bran. J Food Sci. 2014;79(11):2383–2391. doi: 10.1111/1750-3841.1265. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Fang JY, Ng CC. Anti-inflammatory activity of Lactobacillus-fermented adlay-soy milk in LPS-induced macrophages through suppression of NF-κB pathways. Food Res Int. 2013;52:262–268. doi: 10.1016/j.foodres.2013.02.053. [DOI] [Google Scholar]
- Xiao ZJ, Zhao L, Tian L. GC–FID determination of tetramethylpyrazine and acetoin in vinegars and quantifying the dependence of tetramethylpyrazine on acetoin and ammonium. Food Chem. 2018;239:726–732. doi: 10.1016/j.foodchem.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Xu L, Wang P, Ali B, Yang N, Chen YS, Wu FF. Changes of the phenolic compounds and antioxidant activities in germinated adlay seeds. J Sci Food Agric. 2017;97(12):4227. doi: 10.1002/jsfa.8298. [DOI] [PubMed] [Google Scholar]
- Xu L, Chen L. Impact of germination on nutritional and physicochemical properties of adlay seed (Coix lachryma-jobi L.) Int J Food Sci Technol. 2017;53(449–456):9. doi: 10.1111/ijfs.13603. [DOI] [PubMed] [Google Scholar]
- Yin HM, Wang SN, Nie SP, Xie MY. Coix polysaccharides: gut microbiota regulation and immunomodulatory. Bioact Carbohydr Diet Fibre. 2018;21:53–61. doi: 10.1016/j.bcdf.2018.04.002. [DOI] [Google Scholar]
- Zhang J, Ge WP, Chen Y, Zhang Y, Liu LT. Strain improvement of Bacillus subtilis for enhanced production of nattokinase and optimization of solid-state fermentation conditions. Food Sci. 2016;37(3):151–156. [Google Scholar]
- Zhu F. Coix: Chemical composition and health effects. Trends Food Sci Technol. 2017;61:160–175. doi: 10.1016/j.tifs.2016.12.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


