Abstract
The highly perishable nature of the mango fruit which leads to quick deterioration in quality is the major limiting factor for mango growers of north India. Studies were, therefore, planned to determine the role of ‘enhanced freshness formulation’ (EFF) having hexanal as the main component in extending the storage life and quality of mango fruits of cultivar Dashehari. Aqueous formulations of hexanal are known to retard the activity of phospholipase-D thereby increasing the shelf-life and quality of stored fruits. Mango trees were given pre-harvest sprays of hexanal @800 µM, 1200 µM, 1600 µM and 2000 µM at 30 and 15 days before harvesting of the fruit. After harvest at physiologically mature stage, fruits were stored in walk-in-cool chambers at a temperature of 12 ± 2 °C and 85–90% relative humidity. These fruits were analysed for different physiological and biochemical parameters at weekly intervals till the stipulated storage period of 35 days. The results of the research revealed that the hexanal treatments widened the storage period of fruits while maintaining their freshness in comparison to the untreated mango fruits. Hexanal formulation @1600 µM significantly reduced the decay incidence, pectin methyl esterase activity and respiratory rate of mango fruits. These fruits also exhibited increased firmness, total soluble solids, acidity and maintained acceptable palatability up to 28 days of storage. The lower doses of EFF did not showed significant effect in expanding the storage span of fruits beyond 21 days while the higher dose of hexanal @2000 µM did not showed a significant enhancement in quality and storage life of mango fruits in comparison to hexanal @1600 µM.
Keywords: Pre harvest sprays, Hexanal formulations, Mango, Respiration rate, Fruit quality
Introduction
Mango (Mangifera indica Linn.) is a predominant fruit crop which is liked all over the globe due to its excellent aroma and flavour. India is the largest mango producing country in the world due to large area under mango. The production of mangoes was to the tune of 18,642 thousand metric tons from an area of 2208.56 thousand hectares (Indian Horticulture Database 2017). Dashehari is a popular cultivar of north India because of its high yielding capacity and excellent fruit quality. The fruit of this variety is mainly harvested in July month when the temperature and humidity is very high. Moreover, mango being a climacteric fruit needs to be harvested at proper stage of maturity to reduce the postharvest losses. A number of pre and post-harvest factors directly or indirectly affect the quality and the storage life of the mango fruits. Among, the various factors which affect the postharvest life of mango fruits, the most damaging are improper harvesting method and time, ripening conditions, the attack of microorganisms and lack of proper storage facilities. Lack of suitable facilities for postharvest management of fruits result in producing a glut in the market during the peak harvesting season and major portion of the produce is either wasted or has to be sold at very low prices. It was reported by Srinivas et al. (1996) that in India, the total postharvest losses in mango cv. Totapuri and Alphonso are to the tune of 17.9% and 14.4%, respectively. Postharvest losses can sometimes account to be as high as 40% or even higher in some developing countries from harvest to consumption of fruits (James and James 2010).
In mango, the respiratory climacteric begins soon after the fruit is harvested because the tissue no longer receives a substance (anti-ethylene) from the mother plant which probably lowers the sensitivity of the fruit to ethylene (Burg and Burg, 1965). Increase in ethylene production leads to increased respiration, change in texture causing softening of fruits, degradation of chlorophyll and simultaneous carotenoids biosynthesis, metabolic activities leading to changes in composition of carbohydrates, organic acids, lipids, phenolics and volatile compounds resulting in ripening of fruits (Herianus et al. 2003). While excessive softening can lead to spoilage of fruits, however, controlled ripening of fruits can aid in making the produce aesthetically sound for longer duration coinciding with the delayed onset of senescence and undesirable changes. Singh and Singh (2012) have quoted that various techniques such as low temperature storage, MAP, vapour heat treatment; edible coatings and use of fungicides have been considered to maintain the quality and shelf life of mango fruits. Though, the low temperature storage is effective in extending the postharvest life as well as to control the decay incidence but mango being a fruit of tropics cannot tolerate very low temperature storage. Even the rising concern regarding the use of non-biodegradable polythene films in Modified atmosphere packaging (MAP) limits the use of this technology for extending the shelf life of fruits. Synthetic fungicides used for delaying spoilage, ripening and senescence of fruits are again a doubtful method due to environmental and concerns regarding food safety for the consumers (Mari et al. 2012).
Recently, hexanal treatment as pre harvest sprays or postharvest dipping has shown excellent results in extending the shelf life of various fruits such as straw-berry, apple, tomato, cherry, banana and blue berry (Paliyath and Subramanian 2008; Paliyath and Murr 2007). Similar biochemical changes in sugars, firmness, acidity and an increase in anthocyanin and polyphenols have been reported by Sharma et al. (2010) and Cheema et al. (2014) in climacteric and non-climacteric fruits with the hexanal formulations. Recently, a study conducted in mango fruits by Anusuya et al. (2016) revealed significantly reduced postharvest disease incidence with the pre-harvest sprays of 1.6 mM hexanal formulation and fruits remained fresh for longer duration under both room temperature and cold storage conditions. Hence, the present research experiment was formulated to study the role of hexanal formulations in delaying ripening and enhancing the quality and storage life of ‘Dashehari’ mangoes under subtropical conditions.
Materials and methods
Plant material
Mangifera indica L. cv. Dashehari grafted onto desi mango seedlings planted at University Seed Farm, Ladhowal, (lat. 30°58′29"N long. 75°47′15"E) of Punjab Agricultural University (PAU), Ludhiana, India were used in this study for two seasons (2015–2016 and 2016–2017). These trees were planted at a spacing of 9 m × 9 m apart and standard cultural practices for mango as per package of practices of PAU, Ludhiana were followed in both the seasons. Thirty six mango trees were selected considering the uniformity in vegetative growth and crop load after fruit set. Hexanal stock formulation (EFF) consisting of hexanal @1% v/v, ascorbic acid @1% w/v, alpha- tocopherol @1% w/v, geraniol @1% v/v, and surfactant Tween 20 @10% v/v, was prepared by dissolving in ethanol @10% v/v as per procedures given by Paliyath and Murr (2007). As per them, hexanal and geraniol combination act as phospholipase D inhibitor while trans-cinnamic acid has been added since its component of the flavonoid biosynthetic pathway and is involved in flavonoid biosynthesis which enhances or prolongs organoleptic qualities of produce Paliyath and Murr (2007). Ascorbic acid and tocopherol act as antioxidants scavenging the active oxygen species which are major contributor in spoilage of produce. All the ingredients were mixed by stirring and distilled water was used to make the final volume up to one liter. Effective hexanal concentrations of 0.01%, 0.015%, 0.02% and 0.025% on volume/volume basis were prepared by dilution of stock solution with water just before spraying. Corresponding molar concentrations in the final spray solutions of hexanal were 800 µM, 1200 µM, 1600 µM and 2000 µM, respectively. The diluted solution was stirred thoroughly to get homogenous mixture but the diluted solution remained milky white. Fruit trees were sprayed using a knapsack sprayer to the point of dripping to obtain good coverage of the canopy. Selected mango plants were given two superimposed pre harvest sprays of a hexanal solution at 15 and 30 days before harvest. Control trees were sprayed with clean water. Every treatment was replicated thrice with three plants in each replication. Physiologically mature fruit from experimental trees (treated as well as control) was harvested during July. Ten kg fruit was harvested from each tree to store 30 kg fruit per replication which were packed in corrugated fibre board (CFB) boxes of 2 kg capacity (5% vents) lined with newspaper lining. The packed fruit was stored were in walk-in cool chambers (12 ± 2 °C and 85–90% RH). Separate boxes of mango fruits were kept for spoilage and weight loss studies with 100 fruits per replication to assess them at different storage intervals. Ten fruits per replication were removed from the cold storage at 7, 14, 21, 28 and 35 days after storage for analyzing different physiological and biochemical parameters.
Respiration rate
For Respiratory quotient measurements, 3 replicate samples of 1 fruits each were placed in 1.9 L sealed glass jars. A rubber septum was tightly inserted by drilling a hole (12 mm diameter) in the lid of the jar which was used for inserting needles for taking gas samples from the jars. After each storage interval the fruit samples were removed from the cold storage and gas chromatograph was used to determine the respiration rate. One milliliter of certified standard gas was used to calibrate the instrument. One milliliter of the headspace gas sample was injected into a gas chromatograph (Nucon 5700, New Delhi, India) fitted with a dual column. The amount of CO2 was expressed as mL kg−1 h −1.
Physicochemical parameters recorded
The physiological loss in weight (PLW) of mango fruits was calculated by subtracting final weight from initial weight of the fruit and then expressed as percentage weight loss with reference to the initial weight. The firmness of the fruit was measured with hand held penetrometer (Model FT-327) made in Italy and supplied by Elixir Technologies, Bangalore having plunger diameter of 8 mm, and expressed in terms of kg/cm2. The total soluble solids (TSS) were recorded in juice from whole fruit with handheld refractometer (make Atago, Tokyo, Japan; measurement range of 0–53%) calibrated at 20 °C and expressed as percentage soluble solids (Padda et al. 2011). The titratable acidity in juice as citric acid was determined by titrating a known volume of juice with 0.1 N NaOH using phenolphthalein as an indicator. Spoilage percentage was calculated by counting the number of spoiled fruits from a total of 100 fruits per replication. Organoleptic score was determined on the basis of texture and flavour of fruit by a panel of 10 expert judges according to Hedonic scale (1–9 points) as described by Amerine et al. (1965). The total sugars were estimated by the method described by Lane and Eynon (AOAC 2000).
Pectin methyl esterase activity
20 g of fruit pulp was, taken for enzyme extraction immediately after removing the fruits from cold storage was homogenized in 80 to100 ml solution of NaCl (0.15 M). The pulp + NaCl solution was then filtered through four layered muslin-cloth and centrifuged for half an hour at 2000 rpm and 5 °C temperature to remove the debris. This supernatant was used as an enzyme source for the estimation of its activity. For maximum PME activity, the pH for 0.15 M solution of NaCl was maintained at 7.0 as reported by Denes et al. (2000). The increase in acidity after the hydrolysis of pectin was measured to determine the activity of Pectin Methyl Esterase enzyme. In a beaker (50 ml), 20 ml of solution whose pH was adjusted to 7.0 (by adding 1 N NaOH) was taken and this was considered as the zero time. Subsequently, the beaker was placed in water bath for 15 min at 30 °C. The pH was constantly monitored and regulated to 7.0 after every 15 min by using 0.02 N NaOH, and the volume of alkali used was noted at each interval (Mahadevan and Sridhar 1982). PME activity was expressed as (units/ml juice) representing milli equivalents of esters hydrolyzed per minute per ml of juice. The units per milliliter were multiplied by 1000 for easy interpretation as described by Balaban et al. (1991).
Experimental design and statistics
The experiment was laid out as per Randomized Block Design (RBD) with Factorial arrangement considering two factors and two treatments (EFF dose) and length of storage (days) with three replications. The data of two seasons was pooled and subjected to analysis for variance using SAS package (Version 9.3 SAS Institute Inc., USA) to find significance of different treatments at p ≤ 0.05.
Result and discussion
Respiration rate
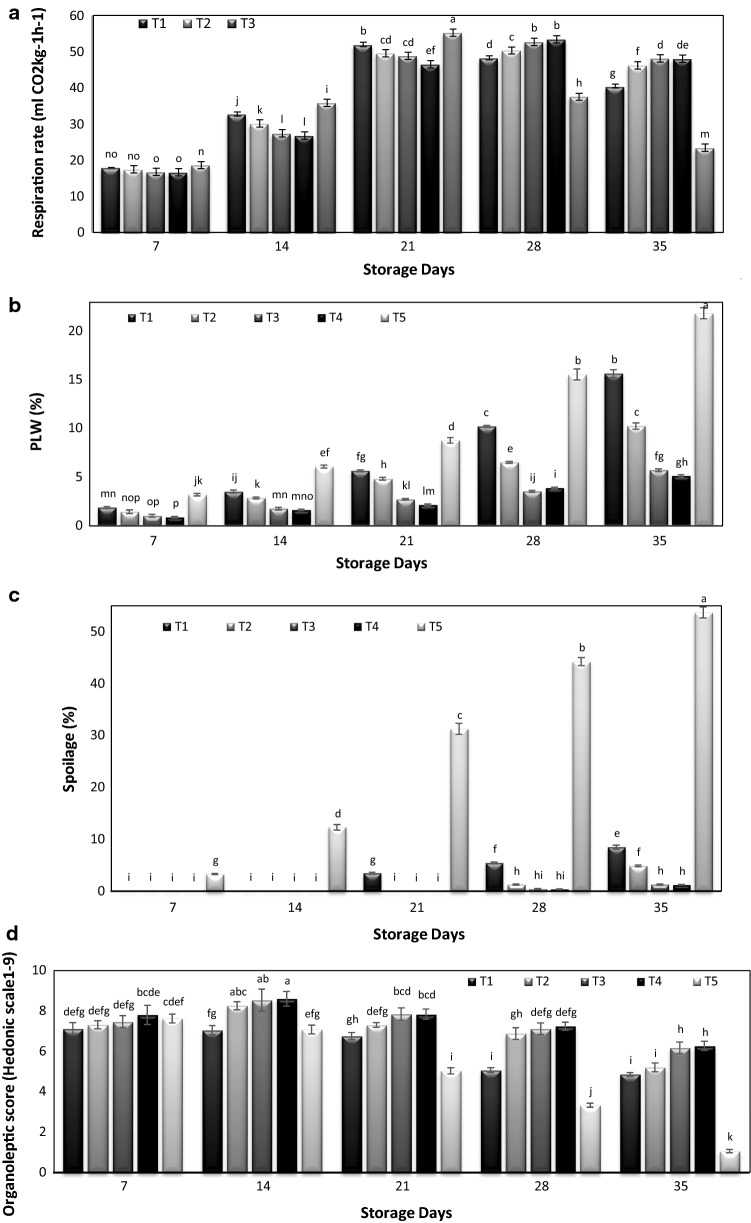
The rate of the respiration activity of fruits increased during storage with the increase in the period of storage interval up till 21 days with a progressive drop in rate thereafter. The highest respiration rate (55.2 ml CO2 kg−1 h−1) was observed in control fruits after 21 days in storage (Fig. 1a). This coincided with the respiratory peak in case of control fruits closely followed by hexanal @ 800 µM. However, in case of fruits treated with hexanal @1200 µM, 1600 µM and 2000 µM the respiratory peak was recorded after 28 days of storage. Mango fruits sprayed with hexanal @ 1600 µM and 2000 µM exhibited a gradual increase in respiratory rate from 7 to 35 days of storage. The rate of respiration decreased gradually after the respiratory peak which might also be due to increase in carbon dioxide concentration and decrease in oxygen concentration within the storage chamber along with the loss of substrate. It was quite evident that the fruits treated with hexanal formulations exhibited a progressive enhancement in respiratory rate while it was at a very higher rate in the control fruits. Our data closely conform to the outcome of studies conducted by Anusuya et al. (2016) that the mango fruits from hexanal treated trees had reduced respiratory and ethylene production rates which are indicative of delayed ripening. Further, the retardation of phospholipase-D activity slows down ethylene evolution in case of temperate fruits as reported by Sharma et al. (2010) and it seems that the similar effect might be one of the contributing factors for extending the shelf-life of mango fruits.
Fig. 1.
Effect of pre-harvest Hexanal sprays and storage intervals on a respiration rate (ml CO2kg−1 h−1), b Physiological loss in weight (%), c Spoilage (%), d Organoleptic score (Hedonic scale1-9) of mango cv. ‘Dashehari’. T1 = Hexanal @ 800 µM, T2 = Hexanal @ 1200 µM, T3 = Hexanal @1600 µM, T4 = Hexanal @2000 µM and T5 = Control. Bars represent ± SE. Values are means of 2 years
Physiological loss in weight (PLW)
Mango fruits cv. Dashehari exhibited a gradual increase in physiological weight loss in all the treatments with the corresponding increase in storage period (Fig. 1b). The highest weight loss percentage (21.89) was recorded under untreated mango fruits; whereas the mango fruits treated with hexanal @ 1600 µM (5.14%) closely followed by treatment with hexanal @ 2000 µM (5.73%) recorded a minimum loss in weight towards the end of storage period. Weight loss percentage in mango fruits treated with hexanal @ 800 µM and 1200 µM was significantly higher than the higher concentrations of hexanal formulations (p ≤ 0.05). Loss in fruit weight is considered as one of the principal parameter which is indicative of the freshness of fruits during storage. EFF sprayed mango fruits even after 4 weeks of storage showed less than 5% weight loss, whereas, more than 15% weight loss was registered in control fruits after 3 weeks of storage. Thus it is quite evident from the data that hexanal formulations are very beneficial to retain the freshness and preventing the shriveling which limits the postharvest quality of fruit. The exogenous application of hexanal might have facilitated in delaying the ripening and reducing the physiological loss in weight due to slow down of activity of lipogenases present in the skin of the fruits. Tiwari and Paliyath (2011) reported that the cell structure and cell membrane integrity might have preserved due to reduced catabolic processes with the application of the hexanal formulations.
Spoilage
Mango fruits are highly perishable owing to the rapid ripening and attack by various pathogens. Decay incidence to the tune of 3.29% in untreated mango fruits was observed after 7 days of storage and thereafter increased sharply up to 53.71% with progressive advancement in the storage time (Fig. 1c). Fruits treated with Hexanal @ 1600 µM and 2000 µM recorded a negligible decay incidence score of 1.29% and 1.22%, respectively after 28 days of storage period. Hexanal along with other constituents of Enhanced Freshness Formulations might have helped in maintaining the structure of the membrane thereby shielding from the oxidative damage. Hexanal treated Apples @ 0.01% v/v or 0.02% v/v showed reduced oxidative stress induced physiological disorders (Paliyath and Subramanian 2008). Song et al (2010) reported decay reductions of up to 70% in blueberries with Hexanal vapor treatment prior to prolonged storage periods. Pre-harvest sprays of hexanal have been reported by Anusuya et al (2016) to help in generating a significant benefit in terms of economics to the mango farmers by enhancing the on tree retention of mango fruits for a period of about two to three weeks.
Organoleptic rating
Organoleptic score increased up till 14 days of storage period in mango fruits treated with different doses of hexanal, and subsequently, the sensory scores declined gradually (Fig. 1d) with the extension of storage period. The untreated mango fruits displayed maximum organoleptic score (7.45) after 1 week under cold storage. These fruits were subsequently rated desirable up till a storage period of 14 days, and thereafter evaluated as not suitable for consumption with the increase in storage period. However, hexanal treated fruits @ 1600 µM and 2000 µM up to 28 days of storage were assessed as moderately desirable with the organoleptic rating of 7.12 and 7.25, respectively (Fig. 1d). The initially enhanced palatability rating of mango fruits after 1 week in storage could be attributed to the increase in the sugars:acid ratio as a result of breakdown of starch and other complex sugars into simple sugars and the utilizations of acids leading to the improvement in flavor and taste. However, panel of judges was unable to establish a significant difference in odour between the treatment and control samples which was probably due to the fact that the components of EFF are mostly naturally occurring compounds. Also, antioxidants such as alpha-tocopherol, ascorbic acid and combinations of the two are somewhat effective in preventing oxidative off-flavour development. Paliyath and Subramanian (2008) also reported significant enhancement in the quality of fruits such as cherry, banana, apple, strawberry and pear in terms of different biochemical parameters with Enhanced Freshness Formulation treatments.
Total soluble solids
Total soluble solids (TSS) in hexanal treated mango fruits showed a gradual increase up to 21 days of storage, and the levels reduced thereafter with the progressive advancement in storage intervals (Table 1). Among the different hexanal concentrations, highest total soluble content was observed in hexanal treated fruits @ 1600 µM (21.02%) closely followed by 2000 µM (20.94%), respectively. After 14 days of low temperature storage, highest TSS (21.26%) content was noticed in untreated mango fruits followed by a sharp reduction in levels with the increase in storage period. The hydrolysis of starch into simple sugars might be a contributing factor for the initial rise in TSS content during storage. After the total hydrolysis of starch, no further enhancement in sugar levels occur due to the metabolization of sugars in respiration process and eventually the TSS content decreases (Wills et al. 1980). The above results are consistent with previous work studying the effect of hexanal on fruits such as nectarine, raspberry, mango and guava (Gill et al. 2016; Kumar et al. 2018; El Kayal et al. 2017; Anusuya et al. 2016).
Table 1.
Effect of pre-harvest sprays of aqueous formulations of hexanal and storage intervals (stored at 12 ± 2 °C) on quality parameters of mango fruit cv. ‘Dusheri’. Values are means of 2 years
| Treatments | Storage Interval (days) | Mean ± SE | |||||
|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | ||
| TSS (%) | |||||||
| Hexanal @ 800 µM | 9.3 | 19.53 | 20.84 | 20.01 | 19.47 | 16.86 | 17.67 ± 1.76a |
| Hexanal @ 1200 µM | 8.9 | 19.24 | 20.69 | 20.12 | 19.06 | 17.14 | 17.53 ± 1.79a |
| Hexanal @ 1600 µM | 8.2 | 18.57 | 20.42 | 21.02 | 20.53 | 18.41 | 17.86 ± 1.98a |
| Hexanal @ 2000 µM | 7.8 | 18.58 | 20.33 | 20.94 | 20.48 | 18.39 | 17.75 ± 2.04a |
| Control | 9.8 | 18.39 | 21.26 | 19.54 | 17.61 | 14.07 | 16.78 ± 1.7b |
| Mean ± SE | 8.8 ± 0.36e | 18.86 ± 0.22c | 20.71 ± 0.17a | 20.33 ± 0.28a | 19.43 ± 0.54b | 16.97 ± 0.79d | |
| CD (p ≤ 0.05%) Interaction T*SI = 0.92 | |||||||
| Acidity (%) | |||||||
| Hexanal @ 800 µM | 1.68 | 0.32 | 0.28 | 0.22 | 0.19 | 0.13 | 0.47 ± 0.24 cd |
| Hexanal @ 1200 µM | 1.77 | 0.34 | 0.30 | 0.25 | 0.18 | 0.15 | 0.5 ± 0.26bc |
| Hexanal @ 1600 µM | 1.83 | 0.36 | 0.31 | 0.27 | 0.21 | 0.17 | 0.53 ± 0.26b |
| Hexanal @ 2000 µM | 2.12 | 0.37 | 0.32 | 0.28 | 0.22 | 0.17 | 0.58 ± 0.31a |
| Control | 1.54 | 0.31 | 0.26 | 0.21 | 0.17 | 0.11 | 0.43 ± 0.22d |
| Mean ± SE | 1.79 ± 0.1a | 0.34 ± 0.01b | 0.29 ± 0.01c | 0.25 ± 0.01d | 0.19 ± 0.01e | 0.15 ± 0.01f | |
| CD (p ≤ 0.05%) Interaction T*SI = 0.13 | |||||||
| Total Sugars (%) | |||||||
| Hexanal @ 800 µM | 8.43 | 12.82 | 13.47 | 15.12 | 14.87 | 13.68 | 13.07 ± 0.99a |
| Hexanal @ 1200 µM | 8.27 | 12.54 | 13.18 | 14.05 | 15.08 | 14.94 | 13.01 ± 1.03a |
| Hexanal @ 1600 µM | 7.86 | 12.41 | 13.03 | 13.94 | 15.12 | 15.04 | 12.9 ± 1.1ab |
| Hexanal @ 2000 µM | 7.45 | 12.18 | 12.97 | 13.59 | 14.98 | 15.12 | 12.7 ± 1.15bc |
| Control | 8.76 | 12.96 | 13.88 | 15.47 | 13.75 | 10.41 | 12.54 ± 1.01c |
| Mean ± SE | 8.15 ± 0.23f | 12.58 ± 0.14e | 13.31 ± 0.17d | 14.43 ± 0.36c | 14.76 ± 0.26b | 13.84 ± 0.9a | |
| CD (p ≤ 0.05%) Interaction T*SI = 0.57 | |||||||
| PME Activity (Micro Eq/ml/min) | |||||||
| Hexanal @ 800 µM | 0.19 | 0.36 | 0.41 | 0.53 | 0.49 | 0.46 | 0.41 ± 0.05b |
| Hexanal @ 1200 µM | 0.20 | 0.28 | 0.32 | 0.38 | 0.55 | 0.48 | 0.36 ± 0.03a |
| Hexanal @ 1600 µM | 0.12 | 0.24 | 0.29 | 0.34 | 0.54 | 0.47 | 0.33 ± 0.06c |
| Hexanal @ 2000 µM | 0.10 | 0.23 | 0.27 | 0.34 | 0.52 | 0.50 | 0.33 ± 0.07c |
| Control | 0.23 | 0.48 | 0.52 | 0.48 | 0.37 | 0.29 | 0.4 ± 0.05b |
| Mean ± SE | 0.17 ± 0.2c | 0.32 ± 0.05d | 0.36 ± 0.05c | 0.41 ± 0.04b | 0.49 ± 0.03a | 0.44 ± 0.04b | |
| CD (p ≤ 0.05%) Interaction T*SI = 0.12 | |||||||
Means with the same letter are not significantly different (at p ≤ 0.05)
Acidity
Acid content in mango fruits under all the treatments showed a decreasing trend during the low temperature storage (Table 1). Though, considerably higher levels of acidity was maintained in hexanal treated fruits as compared to untreated mango fruits but a significant difference in acidity among the different hexanal concentrations was not observed. Control fruits exhibited a prompt decline in acid levels, as compared to Hexanal treated fruits. The lowest acidity levels to the tune of 0.11% were recorded in control fruits after a stipulated storage period of 35 days. This decline in acid levels during storage of mango fruits could be ascribed to the utilization of acids as a substrate for the process of respiration (Echeverria and Valich 1989). Hexanal treated fruits retained higher acid content during storage, perhaps due to the curtailment of respiratory rate resulting in slow ripening of fruits. Similar conclusions have been drawn from the studies in cherries (Sharma et al. 2010) and guava (Gill et al. 2016) with hexanal sprays.
Total sugars
The total sugar content of mango fruits cv. Dashehari increased progressively with a corresponding increase in storage period but a drop in levels of total sugars in untreated and fruits treated with lowest concentration of hexanal was observed after 21 days of storage (Table 1). At the initiation of the low temperature storage, total sugars content in hexanal @ 1600 µM and 2000 µM was 7.86 and 7.45%, respectively and reached the maximum value of 15.04 and 15.12% at the termination of storage studies. The hexanal treated fruits maintained slightly lower values in comparison to control fruits which recorded significantly higher total sugar content (15.47%) after storage period of 21 days and thereafter the sugar levels dropped upto 10.41% after 35 days of storage. However, the fruits treated with hexanal @ 1600 µM and 2000 µM registered significantly higher total sugar content than the control fruits after 35 days of the storage. Higher initial increase in sugars noticed in control fruits with a subsequent reduction towards the end of storage period could be ascribed to the disintegration of complex sugar polymers into simple sugars by hydrolytic enzymes which might be further metabolized during subsequent storage period. Thus the present studies confirm that the hexanal treated fruits registered higher total sugars as compared to untreated fruits at the end of the stipulated storage period thereby indicating better quality traits and an overall enhancement in quality of fruits sprayed with hexanal formulation (Anusuya et al. 2016; Gill et al. 2016).
Pectin methyl esterase activity (PME)
PME activity during storage of mango fruits showed a lower initial activity during storage but increased steadily up to ripe stage and finally recorded a declining trend at full ripe stage in all the treatments (Table 1). Even at beginning of storage the Hexanal treated fruit exhibited lower PME activity than the control. At 14 days of storage, peak Pectin methyl esterase activity was noticed in untreated mango fruits and thereafter reported an abrupt decline coinciding with the sharp decline in firmness of the fruits. Hexanal@ 1600 µM and 2000 µM treated fruits showed a slow increase in Pectin Methyl Esterase activity showing a peak after 28 days of storage to the tune of 0.54 and 0.52 micro Eq ml−1 min−1, respectively and declined thereafter. Lower doses of hexanal treated fruits exhibited almost a similar pattern of increase in enzyme activity corresponding with the peak activity occurring at 21 days of storage. Pectin Methyl Esterase catalyzes the process of de-esterification of pectin resulting in short de-methylated pectin chains which are liable to pectin depolymerase assisted degradation resulting in major changes in cell walls of fruits, leading to softening (Vu et al. 2004). The treatment of tomato fruits with hexanal has shown to down regulate the transcript levels of Pectin Methyl Esterase enzyme (Tiwari and Paliyath 2011). A decline in Pectin Methyl Estrase with hexanal treatment along with a decline in depolymerase, leads to decrease in pectin content resulting in enhanced firmness during storage. Pectin methyl esterase enzyme activity is certainly related with ripening of mango fruit (Kumari et al. 2017). However, the degradation of pectin as well as hemicelluloses directly influences the degree of fruit softening, which ultimately results in weakening of cell walls.
Thus it was concluded that the enhanced freshness formulation with hexanal @1600 µM as pre-harvest sprays extends the storage life of mango fruits for a period of 28 days with excellent quality under low temperature storage conditions. The Pectin methyl esterase (PME) enzyme activity which is primarily associated with fruit softening was significantly reduced with hexanal treatment.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amerine MA, Pangborn RM, Roessler EB. Principles of sensory evaluation of food. London: Academic; 1965. p. 5. [Google Scholar]
- Anonymous (2017) Indian Horticulture Data Base. National Horticulture Board, Ministry of Agriculture Government of India. www.nhb.gov.in
- Anusuya P, Nagaraja R, Janavia JG, Subramaniana KS, Paliyath G, Subramanian J. Pre-harvest sprays of hexanal formulation for extending retention and shelf-life of mango (Mangifera indica L.) fruits. Sci Hort. 2016;211:231–240. doi: 10.1016/j.scienta.2016.08.020. [DOI] [Google Scholar]
- AOAC . Official methods of analysis, Association of official analytical chemists. 15. Washington, DC: Benjamin Franklin Station; 2000. [Google Scholar]
- Balaban MO, Arreola AG, Marshall M, Peplow A, Wei CI, Cornel J. Inactivation of pectin esterase in orange juice by supercritical carbon dioxide. J Food Sci. 1991;56:743–746. doi: 10.1111/j.1365-2621.1991.tb05372.x. [DOI] [Google Scholar]
- Burg SP, Burg EA. Ethylene action and the ripening of fruits. Science. 1965;148:1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Cheema A, Padmanabhan P, Subramanian J, Blom T, Paliyath G. Improving quality of greenhouse tomato (Solanum lycopersicum L.) by pre- and postharvest applications of hexanal-containing formulations. Postharvest Biol Tech. 2014;95:13–19. doi: 10.1016/j.postharvbio.2014.03.012. [DOI] [Google Scholar]
- Denes JM, Baron A, Drilleau JF. Purification, properties and heat inactivation of pectin methyl esterase from apple (cv Golden Delicious) J Sci Food Agric. 2000;80:503–509. doi: 10.1002/1097-0010(200008)80:10<1503::AID-JSFA676>3.0.CO;2-U. [DOI] [Google Scholar]
- Echeverria E, Valich J. Enzymes of sugar and acid metabolism in stored "Valencia" oranges. J Am Soc Hort Sci. 1989;114:445–449. [Google Scholar]
- El Kayal W, El-Sharkawy I, Dowling C, Paliyath G, Sullivan JA, Subramanian J. Effect of pre-harvest application of hexanal and growth regulators in enhancing shelf life and regulation of membrane-associated genes in strawberry. Can J Plant Sci. 2017;97:1109–1120. [Google Scholar]
- Gill KS, Dhaliwal HS, Mahajan BVC, Paliyath G, Boora RS. Enhancing postharvest shelf life and quality of guava (Psidium guajava L.) cv. Allahabad Safeda by pre-harvest application of hexanal containing aqueous formulation. Postharvest Biol Tech. 2016;112:224–232. doi: 10.1016/j.postharvbio.2015.09.010. [DOI] [Google Scholar]
- Herianus JD, Singh LZ, Tan SC. Aroma volatiles production during fruit ripening of Kensington Pride mango. Postharvest Biol Technol. 2003;27:323–336. doi: 10.1016/S0925-5214(02)00117-5. [DOI] [Google Scholar]
- James SJ, James C. The food cold-chain and climate change. Food Res Int. 2010;43(7):1944–1956. doi: 10.1016/j.foodres.2010.02.001. [DOI] [Google Scholar]
- Kumar SK, El Kayal W, Sullivan JA, Paliyath G, Jayasankar S. Pre-harvest application of hexanal formulation enhances shelf life and quality of ‘Fantasia’ nectarines by regulating membrane and cell wall catabolism-associated genes. Sci Hort. 2018;229:117–124. doi: 10.1016/j.scienta.2017.10.031. [DOI] [Google Scholar]
- Kumari P, Pilania S, Sarolia DK, Singh V, Mahawer LN, Gupta S. Influence of ripening agent(s) on post-harvest physiology and enzymatic activity of Mango (Mangifera indica) cv. Dashehari. Int J Curr Microbiol App Sci. 2017;6:3915–3920. doi: 10.20546/ijcmas.2017.612.452. [DOI] [Google Scholar]
- Mahadevan A, Sridhar R. Methods in physiological plant pathology. Madras, India: Sivagami Pub; 1982. [Google Scholar]
- Mari M, Martini C, Spadoni A, Rouissi W, Bertolini P. Bio control of apple posthar-vest decay by Aureobasidium pullulans. Postharvest Biol Tech. 2012;73:56–62. doi: 10.1016/j.postharvbio.2012.05.014. [DOI] [Google Scholar]
- Padda MS, Amarante CVT, Garcia RM, Slaughter DC, Mitcham EJ. Methods to analyze physico-chemical changes during mango ripening: a multivariate approach. Postharvest Biol Tech. 2011;62:267–274. doi: 10.1016/j.postharvbio.2011.06.002. [DOI] [Google Scholar]
- Paliyath G, Murr DP (2007) Compositions for the preservation of fruits and vegetables. US patent # 7,198,811B2.
- Paliyath G, Subramanian J. Phospholipase D inhibition technology for enhancing shelf life and quality. In: Paliyath G, Murr DP, Handa AK, Lurie S, editors. Postharvest biology and technology of fruits, vegetables, and flowers. 1. USA: Wiley-Blackwell; 2008. pp. 240–245. [Google Scholar]
- Sharma M, Jacob JK, Subramanian J, Paliyath G. Hexanal and 1–MCP treatments for enhancing the shelf-life and quality of sweet cherry (Prunus avium L.) Sci Hort. 2010;125:239–247. doi: 10.1016/j.scienta.2010.03.020. [DOI] [Google Scholar]
- Singh SP, Singh Z. Postharvest oxidative behaviour of 1-methylcyclopropene treated Japanese plums (Prunus salicina Lindell) during storage under controlled and modified atmospheres. Postharvest Biol Technol. 2012;74:26–35. doi: 10.1016/j.postharvbio.2012.06.012. [DOI] [Google Scholar]
- Song J, Fan L, Forney C, Campbell-Palmer L, Fillmore S. Effect of hexanal vapor to control postharvest decay and extend shelf-life of high bush blueberry fruit during controlled atmosphere storage. Can J Plant Sci. 2010;90:359–366. doi: 10.4141/CJPS09135. [DOI] [Google Scholar]
- Srinivas RN, Reddy TV, Ravi PC, Reddy BVC. Postharvest loss assessment of ‘Totapuri’ and ‘Alphonso’ mangoes. J Food Sci Tech. 1996;34:70–72. [Google Scholar]
- Tiwari K, Paliyath G. Micro array analysis of ripening regulated gene expression and its modulation by 1-MCP and hexanal. Plant Physiol Biochem. 2011;49:329–340. doi: 10.1016/j.plaphy.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Vu TS, Smout C, Sila DN, LyNguyen B, VanLoey AML, Hendrickx MEG. Effect of preheating on thermal degradation kinetics of carrot texture. Innov Food Sci Emerg Technol. 2004;5:37–44. doi: 10.1016/j.ifset.2003.08.005. [DOI] [Google Scholar]
- Wills RBH, Cambridge PA, Scott KJ. Use of flesh firmness and other objective tests to determine consumer acceptability of delicious apples. Aust J Exp Agric Animal Husb. 1980;20:252–256. doi: 10.1071/EA9800252. [DOI] [Google Scholar]