Abstract
Oil content of almond kernels ranged from 36.7% in the cultivar T12 to 79.0% in genotype T27. The major fatty acid in almond oil is oleic (62.43% in T7-76.34% in T4) followed by linoleic (13.97% in T4-29.55% in T3) and palmitic (4.97% in T2-7.51% inT3). The main tocopherol in almond oil was α-tocopherol (44.25 mg/100 g in T25-75.56 mg/100 g in T13) that was 44 folds higher than other tocopherols in the oil. Total tocopherol contents of almond oils ranged between 47.42 mg/100 g (T14) and 80.15 mg/100 g (T16). Among macro minerals, K was the highest (5238–14,683 mg/kg), followed by P (3475–11,123 mgkg), Ca (1798–5946 mg/kg), and Mg (2192–3591 mg/kg), whereas Na was the least (334–786 mg/kg) in almond kernel. The total polyphenol was observed in T16 (98.67 mg GAE/100 g), while the least was found in T24 (23.75 mg GAE/100 g). Antioxidant activity was high in T7 (91.18%) and low in T12 (44.59%).
Keywords: Almond cultivars, Almond oil, Fatty acid, Tocopherol, Mineral, Total phenol, Antioxidant activity, GC-FID, HPLC, ICP-AES
Introduction
Almonds (Prunus amygdalus L.) belong to the Rosaceae family and Prunus genus and can be found in temperate environment around the globe (Moayedi et al. 2011; Izaddost et al. 2013; Čolić et al. 2019). The almonds have numerous nutritional benefits as well as industrial and medicinal importance as they contain good quality oil, protein, fiber, mineral and bioactive compounds. The consumption of nuts is greatly increased globally due to their high health benefits. Among nuts, almonds received great attention around the globe due to their wide food, medicinal, and cosmetic applications. They are frequently used as ingredients in the manufacture of snacks and other processed foods. However, the oil content and composition of food ingredients such as almond in confectionary industry are very important because high oil content reduce the water absorption and affect the product quality. Nutritionally almonds are considered as excellent sources of energy, fats, proteins, essential minerals, carbohydrates, unsaturated fatty acid (linoleic acid and γ-linoleic acids), phenolic compounds and vitamins (Welna et al. 2008). There is lot of work underway to improve the genetic, agronomic and climate adoptability features of almonds. However, the biochemical composition of newly developed almond genotypes yet needed to be explored (Moayedi et al. 2011; Kodad et al. 2008). The ratio of oleic acid to linoleic acid is suggested to be used as a tool for highlighting oil stability and fatty acid profiling of the almond kernels (Kodad et al. 2008). The objective of the current study was to investigate effect of almond genotypes on fatty acid composition, tocopherols and mineral contents and bioactive properties of and oils of thirty-one different sweet almond (Prunus amygdalus Batsch spp. dulce) growing in the Mersin (Büyükeceli-Gülnar) district of Turkey.
Materials and methods
Material
Almond fruits of thirtyone genotypes were collected during the full maturated stage from the Mersin (Büyükeceli-Gülnar) province in Turkey during 2018 season. Precipitation was about 702 mm per year and maximum air temperature around 28–30 °C during the summer months. In 2018, after almond genotypes were selected, almond genotypes were marked as the native population. Almond fruits from each genotype were harvested on September, and samples were collected from each genotype for analysis. About 25 kg almond were collected from each tree. The fruits were dried at 70 °C in hot air oven. The kernels were ground into fine homogeneous powder, and collected in dark colored bottles. Samples were stored at refrigerated temperature (< 5 °C) until analysis.
Methods
Oil content
The hot extraction method using Soxhlet apparatus was used to extract oil from almond seeds. Briefly, 2 g powder was extracted using petroleum ether for 6 h. After the extraction, the solvent was evaporated from the oil at 40 °C and the obtained oil was stored in vials at refrigerated temperature until used for analysis.
Fatty acid composition of almond oil
The fatty acid profile of oil samples extracted from 31 almond genotypes was determined using a gas chromatography system. The system consisted of a CP-Sil 88 capillary column (100 m long, 0.25 mm ID, and film thickness 0.2 μm) attached to a Varian 5890 gas chromatograph. The fatty acid methyl esters of the samples were injected into this system at 250 °C (Matthäus and Özcan 2006) and the individual fatty acid methyl esters were identified by comparing with the retention time of their respective ester standards.
Tocopherol contents in almond oil
Tocopherol contents in oil samples were determined using HPLC system following the method described earlier (Balz et al. 1992). The oil samples (250 mg) were mixed with 20 mL n-heptane. After that, 20 μL of this mixture was directly injected to a Diol phase HPLC column 25 cm × 4.6 mmID (Merck, Darmstadt, Germany) maintaining 1.3 mL/min flow rate.
Mineral contents in almond kernels
The dried almond powder (0.5 g) was digested by using a mixture of 65% HNO3 (5 ml) and 35% H2O2 (2 ml) in a closed system (Cem-MARS Xpress). The digested mixture was then transferred to a volumetric flask and the volume was raised to 20 ml with deionized water. The diluted samples was injected to ICP AES (Varian-Vista, Australia) and the estimation of a particular mineral content in the sample was made by comparing them with the reference mineral solutions provided by the National Institute of Standard and Technology (NIST; Gaithersburg, MD, USA) (Skujins 1998). The working conditions of ICP-AES used during the experiment were: RF Power kept at 0.7–1.5 kw (1.2–1.3 kw for axial); plasma gas flow rate (Ar) was maintained at 10.5–15 L/min (radial) 15 (axial); auxiliary gas flow rate (Ar) was kept at 1.5; the viewing height was 5–12 mm; the copy and reading time was set at 1–5 s (max. 60 s) and the copy time was kept at 3 s (max. 100 s).
Total phenolic content
Total phenolic contents of obtained almond extracts were found by using the Folin-Ciocalteu (FC) reagent as applied by Yoo et al. (2004) with some modifications. 1 ml of FC was added and mixed for five minutes. Following the addition of 10 mL of 7.5% Na2CO3 solution tubes were mixed and the final volume was completed to 25 ml with deionised water. At the end of 1 h, total phenol content was determined as 750 nm wavelength in spectrophotometer. The results were given as mg gallic acid equivalent (GAE)/100 g of fresh weight.
Antioxidant activity
The free radical scavenging activity of almond samples was determined using DPPH(1,1-diphenyl-2-picrylhydrazyl) according to Lee et al. (1998). The extract was mixed with 2 mL methanolic solution of DPPH. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min and the absorbance was recorded at 517 nm by using a spectrophotometer. All determinations were performed in triplicate.
Statistical analysis
All the treatments and measurements were replicated three times and the data was subjected to analysis of variance (ANOVA) using the MSTAT C software (Püskülcü and İkiz 1989). The results were presented as mean ± standard deviation and significance was considered at the probability level of 0.05. Principle component analysis was performed using MULTBIPLOT software as described previously (Vicente-Villardon 2010)
Results and discussion
The oil percentage and fatty acid composition of almond kernels collected from thirty-one genotype of naturally growing almond trees in Mersin province, Turkey are given in Table 1. The results showed high variations in oil contents and fatty acid profiles among almond genotype (P < 0.05). The highest oil content was noticed in the kernel of the cultivar T27 (79%) whereas the lowest value was observed in the cultivar T12 (36.7%). With exception of the genotype T1, T2, T3, T4, T5, T11, T12, T14, T16, and T18, the oil content of all other almond cultivars was higher than 50% suggesting that these almond genotypes are rich sources of vegetable oil. The commercial production of oil from Prunus genus is possibly due to its higher oil yields compared to other commercially used oilseeds such as rapeseed and sunflower seeds (Aşkın et al. 2007).The oil contents found in this study are comparable to those reported previously in different almond species (44.4% in A. Scoparia and 51.4% in A. dulcis (Moayedi et al. 2011). In another study, Kırbaşlar et al. (2012) reported that almond kernel contained 55.86% oil. In addition, Sathe et al. (2008) reported that almond kernels collected from 12 different locations contained 52.51–61.92% oil. Moreover, Martins et al. (2000) stated that the oil of 12 kinds of almond in Portugal ranged between 30.01 and 51%. The cultivation of almond genotypes is restricted to areas with hot and dry conditions where almond trees produce the highest yields of high-quality kernels (Rabadan et al. 2018). Rabadan et al. (2018) reported that although significant variability was reported in almond oils as a result of the crop year and the interaction between crop year and genotype. The variability in the content of oil in nuts was lower than for previous components; but, it was higher in almonds than walnuts and pistachios. The genotypes were considered for the nuts as the main source of variability for this parameter (Rabadan et al. 2019). Apparently the variation in oil contents between these studies could be attributed to the differences in almond genotype, growing conditions, and environmental factors. Zhu et al. (2015) stated that the humid depressed almond kernel lipid synthesis regardless of the imposed deficit treatment. In addition, in average season like that 2011/2012, with generally higher lipid content, moderate water deficiency was not detrimental to almond lipid content but severe deficit irrigation was (Zhu et al. 2015).
Table 1.
Oil content and fatty acid composition of different almond kernel and oils (%)
| Almonds | Oil | Palmitic | Palmitoleic | Stearic | Oleic | Vaccenic | Linoleic | Linolenic | Arachidic | Eicosenoic | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | 45.0 ± 1.13*l | 6.17 ± 0.11 g | 0.27 ± 0.01ı | 1.97 ± 0.17e | 63.15 ± 0.71i | 0.95 ± 0.05 g | 27.49 ± 0.37b | –*** | – | – | 100.0 |
| T2 | 43.1 ± 0.98n** | 4.97 ± 0.07i | 0.26 ± 0.03ı | 1.46 ± 0.21ı | 72.28 ± 1.16e | 1.43 ± 0.11b | 17.25 ± 0.61j | 1.42 ± 0.11a | 0.15 ± 0.03a | 0.28 ± 0.09a | 99.51 |
| T3 | 48.6 ± 0.62j | 7.51 ± 0.21a | 0.39 ± 0.05e | 1.56 ± 0.13 h | 58.89 ± 1.11 k | 1.31 ± 0.09c | 29.55 ± 0.43a | 0.20 ± 0.03b | 0.12 ± 0.01c | 0.12 ± 0.05b | 99.64 |
| T4 | 46.3 ± 0.76 k | 5.92 ± 0.14f | 0.37 ± 0.01f | 2.22 ± 0.24c | 76.34 ± 0.38a | 1.02 ± 0.07f | 13.97 ± 0.27n | 0.16 ± 0.01c | – | – | 100.00 |
| T5 | 46.7 ± 1.23 k | 5.37 ± 0.09ı | 0.38 ± 0.07f | 2.41 ± 0.19b | 74.51 ± 0.21c | 1.06 ± 0.03f | 15.76 ± 1.18 l | 0.11 ± 0.01e | 0.14 ± 0.01b | 0.11 ± 0.03c | 99.85 |
| T6 | 55.4 ± 1.17ef | 5.57 ± 0.07ı | 0.35 ± 0.01ef | 1.62 ± 0.09 h | 72.72 ± 0.56e | 1.14 ± 0.01e | 18.05 ± 0.98i | 0.14 ± 0.03d | 0.10 ± 0.01 | 0.11 ± 0.07c | 99.80 |
| T7 | 54.4 ± 1.21 g | 7.34 ± 0.03c | 0.46 ± 0.09d | 1.98 ± 0.11e | 62.43 ± 0.74j | 1.21 ± 0.09e | 26.36 ± 1.32c | 0.10 ± 0.01e | – | – | 99.89 |
| T8 | 55.8 ± 1.11ef | 6.64 ± 0.15f | 0.52 ± 0.11c | 1.58 ± 0.17 h | 71.62 ± 0.89f | 1.41 ± 0.07b | 17.64 ± 1.35j | 0.10 ± 0.03e | 0.08 ± 0.01 g | 0.08 ± 0.01f | 99.67 |
| T9 | 53.3 ± 1.09 h | 5.49 ± 0.11ı | 0.33 ± 0.01 g | 1.61 ± 0.07 h | 73.48 ± 0.81d | 1.12 ± 0.05e | 17.33 ± 0.67j | 0.10 ± 0.01e | 0.11 ± 0.03d | 0.10 ± 0.03d | 99.68 |
| T10 | 54.6 ± 2.56 g | 6.92 ± 0.07d | 0.35 ± 0.03 g | 1.63 ± 0.05 h | 66.75 ± 0.93 h | 1.16 ± 0.11e | 22.21 ± 0.86f | 0.07 ± 0.01 g | 0.10 ± 0.01e | 0.10 ± 0.01d | 99.29 |
| T11 | 44.3 ± 1.78 m | 6.64 ± 0.21f | 0.63 ± 0.13a | 2.01 ± 0.27d | 70.78 ± 0.67 g | 1.35 ± 0.17c | 17.85 ± 0.71j | 0.10 ± 0.03e | 0.11 ± 0.05d | 0.09 ± 0.01e | 99.56 |
| T12 | 36.7 ± 0.87ö | 7.49 ± 0.18ab | 0.46 ± 0.09d | 1.85 ± 0.18f | 62.88 ± 0.82j | 1.20 ± 0.19e | 25.60 ± 1.29d | 0.08 ± 0.01f | 0.09 ± 0.01f | 0.07 ± 0.01 g | 99.71 |
| T13 | 55.6 ± 1.61ef | 6.52 ± 0.15f | 0.30 ± 0.07 g | 2.32 ± 0.21b | 63.93 ± 0.98i | 0.91 ± 0.03 g | 25.55 ± 0.89d | 0.08 ± 0.01f | 0.12 ± 0.03c | 0.08 ± 0.01f | 99.80 |
| T14 | 44.5 ± 0.55 m | 5.44 ± 0.21ı | 0.39 ± 0.03e | 1.89 ± 0.11f | 75.49 ± 0.54b | 1.10 ± 0.07e | 14.82 ± 0.75 m | – | 0.09 ± 0.01f | 0.09 ± 0.03e | 99.31 |
| T15 | 57.3 ± 0.61d | 6.06 ± 0.16 g | 0.34 ± 0.01 g | 1.57 ± 0.13 h | 72.60 ± 0.39e | 1.13 ± 0.13e | 18.03 ± 0.68i | – | 0.10 ± 0.03e | 0.10 ± 0.05d | 99.92 |
| T16 | 44.5 ± 0.83 m | 5.85 ± 0.09hf | 0.33 ± 0.05 g | 1.76 ± 0.32 g | 65.68 ± 0.43ı | 1.12 ± 0.15e | 24.77 ± 1.27e | 0.14 ± 0.05d | 0.10 ± 0.03e | 0.10 ± 0.01d | 99.85 |
| T17 | 53.2 ± 0.78 h | 5.73 ± 0.07ı | 0.35 ± 0.07 g | 2.26 ± 0.21c | 71.78 ± 0.48f | 0.95 ± 0.03 g | 18.49 ± 1.13i | 0.08 ± 0.03f | 0.11 ± 0.07d | 0.08 ± 0.01f | 99.84 |
| T18 | 44.4 ± 0.96 m | 6.67 ± 0.14e | 0.52 ± 0.09c | 1.74 ± 0.27 g | 69.19 ± 0.51 h | 1.28 ± 0.07d | 20.33 ± 1.19 h | – | 0.11 ± 0.01d | 0.08 ± 0.03f | 99.90 |
| T19 | 59.7 ± 1.33d | 6.58 ± 0.21e | 0.50 ± 0.05c | 1.89 ± 0.18f | 69.01 ± 0.37 h | 1.28 ± 0.03d | 20.42 ± 1.32 h | 0.06 ± 0.01 g | 0.09 ± 0.03f | 0.08 ± 0.03f | 99.91 |
| T20 | 63.5 ± 1.27c | 6.21 ± 0.17 g | 0.52 ± 0.03c | 2.09 ± 0.21d | 67.42 ± 0.73 g | 1.27 ± 0.05d | 22.23 ± 1.56f | – | 0.10 ± 0.05e | 0.08 ± 0.03f | 99.92 |
| T21 | 56.1 ± 1.16e | 5.37 ± 0.13ı | 0.41 ± 0.07d | 2.08 ± 0.13d | 74.14 ± 0.69c | 1.06 ± 0.01f | 16.65 ± 1.63 k | – | 0.11 ± 0.01d | 0.09 ± 0.01e | 99.91 |
| T22 | 59.6 ± 1.18d | 6.90 ± 0.19d | 0.52 ± 0.13c | 2.02 ± 0.17d | 70.11 ± 0.81 g | 1.29 ± 0.07d | 18.86 ± 0.58i | – | 0.12 ± 0.03c | 0.08 ± 0.03f | 99.90 |
| T23 | 52.1 ± 1.56ı | 5.31 ± 0.22ı | 0.29 ± 0.03 h | 1.80 ± 0.19f | 74.23 ± 0.48c | 0.97 ± 0.03d | 17.13 ± 0.27j | – | 0.09 ± 0.01f | 0.09 ± 0.01e | 99.91 |
| T24 | 50.2 ± 0.77i | 7.37 ± 0.05c | 0.63 ± 0.11a | 1.09 ± 0.07i | 68.07 ± 0.54f | 1.57 ± 0.09a | 20.90 ± 0.38 h | 0.08 ± 0.01f | 0.11 ± 0.03d | 0.09 ± 0.03e | 99.90 |
| T25 | 52.6 ± 0.68ı | 7.12 ± 0.03 cd | 0.55 ± 0.03b | 2.72 ± 0.05a | 62.83 ± 0.72j | 1.19 ± 0.03d | 25.41 ± 0.46d | – | 0.11 ± 0.01d | – | 99.92 |
| T26 | 56.7 ± 0.94e | 5.91 ± 0.17 h | 0.31 ± 0.05 g | 2.25 ± 0.03c | 69.29 ± 0.84 h | 0.93 ± 0.07 g | 21.30 ± 0.75 g | – | – | – | 100.00 |
| T27 | 79.0 ± 2.71a | 6.91 ± 0.32d | 0.48 ± 0.01d | 1.99 ± 0.11e | 69.74 ± 0.43 h | 1.24 ± 0.05d | 19.53 ± 0.36ı | – | 0.11 ± 0.03d | – | 100.00 |
| T28 | 54.8 ± 1.32 g | 6.93 ± 0.18d | 0.60 ± 0.13ab | 1.70 ± 0.09 g | 63.78 ± 0.48i | 1.41 ± 0.06b | 25.34 ± 1.43d | – | 0.07 ± 0.01 h | 0.07 ± 0.01 g | 99.91 |
| T29 | 53.8 ± 1.19 h | 7.01 ± 0.34 cd | 0.46 ± 0.03d | 2.50 ± 0.21b | 64.58 ± 0.51i | 1.09 ± 0.03f | 24.07 ± 1.38e | – | 0.13 ± 0.05c | 0.08 ± 0.01f | 99.92 |
| T30 | 67.4 ± 2.58b | 5.97 ± 0.13 h | 0.42 ± 0.03d | 1.78 ± 0.27 g | 71.25 ± 0.67f | 1.21 ± 0.07d | 19.01 ± 0.85ı | 0.05 ± 0.01 h | 0.11 ± 0.01d | 0.10 ± 0.03d | 99.91 |
| T31 | 57.6 ± 1.53d | 5.85 ± 0.09hf | 0.37 ± 0.01 g | 1.66 ± 0.18 h | 73.36 ± 0.86d | 1.13 ± 0.09e | 17.25 ± 0.77j | 0.08 ± 0.01f | 0.11 ± 0.03d | 0.10 ± 0.01d | 99.91 |
*Standard deviation; **values within each column followed by different letters are significantly different at p < 0.05; ***nonidentified
The fatty acids profiles also differed significantly (p < 0.05) among the almond genotype. The major fatty acid in almond genotypes was oleic (62.43% in T8 to 76.34% in T4), followed by linoleic (13.97% in T4 to 29.55% in T3), and palmitic (4.97% in T2 to 7.51 in T3), whereas the lowest proportion was found for arachidic acid (0.07% in T28 to 0.15% in T2). The palmitic, palmitoleic, stearic, oleic, vaccenic, and linoleic acids were observed in all almond cultivars with different quantities, whereas, linolenic, arachidic and eicosenoic acids were not detected in some genotypes. These findings suggested that the fatty acid composition of almond oil depends mainly on the genotype as all these genotypes were collected from same location. Previous studies indicated that fatty acid composition of almond differed among genotype and growing environments. It was observed statistically significant differences among fatty acid compositions of almond kernel oils (p < 0.05). Kırbaşlar et al. (2012) studied the fatty acid compositions of the almond oil and found palmitic (0.39%), palmitoleic (0.56%), stearic (1.20%), oleic (71.98%), linoleic (20.37%) acids as the main fatty acids in almond oil. In addition, Sathe et al. (2008) reported that oleic acid content of eight almond genotype varied between 57.54% and 73.94%. The oleic acid content in almond oils in the current study was in agreement with those reported previously by Maguire et al. (2004) and Mehran and Filsoof (1974) for different types of commercially available almonds. Piscopo et al. (2010) reported the presence of higher oleic and lower linoleic acid in an almond variety in the month of August. The oleic acid content of some of the of almond species have been reported to be in between 66.7 and 69.7%, and the linoleic acid content has been detected in the range of 18.2–23.0% (Moayedi et al. 2011). Karatay et al. (2014) reported 74.46% oleic, 0.70% palmitoleic, 17.89% linoleic, 5.34% palmitic, 0.85% stearic and 0.75% linolenic acid as an average of a total of 32 different almond varieties. The presence of oleic (72.5–79.9%), linoleic (13.5–19.8%) and palmitic (5.9–6.7%) acids have been reported in different cultivars of almonds (Özcan et al. 2011). In another report about different cultivars of almond oils, oleic contents ranged between 50.41 and 81.20%, linoleic between 6.21 and 37.13%, palmitic between 5.46 and 15.78%, stearic 0.80 and 3.83% and palmitoleic between 0.36 and 2.52% (Aşkın et al. (2007). The oleic acid contents have been reported to be 60.9%, 62.9% and 61.0% in different studies carried out with domestically used almonds. Still higher oleic acid contents have been reported by Nanos et al. (2002) (72–80%) and Kodad et al. (2008) (69–78%) for domestically used kernels. Karatay et al. (2014) reported that average palmitic, palmitoleic, stearic, oleic, linoleic and linolenic acid amounts of 32 almond oils were found 5.34%, 0.70%, 0.85%, 74.46%, 17.89% and 0.75%, respectively. Rabadán et al. (2017) determined 5.87–7.64% palmitic, 0.54–0.86% palmitoleic, 1.97–3.21% stearic, 48.19–56.88% oil, 65.37–72.99% oleic, 16.90–23.54% linoleic acids in almond kernels. Zhu et al. (2015) determined 48.99–54.55% and 50.64–57.32% oil, 6.76–7.04% and 6.80–7.60% palmitic, 61.85–62.80% and 57.13–64.30% oleic and 25.32–26.09% and 24.03–30.23% linoleic acids in the oils of almonds collected in 2010/2011 and 2011/2012 harvest seasons, respectively. The almond oil fatty acid compositions do not vary widely such as for the results about A. scorparia almond oils as reported by Farhoosh and Tavakoli (2008). Different type of reports about oils obtained from almond kernels pointed out that the oleic and linoleic acids are the predominant fatty acids in almond oils. There is a wide variation in fatty acid composition of oils obtained from different almond seeds and the presence of some characteristic fatty acids can help in differentiating different plant species and their respective families (Aitzetmuller 1993). There are various factors that can affect the fatty acid composition of oils obtained from different almond seeds and these factors include species, variety, cultivation and climatic conditions, and the harvesting time and conditions (Kritsakis and Markakis 1984). Rabadán et al. (2017) reported that almond oil yield was similar for all the selected 10 cultivars, and significant differences were observed in fatty acid profile, including essential fatty acids of main nutritional interest. Linoleic acid contents illustrate high variability (13.97–29.55%), becoming a parameter of main nutritional interest as it is the main essential fatty acid found in almond oil. On the other hand, stearic and palmitic acids are the most common saturated fatty acids in almond oil, with percentages in our samples below 2.72 and 7.11%, respectively. Rabadán et al. (2017) reported that the nutritional value of almond oil is highly influenced by the high presence of unsaturated fatty acids and although all cultivars show a health fatty acid profile. Nanos et al. (2002) reported that oleic acid levels were higher in irrigated than non-irrigated almonds (Ferragnes and Texas cv).
The results in Table 2 show the tocopherol contents in 31 almond genotypes. The results showed significant variations in tocopherol contents and composition among almond genotype. The total tocopherols content was ranged between 47.42 mg/100 g in T14 and 80.15 mg/100 g in T16. Among all detected tocopherols, α-tocopherol was the highest (44.25 mg/100 g in T5 to 75.56 mg/100 g in T13), followed by γ-tocopherol (0.63 mg/100 g in T10 to 7.51 mg/100 g in T20). With few exceptions, most of the almond kernel oils were found to have more than 50.0% of the α-tocopherol contents. α-Tocotrienol contents of almond oils varied between 0.44 mg/100 g (T22) and 1.35 mg/100 g (T 28). β-tocopherol contents of almond oils were varied between 0.48 mg/100 g (T31) and 1.73 mg/100 g (T26). γ-Tocotrienol contents (0.23 and 0.46 mg/100 g) were found in only T17 and T27 almond oil, while δ-tocopherol (0.24 mg/100 g) was found only in T27 almond oil. It was observed statistically significant differences were observed among tocopherol contents of almond kernel oils (p < 0.05). Several researchers have also reported similar ranges of tocopherols in almond oils from different locations (Sathe et al. 2008; Moayedi et al. 2011; Gupta et al. 2012). The concentration of α-tocopherols was reported between 187 and 490 g/kg of almond oil (Kodad 2017). The range of variability for the different tocopherol homologues is of 335–657 mg/kg of almond oil for α-, 2–50 for γ-, and 0.1–22 for β-tocopherol (Kodad et al. 2018). Almond oil contained 97.3 mg/kg α-tocopherol and 2.8 mg/kg γ-tocopherol as reported by Fernandes et al. (2017). In another study by Lopez Ortiz et al. (2006), the α, γ and γ-tocopherol have been detected in the ranges of 23.5–44.9, 2.90–6.15 and 1.27–8.06 mg/100 g, respectively. Kornsteiner et al. (2006) reported that almond oil had 24.2 mg/100 g α-tocopherol content. According to Kodad et al. (2006), concentration of α-tocopherol in almond oil varied between 187 and 490 mg/kg of oil. The higher content of α- and γ-tocopherol contents in the almond oil could likely enhanced the oil resistance to oxidation because tocopherols can helps to protect the peroxidation of polyunsaturated fatty acids (Beringer and Dompert 1976; Kamal-Eldin and Andersson 1977). The oil products having higher amount of fatty acids and tocopherols are considered good from the health point of view (Oomah et al. 2000). Kodad et al. (2008) reported that environmental factors like temperature have significant effects on the concentration of tocopherols in almond. It is thought that higher concentration of tocopherols in almond oil helps its long term storage with no adverse effects on the quality of oil (Filsoof et al. 1976; Garcia-Pascual et al. 2003). One of the parameters greatly affected by genotype, crop year and the interaction of both was the energy value of nuts (Rabadan et al. 2019).
Table 2.
Tocopherol contents of different almond type kernel oils (mg/100 g)
| Almonds | α-tocopherol | α-tocotrienol | β-tocopherol | γ-tocopherol | β-tocotrienol | P8 | γ-tocotrienol | δ-tocopherol | Total |
|---|---|---|---|---|---|---|---|---|---|
| T1 | 56.94 ± 0.67*i | 0.52 ± 0.09ı | 0.74 ± 0.03 h | 3.33 ± 0.37c | 0.61 ± 0.03 g | – | – | – | 62.15 |
| T2 | 49.79 ± 0.81l** | 0.65 ± 0.05g | 0.82 ± 0.09g | 1.08 ± 0.19f | 2.94 ± 0.11a | – | – | – | 55.28 |
| T3 | 62.54 ± 0.47fg | 1.10 ± 0.13d | 0.87 ± 0.01g | 5.00 ± 0.21b | 2.68 ± 0.18b | 1.27 ± 0.13a | – | – | 73.46 |
| T4 | 47.18 ± 0.24m | 0.75 ± 0.09g | 0.90 ± 0.13f | 1.37 ± 0.15ef | 0.15 ± 0.03i | 0.38 ± 0.05 h | – | – | 50.74 |
| T5 | 44.25 ± 0.32n | 1.01 ± 0.07d | 1.12 ± 0.09e | 1.07 ± 0.09f | 0.32 ± 0.07ı | 0.50 ± 0.09f | – | – | 48.26 |
| T6 | 44.95 ± 0.51n | 0.45 ± 0.01i | 0.80 ± 0.07 g | 0.96 ± 0.13 g | 0.58 ± 0.09 h | – | – | – | 47.74 |
| T7 | 59.93 ± 0.63h | 0.66 ± 0.03g | 1.27 ± 0.03cd | 2.48 ± 0.32d | 2.28 ± 0.17c | – | – | – | 66.61 |
| T8 | 48.94 ± 0.88lm | 0.83 ± 0.11f | 1.26 ± 0.09 cd | 1.21 ± 0.19ef | 1.51 ± 0.21e | 0.31 ± 0.03ı | – | – | 54.06 |
| T9 | 50.68 ± 0.76kl | 0.53 ± 0.07 h | 0.91 ± 0.07f | 2.09 ± 0.27de | 1.82 ± 0.23d | – | – | – | 56.03 |
| T10 | 51.11 ± 0.62k | 0.69 ± 0.03g | 1.01 ± 0.03e | 0.63 ± 0.03h | 1.12 ± 0.18f | – | – | – | 54.56 |
| T11 | 60.58 ± 0.54g | 0.72 ± 0.09g | 1.33 ± 0.17c | 2.49 ± 0.11d | –*** | – | – | – | 65.12 |
| T12 | 68.63 ± 0.29e | 0.90 ± 0.12e | 1.23 ± 0.19cd | 2.55 ± 0.33d | – | – | – | – | 73.32 |
| T13 | 75.56 ± 0.31a | 0.68 ± 0.11g | 1.10 ± 0.03e | 2.31 ± 0.28d | – | 0.28 ± 0.01j | – | – | 79.92 |
| T14 | 44.72 ± 0.47n | 0.83 ± 0.17f | 0.84 ± 0.15g | 0.67 ± 0.09 h | – | 0.36 ± 0.03h | – | – | 47.42 |
| T15 | 69.84 ± 0.98dg | 0.80 ± 0.15f | 1.02 ± 0.09e | 3.02 ± 0.42c | – | – | – | – | 74.67 |
| T16 | 74.38 ± 0.77b | 1.15 ± 0.09c | 1.62 ± 0.21b | 2.31 ± 0.19d | – | 0.68 ± 0.05d | – | – | 80.15 |
| T17 | 60.19 ± 0.69g | 0.88 ± 0.13f | 1.20 ± 0.18 cd | 1.36 ± 0.21ef | – | 0.27 ± 0.09k | 0.23 ± 0.07b | – | 64.13 |
| T18 | 58.61 ± 0.81ıı | 0.58 ± 0.07 h | 1.28 ± 0.13cd | 1.62 ± 0.27e | – | – | – | – | 62.09 |
| T19 | 54.64 ± 0.95j | 0.95 ± 0.19e | 0.52 ± 0.09ı | 1.07 ± 0.09f | – | – | – | – | 57.19 |
| T20 | 56.34 ± 0.47i | 1.16 ± 0.07c | 1.23 ± 0.07cd | 7.51 ± 0.39a | – | 0.28 ± 0.03j | – | – | 66.81 |
| T21 | 54.19 ± 0.27j | 0.64 ± 0.03 g | 1.20 ± 0.03cd | 1.79 ± 0.25e | – | 0.22 ± 0.07l | – | – | 58.05 |
| T22 | 53.24 ± 0.13jk | 0.44 ± 0.03i | 1.50 ± 0.11b | 2.23 ± 0.17d | – | – | – | – | 57.40 |
| T23 | 61.34 ± 0.38g | 0.76 ± 0.09 g | 1.37 ± 0.09c | 1.00 ± 0.09f | – | 0.81 ± 0.09b | – | – | 65.28 |
| T24 | 57.08 ± 0.17i | 0.47 ± 0.01i | 0.96 ± 0.03f | 2.78 ± 0.18d | – | 0.73 ± 0.07c | – | – | 62.01 |
| T25 | 63.55 ± 0.21f | 0.67 ± 0.03 g | 0.64 ± 0.15ı | 5.23 ± 0.46b | – | 0.26 ± 0.03f | – | – | 70.34 |
| T26 | 70.69 ± 0.28c | 0.94 ± 0.15e | 1.73 ± 0.27a | 2.17 ± 0.38d | – | 0.50 ± 0.03f | – | – | 76.03 |
| T27 | 69.30 ± 0.43d | 1.18 ± 0.13b | 1.20 ± 0.18cd | 3.15 ± 0.23c | – | 0.45 ± 0.01g | 0.46 ± 0.03a | 0.24 ± 0.03 | 75.97 |
| T28 | 64.92 ± 0.86f | 1.35 ± 0.17a | 0.59 ± 0.07ı | 5.14 ± 0.38b | – | 0.62 ± 0.09e | – | – | 72.62 |
| T29 | 62.24 ± 0.84fg | 0.70 ± 0.09 g | 0.79 ± 0.11h | 0.83 ± 0.03g | – | 0.60 ± 0.07e | – | – | 65.16 |
| T30 | 45.62 ± 0.57mn | 0.54 ± 0.03 h | 0.68 ± 0.09ı | 1.55 ± 0.11e | – | 0.31 ± 0.01i | – | – | 48.70 |
| T31 | 45.59 ± 0.65mn | 0.58 ± 0.07 h | 0.48 ± 0.03i | 1.11 ± 0.07f | – | 0.50 ± 0.09f | – | – | 48.26 |
*Standard deviation; **values within each column followed by different letters are significantly different at p < 0.05; ***nonidentified
Mineral contents of 31 almond samples collected from Mersin province, Turkey are presented in Table 3. Significant variations in mineral contents and composition were observed among several almond genotype suggesting the influence of genetic makeup on the mineral composition of almond kernels. Noticeably, there are big variations in the macro and trace mineral content in almond genotypes. Among macro minerals, potassium (5238 mg/kg in T6–14,683 mg/kg in T27) represent the highest followed by phosphorous (3475 mg/kg in T31–11,123 mg/kg in T27), calcium (1798 mg/kg in T24–5946 mg/kg in T27) and magnesium (2192 mg/kg in T22–3591 mg/kg in T27), whereas sodium showed the lowest content (334 mg/kg in T18–786 mg/kg in T27). Among the trace minerals, zinc (32.9 mg/kg in T6–87.4 mg/kg in T27) was the major mineral in almond kernel, followed by iron (34.7 mg/kg in T5–83.5 mg/kg in T31), aluminum (7.0 mg/kg in T30–28.0 mg/kg in T11), copper (6.6 mg/kg in T31–26.6 mg/kg in T27), and manganese (6.2 mg/kg in T15–24.7 mg/kg in T27), while, chromium (0.19 mg/kg in T23–0.58 mg/kg in T27) was found as the least trace mineral in almond kernel. Interestingly, with view exceptions, the cultivar T27 outscores all other genotypes in quantity of most minerals indicating that the kernel of this cultivar is of great importance from nutritional stand point. It was observed statistically significant differences were observed among mineral contents of almond kernels (p < 0.05). Özcan et al. (2011) reported that some almond kernels contained 2.98-4.04 mg/g Mg, 0.29-0.38 mg/g Na, 7.93–9.38 mg/gP, 13.14–15.10 mg/g K, 1.83–2.94 mg/g Ca, 0.20–0.27 mg/g Fe and 0.04–0.06 mg/g Zn. In a previous study, almond kernels contained 1546–1685 mg/100 g K, 253–259 mg/100 g P, 640–678 mg/100 g Ca, 447–496 mg/100 g Mg, 24.30–25.80 mg/100 g Cu, 76.33–80.50 mg/100 g Zn, 54.83–65.33 mg/100 g Fe and 37.67–37.83 mg/100 g Mn (Barbara et al. 1994). Aslantas et al. (2001) reported values of 98.5-187.00 mg/100 g Ca, 360.8-513.4 mg/100 g Mg, 403.9-800 mg/100 g P, 1677.3-2051.1 mg/100 g K, 39.77-146.35 mg/100gFe, 77.86-88.44 mg/100 g Zn, 29.0-33.95 mg/100gMn, 16.0-23.0 mg/100 g Cu and 56.66-103.88 mg/100 g Na in selected almond cultivars naturally grown in Kemaliye district of Erzincan in Turkey. Schirra et al. (1994) determined 1050 mg/100 g K, 300 mg/100 g P, 467 mg/100 g Ca, 30 mg/100 g Mg, 5 ppm Cu 34 ppm Zn and 70 ppm Fe in Texas almonds during fruit growth and ripening. Significant differences were observed in the mineral composition of almonds by several researchers (Saura Calixto et al. 1981; Özcan et al. 2011). The difference in mineral contents and composition between the almond fruits may be due to the kind and genetic structure of the fruit, variety, genetic factors, ecological conditions and different ripening stages. In addition, harvesting time is generally affects the chemical contents of almond kernel. The current investigations showed that the almond kernels were found to be rich in most of the essential elements. The mineral elements present in the dry nuts have a pivotal role in human nutrition. The high levels of macro (Ca, K, P, Mg, and Na) and micro (Zn, Fe, Cu, and Mn) elements demonstrated that almond kernels in the current study could serve as excellent sources of these essential minerals in the human diet.
Table 3.
Mineral contents of different almond type kernels (mg/kg)
| Almonds | Al | Ca | B | Cr | Cu | Fe | K |
|---|---|---|---|---|---|---|---|
| T1 | 8.6 ± 0.3*k | 2485 ± 261 | 13.0 ± 0.2h | 0.34 ± 0.02f | 13.0 ± 1.3f | 41.0 ± 1.2 m | 7798 ± 169de |
| T2 | 12.7 ± 0.1g** | 2292 ± 140 | 13.9 ± 1.1h | 0.34 ± 0.05f | 13.2 ± 1.1f | 39.7 ± 1.6ö | 7574 ± 74ef |
| T3 | 12.5 ± 0.0g | 3940 ± 29 | 14.6 ± 0.5g | 0.33 ± 0.01f | 11.0 ± 0.0h | 45.7 ± 0.3j | 7692 ± 52e |
| T4 | 10.9 ± 0.9i | 2216 ± 134 | 19.1 ± 0.3c | 0.39 ± 0.02f | 11.2 ± 1.1h | 39.3 ± 2.8ö | 7402 ± 5f |
| T5 | 9.3 ± 0.9j | 3286 ± 150 | 17.1 ± 1.1d | 0.29 ± 0.01g | 13.0 ± 0.6f | 34.7 ± 2.2 s | 9324 ± 57b |
| T6 | 8.4 ± 1.0k | 2866 ± 186 | 12.4 ± 0.9ı | 0.36 ± 0.02f | 12.2 ± 1.4g | 40.3 ± 3.7n | 5238 ± 132 m |
| T7 | 8.8 ± 0.3k | 2585 ± 175 | 11.8 ± 0.0i | 0.47 ± 0.08c | 14.8 ± 0.8e | 41.5 ± 1.8 m | 7082 ± 43g |
| T8 | 8.8 ± 0.7k | 2148 ± 122 | 14.4 ± 0.3g | 0.32 ± 0.04f | 14.4 ± 0.6e | 37.4 ± 0.9p | 6777 ± 81g |
| T9 | 14.4 ± 0.4e | 2637 ± 22 | 16.9 ± 0.1e | 0.49 ± 0.11c | 17.0 ± 0.0b | 46.4 ± 1.4i | 6200 ± 1i |
| T10 | 16.7 ± 0.0c | 2612 ± 150 | 11.1 ± 0.4i | 0.51 ± 0.08b | 16.7 ± 0.1c | 64.8 ± 5.6d | 6336 ± 42ı |
| T11 | 28.0 ± 1.6a | 3092 ± 80 | 15.1 ± 1.0f | 0.46 ± 0.05d | 13.9 ± 0.5f | 42.7 ± 2.3l | 7124 ± 347 fg |
| T12 | 13.1 ± 0.8f | 2695 ± 236 | 20.3 ± 1.2b | 0.41 ± 0.07e | 11.8 ± 0.8h | 44.2 ± 1.7k | 5704 ± 350l |
| T13 | 13.2 ± 0.7f | 3307 ± 191 | 21.9 ± 0.9a | 0.41 ± 0.04e | 11.6 ± 0.7h | 39.0 ± 1.5ö | 6743 ± 307g |
| T14 | 9.3 ± 0.7j | 4252 ± 95 | 16.1 ± 0.8e | 0.37 ± 0.01f | 9.8 ± 0.4j | 39.2 ± 0.4ö | 7987 ± 282 cd |
| T15 | 12.4 ± 0.2g | 2021 ± 36 | 10.6 ± 0.4j | 0.34 ± 0.02f | 14.0 ± 0.2e | 45.6 ± 1.2j | 7167 ± 130 fg |
| T16 | 12.0 ± 1.2g | 3118 ± 206 | 6.8 ± 0.1l | 0.27 ± 0.02g | 12.9 ± 0.4g | 42.1 ± 1.2l | 6763 ± 74g |
| T17 | 14.2 ± 1.0e | 3490 ± 38 | 16.6 ± 0.1e | 0.40 ± 0.0e7 | 15.1 ± 0.3d | 48.1 ± 3.0g | 7613 ± 303e |
| T18 | 8.0 ± 1.1k | 3770 ± 120 | 12.8 ± 0.7ı | 0.21 ± 0.02ı | 10.4 ± 0.3i | 52.8 ± 5.3f | 8072 ± 194d |
| T19 | 12.6 ± 0.2g | 3739 ± 200 | 14.4 ± 0.7g | 0.45 ± 0.04c | 12.5 ± 1.1g | 47.2 ± 1.1h | 7929 ± 404 cd |
| T20 | 8.9 ± 0.1k | 2590 ± 3 | 11.0 ± 1.2i | 0.41 ± 0.02e | 15.2 ± 0.1d | 42.8 ± 1.8l | 6404 ± 91h |
| T21 | 15.1 ± 0.5d | 2946 ± 53 | 12.9 ± 0.0ı | 0.43 ± 0.04c | 9.4 ± 0.1j | 56.9 ± 2.0e | 6691 ± 104gh |
| T22 | 12.8 ± 1.1g | 2037 ± 144 | 4.6 ± 0.1n | 0.41 ± 0.01e | 10.5 ± 0.5i | 36.9 ± 4.0r | 7536 ± 34ef |
| T23 | 12.8 ± 0.2g | 2754 ± 37 | 6.1 ± 0.5l | 0.19 ± 0.01h | 13.7 ± 0.1f | 95.0 ± 1.2a | 6083 ± 71k |
| T24 | 10.7 ± 0.3i | 1798 ± 133 | 12.4 ± 1.0ı | 0.32 ± 0.03f | 15.9 ± 0.9d | 40.8 ± 0.1n | 6243 ± 14j |
| T25 | 17.8 ± 4.0b | 2504 ± 128 | 5.7 ± 0.7 m | 0.41 ± 0.07e | 13.7 ± 0.2f | 47.4 ± 2.3h | 9154 ± 130b |
| T26 | 17.3 ± 5.6b | 3493 ± 229 | 4.6 ± 0.6n | 0.40 ± 0.04e | 13.2 ± 0.1 | 47.9 ± 1.8h | 6744 ± 622g |
| T27 | 15.7 ± 0.1d | 5946 ± 21 | 15.0 ± 0.3f | 0.58 ± 0.02a | 26.6 ± 0.2a | 70.9 ± 0.2c | 14,683 ± 69a |
| T28 | 8.3 ± 1.0k | 2538 ± 33 | 10.2 ± 0.8 | 0.31 ± 0.04f | 17.5 ± 1.6b | 37.8 ± 4.1p | 7626 ± 487e |
| T29 | 11.6 ± 0.4h | 1906 ± 93 | 8.8 ± 0.5j | 0.38 ± 0.02f | 9.2 ± 0.8j | 44.0 ± 4.4k | 7539 ± 425ef |
| T30 | 7.0 ± 0.6l | 1970 ± 62 | 7.8 ± 0.4k | 0.27 ± 0.03g | 8.6 ± 0.0k | 37.4 ± 2.8p | 6254 ± 327j |
| T31 | 9.3 ± 0.1j | 2040 ± 17 | 7.0 ± 0.6k | 0.45 ± 0.04c | 6.6 ± 0.2l | 83.5 ± 4.1b | 8494 ± 562c |
| Almonds | Mg | Mn | Na | P | S | Zn |
|---|---|---|---|---|---|---|
| T1 | 2514 ± 40 | 11.4 ± 0.2f | 563 ± 7d | 4507 ± 42l | 1232 ± 23d | 51.1 ± 0.9b |
| T2 | 2502 ± 29b | 15.4 ± 1.0b | 632 ± 41c | 5621 ± 465c | 1338 ± 32c | 47.6 ± 0.8d |
| T3 | 2631 ± 15b | 14.9 ± 0.2c | 710 ± 5b | 5848 ± 77b | 1332 ± 1c | 48.3 ± 0.1 cd |
| T4 | 2572 ± 33b | 15.9 ± 0.1b | 530 ± 40d | 5185 ± 30h | 1378 ± 11c | 39.3 ± 2.1j |
| T5 | 2442 ± 8c | 15.3 ± 0.1b | 527 ± 17d | 5163 ± 437h | 1345 ± 11c | 37.1 ± 1.2k |
| T6 | 2338 ± 68d | 8.0 ± 0.5i | 555 ± 53d | 4458 ± 170 m | 975 ± 27g | 32.9 ± 2.9n |
| T7 | 2395 ± 59d | 6.9 ± 0.3k | 630 ± 42c | 5506 ± 101d | 871 ± 6j | 36.6 ± 0.1lm |
| T8 | 2473 ± 34c | 9.7 ± 0.2h | 489 ± 47e | 5975 ± 109a | 908 ± 21i | 37.8 ± 1.9k |
| T9 | 2424 ± 10c | 7.0 ± 0.1j | 527 ± 5d | 5118 ± 20h | 1210 ± 19 | 39.5 ± 0.2j |
| T10 | 2384 ± 3d | 10.1 ± 0.3g | 540 ± 23d | 4596 ± 229l | 1256 ± 46d | 37.7 ± 0.1k |
| T11 | 2279 ± 81e | 10.5 ± 0.2g | 556 ± 52d | 5517 ± 464d | 1113 ± 79e | 38.0 ± 1.4jk |
| T12 | 2546 ± 91b | 9.0 ± 0.0h | 535 ± 29d | 5098 ± 357i | 1221 ± 52d | 42.1 ± 1.7g |
| T13 | 2462 ± 70c | 10.9 ± 0.5g | 539 ± 22d | 5968 ± 179a | 1178 ± 60e | 38.0 ± 1.9l |
| T14 | 2234 ± 61e | 12.3 ± 0.0e | 525 ± 5d | 5981 ± 191a | 1469 ± 48b | 46.5 ± 0.2e |
| T15 | 2510 ± 34b | 6.2 ± 0.1k | 484 ± 9f | 5103 ± 139h | 936 ± 17h | 35.1 ± 0.4lm |
| T16 | 2293 ± 9e | 11.9 ± 1.4f | 678 ± 14c | 4642 ± 16k | 1114 ± 0e | 42.2 ± 2.5g |
| T17 | 2356 ± 33d | 8.8 ± 1.2i | 709 ± 14b | 5824 ± 318b | 1531 ± 12ab | 48.2 ± 2.0 cd |
| T18 | 2383 ± 52d | 12.7 ± 0.4e | 334 ± 10f | 5213 ± 185g | 1046 ± 36f | 42.0 ± 0.7g |
| T19 | 2319 ± 46d | 15.0 ± 0.2b | 572 ± 17d | 5101 ± 431h | 1060 ± 40f | 41.5 ± 0.1h |
| T20 | 2435 ± 56c | 6.5 ± 0.5k | 527 ± 41d | 5353 ± 79f | 1066 ± 23f | 42.3 ± 0.4g |
| T21 | 2564 ± 39b | 13.8 ± 0.3d | 562 ± 9d | 5843 ± 129b | 1137 ± 11e | 47.9 ± 0.6d |
| T22 | 2192 ± 9f | 6.5 ± 0.4k | 542 ± 14d | 5894 ± 11b | 1152 ± 0e | 39.1 ± 4.8j |
| T23 | 2508 ± 34b | 10.7 ± 0.2g | 349 ± 4f | 4755 ± 105j | 1346 ± 28c | 40.7 ± 0.4i |
| T24 | 2474 ± 22c | 12.9 ± 0.8e | 522 ± 42d | 5511 ± 116d | 1562 ± 24a | 42.0 ± 0.2g |
| T25 | 2437 ± 11c | 15.7 ± 1.9b | 520 ± 20d | 5483 ± 39e | 1238 ± 39d | 49.5 ± 0.9c |
| T26 | 2324 ± 36d | 10.2 ± 1.0g | 643 ± 17c | 5397 ± 420f | 1274 ± 80d | 40.3 ± 2.9i |
| T27 | 3591 ± 29a | 24.7 ± 0.0a | 786 ± 5a | 11,123 ± 127 | 2594 ± 23a | 87.4 ± 0.4a |
| T28 | 2332 ± 74d | 12.6 ± 1.9e | 532 ± 12d | 5357 ± 145f | 1423 ± 18b | 47.2 ± 3.7d |
| T29 | 2448 ± 65c | 10.6 ± 0.7g | 526 ± 11d | 5910 ± 463a | 1441 ± 23b | 47.2 ± 4.5d |
| T30 | 2440 ± 17c | 8.6 ± 0.6i | 561 ± 37d | 5419 ± 244e | 1265 ± 22d | 43.3 ± 3.6f |
| T31 | 2585 ± 80b | 12.1 ± 1.3e | 636 ± 92c | 3475 ± 314n | 1275 ± 40d | 41.2 ± 0.9h |
*Standard deviation; **values within each column followed by different letters are significantly different at p < 0.05
Total phenol and antioxidant activity values of almond kernels collected from naturally growing almond trees in Mersin province are given in Table 4. Significant differences among total phenol and antioxidant activity values were observed among almond kernels (P < 0.05). The total phenol and antioxidant activity values of almond kernels different depending on almond genotype. The highest value of total phenolic was found in T16 (98.67 mg GAE/100 g), whereas the lowest value was observed in T24 (23.75 mg GAE/100 g). The antioxidant activity of almond cultivars ranged from 44.59% (T12) to 91.18 (T23). Statistically significant differences were observed between total phenolic contents and antioxidant activity values of almond kernel (p < 0.05). In previous study, Yıldız et al. (2014) reported that total phenolic contents of almond kernels changed between 45.58 (cv. Primorski) and 93.64 mg GAE/g (cv. Garrigues), respectively. Esfahlan and Jamei (2012) reported total phenolic content of 10 wild almond species kernel extracts between 184 mg GAE/g (A. urumiensis) and 482 mg GAE per g extract for (A. pabotti). T23 genotype in general showed the highest antioxidant activity values in all almonds. In other study, almond extract scavenged 89.50% of the ABTS radical, 66.77% of the hydroxyl radical, and 87.30% of the DPPH radical (Keser et al. 2014). Ten different almond kernels were collected from Spanish almond varieties in the same plot to remove the environmental and agricultural management effects on almond chemical traits (Rabadán et al. 2017). A wide differences of antioxidant activity of almond kernel types were found.
Table 4.
Total phenol and antioxidant activity values of almond kernels
| Almonds | Total phenol mgGAE/100 g) | Antioxidant activity (%) | Almonds | Total phenol mgGAE/100 g) | Antioxidant activity (%) |
|---|---|---|---|---|---|
| T1 | 54.18 ± 1.17*hı | 49.15 ± 0.87no | T17 | 37.93 ± 0.53n | 79.83 ± 3.56g |
| T2 | 38.29 ± 2.37m** | 64.82 ± 3.28jk | T18 | 51.28 ± 1.32i | 81.29 ± 2.87f |
| T3 | 41.57 ± 1.76kl | 59.33 ± 1.76k | T19 | 57.61 ± 1.17ı | 83.47 ± 1.61de |
| T4 | 64.98 ± 2.51f | 47.19 ± 2.13o | T20 | 49.63 ± 0.76j | 85.46 ± 1.19c |
| T5 | 44.71 ± 3.64jk | 51.28 ± 1.18n | T21 | 59.38 ± 0.33fg | 78.32 ± 2.65g |
| T6 | 39.11 ± 0.89m | 73.42 ± 1.78ı | T22 | 47.19 ± 0.45j | 65.38 ± 1.43j |
| T7 | 56.83 ± 0.63h | 65.37 ± 2.65j | T23 | 28.34 ± 0.51p | 91.18 ± 3.76a |
| T8 | 71.19 ± 1.34d | 54.28 ± 2.88 lm | T24 | 23.75 ± 1.15r | 87.35 ± 2.98b |
| T9 | 47.61 ± 1.71j | 76.29 ± 1.89gh | T25 | 42.89 ± 1.13k | 48.53 ± 1.17ö |
| T10 | 67.82 ± 1.57e | 82.38 ± 1.63e | T26 | 37.21 ± 2.38n | 55.38 ± 0.98lm |
| T11 | 88.49 ± 3.87b | 67.17 ± 2.54i | T27 | 56.81 ± 1.68h | 49.21 ± 0.86no |
| T12 | 65.51 ± 1.64f | 44.59 ± 1.35p | T28 | 74.17 ± 3.27c | 56.71 ± 1.67lm |
| T13 | 43.26 ± 1.29k | 71.37 ± 1.08ı | T29 | 43.82 ± 1.56k | 66.98 ± 2.31i |
| T14 | 53.28 ± 0.81ıi | 46.83 ± 2.27o | T30 | 59.48 ± 1.87g | 75.25 ± 3.48h |
| T15 | 41.19 ± 0.45kl | 71.87 ± 3.18ı | T31 | 34.63 ± 0.98ö | 59.77 ± 1.83k |
| T16 | 98.67 ± 1.09a | 65.23 ± 3.76j |
*Standard deviation; **values within each column followed by different letters are significantly different at p < 0.05
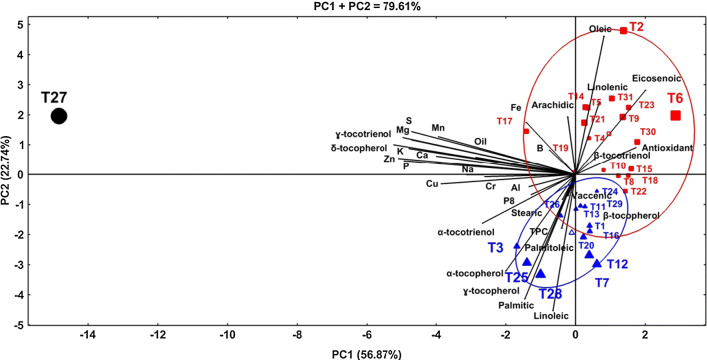
Principle component analysis results clearly indicated the interrelationship between the genotype and their influence on the biochemical composition of almond kernel and oils (Fig. 1). In the HJ-biplot, the short distance between the genotypes indicated their similarity based on the biochemical composition, while long distance indicated dissimilarity. In this sense, the genotype were group into three groups, in which, T27 formed separate group and other genotype formed two groups with some interaction between them. Regarding the traits, the cosine of the angle between the vectors specifies correlations, where, acute angle indicate positive correlation, obtuse and strait angles shows negative correlations, and right angle show no correlations (Yan and Fregeau-Reid, 2008). In this regard, strong positive correlations were observed among minerals, tocopherols, and fatty acids. The first group (upper left, circle symbol) composed of the cultivar T27 which is characterized by high levels of minerals (mainly Mn, S, Mg, K, Ca, Zn, P, Na, Cu, Cr, and Al), oil, γ-tocotrienol, δ-tocopherol, P8, and α-tocotrienol. The second group (upper right, squire symbols) is consisted of the cultivars T2, T4, T5, T6, T8, T9, T10, T14, T15, T17, T18, T19, T21, T22, T23, T30 and T31. This group is characterized by high levels of antioxidant, Fe, oleic, linolenic, arachidic, eicosenoic, and β-tocotrienol. Within this group, the cultivars T2 and T6 contributed more to these parameters than other cultivars. The third group (lower of the graph, tringle symbol) consisted of the cultivars T1, T3, T7, T11, T12, T13, T16, T20, T24, T25, T26, T28, and T29. This group is characterized by great contents of total polyphenols (TPC), tocopherols (α-, β-, and γ- tocopherols), stearic, palmitic, linoleic, palmitoleic, and vaccenic acids. Among this group, the cultivars T3, T7, T12, T25, and T28 contributed more to these biochemical properties than other cultivars. Overall, PCA suggested that the genotypes T2, T3, T6, T7, T12, T25, T27 and T28 contains considerable amounts of nutritional compounds and they could thus be recommended for human consumption. Within these cultivars, T27 has a higher nutritional quality than the other genotypes.
Fig. 1.
The HJ-biplot based on principal component analysis for biochemical properties of 31 almond genotypes collected from Mersin district of Turkey
Conclusion
In the present study, the biochemical composition and antioxidant activity of 31 almond genotypes wildly grown in Turkey was investigated. The results of the present study indicate that the almond kernels constitute a viable source of certain health-beneficial phytochemical compounds. The results showed wide variations in oil contents, fatty acid composition, tocopherols, minerals, phenolics and antioxidant activity among the kernels and oils of almond genotype. Obtained results from analyzing fatty acid profile revealed that, almond oils contained high amounts of polyunsaturated fatty acids. The oil products having higher amount of good fatty acids and higher tocopherols contents are considered good from the health point of view. The high levels of macro (Ca, K, P, and Mg,) and micro (Zn, Fe, and Cu) minerals in the kernels of almond genotype indicated that they could serve as excellent sources of these elements in the human diet. The current investigations showed that the almond kernels were found to be rich in most of the essential elements. Almond kernels had high total phenolic contents. The total phenol and antioxidant activity values of almond kernels differed depending on almond genotype.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. (RG-1439-080).
Compliance with ethical standard
Conflict of interest
The authors declare that there are no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mehmet Musa Özcan, Email: mozcan@selcuk.edu.tr.
Isam A. Mohamed Ahmed, Email: iali@ksu.edu.sa
References
- Aşkın MA, Balta MF, Tekintaş FE, Kazankaya A, Balta F. Fatty acid composition affected by kernel weight in almond (Prunus dulcis (Mill.) D.A. Webb.) genetic resources. J Food Comp Anal. 2007;20:7–12. doi: 10.1016/j.jfca.2006.06.005. [DOI] [Google Scholar]
- Aslantas R, Güleryüz M, Turan M (2001) Some chemical contents of selected almond (Prunus amygdalus Batsch) types. In: Ak BE (ed). XI GREMPA Seminar on pistachios and almonds. CIHEAM, Zaragoza p 347–350 (Cahiers Options Méditerranéennes; n. 56)
- Balz M, Schulte E, Their HP. Trennung von tocopherolen und tocotrienolen durch HPLC. Fat Sci Technol. 1992;94:209–213. [Google Scholar]
- Barbara G, Martını L, Monastra F. Response of ferragnes and tuono almond cultivars to different environmental conditions in southern Italy. Acta Hort. 1994;373:99–103. [Google Scholar]
- Čolić S, Zec G, Natić M, Fotirić-Akšić M. Almond (Prunus dulcis) oil. In: Ramadan M, editor. Fruit oils: chemistry and functionality. Cham: Springer; 2019. [Google Scholar]
- Esfahlan AJ, Jamei R. Properties of biological activity of ten wild almond (Prunus amygdalus L.) species. Tr J Biol. 2012;36:201. [Google Scholar]
- Fernandes GD, Gómez-Coca RB, Pérez-Camino MC, Moreda W, Barrera-Arellano D. Chemical characterization of major and minor compounds of nut oils: almond, hazelnut, and pecan nut. J Chem. 2017;2017:2609549. doi: 10.1155/2017/2609549. [DOI] [Google Scholar]
- Filsoof M, Mehran M, Farrohi F. Determination and composition oil characteristics in Iranian almond, apricot and peach nuts. Fette Seifen Anstrich. 1976;78:117–150. doi: 10.1002/lipi.19760780403. [DOI] [Google Scholar]
- Garcia-Pascual P, Mateos M, Carbonell V, Salazar DM. Influence of storage conditions on the quality of shelled and roasted almonds. Biosys Eng. 2003;84:201–209. doi: 10.1016/S1537-5110(02)00262-3. [DOI] [Google Scholar]
- Gupta A, Sharma PC, Tilakratne BMKS, Verma AK. Studies on physico-chemical characteristics and fatty acid composition of wild apricot [Prunus armeniaca Linn.] kernel oil. Ind J Nat Prod Res. 2012;3:366–370. [Google Scholar]
- Izaddost M, Imani A, Piri S, Bagiri AM. Oil content, major fatty acids composition, α-tocopherol and nut characteristics of almond at time of harvest. J Basic Appl Sci Res. 2013;3:201–205. [Google Scholar]
- Karatay H, Şahin A, Yılmaz Ö, Aslan A. Major fatty acids composition of 32 almond (Prunus dulcis (Mill.) D.A. Webb.) genotypes distributed in east southeast of Anatolia. Turk J Biochem. 2014;39:307–316. doi: 10.5505/tjb.2014.55477. [DOI] [Google Scholar]
- Keser S, Demir E, Yılmaz O. Phytochemicals and antioxidant activity of the almond kernel from Turkey. J Chem Soc Pakistan. 2014;36(3):534–541. [Google Scholar]
- Kırbaşlar FG, Türker G, Özsoy-Güneş Z, Ünal M, Dülger B, Ertaş E, Kızılkaya B. Evaluation of fatty acid composition, antioxidant and antimicrobial activity, mineral composition and calories values of some nuts and seeds from Turkey. Rec Nat Prod. 2012;6:339–349. [Google Scholar]
- Kodad O (2017) A new late blooming almond cultivar. FAO-CIHEAM-Nucis-Newsletter, Number 17, May 2017
- Kodad O, Socias I, Company R. Variability of oil content and of major fatty acid composition in almond (Prunus amydalus Batsch) and its relationship with kernel quality. J Agric Food Chem. 2008;56:4096–4101. doi: 10.1021/jf8001679. [DOI] [PubMed] [Google Scholar]
- Kodad O, Socias Rafel, Alonso JM. Genotypic and environmental effects on tocopherol content in almond. Antioxidants (Basel) 2018;7(1):6. doi: 10.3390/antiox7010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornsteiner M, Wagner KH, Elmadfa I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006;98:381–387. doi: 10.1016/j.foodchem.2005.07.033. [DOI] [Google Scholar]
- Lee SK, Mbwambo ZH, Chung HS, Luyengi L, Games EJC, Mehta RG. Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- Lopez Ortiz CM, Prats Moya MS, Berenguer Navarro V. A rapid chromatographic method for simultaneous determination of β-sitositerol and tocopherol homologues in vegetable oils. J Food Compos Anal. 2006;19:141–149. doi: 10.1016/j.jfca.2005.06.001. [DOI] [Google Scholar]
- Maguire LS, O’Sullivan SM, Galvin K, O’Connor TP, O’Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55:171–178. doi: 10.1080/09637480410001725175. [DOI] [PubMed] [Google Scholar]
- Martins AN, Gomes C, Ferreira L. Almond production and characteristics in Algarve, Portugal. Nucis Newsl. 2000;9:6–9. [Google Scholar]
- Matthäus B, Özcan MM. Quantitation of fatty acids, sterols, and tocopherols in turpentine (Pistacia terebinthus Chia) growing wild in Turkey. J Agric Food Chem. 2006;54(20):7667–7671. doi: 10.1021/jf060990t. [DOI] [PubMed] [Google Scholar]
- Mehran M, Filsoof M. Characteristics of Iranian almond nuts and oils. J Am Oil Chem Soc. 1974;51:433–434. doi: 10.1007/BF02635147. [DOI] [Google Scholar]
- Moayedi A, Rezaei K, Moini S, Keshavarz B. Chemical composition of oils from several wild almond species. J Am Oil Chem Soc. 2011;88:503–508. doi: 10.1007/s11746-010-1701-z. [DOI] [Google Scholar]
- Nanos GD, Kazantzisb I, Kefalas P, Petrakisb C, Stavroulakisc G. Irrigation and harvest time affect almond kernel quality and composition. Sci Hort. 2002;96:249–256. doi: 10.1016/S0304-4238(02)00078-X. [DOI] [Google Scholar]
- Özcan MM, Ünver A, Erkan E, Arslan D. Characteristics of some almond kernel and oils. Sci Hort. 2011;127:330–333. doi: 10.1016/j.scienta.2010.10.027. [DOI] [Google Scholar]
- Piscopo A, Romeo FW, Petrovicova B, Poiana M. Effect of the harvest time on kernel quality of several almond varieties (Prunus dulcis (Mill.) D.A. Webb) Sci Hortic. 2010;125:41–46. doi: 10.1016/j.scienta.2010.02.015. [DOI] [Google Scholar]
- Püskülcü H, Ikiz F. Introdiction to statistic. Bornova: Bilgehan Presss; 1989. p. 333. [Google Scholar]
- Rabadan A, Alvarez-Orti M, Gomez R, de Miguel C, Pardo J. Influence of genotype and crop year in the chemometrics of almond and pistachio oils. J Sci Food Agric. 2018;98:2402–2410. doi: 10.1002/jsfa.8732. [DOI] [PubMed] [Google Scholar]
- Rabadan A, Alvarez-Orti M, Pardo JE. A comparison of the effect of genotype and weather conditions on the nutritional composition of most important commercial nuts. Sci Hort. 2019;244:218–224. doi: 10.1016/j.scienta.2018.09.064. [DOI] [Google Scholar]
- Rabadán A, Álvarez-Ortí M, Gómez R, Pardo-Giménez A, Pardo JE. Suitability of Spanish almond cultivars for the industrial production of almond oil and defatted flour. Sci Hort. 2017;225:539–546. doi: 10.1016/j.scienta.2017.07.051. [DOI] [Google Scholar]
- Sathe SK, Seeram NP, Kshırsagar HH, Heber D, Lapsley KA. Fatty acid composition of California grown almonds. Food Chem. 2008;73:607–614. doi: 10.1111/j.1750-3841.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- Saura Calixto F, Bauza M, Martinez de Toda F, Argamenteria A. Amino acids, sugars and inorganic elements in the sweet almond (Prunus amygdalus) J Agr Food Chem. 1981;29:509–511. doi: 10.1021/jf00105a018. [DOI] [PubMed] [Google Scholar]
- Schirra M, Mulas M, Nieddu G, Virdis F. Mineral content in Texas almonds during fruit growth and ripening. Acta Hort. 1994;373:207–214. doi: 10.17660/ActaHortic.1994.373.29. [DOI] [Google Scholar]
- Skujins S (1998) Handbook for ICP-AES (Varıan-Vista). A short guide to vista series ICP- AES operation. Varian Int.
- Vicente-Villardon JL. MULTBIPLOT: a package for multivariate analysis using biplots. Salamanca: Department of Statistics, University of Salamanca; 2010. [Google Scholar]
- Welna M, Klimpel M, Zyrnicki W. Investigation of major and trace elements and their distributions between lipid and non-lipid fractions in Brazil nuts by inductively coupled plasma atomic optical spectrometry. Food Chem. 2008;111:1012–1015. doi: 10.1016/j.foodchem.2008.04.067. [DOI] [Google Scholar]
- Yan W, Fregeau-Reid J. Breeding line selection based on multiple traits. Crop Sci. 2008;48(2):417–423. doi: 10.2135/cropsci2007.05.0254. [DOI] [Google Scholar]
- Yıldız H, Atli HS, Tosun M, Ercişli S. Antioxidant activity, total phenolic and flavonoid content of some local and cultivated almonds (Prunus dulcis L.) Oxid Commun. 2014;37(3):733–740. [Google Scholar]
- Yoo KM, Lee KW, Park JB, Lee HJ, Hwang IK. Variation in major antioxidants and total antioxidant activity of Yuzu (Citrus junos Sieb ex Tanaka) during maturation and between cultivars. J Agric Food Chem. 2004;52:5907–5913. doi: 10.1021/jf0498158. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Taylor C, Sommer K, Wilkinson K, Wirthensohn M. Influence of deficit irrigation strategies on fatty acid and tocopherol concentration of almond (Prunus dulcis) Food Chem. 2015;173:821–826. doi: 10.1016/j.foodchem.2014.10.108. [DOI] [PubMed] [Google Scholar]