Abstract
BACKGROUND
Locally advanced rectal cancer is treated using neoadjuvant chemoradiation (nCRT), followed by total mesorectal excision (TME). Tumor regression and pathological post-treatment stage are prognostic for oncological outcomes. There is a significant correlation between markers representing cancer-related inflammation, including high neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte (MLR) and unfavorable oncological outcomes. However, the predictive role of these markers on the effect of chemoradiation is unknown.
AIM
To evaluate the predictive roles of NLR, MLR, and PLR in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiation.
METHODS
Patients (n = 111) with locally advanced rectal cancer who underwent nCRT followed by TME at the Minimally Invasive Surgery Unit, Siriraj Hospital between 2012 and 2018 were retrospectively analyzed. The associations between post-treatment pathological stages, neoadjuvant rectal (NAR) score and the pretreatment ratios of markers of inflammation (NLR, MLR, and PLR) were analyzed.
RESULTS
Clinical stages determined using computed tomography, magnetic resonance imaging, or both were T4 (n = 16), T3 (n = 94), and T2 (n = 1). The NAR scores were categorized as high (score > 16) in 23.4%, intermediate (score 8-16) in 41.4%, and low (score < 8) in 35.2%. The mean values of the NLR, PLR, and MLR correlated with pathological tumor staging (ypT) and the NAR score. The values of NLR, PLR and MLR were higher in patients with advanced pathological stage and high NAR scores, but not statistically significant.
CONCLUSION
In patients with locally advanced rectal cancer, pretreatment NLR, MLR and PLR are higher in those with advanced pathological stage but the differences are not significantly different.
Keywords: Locally advanced rectal cancer, Cancer-related inflammatory markers, Neoadjuvant chemoradiation, Neutrophil-to-lymphocyte ratio, Monocyte-to-lymphocyte ratio, Platelet-to-lymphocyte ratio
Core Tip: Previously, correlations between markers representing cancer-related inflammation, including high neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) and unfavorable oncological outcomes have been reported. The present study demonstrated the predictive role of these markers in patients with locally advanced rectal cancer. Pretreatment NLR, MLR, and PLR were higher in those with advanced pathological stage and high neoadjuvant rectal score, and represented a poor outcome.
INTRODUCTION
In Thailand, colorectal cancer is the third most common cancer, and approximately one-third of Thai patients with colorectal cancer have metastatic disease[1]. Neoadjuvant chemoradiation (nCRT) followed by total mesorectal excision (TME) is the preferred treatment for locally advanced rectal cancer (clinical stage T3 or T4 or node-positive disease). This treatment regimen provides many benefits, including improved local control and reduced toxicity. Although a complete pathological response to this therapeutic regimen (ypT0N0) is a strong prognostic factor, we still lack precise preoperative prognostic and predictive factors for locally advanced rectal cancer. Therefore, surgical removal of the rectum remains the standard treatment and is essential in all patients.
Here we investigated the value of preoperative biochemical markers as prognostic factors and predictive markers for nCRT. Inflammation status correlates with the oncological outcomes of patients with rectal cancer[2-5]. The immune system acts on cancer cells by producing numerous cytokines and mediators of inflammation. The neutrophil-to-lymphocyte ratio (NLR) serves as the primary prognostic marker of white blood cell status. Previous meta-analyses demonstrated the benefit of the NLR as a prognostic factor for worse oncological outcomes in various cancers including endometrial cancer, non-small cell lung cancer, hepatocellular carcinoma, gastric cancer, and colorectal cancer[6,7]. Subsequently, the predictive roles of the monocyte-to-lymphocyte ratio (MLR) and the platelet-to-lymphocyte ratio (PLR) were established[8]. Both pre- and post-chemoradiation NLR were prognostic of worse disease-free and overall survival among locally advanced cancer patients[9,10]. However, the predictive role of these markers in patients receiving nCRT is still inconclusive. Here, we evaluate the predictive roles of pretreatment NLR, MLR, and PLR in patients with locally advanced rectal cancer receiving nCRT.
MATERIALS AND METHODS
The records of 111 patients with locally advanced rectal cancer who underwent nCRT followed by oncological surgical resection at the Minimally Invasive Surgery Unit, Siriraj Hospital between June 2012 and January 2018 were retrospectively analyzed. Thirty-six patients (32.4%) were diagnosed with mid-rectal cancer, defined as a tumor located 5–10 cm from the anal verge, and 75 patients (67.6%) were diagnosed with low-rectal cancer, defined as a tumor located within 5 cm from the anal verge. Clinical staging was performed using magnetic resonance imaging (MRI), computed tomography (CT) or both, with or without endorectal ultrasound (ERUS). Patients with evidence of distant metastasis on preoperative staging were excluded from the study. Demographic data collected included age, sex, clinical tumor and nodal stages, and location of tumors. A complete blood count examination was performed at baseline before starting nCRT in all patients. All patients received standard long-term nCRT comprising 45.0 to 50.4 Gy in twenty-eight fractions, infusion of 5-fluorouracil for five days during the first and fifth weeks of radiation, or continuous oral capecitabine therapy.
All patients underwent oncological resection after completing nCRT at intervals ranging from six to thirteen weeks depending on the clinical response, scheduling, and the surgeon’s preference. High ligation of inferior mesenteric vessels and total mesorectal excision were performed. Pathologists specializing in gastroenterology examined the surgical specimens. Pathological tumor (ypT0-4) and nodal staging (ypN0-2) data were acquired. A pathological complete response was defined as no detectable viable tumor cells in the resected specimen (ypT0) and the resected node (ypN0). The neoadjuvant rectal (NAR) score was calculated according to the data generated using a Valentini nomogram for overall survival, using the clinical tumor stage (cT), pathological tumor stage (pT), and pathological nodal stage (pN)[6,11]. The equation for calculating the NAR score is as follows: NAR = [5ypN – 3(cT – ypT) + 12]2/9.61. NAR scores were categorized into the following levels: High (score > 16), intermediate (score 8-16) and low (score < 8)[11,12]. High NAR scores correlated with poor oncological outcomes. Posttreatment pathological stages and ratios of markers of inflammation (pretreatment NLR, MLR, and PLR ratios) were compared.
Statistical analysis
Statistical analysis of all collected data was performed with SPSS version 19.0 (IBM Corporation, Armonk, NY, United States). All demographic data were analyzed using descriptive statistics. Numerical variables are expressed using frequency. Continuous variables were compared using independent samples Kruskal-Wallis (one-way ANOVA) test. A P value of < 0.05 was considered statistically significant.
RESULTS
Patient demographic data are shown in Table 1. Sixty-six patients were male and 45 were female with a median age of 61 years (range, 22–93 years). The clinical stages determined using CT, MRI, or both were as follows: T4 (n = 16), T3 (n = 94), and T2 (n = 1). Clinically positive lymph nodes were found in 16 patients (14.4%). The NAR scores were categorized as high (> 8), intermediate (8–16), and low (< 8) in 23.4%, 41.4% and 35.2%, respectively.
Table 1.
Demographic data
| n (111) | % | |
| Median age (yr) | 61 | |
| Sex | ||
| Male | 66 | 59.5 |
| Female | 45 | 40.5 |
| Tumor location | ||
| Middle rectum | 36 | 32.4 |
| Lower rectum | 75 | 67.6 |
| Clinical T staging | ||
| cT2 | 1 | 1 |
| cT3 | 94 | 84 |
| cT4 | 16 | 15 |
| Lymph node status | ||
| Positive (cN+) | 16 | 14.4 |
| Negative (cN-) | 95 | 85.6 |
| NAR score | ||
| Low | 26 | 23.4 |
| Intermediate | 46 | 41.4 |
| High | 39 | 35.2 |
NAR: Neoadjuvant rectal.
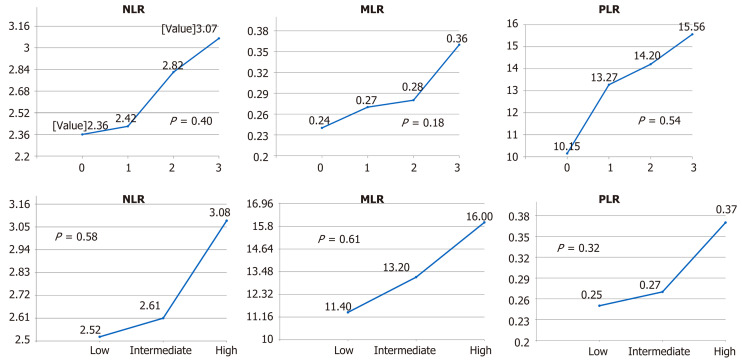
The mean values of the pretreatment NLR, PLR, and MLR correlated with pathologic tumor staging (ypT) and the NAR score. The mean values of the NLR of pathological stages 0, 1, 2, and 3 were 2.36, 2.42, 2.83 and 3.07, respectively (P = 0.40) (Figure 1). The mean values of the MLR of pathological stages 0, 1, 2 and 3 were 0.24, 0.27, 0.28, and 0.36, respectively (P = 0.18). The mean values of the PLR of pathological stages 0, 1, 2, and 3 were 10.15, 13.27, 14.20 and 15.56, respectively (P = 0.54). The mean values of the NLR were 2.52, 2.61, and 3.08 for low, intermediate, and high NAR scores, respectively (P = 0.58). The mean values of the MLR were 0.25, 0.27, and 0.37, respectively (P = 0.32). The mean values of the PLR were 11.40, 13.20, and 16.00, respectively (P = 0.61).
Figure 1.
Mean values of the neutrophil-to-lymphocyte ratio, the monocyte-to-lymphocyte ratio and the platelet-to-lymphocyte ratio by stage. NLR: Neutrophil-to-lymphocyte ratio; MLR: Monocyte-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio.
Twenty patients (76.9%) with low NARs experienced pathological complete responses. Furthermore, 34 patients (73.9%) with intermediate NAR scores and 37 patients (94.9%) with high NAR scores had pathological stages 2 and 3.
DISCUSSION
Our study demonstrated that the mean values of the NLR, PLR, and MLR correlated with pathological tumor staging and the NAR score. The ratios were higher in advanced pathological stages and high NAR scores. However, the differences were not statistically significant among the groups due to the small number of patients which is the main limitation of this study.
The systemic inflammatory response to cancer is increased in various cancers including lung cancer, ovarian cancer, tissue sarcoma, and colorectal cancer[6]. The pathophysiology by which the inflammatory pathway might affect cancer is unknown. There is evidence to indicate an association between inflammation and impaired nutritional status. Inflammatory bowel disease serves as a good example, as colorectal cancer can develop in patients with chronic inflammatory bowel disease[13].
The level of the systemic inflammatory response is determined by the concentration of C-reactive protein, which serves as a marker of acute phase protein stimulated by interleukin (IL)-6[14-16]. The Glasgow prognostic score serves as a prognostic factor[17]. However, these laboratory markers are not routinely measured during the preoperative evaluation of the majority of patients with rectal cancer, but complete blood counts are routinely examined.
T-cell lymphocytes play a major role in the antitumor immune response. The systemic inflammatory response is associated with lymphocytopenia and diminished lymphocyte function caused by the release of numerous proinflammatory cytokines such as IL-10 and transforming growth factor beta. Lymphocytopenia is the major contributor to high NLR, MLR, and PLR values. The low number of lymphocytes in the peripheral circulation may indicate a low antitumor response. CD8+ T lymphocytes play a major role in cancer immunity by killing cancer cells[18]. Tumor–associated macrophages are associated with angiogenesis and tumor invasion[19]. Thrombocytosis reflects cancer-induced inflammation. Furthermore, high concentrations of IL-1 and IL-6 were found in patients’ plasma or cultured supernatants of tumor cells obtained from patients with thrombocytosis and tumors that produce colony-stimulating factor[20]. A meta-analysis of 959 patients suggested that a high NLR is significantly associated with shorter overall survival, disease-free survival, and recurrence-free survival[7].
The NAR score predicts the prognosis of patients with rectal cancer who receive nCRT. According to the NSABP R-04 trial, NAR scores were categorized as low, intermediate, and high, which were significantly associated with overall survival[6]. In the present study, the mean values of pretreatment NLR, MLR, and PLR gradually increased as a function of the NAR score. These scores reflect an association between the levels of these markers and survival, although we did not demonstrate a significant association in the present study. However, in patients with high pretreatment NLR, MLR, and PLR, standard nCRT is warranted. We observed a restricted response to nCRT among patients with with a high NAR score and high ratios of these inflammatory markers. Alternatively, upfront chemotherapy or a total neoadjuvant approach may play an important role in these patients.
The limitations of this study include its retrospective analysis of a small number of patients at a single center.
In summary, pretreatment NLR, MLR, and PLR in patients with locally advanced rectal cancer treated with nCRT were higher in patients with advanced pathological stages, although the differences were not statistically significant. Further studies with larger populations are required to evaluate the role of these inflammatory markers in predicting patient outcomes.
ARTICLE HIGHLIGHTS
Research background
The systemic inflammatory response is increased in cancer. The level of the systemic inflammatory response is indicated by the concentration of C-reactive protein or the Glasgow prognostic score. However, these laboratory markers are not routinely measured. The neutrophil-to-lymphocyte ratio (NLR), the monocyte-to-lymphocyte ratio (MLR) and the platelet-to-lymphocyte ratio (PLR) from the complete blood count are prognostic for various cancers.
Research motivation
The NLR, MLR and PLR are associated with poor oncological outcomes in rectal cancer. However, the predictive role of these markers in patients receiving preoperative chemoradiation is inconclusive.
Research objectives
We evaluated the predictive roles of pretreatment NLR, MLR, and PLR in patients with locally advanced rectal cancer receiving neoadjuvant chemoradiation.
Research methods
The records of patients with locally advanced rectal cancer who underwent neoadjuvant chemoradiation followed by surgical resection at Siriraj Hospital between 2012 and 2018 were retrospectively analyzed. The associations between posttreatment pathological stages, neoadjuvant rectal (NAR) score and the pretreatment ratios of markers of inflammation (NLR, MLR, and PLR) were analyzed.
Research results
Among 111 patients, the clinical stages determined using computed tomography, magnetic resonance imaging, or both were as follows: T4 (n = 16), T3 (n = 94), and T2 (n = 1). The frequency of clinically positive lymph nodes was 14.4%. The NAR scores were categorized as high (> 8) in 23.4%, intermediate (8–16) in 41.4%, and low (< 8) in 35.2%. The mean values of the NLR of pathological stages 0, 1, 2, and 3 were 2.36, 2.42, 2.83 and 3.07, respectively (P = 0.40) (Figure 1). The mean values of the MLR of pathological stages 0, 1, 2 and 3 were 0.24, 0.27, 0.28, and 0.36, respectively (P = 0.18). The mean values of the PLR of pathological stages 0, 1, 2, and 3 were 10.15, 13.27, 14.20 and 15.56, respectively (P = 0.54). The mean values of the NLR were 2.52, 2.61, and 3.08 for low, intermediate, and high NAR scores, respectively (P = 0.58). The mean values of the MLR were 0.25, 0.27, and 0.37, respectively (P = 0.32). The mean values of the PLR were 11.40, 13.20, and 16.00, respectively (P = 0.61).
Research conclusions
The pretreatment NLR, MLR, and PLR of patients with locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy followed by total mesorectal excision were higher in patients with advanced pathological stages, although the differences were not statistically significant.
Research perspectives
This study demonstrated the association between higher numbers of pretreatment inflammatory markers and higher advanced stages. Furthermore, higher numbers of pretreatment NLR, MLR, and PLR were associated with poorer response to neadjuvant chemoradiation (high NAR score). Unfortunately, this is a retrospective analysis of a small number of patients at a single center. Therefore, there were no statistically significant results. Further studies of larger populations are required to evaluate the significance of these inflammatory markers in predicting the response.
ACKNOWLEDGEMENTS
We thank Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study was approved by Siriraj’s institutional review board committee and the committee’s reference number is 335/2561(EC4).
Informed consent statement: All patient information is de-identified.
Conflict-of-interest statement: The authors declare that they have no competing interests
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Peer-review started: February 27, 2020
First decision: April 22, 2020
Article in press: August 16, 2020
P-Reviewer: Burke DA S-Editor: Zhang H L-Editor: Webster JR P-Editor: Zhang YL
Contributor Information
Kitinat Timudom, Department of Surgery, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Thawatchai Akaraviputh, Department of Surgery, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Vitoon Chinswangwatanakul, Department of Surgery, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Ananya Pongpaibul, Department of Pathology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Pornpim Korpraphong, Department of Radiology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Janjira Petsuksiri, Department of Radiology, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Suthinee Ithimakin, Department of Medicine, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand.
Atthaphorn Trakarnsanga, Department of Surgery, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok 10700, Thailand. atthaphorn.tra@mahidol.ac.th.
Data sharing statement
The dataset utilized during the current study is available within the institutional collected data system which was used under Siriraj’s institutional review board committee approval for this study. Data are available upon reasonable request with permission of Siriraj’s institutional review board committee.
References
- 1.Techawathanawanna S, Nimmannit A, Akewanlop C. Clinical characteristics and disease outcome of UICC stages I-III colorectal cancer patients at Siriraj Hospital. J Med Assoc Thai. 2012;95 Suppl 2:S189–S198. [PubMed] [Google Scholar]
- 2.Dudani S, Marginean H, Tang PA, Monzon JG, Raissouni S, Asmis TR, Goodwin RA, Gotfrit J, Cheung WY, Vickers MM. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as predictive and prognostic markers in patients with locally advanced rectal cancer treated with neoadjuvant chemoradiation. BMC Cancer. 2019;19:664. doi: 10.1186/s12885-019-5892-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun LH, Baumann D, Zwirner K, Eipper E, Hauth F, Peter A, Zips D, Gani C. Neutrophil-to-Lymphocyte Ratio in Rectal Cancer-Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable? Int J Mol Sci. 2019;20:2448. doi: 10.3390/ijms20102448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y, Ueno M. Prognostic Impact of Neutrophil-to-Lymphocyte Ratio in Patients with Advanced Low Rectal Cancer Treated with Preoperative Chemoradiotherapy. Dig Surg. 2015;32:496–503. doi: 10.1159/000441396. [DOI] [PubMed] [Google Scholar]
- 5.Shen L, Zhang H, Liang L, Li G, Fan M, Wu Y, Zhu J, Zhang Z. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat Oncol. 2014;9:295. doi: 10.1186/s13014-014-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B, Tannock IF, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 7.Dong YW, Shi YQ, He LW, Su PZ. Prognostic significance of neutrophil-to-lymphocyte ratio in rectal cancer: a meta-analysis. Onco Targets Ther. 2016;9:3127–3134. doi: 10.2147/OTT.S103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee IH, Hwang S, Lee SJ, Kang BW, Baek D, Kim HJ, Park SY, Park JS, Choi GS, Kim JC, Cho SH, Kim JG. Systemic Inflammatory Response After Preoperative Chemoradiotherapy Can Affect Oncologic Outcomes in Locally Advanced Rectal Cancer. Anticancer Res. 2017;37:1459–1465. doi: 10.21873/anticanres.11470. [DOI] [PubMed] [Google Scholar]
- 9.Sung S, Son SH, Park EY, Kay CS. Prognosis of locally advanced rectal cancer can be predicted more accurately using pre- and post-chemoradiotherapy neutrophil-lymphocyte ratios in patients who received preoperative chemoradiotherapy. PLoS One. 2017;12:e0173955. doi: 10.1371/journal.pone.0173955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha YJ, Park EJ, Baik SH, Lee KY, Kang J. Prognostic impact of persistent lower neutrophil-to-lymphocyte ratio during preoperative chemoradiotherapy in locally advanced rectal cancer patients: A propensity score matching analysis. PLoS One. 2019;14:e0214415. doi: 10.1371/journal.pone.0214415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George TJ, Jr, Allegra CJ, Yothers G. Neoadjuvant Rectal (NAR) Score: a New Surrogate Endpoint in Rectal Cancer Clinical Trials. Curr Colorectal Cancer Rep. 2015;11:275–280. doi: 10.1007/s11888-015-0285-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fokas E, Fietkau R, Hartmann A, Hohenberger W, Grützmann R, Ghadimi M, Liersch T, Ströbel P, Grabenbauer GG, Graeven U, Hofheinz RD, Köhne CH, Wittekind C, Sauer R, Kaufmann M, Hothorn T, Rödel C German Rectal Cancer Study Group. Neoadjuvant rectal score as individual-level surrogate for disease-free survival in rectal cancer in the CAO/ARO/AIO-04 randomized phase III trial. Ann Oncol. 2018;29:1521–1527. doi: 10.1093/annonc/mdy143. [DOI] [PubMed] [Google Scholar]
- 13.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World J Gastroenterol. 2014;20:9872–9881. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pathak S, Nunes QM, Daniels IR, Smart NJ. Is C-reactive protein useful in prognostication for colorectal cancer? A systematic review. Colorectal Dis. 2014;16:769–776. doi: 10.1111/codi.12700. [DOI] [PubMed] [Google Scholar]
- 15.Kersten C, Louhimo J, Ålgars A, Lahdesmaki A, Cvancerova M, Stenstedt K, Haglund C, Gunnarsson U. Increased C-reactive protein implies a poorer stage-specific prognosis in colon cancer. Acta Oncol. 2013;52:1691–1698. doi: 10.3109/0284186X.2013.835494. [DOI] [PubMed] [Google Scholar]
- 16.Guthrie GJ, Roxburgh CS, Richards CH, Horgan PG, McMillan DC. Circulating IL-6 concentrations link tumour necrosis and systemic and local inflammatory responses in patients undergoing resection for colorectal cancer. Br J Cancer. 2013;109:131–137. doi: 10.1038/bjc.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann TK, Dworacki G, Tsukihiro T, Meidenbauer N, Gooding W, Johnson JT, Whiteside TL. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8:2553–2562. [PubMed] [Google Scholar]
- 19.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki A, Takahashi T, Nakamura K, Tsuyuoka R, Okuno Y, Enomoto T, Fukumoto M, Imura H. Thrombocytosis in patients with tumors producing colony-stimulating factor. Blood. 1992;80:2052–2059. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset utilized during the current study is available within the institutional collected data system which was used under Siriraj’s institutional review board committee approval for this study. Data are available upon reasonable request with permission of Siriraj’s institutional review board committee.