Abstract
An increased number of patients with skin wounds have been witnessed in the past decades. Among the various kinds of treatments for skin wounds, topical exogenous growth factors are indispensable and have been used in many countries. However, whether they have reliable effects remains controversial, and their application for skin wound treatment needs to be further standardized and optimized in terms of socio-economic considerations. Thus, the Chinese Burn Association developed this guideline indicating efficacy, application details, adverse reactions and precautions of five clinically common topical growth factors using the Grading of Recommendations Assessment Development and Evaluation method to promote the rational application of topical exogenous growth factors in skin wounds and to benefit more patients.
Keywords: Growth factor, Skin wound, Wound healing, GRADE method, Guideline
Highlights.
This is the first guideline for topical application of growth factors in skin wounds.
GRADE method was used to develop the present guideline with emphasis on integration of the scientific evidence and expert opinions.
Recommendations on the effectiveness, application details and precautions of topical growth factors for skin wounds are provided in this guideline.
Background
With the rapid development of the economy, the great changes in people’s lifestyles and the accelerated aging of the population, problems caused by various kinds of skin wounds have multiplied during the past decades. The treatments for these wounds include surgical and non-surgical ones. As a non-surgical treatment, the application of exogenous growth factors (eGFs) is one of the indispensable methods to promote wound healing or to provide healthy wound beds for surgical treatments. eGFs have been used in many countries all over the world and the first report on the successful treatment of wounds with commercial eGFs was published as early as 30 years ago. To date, no obvious toxicity or severe adverse reactions have been reported in the treatment of wounds with eGFs. However, whether the eGFs have reliable effects remains controversial, and the application of eGFs for wound treatment needs to be further standardized and optimized in terms of socio-economic considerations.
The process of wound healing generally consists of three stages, inflammatory reaction, proliferation and tissue reconstruction. It is an elaborate biological process involving multiple factors such as repairing cells, proteins and biotic factors, among which growth factors play a vital role. The growth factors not only promote the proliferation, differentiation and migration of repairing cells such as keratinocytes, fibroblasts and vascular endothelial cells (VECs), but also regulate apoptosis of the repairing cells, the composition of the extracellular matrix (ECM), the synthesis of DNA, RNA and proteins, the process of glycolysis, as well as the remodeling of damaged tissues. It is suggested that decreased activity and/or quantity of growth factors and their receptors is a potential and important pathophysiological basis of refractory non-healing wounds [1]. This lays a theoretical foundation for topical application of eGFs to promote wound healing in various kinds of cases. In order to promote the rational application of eGFs in skin wounds and to benefit more patients, the present guideline was developed after much discussion by writing group of growth factor guideline on behalf of Chinese Burn Association under the guidance of the academician Xiaobing Fu.
Methods
This guideline has been registered in the International Practice Guidelines Registry Platform with the registration number IPGRP-2020CN127. It was developed based on the Grading of Recommendations Assessment Development and Evaluation (GRADE) [2–3]. The recommendations were aimed at indicating the efficacy, application approaches and doses, concentration and treament course of topical eGFs on skin wounds, and adverse reactions and precautions. The process of guidelines development was as follows (Figure 1). (1) Search the Chinese CNKI, CQVIP and WANFANG databases using the key words “basic fibroblast growth factor (bFGF)”, “acidic fibroblast growth factor (aFGF)”, “epidermal growth factor (EGF)”, “granulocyte macrophage colony stimulating growth factor (GM-CSF)”, “platelet-derived growth factor (PDGF)”, “wound”, and “healing/repair” both in Chinese and in English. Meanwhile search PubMed, Web of Science and EMBASE databases using the above key words in English. (2) The guideline supporting group, under the guidance of the experts, reviewed the titles, abstracts and full texts of the obtained literature, determined the articles that should be included in the later literature evaluation based on the inclusion criteria and exclusion criteria, and used an artificial method to grade the quality of evidence forming “high”, “moderate” or “low” classifications. (3) The expert panel reviewed the included literature and, based on comprehensive consideration of benefits and harms, patient values and preferences, economic costs and clinical practice related factors, put forward their own recommendations with “strong” or “weak” as different recommendation strengths.
Figure 1.
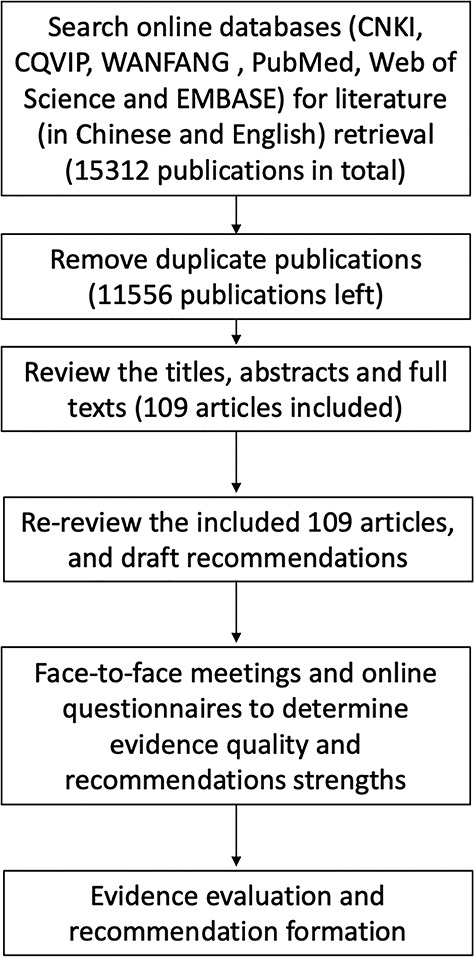
Diagram of the development of clinical guideline on topical growth factors for skin wounds using the Grading of Recommendations Assessment Development and Evaluation method
The inclusion criteria are as follows: (1) clinical trials of topical eGFs for the treatment of skin wounds, or in vitro and in vivo studies involving dose–effect investigations; (2) published from the establishment year of each aforementioned database to 31 July 2019; (3) written in Chinese or English; (4) subjects were patients with skin wounds or in vivo and/or in vitro experimental models; (5) the experimental group was treated with eGFs, while the control group was treated with placebo or standard/routine therapies; (6) outcome index mainly included wound healing data such as wound healing time and wound healing rate. The exclusion criteria include: (1) studies exclusively on non-skin wounds (e.g. oral mucosa); (2) in vitro studies or experimental animal studies which do not involve dose–effect investigations; (3) observational studies with a small sample (n < 30) in each group.
A total of 4253 articles in Chinese and 11 059 articles in English were retrieved, including 3333 articles in Chinese and 8223 in English obtained after repeated examination. After review of the titles, abstracts and full texts, 109 articles were included, of which 78 were in Chinese and 31 in English. Among these, 40 were on bFGF, of which 33 were in Chines and 7 were in English; 7 were on aFGF, of which 6 were in Chines and 1 was in English; 44 were on EGF, of which 35 were in Chinese and 9 were in English; 11 were on GM-CSF, of which 4 were in Chinese and 7 were in English; 7 were on PDGF, all of which were in English.
Recommendations and rationale
Effectiveness of topical growth factors for skin wounds
FGF
FGF is a class of significant growth factors in organisms. It has a wide range of biological effects on tissues and cells derived from mesoderm and neuroectoderm, and is involved in wound repair, nerve repair, metabolic regulation, angiogenesis and embryonic development. In the family of FGFs, topical bFGF and aFGF have been used widely. The possible biological functions of bFGF in promoting wound healing are as follows. (1) bFGF can significantly promote angiogenesis in vivo and in vitro, exert chemotaxis of various kinds of cells involved in angiogenesis, and promote their proliferation and migration, which is one of the main angiogenetic factors. (2) Injury-induced bFGF promotes the aggregation of monocytes, neutrophils, macrophages and fibroblasts via chemotaxis in the injured tissues. It can also promote the formation of granulation tissue, cell division and proliferation related to injury repair and tissue reconstruction, playing a vital role in the repair of tissue damage. aFGF can promote the proliferation and migration of fibroblasts and other skin repair related cells to promote wound healing. Its underlying mechanisms include mitogenic activity, which mainly demonstrates promotion of cell proliferation and division, and non-mitogenic activity such as reducing local ischemia. aFGF is a mitogen in a variety of cells and thus participates in multiple processes of tissue repair. The evidence grades and expert recommendations for FGF to promote the healing of different types of wounds [4–46] are shown in Tables 1 and 2.
Table 1.
Quality of evidence and Grading of Recommendations Assessment Development and Evaluation (GRADE) recommendations for topical application of basic fibroblast growth factor in different types of wounds
| Wound type | Quality of evidence | GRADE recommendation | References |
|---|---|---|---|
| Superficial partial-thickness burns | High | Strong | 4–11 |
| Deep partial-thickness burns | High | Strong | 4–14 |
| Fresh traumatic wounds | Moderate | Strong | 4, 15–19 |
| Donor sites | Moderate | Weak | 4, 6, 7, 20–23 |
| Chronic wounds | Moderate | Weak | 4, 5, 20, 24–29 |
| Diabetic ulcers | Moderate | Weak | 30–32 |
| Pressive ulcers | Moderate | Weak | 33–36 |
| Grafted wounds | Low | Weak | 37, 38 |
| Redisual granulation wounds after burns | Low | Weak | 4, 7, 39 |
| Chronic ulcers after burns | Low | Weak | 39 |
| CO2 laser treated wounds | Low | Weak | 40 |
| Radiative dermatitis wounds | Low | Weak | 41 |
Table 2.
Quality of evidence and Grading of Recommendations Assessment Development and Evaluation (GRADE) recommendations for topical application of basic fibroblast growth factor in different types of wounds
EGF
EGF can be found in almost all kinds of body fluids, secretions and most tissues. EGF receptor (EGFR) is mainly expressed by keratinocytes. Other EGFR-expressing cells include fibroblasts, glial cells, smooth muscle cells (SMCs) and chondrocytes.
EGF exerts chemotaxis and mitogenic effects mainly by binding to receptors on the cell membrane and forming a complex metabolic network during its intracellular transmission, resulting in the regulation of cell metabolism, differentiation and other biological activities. Based on the literature, experts suggest that EGF can promote the healing of various kinds of skin wounds. The evidence grades that EGF promotes healing of different types of wounds and recommendations [47–78] are shown in Table 3.
Table 3.
Quality of evidence and Grading of Recommendations Assessment Development and Evaluation (GRADE) recommendations for topical application of epidermal growth factor in different types of wounds
| Wound type | Quality of evidence | GRADE recommendation | References |
|---|---|---|---|
| Superficial partial-thickness burns | Moderate | Weak | 47–59 |
| Deep partial-thickness burns | Moderate | Weak | 47–62 |
| Donor sites | Moderate | Weak | 47–51 |
| Redisual granulation wounds after burns | Moderate | Weak | 48,50,51,53 |
| Diabetic foot ulcers | High | Strong | 47,63–70 |
| Venous ulcers | Moderate | Weak | 71–73 |
| CO2 laser treated wounds | Moderate | Weak | 74–76 |
| Grafted wounds | Low | Weak | 48 |
| Chronic ulcers after burns | Low | Weak | 48,53 |
| Radiative dermatitis wounds | Low | Weak | 77 |
| Leg ulcers | Low | Weak | 78 |
GM-CSF
GM-CSF is a family of specific cytokines. Not only can it promote the proliferation of skin repairing cells through autocrine, it also plays a role in promoting wound healing by mediating several other cytokines. Before 2008, GM-CSF had been applied mainly by injection as a general clinical drug for nearly 15 years. It could be used for more than 7 days at a safe dose of 3 μg·kg−1·d−1. Subsequently, recombinant human GM-CSF (rhGM-CSF) gel was developed and applied to clinical wound therapy.
In 1992, Kaplan first reported the introduction of GM-CSF into the treatment of acute skin wounds. It was proved that rhGM-CSF injection could promote wound healing [79]. GM-CSF is involved in a series of processes of wound repair, including activation of T lymphocytes, dendritic cells, macrophages, endothelial cells and fibroblasts, etc. GM-CSF can enhance the function of many kinds of cells necessary for wound healing, such as activation of neutrophils and macrophages, increased migration and proliferation of epithelial cells, regulation of fibroblasts phenotypes, etc. The grades of evidence and expert recommendations for GM-CSF to promote wound [80–89] healing in different types of wounds are shown in Table 4.
Table 4.
Quality of evidence and Grading of Recommendations Assessment Development and Evaluation (GRADE) recommendations for topical application of granulocyte macrophage colony stimulating growth factor in different types of wounds
PDGF
PDGF possesses a wide range of biological activities. It acts on the membrane receptors of target cells, producing a series of biological effects that play an important role in the physiological and pathological processes of tissue repair. These include chemotaxis of inflammatory cells and repairing cells to the wound, promoting the mitosis of VECs, fibroblasts, SMCs and epithelial cells, promoting the formation and reconstruction of vascular regeneration of ECM, forming granulation tissue, and promoting re-epithelialization of the wound. The grades of evidence and expert recommendations for PDGF to promote wound healing in different types of wounds [90–95] are shown in Table 5.
Table 5.
Quality of evidence and Grading of Recommendations Assessment Development and Evaluation (GRADE) recommendations for topical application of platelet-derived growth factor in different types of wounds
Application of topical growth factors for skin wounds
As biological agents, eGFs are recommended to be applied to wounds according to the manufacturer’s instructions. This is because there are stringent requirements for application environment and preservation conditions as well as various forms of different eGFs.
Experts especially stress that the application of eGFs must be based on the specific condition of the wound. The precondition for the effective application of eGFs on all kinds of wounds is debridement. The necrotic tissue of the wound must be removed, and severe infections of the wounds should be effectively controlled before application of eGFs. The application methods of various growth factors are basically similar, and the forms of growth factors mainly include dry powder for the preparation of solutions and gels. The former can be dissolved in injection water or saline and applied directly or sprayed via a sprayer. Another way of application for eGFs is to drip the preparation solution evenly onto a suitably sized piece of gauze, which is then used to cover the wound. A gel, such as bFGF, can be evenly applied to the debrided wound or smeared evenly on a suitably sized piece of gauze, which is then used to cover the wound, with subsequently conventional bandaging (outer gauze) [4–6, 8,9,13,16,24,27]) (GRADE recommendation: weak; quality of evidence: low). The doses and concentrations of various growth factors are determined mainly according to the manufacturer’s instructions [4–6,8,9,13,14,16,21,24,27,42,43,45,60,67,80–83,89–108], which are shown in Table 6. However, there is also literature supporting different methods of application, such as a combination of EGF and saline soaking or infrared ray therapy [101,104,109] (GRADE recommendation: weak; quality of evidence: low). As for the course of treatment, experts agree that one or more times a day is appropriate because growth factors exert their curative effects by rapid contact with receptors. Given the common frequency of clinical dressing changes, once a day is also reasonable. In addition, since application of eGFs is to promote wound healing, they can be used until the wound bed becomes appropriate for skin grafting or wound healing, as long as the patient has sufficient financial support.
Table 6.
Application parameters of topical growth factors for skin wounds
| Growth factor | Dosage form | Concentration | Common dose | Frequency | References |
|---|---|---|---|---|---|
| bFGF | Powder | Adjustable preparing solution | 150 IU/cm2 or 1 μg/cm2 | Once or twice per day | 4–6,8,9,13,14,16,24,27 |
| Gel | 2100 IU/g | 150 IU/cm2 | Once per day | 96–99 | |
| aFGF | Powder | Adjustable preparing solution, e.g. 1000 IU/mL | 100 IU/cm2 | Once per day | 42–43,45 |
| EGF | Powder or solution | Adjustable preparing solution, e.g. 2000 IU/mL or 2.5 to 5.0 μg/mL or 10.0 μg/g | 400 IU/cm2 or 80 mg/cm2 | Once per day | 21,100–103 |
| Gel or cream | 10 μg/g or 20 to 40 μg/g | 1 μg/cm2 or 50 000 IU/cm2 or 80 mg/cm2 | Once every 1–4 days | 60,67,104–107 | |
| GM-CSF | Powder | Adjustable preparing solution | 5 μg/cm2 | Once per day | 108 |
| Gel | 10 μg/g | 1 μg/cm2 or 10 μg/cm2 | Once per day | 80–83,89 | |
| PDGF | Powder | Adjustable preparing solution, e.g. 100 μg/mL | 10 μg/cm2 | Once per day | 93 |
| Gel | 10 μg/g | 10 μg/cm2 | Once per day | 90–92,94–95 |
bFGF basic fibroblast growth factor, aFGF acidic fibroblast growth factor, EGF epidermal growth factor, GM-CSF granulocyte macrophage colony stimulating growth factor, PDGF platelet-derived growth factor
Supplementary recommendations are as follows. (1) bFGF can effectively promote wound healing in a certain dose range (75–300 IU/cm2) while ensuring that the wound is clean. Increasing the number of applications or prolonging the moisturizing time can significantly shorten the wound healing time at a constant dosage of eGFs [15, 22–23] (GRADE recommendation: weak; quality of evidence: moderate). (2) The commonly used forms of topical rhEGF include solution, cream and hydrogel, of which hydrogel is the most effective in terms of wound healing [105] and can reduce the pain and the workload of dressing changes [60] (GRADE recommendation: weak; quality of evidence: moderate). (3) Literature on the application of growth factors for skin wounds in pediatric patients is insufficient at present. According to the existing scientific reports and expert opinions, bFGF [8, 14], aFGF [96, 97] and EGF [100] can be applied on superficial and deep second-degree burn wounds in children.
Adverse reactions and precautions for topical growth factors for skin wounds
So far, no toxic effects or adverse reactions have been reported to be caused directly by the application of eGFs onto human skin wounds [4, 42, 44, 45, 110–111] (GRADE recommendation: weak; quality of evidence: low). Possible adverse effects include local allergic reactions such as redness, swelling, pruritus, transient pain, etc. which will disappear after withdrawal of growth factors. Few reports have implicated eGFs in the promotion of scar development while one trial suggested that eGFs may reduce scar formation [61].
Precautions to be taken with the use of topical growth factors for skin wounds are as follows:
(1) There is a lack of adequate evidence on the safety of topical eGFs for pregnant or lactating women, children and the elderly (>65 years old).
(2) The premise of topical application of eGFs is thorough debridement which resulted in relatively clean wounds, and eGFs should not be used on contaminated or infected wounds.
(3) It is not recommended to apply eGFs on wounds with severe peripheral inflammatory reaction and exudation.
(4) Dose-effects of eGFs within a concentration range of 100-1000 IU/cm2 have been supported by currently published data.
(5) The frequency of application of eGFs is generally more than once a day when clinical condition permits.
(6) The eGFs treatment can be maintained until the wound is healed or the wound bed is appropriate for skin grafting.
(7) The effectiveness of combined use of multiple growth factors at different healing stages and different conditions needs to be confirmed by further high-quality evidence. The current literature suggests that the combined use of multiple growth factors may contribute to wound repair, but it is necessary to balance the benefits and costs for the patients.
(8) Current data show that combined application of eGFs with other treatments, such as alginate dressing and vacuum sealing drainage (VSD), can accelerate wound healing, but the dose-effects of eGFs should be considered, and it still needs further substantial clinical observation and verification.
(9) In order to prevent reduced effects of eGFs, combining topical eGFs with solvents attenuating protein activities (such as ethanol solution, hydrogen peroxide solution, etc.) or heavy-metal preparations (such as silver ion products) should be avoided.
(10) Since growth factors can promote the proliferation and growth of tumor cells, they should not be used on cancerous wounds, skin wounds in patients with cachexia or malignant ulcer wounds.
The application of rhEGF is cost-effective for diabetic foot ulcer and Wagner’s stage III or IV wounds while PDGF has been shown to be cost-effective for the treatment of pressure injuries [112, 113]. However, the decision should be made by patients and their family according to their preferences and values, economic and health insurance states.
Conclusions
Topical application of growth factors are promising in treating various kinds of skin wounds, and the effectiveness of several growth factors has been confirmed in certain conditions based on current scientific evidence. Their rational application leads to more reliable effects and fewer unexpected results. Recommendations concerning the effectiveness, standardized application and precautions are provided in this guideline based on the GRADE system and expert opinions. More clinical trials are expected to further determine the efficacy, optimization and cost-effectiveness of different growth factors and their combinations with other treatments in different conditions in the future.
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
The authors acknowledge the following contributors: Consultants: Xiao-bing Fu, Chinese PLA General Hospital and Zhao-fan Xia, Changhai Hospital of Shanghai provided valuable advice for the development of the guidelines. Xiao-bing Fu, Chinese PLA General Hospital, Yuesheng Huang, Southwest Hospital, Third Military Medical University (Army Medical University) and Yizhi Peng, Southwest Hospital, Third Military Medical University (Army Medical University) helped with revision of the manuscript. The writing group of growth factor guideline on behalf of Chinese Burn Association: Chiyu Jia, The 8th Medical Center of Chinese PLA General Hospital; Dahai Hu, Xijing Hospital, Air Force Military Medical University; Gaoxing Luo, Southwest Hospital, Third Military Medical University (Army Medical University); Guanghua Guo, First Affiliated Hospital of Nanchang University; Guo’an Zhang, Beijing Jishuitan Hospital; Guozhong Lv, Wuxi Third People’s Hospital; Hongming Yang, Fourth Medical Center of the Chinese PLA General Hospital; Hongtai Tang, Changhai Hospital of Shanghai; Hongyan Zhang, First Affiliated Hospital of Nanchang University; Huade Chen, Guangdong Provincial Peoples Hospital; Jin Lei, Burn Care Center of Shanxi Province; Jinfeng Fu, The Second Affiliated Hospital of Kunming Medical University; Jingning Huan, Ruijin Hospital Affiliated to School of Medicine of Shanghai Jiaotong University; Jun Wu, The First Affiliated Hospital of Sun Yat-sen University; Kunwu Fan, Shenzhen Second People’s Hospital; Pihong Zhang, Xiangya Hospital, Central South University; Qian Tan, The Affiliated Hospital of Nanjing University Medical School (Nanjing Drum Tower Hospital); Qinglian Xu, The First Affiliated Hospital of Anhui Medical University; Shaohai Qi, The First Affiliated Hospital of Sun Yat-sen University; Shuliang Lu, Ruijin Hospital Affiliated to School of Medicine of Shanghai Jiaotong University; Weiguo Xie, Tongren Hospital of Wuhan University and Wuhan Third Hospital; Wen Lai, Guangdong Provincial Peoples Hospital; Xiaobing Fu, Chinese PLA General Hospital; Xiaojian Li, Guangzhou Red Cross Hospital, Jinan University; Xiaoyuan Huang, Xiangya Hospital, Central South University; Xihua Niu, Zhengzhou Zhengzhou First People’s Hospital; Xusheng Liu, The First Affiliated Hospital of Sun Yat-sen University; Yi Liu, General Hospital of Lanzhou Command of PLA; Yibing Wang, Provincial Hospital Affiliated to Shandong University; Yizhi Peng, Southwest Hospital, Third Military Medical University (Army Medical University); Yongming Yao, Fourth Medical Center of the Chinese PLA General Hospital; Yuesheng Huang, Southwest Hospital, Third Military Medical University (Army Medical University); Zhaofan Xia, Changhai Hospital of Shanghai; Zongyu Li, Harbin Fifth Hospital; Supportive group: Qiong Li, Songxue Guo, Xingang Wang, Fengping Liu, Chuangang You, Haitao Ren, Xuanliang Pan and Hang Hu, The Second Affiliated Hospital of Zhejiang University School of Medicine.
Contributor Information
Chun-mao Han, Department of Burns & Wound Care Center, the Second Affiliated Hospital of Zhejiang University School of Medicine, No. 88 Jiefang Road, Hangzhou 310009, China.
Biao Cheng, Department of Burns & Plastic Surgery, General Hospital of Southern Theater Command, PLA, No. 111 Liuhua Road, Guangzhou 510000, China.
Pan Wu, Department of Burns & Wound Care Center, the Second Affiliated Hospital of Zhejiang University School of Medicine, No. 88 Jiefang Road, Hangzhou 310009, China.
Authors’ contributions
CMH made major contributions to conception and design. CMH, BC and PW did the literature research for clinical data, most of the work in analysis, interpretation of data, and drafted the manuscript. All authors from writing group of growth factor guideline on behalf of Chinese Burn Association substantially contributed to the literature review, participating in meetings and formation of recommendations.
References
- 1. Sun X, Xie T. Research and related therapies of growth factors in wound healing (Chinese) [J]. J Trauma Surg. 2016; 18: 190–3. [Google Scholar]
- 2. Chen Y, Yao L, Norris S, Du L, Chen H, Zeng X, et al. Application of GRADE in systematic reviews: necessity, frequently asked questions and concerns (Chinese)[J]. Chin J Evid-based Med. 2013; 13: 1401–4. [Google Scholar]
- 3. Zeng X, Leng W, Li S, Guo Y, Wang P. How to understand and use GRADE system correctly? A briefly outline (Chinese) [J]. Chin J Evid-based Med. 2011; 11: 985–90. [Google Scholar]
- 4. Wang S, Cui X, Fu X, Zhou L, Ma J, Yan S. Recombinant human basic fibroblast growth factor in treating burns and other skin injuries (Chinese) [J]. Chin J Traumatol. 1999; 15: 196–9. [Google Scholar]
- 5. Zhang C, Hong S, Gu C, Huang Z, Du S. Clinical observation of recombinant bovine basic fibroblast growth factor in treating second degree burn wounds (Chinese) [J]. Chin J Burns. 2001; 17: 246–6. [Google Scholar]
- 6. Li X, Hong A, Xu H, Yao C, Fu X, Lin J. The clinical study of recombinant bovine basic fibroblast growth factor on wound healing (Chinese) [J]. Journal of Jinan University (Medicine Edition) 2002; 23: 22–7. [Google Scholar]
- 7. Li Z, Huang L, Yang X, Wang C. Application of basic fibroblast growth factor on burn surface (Chinese) [J]. Acad J Sec Mil Med Univ. 2004; 25: 349–9. [Google Scholar]
- 8. Liu Y, Fu Y. Healing effect of bFGF on burn wound degree II of children (Chinese) [J]. Journal of Pediatric Pharmacy. 2005; 4: 20–1. [Google Scholar]
- 9. Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, et al. Randomised placebo-controlled trial of use of topical recombinant bovine basic fibroblast growth factor for second-degree burns [J]. Lancet. 1998; 352: 1661–4. [DOI] [PubMed] [Google Scholar]
- 10. Ye L, Ma J. Therapeutic effects of Befuji (recombinant bovine bFGF solution) on skin wounds and major joint mobility of burn patients (Chinese) [J]. Chinese Journal of Clinical Rehabilitation. 2002; 6: 543–3. [Google Scholar]
- 11. Sun R, Zhao L, Sun J, Li D, Huai Q, Xu L. Clinical observation of modified chitin combined with recombinant human basic fibroblast growth factor in treating superficial partial-thickness burn wound (Chinese) [J]. Chin J Injury Repair and Wound Healing 2018; 13: 269–72. [Google Scholar]
- 12. Liu H, Chen W, Xu H, Hu J, Zhang X. Combined application of EGF and bFGF on early deep partial-thickness burns (Chinese) [J]. Guangdong Medical Journal. 2009; 30: 800–1. [Google Scholar]
- 13. Nie K, Li P, Zeng X, Sun G, Jin W, Wei Z, et al. Clinical observation of basic fibroblast growth factor combined with topical oxygen therapy in enhancing burn wound healing (Chinese) [J]. Chinese Journal of Reparative and Reconstructive Surgery. 2010; 24: 643–6. [PubMed] [Google Scholar]
- 14. Hayashida K, Akita S. Quality of pediatric second-degree burn wound scars following the application of basic fibroblast growth factor: results of a randomized, controlled pilot study [J]. Ostomy Wound Manage. 2012; 58: 32–6. [PubMed] [Google Scholar]
- 15. Yao Y, Fei C, Li Z, Wei W, Wang Y. A comparative study on wound healing treated by different doses of bovine basic fibroblast growth factor (Chinese) [J]. Chin J Burns 2001; 17: 10–2. [PubMed] [Google Scholar]
- 16. Hu Y, Guo S, Lu K, Yang Z. Application of basic fibroblast growth factor on the abrasion of face (Chinese) [J]. Chinese Journal of Aesthetic Medicine. 2001; 10: 114–5. [Google Scholar]
- 17. Sone P, Xue B, Ding Y, Yang J. Comparison of three different treatments for unhealing abdominal incisions (Chinese) [J]. Journal of Chongqing Medical University. 2011; 36: 1400–1. [Google Scholar]
- 18. Zhao Z, Jing Z, Bao J, Zhao J, Qu L, Lu Q. Effect of basic fibroblast growth factor on the incision healing of vascular surgery (Chinese) [J]. Chin J Gen Surg. 2001; 16: 27–8. [Google Scholar]
- 19. Cheng W, Wang Z, Yang W, He J, Yuan J. Therapeutic effects of topical recombinant bovine basic fibroblast growth factor spray on maxillofacial soft tissue abrasion (Chinese) [J]. Clinical Medicine. 2011; 31: 37–8. [Google Scholar]
- 20. Fu X, Shen Z, Chen Y, Xie J, Guo Z, Zhang M, et al. Basic fibroblast growth factor (bFGF) and wound healing, a multi-center and controlled clinical trial in 1024 cases (Chinese) [J]. Chinese J Reparative and Reconstructive Surgery. 1998; 12:209–11. [PubMed] [Google Scholar]
- 21. Chen Q, Li J, Yao J, Wu S, Shen X, Song Z, et al. The clinical observation of the basic fibroblast growth factor on skin graft donor site (Chinese) [J]. Journal of Practical Aesthetic and Plastic Surgery. 2001; 12: 302–4. [Google Scholar]
- 22. Wu J, Fu Z, Chen W, Gu S, Yang J. Therapeutic effect of basic fibroblast growth factor on the wound healing of skin graft donor site (Chinese) [J]. The Journal of Practical Medicine. 2003; 19: 1145–6. [Google Scholar]
- 23. Wang Q, Hu Q, Liu D. Effect of basic fibroblast growth factor treatment on skin graft donor site with different application methods (Chinese) [J]. Sichuan Medical Journal. 2010; 31: 214–5. [Google Scholar]
- 24. Fu X, Guo Z, Sheng Z. Effects of basic fibroblast growth factor on the healing of cutaneous chronic wounds (Chinese) [J]. Chinese J Reparative and Reconstructive Surgery. 1999; 13: 270–2. [PubMed] [Google Scholar]
- 25. Rong Z, Wang L, Ba T. Application of basic fibroblast growth factor on chronic refractory wound (Chinese) [J]. Chin J Exp Clin Infect Dis (Electronic Edition) 2012; 6: 580–2. [Google Scholar]
- 26. Pan D, Ye G, Wu C, Xu S, Wang S, Qin Y. Prosthetic effect of recombinant human basic fibroblast growth factor combined with silvadene cream on the healing of chronic and refractory wound (Chinese) [J]. Journal of Wenzhou Medical University. 2014; 44: 294–6. [Google Scholar]
- 27. Fu X, Shen Z, Guo Z, Zhang M, Sheng Z. Healing of chronic cutaneous wounds by topical treatment with basic fibroblast growth factor (Chinese) [J]. Chin Med J (English). 2002; 115: 331–5. [PubMed] [Google Scholar]
- 28. Le J, Jiang J, Wu J. Effect of basic fibroblast growth factor on the healing of cutaneous burn wounds in diabetes patients (Chinese) [J]. Acta Univ Med Tongji 2000; 29: 545–6. [Google Scholar]
- 29. Chen Q, Ou J, Xin W, Bi Y. Effects of recombinant bovine basic fibroblast growth factor combined with alginate dressing on chronic ulcers of elderly patients (Chinese) [J]. Modern Medical Journal. 2011; 39: 412–6. [Google Scholar]
- 30. Zheng H, Fang F, Chen W, Liu L, Feng X, Xu J. Treatment of diabetic foot disease by recombinant bovine basic fibroblast growth factor: randomized controlled observation on the therapeutic effect (Chinese) [J]. Chinese Journal of Clinical Rehabilitation. 2004; 8: 6564–5. [Google Scholar]
- 31. Richard J, Parer-Richard C, Daures J, Clouet S, Vannereau D, Bringer J, et al. Effect of topical basic fibroblast growth factor on the healing of chronic diabetic neuropathic ulcer of the foot. A pilot, randomized, double-blind, placebo-controlled study [J]. Diabetes Care. 1995; 18: 64–9. [DOI] [PubMed] [Google Scholar]
- 32. Uchi H, Igarashi A, Urabe K, Koga T, Nakayama J, Kawamori R, et al. Clinical efficacy of basic fibroblast growth factor (bFGF) for diabetic ulcer. Eur J Dermatol. 2009; 19: 461–8. [DOI] [PubMed] [Google Scholar]
- 33. Zhang C, Yang J, Feng Y, Wang H, Li X, Chen N. Clinical observation of negative pressure wound therapy and basic fibroblast growth factor in the treatment of intractable pressure ulcer (Chinese) [J]. Natl Med J China. 2012; 92: 2862–4. [PubMed] [Google Scholar]
- 34. Robson M, Phillips L, Lawrence W, Bishop J, Youngerman J, Hayward P, et al. The safety and effect of topically applied recombinant basic fibroblast growth factor on the healing of chronic pressure sores[J]. Ann Surg. 1992; 216: 401–6discussion 406-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ohura T, Nakajo T, Moriguchi T, Oka H, Tachi M, Ohura N Jr et al. Clinical efficacy of basic fibroblast growth factor on pressure ulcers: case-control pairing study using a new evaluation method [J]. Wound Repair Regen. 2011; 19: 542–51. [DOI] [PubMed] [Google Scholar]
- 36. Robson M, Hill D, Smith P, Wang X, Meyer-Siegler K, Ko F, et al. Sequential cytokine therapy for pressure ulcers: clinical and mechanistic response [J]. Ann Surg. 2000; 231: 600–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gan L, Fu Y. Healing effect of bFGF on thin-partial-thickness grafting of children (Chinese) [J]. Journal of Pediatric Pharmacy. 2005; 11: 19–20. [Google Scholar]
- 38. He X, Zeng X. Application of Beifuji on split thin-partial-thickness autografting of medium thickness skin donor sites (basic fibroblast growth factor) (Chinese) [J]. Practical Clinical Medicine. 2008; 9: 65–6. [Google Scholar]
- 39. He L, Liu H, Xu Y. Li and Sun D. treatment of basic fibroblast growth factor on skin wounds in 65 cases (Chinese) [J]. Chin J Traumatol. 2000; 16: 135. [Google Scholar]
- 40. Zhang S, Han C. Therapeutic effects of basic fibroblast growth factor on CO2 laser treated wounds (Chinese) [J]. Chin J Dermatol. 2001; 34: 141–2. [Google Scholar]
- 41. Dai X. Recombinant basic fibroblast growth factor in the treatment of II° acute radiodermatitis (Chinese) [J]. Journal of Chongqing Medical University. 2001; 26: 337. [Google Scholar]
- 42. Ma B, Cheng D, Xia Z, Ben D, Lu W, Cao Z, et al. Randomized, multicenter, double-blind, and placebo-controlled trial using topical recombinant human acidic fibroblast growth factor for deep partial-thickness burns and skin graft donor site [J]. Wound Repair Regen. 2007; 15: 795–9. [DOI] [PubMed] [Google Scholar]
- 43. Cui F, Xu Q, Zheng H. Treatment of acidic fibroblast growth factor on medium and small sized deep-partial-thickness burns (Chinese) [J]. Chin J Hemorh. 2012; 22: 291. [Google Scholar]
- 44. Chai J, Sun Y, Xia Z, Liao Z, Chen H, Shen C, et al. A randomized, multi-center and parallel control trial for acidic fibroblast growth factor in the treatment of deep second degree burn (Chinese) [J]. China Pharmacist. 2015; 18: 589–91. [Google Scholar]
- 45. Zhang J, Xu Q, Zhang F. Application of acidic fibroblast growth factor on residual burn wounds (Chinese) [J]. Chin J Hemorh. 2012; 22: 292–3. [Google Scholar]
- 46. Liu F, Jiang Z, Yang J, Geng S, Zhang S, Yin L, et al. Treatment of human acidic fibroblast growth factor on diabetic foot ulcers in 23 cases (Chinese) [J]. Chinese Journal of Surgery of Integrated Traditional and Western Medicine. 2004; 10: 424–5. [Google Scholar]
- 47. Li H, Liang Z, Meng C, Li D. The efficacy and safety of recombinant human epidermal growth factor in 4 types of wounds (Chinese) [J]. Chinese Journal of New Drugs. 2003; 13: 1048–50. [Google Scholar]
- 48. Wang S, Guo Z, Zhou L, Zhou Y, Liu X, Zhou H, et al. Effects of recombinant human epidermal growth factor on the healing of burn wounds (Chinese) [J]. Chin J Clin Pharmacol. 1998; 14: 150–4. [Google Scholar]
- 49. Li C, Yu D, Qin F, Chen Z, Sun Y. Clinical experiment study of epidermal growth factor (EGF) on the promotion of burns wound healing (Chinese) [J]. Chin J Clin Pharmacol. 2001; 17: 27–30. [Google Scholar]
- 50. Zhou L, Wang S, Ma J, Chai J, Xu M. Recombinant human epidermal growth factor in treatment of 100 cases of burn wounds (Chinese) [J]. Chinese New Drugs Journal. 2000; 9: 399–401. [Google Scholar]
- 51. Zhou L, Wang S, Ma J, Chai J, Li L. A multi-center clinical trial of topical recombinant human epidermal growth factor on burn wounds (Chinese) [J]. Chin J New Dugs Clin Rem. 2000; 1: 337–40. [Google Scholar]
- 52. Guo X, Tan M, Guo L, Xiong A, Li Y, He X. Clinical study on repair of burn wounds of degree II with recombinant human epidermal growth factor in elderly patients (Chinese) [J]. Chin J Reparative Reconstructive Surgery. 2010; 24: 462–4. [PubMed] [Google Scholar]
- 53. Li X, Zhao B, Qian L, Wu Z. Clinical observation of healing efficacy of recombinant human epidermal growth factor in treatment of burn wounds (Chinese) [J]. China Journal of Modern Medicine. 2005; 15: 2683–5. [Google Scholar]
- 54. Peng W, Yu J, Shu H. Effects of recombinant human epidermal growth factor on the healing of second degree burn wounds (Chinese) [J]. Guangdong Medical Journal. 2002; 23: 75–6. [Google Scholar]
- 55. Tan J, Zhang B, Yu L, Li W. The role of topical recombinant human epidermal growth factor on the healing of second degree burn wounds (Chinese) [J]. Journal of Practical Medicine. 2001; 17: 872–3. [Google Scholar]
- 56. Wang J, Li J, Zhang G, Wu X, Wei T, Chen J. Clinical observation of epidermal growth factor on wound repair (Chinese) [J]. Chin Hosp Pharm J. 2001; 21: 296–7. [Google Scholar]
- 57. Liu X, Huang Y, Wang J, Yang Z. Relationship between improving burn wound management and raising cure rate (Chinese) [J]. Acta Academiae Medicinae Militaris Tertiae. 2000; 22: 1197–200. [Google Scholar]
- 58. Liang Z, Li H, Meng C, Sun X. A comparison of repaired effect of recombinant human epidermal growth factor for the facial degree II burn wounds (Chinese) [J]. Chinese Journal of New Drugs. 2006; 15: 812–4. [Google Scholar]
- 59. Yi C, Chen Y, Wei D, Lu W, Huan J, Ben D. Acceleration of II degree burn wound healing with topical application of recombinant human epidermal growth factor (Chinese) [J]. Chinese Journal of Traumatology. 1998; 14: 350–2. [Google Scholar]
- 60. Wang Z, Ge L, Rong X, Wei H, Liu L, Yang R, et al. Irradiated pigskin graft dressing and recombinant human epidermal growth factor gel application in treatment of small-area partial-thickness burn: report of 135 cases (Chinese) [J]. Acta Academiae Medicinae Militaris Tertiae. 2010; 32: 71–3. [Google Scholar]
- 61. Wang G, Xia Z, Zhu S, Tang H, Huan J, Chen Y, et al. Clinical observation of the long-term effects of rhEGF on deep partial-thickness burn wounds (Chinese) [J]. Chin J Burns. 2003; 19: 167–8. [PubMed] [Google Scholar]
- 62. Zhou Z, Huang X. Effect of recombinant human epidermal growth factor gel combined with nano silver dressing on total bacterial culture positive rate and healing time in patients with deep second degree burn wounds. Chin J Ctrl Endem Dis. 2017; 32: 1400. [Google Scholar]
- 63. Zhang X, Ding X, Lin X, Yang P, Lin K. Clinical observation of recombinant human epidermal growth factor combined with lipoic acid in the treatment of diabetic foot (Chinese) [J]. China Pharmacy. 2016; 27: 4147–9. [Google Scholar]
- 64. Wei L, Sun S, Song G, Chen S. The efficacy and safety of recombinant human epidermal growth factor combined with ionic silver dressing intreatment of diabetic foot ulcers (Chinese) [J]. Chinese General Practice. 2011; 14: 1637–9. [Google Scholar]
- 65. Afshari M, Larijani B, Fadayee M, Ghahary A, Pajouhi M, Bastanhagh M, et al. Efficacy of topical epidermal growth factor in healing diabetic foot ulcers [J]. Therapy. 2005; 2: 759–65. [Google Scholar]
- 66. Gomez-Villa R, Aguilar-Rebolledo F, Lozano-Platonoff A, Teran-Soto J, Fabian-Victoriano M, Kresch-Tronik N, et al. Efficacy of intralesional recombinant human epidermal growth factor in diabetic foot ulcers in Mexican patients: a randomized double-blinded controlled trial [J]. Wound Repair Regen. 2014; 22: 497–503. [DOI] [PubMed] [Google Scholar]
- 67. Tsang M, Wong W, Hung C, Lai K, Tang W, Cheung E, et al. Human epidermal growth factor enhances healing of diabetic foot ulcers [J]. Diabetes Care. 2003; 26: 1856–61. [DOI] [PubMed] [Google Scholar]
- 68. Song R, Shen K, Ma H, Ren L. Epidermal growth factor combined with silver ion dressing treatment for diabetic foot infections in clinical research (Chinese) [J]. Chinese Journal of Nosocomiology. 2014; 24: 4033–5. [Google Scholar]
- 69. Liu K, Xu L, Wang L. Observing the clinical effect of rhEGF for treating diabetes patients skin infection (Chinese) [J]. Chinese Pharmacological Bulletin. 2005; 21: 1535–6. [Google Scholar]
- 70. Park K, Han SH, Hong J, Han SK, Lee D, Kim B, et al. Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: a phase III multicenter, double-blind, randomized, placebo-controlled trial [J]. Diabetes Research and Clinical Practice. 2018; 142: 335–44. [DOI] [PubMed] [Google Scholar]
- 71. Xue L, Lu M, Feng L, Zhu W, Li W, Jiang M, et al. Local treatment of venous leg ulceration with rhEGF (Chinese) [J]. Chinese Journal of Experimental Surgery. 1998; 15: 124–5. [Google Scholar]
- 72. Falanga V, Eaglstein W, Bucalo B, Katz M, Harris B, Carson P. Topical use of human recombinant epidermal growth factor (h-EGF) in venous ulcers [J]. J Dermatol Surg Oncol. 1992; 18: 604–6. [DOI] [PubMed] [Google Scholar]
- 73. Doerler M, Eming S, Dissemond J, Wolter A, Stoffels-Weindorf M, Reich-Schupke S, et al. A novel epidermal growth factor-containing wound dressing for the treatment of hard-to-heal venous leg ulcers [J]. Adv Skin Wound Care. 2014; 27: 456–60. [DOI] [PubMed] [Google Scholar]
- 74. Yu W, Liu C. Effects of recombinant human epidermal growth factor on the healing of ota mole laser-treated wounds (Chinese) [J]. Guangdong Medical Journal. 2003; 24: 270–1. [Google Scholar]
- 75. Ju M, Zheng Z, Zhang X, Chen Q, Yang X, Cao N, et al. Clinical observation of recombinant human epidermal growth factor on wound healing (Chinese) [J]. Chinese Journal of Dermatology. 2002; 35: 237. [Google Scholar]
- 76. Techapichetvanich T, Wanitphakdeedecha R, Iamphonrat T, Phothong W, Eimpunth S, Hidajat I, et al. The effects of recombinant human epidermal growth factor containing ointment on wound healing and post inflammatory hyperpigmentation prevention after fractional ablative skin resurfacing: a split-face randomized controlled study [J]. Journal of Cosmetic Dermatology. 2018; 17: 756–61. [DOI] [PubMed] [Google Scholar]
- 77. Li S, Gao L, Yin W, Xu G, Xiao G. Effect of gene time on acute radiation mucositis and dermatitis (Chinese) [J]. Chinese Jounal of Radiation Oncology. 2002; 11: 30–2. [Google Scholar]
- 78. Hemanthi R. Role of recombinant human epidermal growth factor in wound healing of chronic leg ulcers [J]. International Journal of Scientific Research. 2018; 7: 59. [Google Scholar]
- 79.Kaplan G, Walsh G, Guido LS, Meyn P, Burkhardt RA, Abalos RM, et al. Novel responses of human skin to intradermal recombinant granulocyte/macrophage-colony-stimulating factor: Langerhans cell recruitment, keratinocyte growth, and enhanced wound healing. J Exp Med. 1992; 175(6): 1717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yan H, Chen J, Peng X. Recombinant human granulocyte macrophage colony-stimulating factor hydrogel promotes healing of deep partial thickness burn wounds [J]. Burns. 2012; 38: 877–81. [DOI] [PubMed] [Google Scholar]
- 81. Zhang L, Chen J, Han C. A multicenter clinical trial of recombinant human GM-CSF hydrogel for the treatment of deep second-degree burns [J]. Wound Repair Regen. 2009; 17: 685–9. [DOI] [PubMed] [Google Scholar]
- 82. Chi Y, Chai J, Luo H, Zhang Q, Feng R. Safety of recombinant human granulocyte-macrophage colony-stimulating factor in healing pediatric severe burns [J]. Genet Mol Res. 2015; 14: 2735–41. [DOI] [PubMed] [Google Scholar]
- 83. Wang Z, Zhang Q, Liao Z, Han C, Lv G, Luo C, et al. Effect of recombinant human granulocyte-macrophage colony stimulating factor on wound healing in patients with deep partial thickness bum (Chinese) [J]. Chin J Burns. 2008; 24: 107–10. [PubMed] [Google Scholar]
- 84. Lin Y, Chen M, Ding F, Wang R, Liang Z, Meng C, et al. Study of the use of recombinant human granulocyte-macrophage colony-stimulating factor hydrogel externally to treat residual wounds of extensive deep partial-thickness burn [J]. Burns. 2015; 41: 1086–91. [DOI] [PubMed] [Google Scholar]
- 85. Qu K. Effects of recombinant human granulocyte-macrophage colony-stimulating factor hydrogel on healing of deep partial-thickness burn wounds and its mechanism analysis (Chinese) [D]. Qingdao, China: University of Qingdao, 2017. [Google Scholar]
- 86. Yan D, Liu S, Zhao X, Bian H, Yao X, Xing J, et al. Recombinant human granulocyte macrophage colony stimulating factor in deep second-degree burn wound healing [J]. Medicine. 2017; 96: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Qiu X, Wang J, Yang L, Ren J. A comparative study of topical rhGM-CSF with rhEGF on residual burn wounds (Chinese) [J]. Guangdong Medical Journal. 2013; 34: 956–8. [Google Scholar]
- 88. Shi C. Recombinant human granulomacrophage colony-stimulating factor gel on residual burn wounds (Chinese) [J]. Shanxi Medical Journal. 2012; 41: 882–3. [Google Scholar]
- 89. Huang G, Sun T, Zhang L, Wu Q, Zhang K, Tian Q, et al. Combined application of alginate dressing and human granulocyte-macrophage colony stimulating factor promotes healing in refractory chronic skin ulcers [J]. Exp Ther Med. 2014; 7: 1772–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Steed D. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic ulcer study group [J]. J Vasc Surg. 1995; 21: 71–8discussion 79-81. [DOI] [PubMed] [Google Scholar]
- 91. Wieman T and Becaplermin Gel Studies Group. Clinical efficacy of becaplermin (rhPDGF-BB) gel [J]. Am J Surg. 1998; 176: 74–9. [DOI] [PubMed] [Google Scholar]
- 92. Jaiswal S, Gambhir R, Agrawal A, Harish S. Efficacy of topical recombinant human platelet derived growth factor on wound healing in patients with chronic diabetic lower limb ulcers [J]. Indian J Surg. 2010; 72: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mustoe T, Cutler N, Allman R, Goode P, Deuel T, Prause J, et al. A phase II study to evaluate recombinant platelet-derived growth factor-bb in the treatment of stage 3 and 4 pressure ulcers [J]. Arch Surg. 1994; 129: 213–9. [DOI] [PubMed] [Google Scholar]
- 94. Rees R, Robson M, Smiell J, Perry B. Becaplermin gel in the treatment of pressure ulcers: a phase II randomized, double-blind, placebo-controlled study [J]. Wound Repair Regen. 1999; 7: 141–7. [DOI] [PubMed] [Google Scholar]
- 95. Robson M, Phillips L, Thomason A, Robson L, Pierce G. Platelet-derived growth factor bb for the treatment of chronic pressure ulcers [J]. Lancet. 1992; 339: 23–5. [DOI] [PubMed] [Google Scholar]
- 96. Zhang M. The study of the clinical effects of external use of recombinant human acidic fibroblast growth factor (rhaFGF) on the burn and poor healing wound in children (Chinese) [J]. China & Foreign Medical Treatment. 2014; 10: 29–30. [Google Scholar]
- 97. Liu Y, Liu J, Li Y. Study on promotion effect of recombinant human acidic fibroblast growth factor on wound healing in children by multicenter randomized controlled trials (Chinese) [J]. China Pharmacist 2015; 18: 77–9. [Google Scholar]
- 98. Xu F, He M, Yang F. The clinical effect of recombinant bovine basic fibroblast growth factor promoting wound healing after burn (Chinese) [J]. Chin J of Clinical Rational Drug Use. 2016; 9: 35–6. [Google Scholar]
- 99. Liu Z, Xia Z, Tang H, Yang Y. Curative effects of recombinant bovine basic fibroblast growth factor gel on II degree burn wound (Chinese) [J]. Academic Journal of Second Military Medical University. 2004; 25: 1270–1. [Google Scholar]
- 100. Zheng Z, Liu J, Xie W, Wu R, Zhou H, Wan S. Application of recombinant human epidermal growth factor in the treatment of children with II degree burn (Chinese) [J]. Journal of Huazhong University of Science and Technology (Health Sciences) 2003; 32: 667–8. [Google Scholar]
- 101. Li W, Deng L. Clinical efficacy of the usage of rhEGF in combination with recombinant b-FGF on the treatment of patients with pressure ulcer (Chinese) [J]. Chinese Journal of Hospital Pharmacy. 2013; 33: 1517–9. [Google Scholar]
- 102. Tang Y, Zhang C, Liu Y, Tian Y. Observation of treatment of recombinant human epidermal growth factor combined with Rifampin Vaseline gauze on residual burn wounds (Chinese) [J]. Guangdong Medical Journal. 2009; 30: 1730–2. [Google Scholar]
- 103. Liao Y, Guo L, Ding E, He X, Xie X, Xia D. A comparative study on burn wound healing treated by different methods of recombinant human epidermal growth factor (Chinese) [J]. Chin J Reparative and Reconstructive Surgery. 2003; 17: 301–2. [PubMed] [Google Scholar]
- 104. Zhang X, Li H, Xu B, Liu S, Zhou X. The effectiveness of recombinant human epidermal growth factor gel together with infrared radiation in the treatment of pressure ulcer (Chinese) [J]. Chinese Journal of Nursing. 2009; 44: 937–8. [Google Scholar]
- 105. Wang S, Deng S, Ma J, Zhu J, Li L, Liao Z, et al. To compare the clinical healing efficacy of recombinant human epidermal growth factor of different form of drug in treatment of burn wounds (Chinese) [J]. The Chinese Journal of Clinical Pharmacology. 2002; 18: 12–4. [Google Scholar]
- 106. Wang S, Ma J, Zhou L, Liao Z, Deng S, Li L, et al. Effect of recombinant Hunan epidermal growth factor (EGF) cream on EGF concentration in serum of patients (Chinese) [J]. The Chinese Journal of Clinical Pharmacology. 2003; 19: 174–7. [Google Scholar]
- 107. Wang S, Ma J, Chai J, Zhou L, Liao Z, Huang Y, et al. Acceleration of burn wound healing with topical application of recombinant human epidermal growth factor ointments (Chinese) [J]. Chin J Reparative Reconstructive Surgery. 2002; 16: 173–6. [PubMed] [Google Scholar]
- 108. Jaschke E, Zabernigg A, Gattringer C. Recombinant human granulocyte-macrophage colony-stimulating factor applied locally in low doses enhances healing and prevents recurrence of chronic venous ulcers [J]. Int J Dermatol. 1999; 38: 380–6. [DOI] [PubMed] [Google Scholar]
- 109. Zeng H. Effects of recombinant human epidermal growth factor wet dressing on II-III stage pressure sore after stroke (Chinese) [J]. Chinese Journal of Rehabilitation Theory and Practice. 2011; 17: 292–3. [Google Scholar]
- 110. Wang S, Zhou L, Ma J. Experimental observation on ADRs of recombinant human epidermal growth factor (rhEGF) in healthy and burned volunteers (Chinese) [J]. Chinese Journal of New Drugs. 2000; 9: 34–6. [Google Scholar]
- 111. Wang S, Zhou L, Li L, Ma J, Chai J, Deng S, et al. Safety researches of recombinant human epidermal growth factor (rhEGF) cream in treating burns (Chinese) [J]. The Chinese Journal of Clinical Pharmacology. 2001; 17: 268–71. [Google Scholar]
- 112. Prada M, Roa C, Alfonso P, Acero G, Huérfano L, Vivas-Consuelo D. Cost-effectiveness analysis of the human recombinant epidermal growth factor in the management of patients with diabetic foot ulcers [J]. Diabetic Foot & Ankle 2019; 1480249: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Gilligan A, Waycaster C, Milne C. Cost-effectiveness of becaplermin gel on wound closure for the treatment of pressure injuries [J]. Wounds. 2018; 30: 174–81. [PubMed] [Google Scholar]


