Abstract
The current coronavirus pandemic is imposing unpreceded challenges to the practice of pediatric gastroenterology. These are highlighted in their impact on performing aerosol-generating endoscopy procedures and the need to accommodate longer room turnaround time for disinfection, ensuring appropriate and consistent safety measures for patients, staff and providers, and emphasizing the importance for screening patients for active coronavirus disease (COVID) infection before endoscopy when possible. Pediatric patients are less likely to exhibit severe COVID-related symptoms so survey-based screening would not be a sensitive measure to identify patients with active infections. To address the restrictions of patients coming for face to face clinic encounters, there has been rapid expansion of telehealth services in a very short time period with several difficulties encountered. To survive these challenges, pediatric gastroenterology practices need to adapt and accept flexibility in clinical operations with ongoing commitment to safety for patients and healthcare workers.
Keywords: Coronavirus, Pandemic, Pediatric, Gastroenterology, Endoscopy, Screening, Telehealth
Core Tip: This article highlights the impact of the current coronavirus pandemic on the field of pediatric gastroenterology. We present the available data on infection incidence, symptomatology and screening in children. We focus on the difficulties noted in offering endoscopy services and the accommodations needed to achieve that while maintaining safety for patients and providers. We also describe our experience with the rapid expansion of telehealth services in light of restrictions in face to face clinic visits and the challenges associated with that.
INTRODUCTION
The world has been grappling with the consequences of the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 2019 (COVID-19) disease which started in Wuhan, China in December 2019, and quickly spread across the world. It was obvious that there was inadequate preparation to face the pandemic challenges in most countries. The mortality rates have varied between countries with suspected risk factors including older patients’ age and preexisting conditions. Globally, total cases up to May 17, 2020, have exceeded 4.6 million, with more than 312000 deaths. Cases in the United States (USA) have surpassed 1.4 million with more than 88000 deaths reported, highlighting the huge burden on healthcare systems. Within the USA, the incidence and mortality rates have differed between states, possibly related to the wide range of approaches implemented between states addressing rules on social distancing and lock down and variable degree of investigating infection spread. Wide variability in reported cases and deaths was evident between regions across the country and between counties within the same state. Large clusters were encountered in the states of New York (Northeast), Illinois (Midwest), Louisiana (South) and Washington and California (West).
To limit the spread of the virus, hospitals and clinical practices across the USA took aggressive measures to protect patients and healthcare workers through a variety of processes including limiting patient access and canceling elective medical services. The pandemic has had an impact on every aspect of the pediatric gastroenterology practice requiring rapid adjustments with more challenges noted in endoscopy units, where many aerosol generating procedures take place. To compensate for the restrictions in face to face clinic encounters, there was an urgent need to roll out telehealth care which was not commonly utilized in pediatric gastroenterology. A pathway for conducting safe clinic visits and endoscopy had to be considered, weighing the potential risks of postponing visits and endoscopy.
Initial reports on COVID-19 disease focused mainly on respiratory symptoms including acute respiratory failure. However later data showed that the infection can affect other systems including the gastrointestinal (GI) system[1]. This highlights the importance of considering COVID-19 infection in patients presenting with GI symptoms to general practitioners and gastroenterologists.
COVID-19 IN CHILDREN
Incidence and general symptoms
Initially the emphasis on COVID-19 related publications was almost exclusively in adults, but more studies are now emerging in children. In China, the first pediatric COVID-19 case was reported on January 20, 2020 with 2.2% of confirmed cases noted to be among individuals < 19 years of age[2]. In the USA, the first pediatric case was on March 2, 2020 with pediatric cases, categorized as those < 18 years of age, now accounting for 3.1% of all cases according to Centers for Disease Control and Prevention (CDC) website[3].
There is no evidence to suggest that children are less susceptible to this infection though they seem to have less severe symptoms[4,5]. Severe complications including acute respiratory distress syndrome and septic shock have been noted across the pediatric age range. Infants (< 1 year of age), which accounted for 15% of pediatric COVID-19 cases in USA, have had the highest proportion of severe and critical cases[6,7]. However, this age group is thought to be underrepresented.
Presenting symptoms in children have included cough, diarrhea, rhinorrhea, sore throat, vomiting, tachypnea and fatigue[8]. Overall, fever, cough, and shortness of breath occurred at a lower frequency in children (73%) as compared to adults (93%)[3]. Infants born to COVID-19 positive mothers experienced shortness of breath, cyanosis, vomiting, feeding intolerance, tachycardia and rashes. Infants experiencing late onset symptoms, several weeks after birth, have also been reported[9]. Recently reports of multisystem inflammatory syndrome, similar to Kawasaki disease, have emerged in children[10]. Presentations included persistent fever, elevated inflammatory markers and evidence for multiorgan involvement of cardiac, renal, neurologic and GI systems[11].
Emphasis on gastrointestinal system
The most frequent GI related signs and symptoms in children with COVID-19 infection included diarrhea (13%), nausea/vomiting (11%) and abdominal pain (6%)3. Other GI symptoms noted in adults include anorexia, anosmia and GI bleeding. Diarrhea lasted a mean duration of 4.1 d and was noted either before or after a COVID-19 diagnosis[12]. In both children and adults, GI signs and symptoms were reported less frequently when compared to respiratory symptoms but this may be an underestimation. Vomiting was noted more frequently among children compared to adults. It has been shown that the virus is detectable in stool of patients diagnosed with COVID-19, which raised concerns about the potential transmission by the oral fecal route[13,14]. In fact, fecal viral excretion persisted beyond respiratory excretion in up to 82% patients for up to 11 d[15].
The potential concern for GI involvement stems from the fact that the virus uses angiotensin-converting enzyme-2 (ACE2) for cell entry[16]. This is highly expressed in the epithelial cells of the colon, ileum and esophagus[17]. ACE2 is also highly expressed in cholangiocytes, more than hepatocytes, which may explain biliary and liver involvement reported in some studies[18,19]. Incidence of liver injury ranges from 14% to 53% in hospitalized patients. Liver injury can manifest as elevated transaminases and mildly elevated bilirubin[4]. Low albumin was found to have poor prognosis[20]. Liver specimens from one COVID-19 positive patient showed moderated microvascular steatosis with mild lobular and portal injury[21].
Hospitalization and outcomes
Hospitalization rates of children with COVID-19 were in the range of 5.7%-20% (highest among infants) with intensive care unit admission rates in the range of 0.58%-2%[3]. Majority of hospitalized children had at least one underlying condition including asthma, cardiovascular disease or immunosuppression[3]. According to CDC website, hospitalization rates up until May 9, 2020 for the age groups 0-4 years and 5-17 years were 3 and 1.4 per 100000 respectively. A multicenter study of COVID-19 positive children admitted to pediatric intensive care units in the USA and Canada found that 38% needed invasive ventilation and the majority survived. The fatality rate was 4.2% in that cohort with 2 patient deaths (both had preexisting comorbidities)[22]. One death was also reported in an infant, attributed to gastric bleeding and shock[7].
Telehealth care
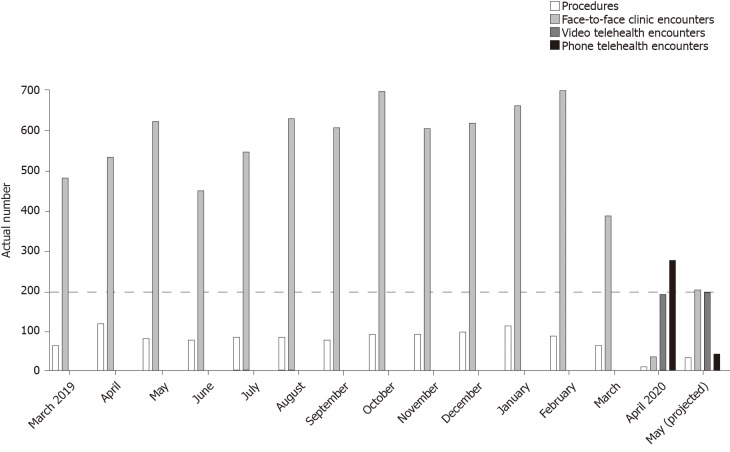
With attempts to limit the number of patients physically coming to clinics, the need to utilize telehealth technology and enable virtual patient care became a reality. This is consistent with data from the Pediatric Gastroenterology division at the University of Iowa Children's Hospital, which is the only comprehensive children’s hospital in the State of Iowa in the Midwest, USA (Figure 1). A 20% drop in face-to-face clinic encounters, down to 388, was noted in March 2020 as compared to March 2019. A more drastic drop in numbers of face-to-face clinic encounters and procedures, down to 39 and 13 respectively, was seen in April 2020 (corresponding to 93% and 89% reductions respectively) as compared to the same period the prior year. Telehealth medicine had not been utilized by the division prior to the pandemic with a rapid rise in pediatric gastroenterology telehealth services, reaching a total of 472 encounters, was noted in April 2020. By end of May 2020 (data available through May 20), the projected number of face-to-face clinic encounters and procedures had started to increase but the numbers remain below those of May 2019 (Figure 2). The number of telehealth encounters in May dropped slightly but are anticipated to continue being offered long term due to conveniences offered to families living in remote locations and those with mobility/transportation difficulties.
Figure 1.
Impact of coronavirus disease 2019 pandemic on actual number of pediatric gastroenterology encounters. Drastic reductions in procedures (black) and face-to-face clinic encounters (red) and with surge in telehealth encounters (green and blue) noted in April 2020.
Figure 2.
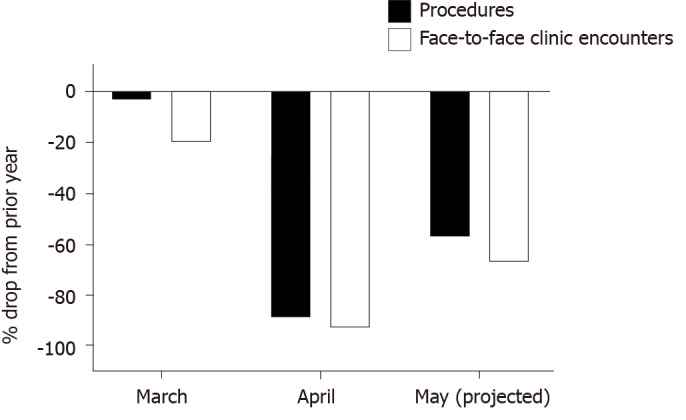
Percent changes in pediatric gastroenterology procedures and face-to-face encounters due to the coronavirus disease 2019 pandemic as compared to same period the prior year.
Several challenges were encountered in rolling out telehealth services in a short time period especially for video-based telehealth encounters. These challenges included: (1) Practice/provider access to electronic devices with microphones and cameras; (2) Practice access to telehealth platform that abides by Health Insurance Portability and Accountability Act compliance rules (maintaining patient confidently); (3) Guide and support for patients and their families to activate telehealth technology with reliably functioning video connection and proper onboarding into each encounter; and (4) Uncertainty regarding professional reimbursement for phone telehealth care and loss of facility fee reimbursement for both phone and video telehealth care.
COVID-19 IMPACT ON THE ENDOSCOPY UNIT
Endoscopy related risk
The endoscopy unit is a high-risk environment for COVID-19 transmission due to aerosolization and exposure during upper endoscopy procedures. In addition, due to concerns about detectable virus in GI secretions, both upper endoscopy and colonoscopy can exacerbate the risk of transmission. This can result in contamination of surfaces throughout the procedure room, which requires appropriate disinfection. This impacts procedural room turnover and patient flow through the endoscopy unit.
Procedure prioritization
In the COVID-19 containment stage, several GI societies published guidelines suggested focusing on urgent and emergent endoscopy cases while postponing elective cases[18]. In children, endoscopic procedure indications that fit that category include GI bleeding, foreign body ingestion, biliary pancreatitis and obstructive jaundice.
If endoscopy is needed on a suspected or confirmed COVID positive patient, the procedure should be done in a dedicated negative pressure room. Staff should wear proper personal protective equipment (PPE) for airborne, droplets, and contact precautions including N95 masks and water resistance gowns[23,24]. Proper hand hygiene should be practiced, and monitoring protocols should be in place.
Patient screening
Screening by phone for respiratory and GI symptoms associated with COVID-19 infection should be utilized before endoscopic procedures; however, these are not likely to be sensitive enough in children due to lower frequency of reported symptoms. This poses a significant limitation if screening is solely based on reported symptoms. This highlights the need to test all pediatric patients to determine COVID-19 status before endoscopy when possible. There is no data currently to guide stratifying screening by age (infants, vs toddlers vs older children). Screening can potentially occur the day before the endoscopy for non-emergent cases either in local settings or drive-through testing services. If screening is happening on site just before the procedure, the reception area should include screening area before the patient enters the waiting room.
A determination about whether the procedure can be postponed should be undertaken for patients who test positive. It is expected that some positive cases will still require endoscopy and those should be performed with adequate preparation to limit risk for healthcare providers and other patients utilizing the same endoscopy unit.
Processes to minimize risk
Endoscopy unit setup: Some authors proposed creating three zones and two passages in addition to having negative pressure operating room (OR) and endoscopy suites to decrease contamination risk[25]. Use of negative pressure rooms can serve towards the containment of airborne contaminants within the room. Duct systems within endoscopy suites may be reworked to accommodate special air pressure needs in consultation with hospital epidemiology and engineering services.
For suspected or known COVID-19 positive patients, the number of healthcare providers participating in the procedure should be limited to those absolutely necessary. This should include anesthesiology staffs that are frequently utilized in pediatric endoscopy. This will likely limit the presence of trainees and nonessential endoscopy assistants[26-28]. The number of accompanying family members or legal guardians should be minimized whenever possible and, and visitation should not be allowed at this time[29,30]. A dedicated recovery room for COVID-19 patients recovering from endoscopy should be available.
Proper personal protective equipment use: Measures to minimize the transmission risk should be applied including adequate PPE access and use with appropriate don and duff practice[23,26]. Hospitals should take every effort to make sure proper PPE supplies are available for healthcare workers involved in endoscopy procedures[29]. Processes can be implemented to decontaminate and reuse certain PPE (such as N95 masks) to maintain adequate supplies. All staff should go through training and proper education on how to don and doff PPE in designated areas within the endoscopy unit. Respirator fit testing should be in place according to institutional policies[31].
Cleaning and room turnover: Adequate time for endoscopy room cleaning and disinfecting will be needed for each case suspected or known to be COVID positive so slower room turnover is anticipated. The procedure room should be cleaned right after every case, and that process should include all surfaces including endoscopy tower, trays, bed rails, tables, chairs, computers, phones, and the floor. Alcohol-based or chlorine-based solutions with proven efficacy should be used for cleaning[32]. Disinfection of endoscopes with the current disinfection protocols seems to be adequate based on findings from a study after Middle East respiratory syndrome coronavirus (MERS-CoV) and other SARS-CoV-1 outbreak[33,34]. Training should take place to ensure that the staffs adhere with appropriate disinfection protocols.
Resumption of elective endoscopy procedures: As the rates of new COVID-19 diagnosis plateau, healthcare facilities will expand access to services including elective endoscopy. Accommodating more patients in the endoscopy suites should be done in a thoughtful and safe manner for both patients and healthcare workers. Steps to follow should include:
Patient related: (1) Patients should be evaluated for active COVID-19 infection by PCR-based testing within 48 h of endoscopy (similar to current process with increased testing capability); (2) Patients who cannot undergo PCR-based testing prior to endoscopy, should be treated as presumed positive with proper safety precautions and post procedure disinfection; and (3) Parents or legal guardians who plan to accompany patients on the day of endoscopy should be screened for COVID-19 symptoms (if positive screen noted, they should not attend and be referred to their primary provider for testing).
Endoscopy unit related: (1) Endoscopy suite employees should be screened daily for COVID-19 signs and symptoms including fever and respiratory symptoms; (2) Endoscopy suite employees, patients and accompanying parents or legal guardians should wear masks; (3) Limit number of accompanying parents or legal guardians to a minimum (preferably one per patient); (4) Space out chairs in the waiting room area; (5) Space out beds in endoscopy unit preparation and recovery areas; (6) Space out workstations for employees; and (7) Avoid routine endotracheal intubation when possible.
CONCLUSION
We are still learning about this virus and how it affects patients especially in children. Dealing with the pandemic’s challenges and uncertainties has led to changes in how we practice medicine in the containment phase with more challenges excepted as healthcare services reopen to accommodate more patients. Medical practitioners, and healthcare industries, need to adjust the way they run their business especially with the expectation that dealing with this infection will be long term at least until an effective vaccine is available. Flexibility in clinical operations, such as expanding beyond routine work hours, should be considered to address the backlog of patients. To address the financial downfall, some practices may need to consider staff furloughs and layoffs. In the short term, gastroenterology practices (both adult and pediatric) should apply and benefit from financial relief available in some countries such as the Coronavirus Aid, Relief and Economic Security (CARES) Act in the US that includes support for hospitals and physician practices. Telehealth services are likely here to stay due to the many benefits they offer but this may be hindered by reimbursement restrictions that maybe imposed in the future. We are ultimately responsible to ensure the safety of our patients and staff as well the financial survival of our practices.
Footnotes
Conflict-of-interest statement: The authors declare no conflict of interests.
Manuscript source: Unsolicited manuscript
Peer-review started: May 7, 2020
First decision: May 15, 2020
Article in press: September 11, 2020
Specialty type: Pediatrics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Choe BH, De Rose DU, Sergi C S-Editor: Wang DM L-Editor: A E-Editor:Ma YJ
Contributor Information
Jamal Kriem, Department of Pediatrics, Central Michigan University/Covenant Medical Center, Saginaw, MI 48602, United States. jkriem@gmail.com.
Riad Rahhal, Division of Pediatric Gastroenterology, University of Iowa Children’s Hospital, Iowa, IA 52242, United States.
References
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;Feb 24 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am J Gastroenterol. 2020;115:766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team. Coronavirus Disease 2019 in Children-United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. COVIDView: A Weekly Surveillance Summary of US COVID-19 Activities. Available from: URL: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html. [Google Scholar]
- 5.Hasan A, Mehmood N, Fergie J. Coronavirus Disease (COVID-19) and Pediatric Patients: A Review of Epidemiology, Symptomatology, Laboratory and Imaging Results to Guide the Development of a Management Algorithm. Cureus. 2020;12:e7485. doi: 10.7759/cureus.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S. Epidemiology of COVID-19 Among Children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 7.De Rose DU, Piersigilli F, Ronchetti MP, Santisi A, Bersani I, Dotta A, Danhaive O, Auriti C Study Group of Neonatal Infectious Diseases of The Italian Society of Neonatology (SIN) Novel Coronavirus disease (COVID-19) in newborns and infants: what we know so far. Ital J Pediatr. 2020;46:56. doi: 10.1186/s13052-020-0820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonsenso D, Costa S, Sanguinetti M, Cattani P, Posteraro B, Marchetti S, Carducci B, Lanzone A, Tamburrini E, Vento G, Valentini P. Neonatal Late Onset Infection with Severe Acute Respiratory Syndrome Coronavirus 2. Am J Perinatol. 2020;37:869–872. doi: 10.1055/s-0040-1710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahase E. Covid-19: concerns grow over inflammatory syndrome emerging in children. BMJ. 2020;369:m1710. doi: 10.1136/bmj.m1710. [DOI] [PubMed] [Google Scholar]
- 11.CDC. Information for Pediatric Healthcare Providers. Available from: https://www.cdc.gov/coronavirus/2019ncov/hcp/pediatrichcp.html#anchor_1589580133375. [Google Scholar]
- 12.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus-infected pneumonia. J Med Virol. 2020;92:680–682. doi: 10.1002/jmv.25742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu J, Han B, Wang J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng S, Fan J, Yu F, Feng B, Lou B, Zou Q, Xie G, Lin S, Wang R, Yang X, Chen W, Wang Q, Zhang D, Liu Y, Gong R, Ma Z, Lu S, Xiao Y, Gu Y, Zhang J, Yao H, Xu K, Lu X, Wei G, Zhou J, Fang Q, Cai H, Qiu Y, Sheng J, Chen Y, Liang T. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. 2020;525(1):135–140. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Cui X, Xiao J, Meng T, Zhou W, Liu J, Xu H. The digestive system is a potential route of 2019-nCov infection: a bioinformatics analysis based on single-cell transcriptomes. BioRxiv [Google Scholar]
- 18.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, Zhou J, Shi G, Fang N, Fan J, Cai J, Fan J, Lan F. Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019-nCoV Infection. BioRxiv [Google Scholar]
- 20.Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Yang M, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shekerdemian LS, Mahmood NR, Wolfe KK, Riggs BJ, Ross CE, McKiernan CA, Heidemann SM, Kleinman LC, Sen AI, Hall MW, Priestley MA, McGuire JK, Boukas K, Sharron MP, Burns JP International COVID-19 PICU Collaborative. Characteristics and Outcomes of Children With Coronavirus Disease 2019 (COVID-19) Infection Admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh CM, Fishman DS, Lerner DG NASPGHAN Endoscopy and Procedures Committee#. Pediatric Endoscopy in the Era of Coronavirus Disease 2019: A North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Position Paper. J Pediatr Gastroenterol Nutr. 2020;70:741–750. doi: 10.1097/MPG.0000000000002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brewster DJ, Chrimes N, Do TB, Fraser K, Groombridge CJ, Higgs A, Humar MJ, Leeuwenburg TJ, McGloughlin S, Newman FG, Nickson CP, Rehak A, Vokes D, Gatward JJ. Consensus statement: Safe Airway Society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020;212:472–481. doi: 10.5694/mja2.50598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. What to do if you are sick with coronavirus disease 2019 (COVID-19) Available from: https://www.cdc.gov/coronavirus/2019-ncov/downloads/sick-with-2019-nCoV-fact- sheet.pdf.
- 26.SAGES. SAGES Recommendations regarding surgical response to COVID-19 Crisis. Available from: https://www.sages.org/recommendations-surgical-response-covid-19. [Google Scholar]
- 27.Lui RN, Wong SH, Sánchez-Luna SA, Pellino G, Bollipo S, Wong MY, Chiu PWY, Sung JJY. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol. 2020;35:749–759. doi: 10.1111/jgh.15053. [DOI] [PubMed] [Google Scholar]
- 28.Potts JR., 3rd Residency and Fellowship Program Accreditation: Effects of the Novel Coronavirus (COVID-19) Pandemic. J Am Coll Surg. 2020;230:1094–1097. doi: 10.1016/j.jamcollsurg.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soetikno R, Teoh AYB, Kaltenbach T, Lau JYW, Asokkumar R, Cabral-Prodigalidad P, Shergill A. Considerations in performing endoscopy during the COVID-19 pandemic. Gastrointest Endosc. 2020;92:176–183. doi: 10.1016/j.gie.2020.03.3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joint GI Society Statement: Use of Personal Protective Equipment in GI Endoscopy. Available from: URL: https://gi.org/2020/04/01/joint-gi-society-message-on-ppe-during-covid-19/ [Google Scholar]
- 31.Suen LKP, Guo YP, Tong DWK, Leung PHM, Lung D, Ng MSP, Lai TKH, Lo KYK, Au-Yeung CH, Yu W. Self-contamination during doffing of personal protective equipment by healthcare workers to prevent Ebola transmission. Antimicrob Resist Infect Control. 2018;7:157. doi: 10.1186/s13756-018-0433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong SWX, Tan YK, Chia PY, Lee TH, Ng OT, Wong MSY, Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020;323(16):1610–1612:. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabenau HF, Kampf G, Cinatl J, Doerr HW. Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect. 2005;61:107–111. doi: 10.1016/j.jhin.2004.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson DB, Muscarella LF. Current issues in endoscope reprocessing and infection control during gastrointestinal endoscopy. World J Gastroenterol. 2006;12:3953–3964. doi: 10.3748/wjg.v12.i25.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]