Abstract
BACKGROUND
Infliximab and other intravenous biologic infusions are increasingly used for chronic disorders like inflammatory bowel disease (IBD). Rapid infliximab and home-based infusions are attractive solutions to address resource and capacity issues for infusion centres, yet infliximab infusion reactions reportedly occur in up to 25% of patients with IBD, even at the manufacturers’ recommended infusion duration of 2 h.
AIM
To evaluate the safety, cost and patient satisfaction of transitioning from hospital-based, standard 2 h to rapid home-based, 30-min infliximab infusions.
METHODS
All patients receiving rapid infliximab infusions for IBD between 2014 to 2017 (39 mo) were compared with those who received standard two-hour IFX infusions between 2005-2013 (96 mo) at a single IBD centre. Data (per-infusion and per-individual) including adverse drug reactions (ADR), duration (based on needle-departure time) and other clinical data were extracted from electronic medical records. Multivariable logistical regression analysis assessed factors potentially associated with increased risk of ADRs to rapid infusions. The primary outcome was the safety [as per relative risk (RR) of ADR] of (1) rapid 30 m infusions (both hospital- and home-based) vs standard 2 h infliximab infusions. Also, relative cost per infusion and patient satisfaction and productivity were evaluated in rapid infusion recipients who transitioned to home-based infusions.
RESULTS
Of 129 patients who received 1461 rapid IFX infusions (2014-2017) were compared with 169 patients who received 2214 standard IFX infusions (2005-2013). Within the rapid cohort, 55 (42.6%) were males, median age 42 years (range 18, 86), 114 (84%) had Crohn’s disease (CD) with a median disease duration 5 years (0, 36). Median needle to departure time was higher in the standard than the rapid protocol group, 108 (70, 253) vs 50 (33, 90) min, P < 0.001), with a per infusion cost of $AUD 107.50 vs $49.77, respectively (both P < 0.001). There was no difference in median infusion duration or costs between rapid home vs hospital-based infusions (P = 0.21). 8 patients in the rapid infliximab cohort had an ADR compared with 23 standard infliximab recipients (RR 0.55% vs 1.04% respectively), hence a higher likelihood of ADR with standard compared to rapid infusions [RR 3.0, 95%CI (1.2, 7.7), P = 0.02]. No ADRs were observed in 405 rapid home-based infusions. A lower body mass index (< 22 kg/m2), presence of one or more extra intestinal manifestations, longer disease duration (> 3 years) and previous exposure to another biologic were each independently associated with a higher likelihood of reaction (s) to rapid infusions. All (100%) survey respondents preferred the rapid vs standard infusions, however within rapid infusion recipients, 61.3% found home based infusions more inconvenient than hospital-based infusions despite a median of 0 h per week missed from paid work and no self-reported loss of work productivity.
CONCLUSION
Transitioning to rapid infliximab infusions appears very safe with significant cost benefit, patient satisfaction and avails the provision of safe, efficient, home-based infliximab infusions by IBD centres worldwide.
Keywords: Inflammatory bowel disease, Infliximab, Safety, Cost, Therapy, Drug reaction
Core Tip: Home-based infliximab infusions are a potential avenue to address overburdened infusion centres, yet enhance patient convenience. However, this depends on more rapid infusions, minimal risk of reactions and at no increased cost. This study provides a safety-centric, how-to guide for transitioning from standard 2-h hospital-based to rapid 30-min home-based infliximab infusions. An additional layer of safety is provided by careful patient selection; this study found that lower body mass index (< 22 kg/m2), presence of extraintestinal manifestation (s), longer disease duration (> 3 years) and previous biologic exposure were associated with an increased likelihood of reaction (s) to rapid maintenance infliximab infusions.
INTRODUCTION
Infliximab is a biologic monoclonal IgG antibody against tumour necrosis alpha (anti-TNF). It is a highly effective induction and maintenance treatment for multiple immune-mediated inflammatory disorders, including inflammatory bowel diseases (IBD)[1-3]. Recently in Australia and many countries worldwide, the accessibility of infliximab for indications within IBD has increased, alongside an increasing trend to use accelerated doses and shorter dose intervals to regain and/or achieve remission in certain disease subtypes[4,5]. Hence, despite a growing number of biologic options, many of which are subcutaneously administered, there has still been an increased demand for infliximab infusions over recent years. As a maintenance therapy, infliximab (and other infusion-based biologic agents) are often prescribed on a long-term basis[6-8]. Allocation of funding to resource-intensive infusion centres may not always a priority for hospital administrators, given the traditional hospital model of care is typically focussed on acute inpatient care resulting in continual pressure on infusion centres already at, or near capacity. Additionally, the cost of infusion-based treatment is expensive, with some studies suggesting that the cost of the administering infliximab is approximately 9% to 12% of the total cost of treatment[9,10].
In an effort to address these capacity and cost issues for infusion centres shortening the infusion time is one potential solution. Rapid infliximab infusions (at typically 30-60 min’ duration per infusion) have been previously identified in IBD and other indications as safe, with the potential for increased capacity yet reduce cost, plus apparently improve patient satisfaction[9,11]. Despite this, with the recent further exponential growth of infusion-based therapies for various chronic diseases, improving efficiencies within infusion centres does not appear sufficient and there is still an imperative to explore further alternatives.
Home-based infusions are therefore a logical extension from hospital or infusion-centre based infusions and are potentially appealing to both patients and health providers. Home-based infusions of many drug therapies have been demonstrated to be cost-saving and are used in many jurisdictions worldwide[12,13]. However, home-based infliximab poses a challenge given the relatively high rate of infusion reactions, including anaphylaxis and anaphylactoid reactions[14,15], the need to dilute and refrigerate the medication and the two hours required for the infusion and one hour observation period thereafter[16]. Some of these problems can be overcome to allow patients to have their infusions at home. For example, initial therapy can be completed in the hospital to monitor for any reactions and then subsequent infusions can be administered at home. Rapid infusions and home-based infusions of infliximab are currently undertaken at our hospital. The safety of this approach is yet to be comprehensively evaluated. Therefore, we aimed to evaluate the safety of transition from standard hospital-based infliximab infusions to rapid infusions and thence to home-based infusions in a protocolised manner, while also assessing efficiency, cost savings and patient satisfaction with this process.
MATERIALS AND METHODS
Patient selection and data collection
All consecutive patients with confirmed IBD attending two Australian tertiary inflammatory bowel disease centres over a 12-year period from January 2005 to March 2017 who were prescribed infliximab and commenced infusions at the study centres were identified via an IBD database and/or pharmacy dispensing records, then prospectively followed. Inpatients receiving infliximab (for example for acute severe colitis) were excluded from this analysis. All patients underwent standard dosing of infliximab 5 mg per kilogram of body weight for induction at week 0, 2 and 6 followed by maintenance infusions, where dosing/dosage interval may have been altered as per the treating clinician’s discretion, predominantly to address secondary loss of response.
Data including baseline demographic data, IBD data including disease distribution duration and complications, therapeutic data including adverse drug reactions (ADRs) and location of infliximab administration, were extracted from medical records. The severity of infliximab infusion reactions were graded retrospectively according to the Common Toxicity Criteria (CTC) version 2.0[17] from 1 to 4, with a CTC score of 1-2 graded arbitrarily defined as “mild” and 3-4 as “severe” reactions respectively.
Inclusion criteria: (1) Aged 18 and above; and (2) Received maintenance therapy infliximab between January 2005 and March 2017 for an IBD indication.
Exclusion criteria: (1) Less than age 18; (2) Received infliximab for a non – IBD indication; and (3) received infliximab as an inpatient.
Study outcome measures
The primary outcome measure in this study was the safety of infliximab infusions, with the standard infusion protocol as per manufacturer’s guidelines as the reference, compared to (1) a rapid infusion protocol; and (2) a rapid infusion protocol administered via a home-based service, comparing relative incidence of serious adverse events. Secondary outcomes assessed included the relative cost of infusion centre and home-based infliximab infusions and factors associated with a higher risk of infusion reactions in order to risk stratify patients prior to referral for home-based infusions.
Initial standard 2-h infusion data was available from patients infused between January 1, 2005 to December 31, 2013, inclusive. Rapid 30-min infliximab infusion data was available from patients infused between January 1, 2014 and March 31, 2017 inclusive and home-based rapid infliximab infusion data was available from patients infused between October 1, 2017 and December 31, 2019 inclusive. In each case, needle to departure times were collected for each patient for time spent in at an infusion centre and compared between the standard and rapid infusion groups.
Cost analysis
Moreover, a cost analysis was performed for infusion centre and home-based delivery of infliximab, comprising study centre staff costs (per unit time) and infusion-related consumables (per unit), per infusion. Total infusion time duration was measured from time of cannula insertion to the patient’s time of departure from the infusion centre, or the time of the nurse’s departure from the home setting (i.e., needle to departure time), given these events were accurately recorded in the medical records for all patients.
Satisfaction and productivity survey relating to rapid/ home-based infusions
Patients who were transitioned to home-based infusions were asked to complete a survey comprising items assessing their satisfaction regarding rapid infusions compared to standard infusions and rapid infusions administered in infusion centre-based vs home-based settings (see Appendix). The items included were customised based on feedback via qualitative interviews of a subgroup of rapid infusion patients (by investigators DvL and QaR), given no suitable, previously validated surveys were found in the literature. Responses to each item were recorded across a numerical scale from 1 (most negative) to 5 (most positive) with 3 denoted as neutral. Furthermore, the short IBD Questionnaire (sIBDQ), to measure concurrent health-related quality of life and the Work Productivity and Activity Impairment in IBD Questionnaire (WPAI-IBD) were also administered to examine perceived effects of rapid infliximab infusions (including home-based) on these domains[18]. Based on the cut-off derived by Swart et al[19], a sIBDQ score of greater than 55 (total 70 indicating excellent quality of life) was deemed to represent a “satisfactory/normal” quality of life[19,20]. Loss of work productivity and daily activity impairment (expressed as a percentage, 100% indicating complete loss/impairment) were derived from the WPAI-IBD as per Reilly et al[20]. The surveys were applied to a seven-day period including a home-based infliximab infusion.
Infliximab infusion protocols used
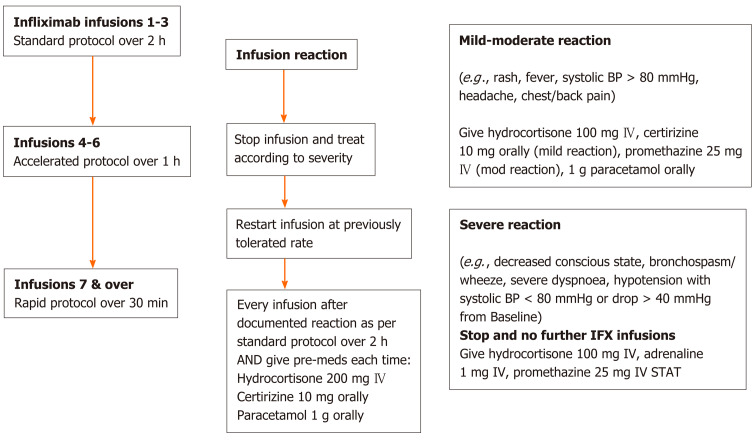
The rapid infusion protocol as applied is shown in Figure 1. Patients underwent their first 3 infusions via a standard 2-h infusion protocol, then progressed onto an “accelerated” 1-h infusion for infusion 4-6 if there were no adverse reactions and from the 7th infusion onwards then received “rapid” infusions over 30 min thereafter assuming no further reactions. ADRs were treated immediately according to severity and only those graded as mild-moderate had a subsequent re-trial of infliximab at the last tolerated infusion rate. A standardised pre-medication regimen of 200 mg intravenous hydrocortisone, 10 mg oral cetirizine and 1 g oral paracetamol was administered to all patients prior to their first three standard infusions and also immediately in the case of ADRs, as per the nursing and/or medical staff’s discretion at the time.
Figure 1.
Rapid infliximab infusion protocol. BP: Blood pressure; IV: Intravenous; IFX: Infliximab.
Subsequently, those who tolerated at least three rapid infusions without ADRs were offered the opportunity to transition their infusion to home-based infusions. Patients with known active psychiatric comorbidities, unsafe (i.e., for visiting nursing staff) or itinerant living conditions and/or previous poor adherence to medications, infusion and/or clinic visits were deemed unsuitable candidates for home-based infusions and were not offered this option.
Ethics approval
The Eastern Health Human Research Ethics Committee approved the study as a low risk audit (LR 64/2017), thus informed consent was deemed not required.
Statistical analysis
Comparisons between the infusion groups were performed using non-parametric statistics (assuming non-normal distributions) with medians (ranges) presented and Mann-Whitney tests calculated where appropriate. Proportions were expressed as percentages and compared using Fisher exact tests, including relative risks (RR) of infusion reactions/ADRs which were presented for both per infusion and per patient.
In order to determine factors potentially associated with the occurrence of one or more infusion reaction(s) to rapid infliximab infusion(s), univariable analyses [expressed as odds ratios (OR) for categorical variables, with 95% confidence intervals displayed] were performed. Then a multivariable logistic regression analysis was conducted with the occurrence of an infusion reaction(s) due to rapid infusion as the dependent variable. Multiple exploratory models were initially undertaken and forced entry models were always used, with the final model chosen on the basis of effect size and goodness of fit of the overall model. All variables with significant or those trending to significance were included in the multivariable analysis plus other putatively important factors were included, such as age and body mass index (BMI) (dichotomised by cut-off < 22 kg/m2 representing the lowest quartile in this cohort). Those factors exerting minimal or no effect size within the model (as per standardised B values) were excluded from the final model. A P value of < 0.05 was deemed to be statistically significant throughout the study.
RESULTS
Baseline demographics
The standard infusion cohort comprised 169 patients who received a total of 2214 infliximab infusions over an 8-year period (2006 to 2013 inclusive) and the rapid infusion cohort comprised 129 patients who received a total of 1461 infusions over a 4-year period (2014-2017 inclusive). Home-based rapid infusions were administered to 32 patients, with a total of 405 infusions over 2.2 years (October 2017 to December 2019 inclusive). The baseline characteristics of the standard and rapid infusion cohorts are represented in Table 1.
Table 1.
Baseline characteristics comparing the standard infusion (n = 169) and rapid infusion (n = 129) cohorts
| Variable | Standard, n (%) | Rapid, n (%) | P value |
| Male sex (%) | 76 (45.0) | 55 (42.6) | 0.72 |
| Age (yr) (median, range) | 39 (20-88) | 42 (18-86) | 0.86 |
| Low BMI (< 22 kg/m2) | 17 (10.1) | 13 (10.1) | 0.84 |
| High BMI (> 30 kg/m2) | 28 (16.6) | 31 (24.0) | 0.14 |
| Current smoker | 28 (16.6) | 26 (20.2) | 0.45 |
| Disease duration (yr) (median, range) | 7 (0-49) | 5 (0-36) | 0.78 |
| Disease type | |||
| CD | 126 (74.6) | 114 (88.4) | < 0.01 |
| UC | 43 (25.4) | 14 (10.9) | < 0.01 |
| Indeterminate | 0 (0.0) | 1 (0.8) | 0.43 |
| Extra-intestinal manifestation(s) documented | 46 (27.2) | 29 (22.5) | 0.42 |
| Psychiatric comorbidity documented | 24 (14.2) | 45 (34.9) | < 0.01 |
| Concomitant medications | |||
| Corticosteroid | 11 (6.5) | 11 (8.5) | 0.51 |
| Immunomodulator | 114 (67.5) | 104 (80.6) | 0.01 |
| Thiopurine | 91 (79.8) | 70 (67.3) | 0.04 |
| Thiopurine ADR | 32 (35.2) | 32 (45.7) | 0.20 |
| Methotrexate | 23 (20.2) | 34 (32.7) | 0.04 |
| Methotrexate ADR | 12 (52.2) | 10 (29.4) | 0.10 |
| Prior biologic | 21 (12.4) | 20 (15.5) | 0.40 |
| Other anti-TNF | 18 (85.7) | 18 (90.0) | 1.00 |
BMI: Body mass index; CD: Crohn’s disease; UC: Ulcerative colitis; ADR: Adverse drug reaction; anti-TNF: Anti-tumour necrosis factor.
Infliximab infusion ADRs and outcome of rechallenges in standard vs rapid infusion cohorts
In the standard infusion cohort, a total of 23 patients experienced ADRs directly related to infliximab infusion(s), with 16 of these graded as mild according to CTC. All 16 patients were rechallenged with infliximab of which 15 tolerated the subsequent infusion with the aid of premedications and a slower infusion rate. Seven patients had severe reactions and were not rechallenged.
In the rapid infusion cohort, 8 patients experienced ADRs due to infliximab, all of which (8/8) were mild as per the CTC. Of these, 7 patients were rechallenged with concurrent premedications administered and all seven tolerated the subsequent infusion; four patients returned to rapid infusions, two remained on accelerated infusions and one returned to the standard infusion protocol. All of the eight patients described had previously documented ADRs to standard protocol infusions.
Hence per infliximab infusion administered, the relative risk (RR) for a mild reaction was 0.8% and 0.7% and for a severe reaction was 0.2% and 0.0% in the standard infusion and rapid infusion cohorts respectively. Per patient, the overall RR for a mild reaction was 10.7% and 7.8% and for a severe reaction 3.0% and 0.0% within the standard and rapid infusion cohorts respectively, as shown in Table 2.
Table 2.
Infusion reactions per cohort, type and severity
|
Standard cohort, n (%) |
Rapid cohort, n (%) |
|
| 2214 infusions/169 patients | 1461 infusions/129 patients | |
| Mild reaction1 | ||
| RR per infusion | 0.8% | 0.7% |
| RR per patient | 10.7% | 7.8% |
| Severe reaction1 | ||
| RR per infusion | 0.2% | 0.00% |
| RR per patients | 3.0% | 0.00% |
| Total ADRs to infliximab | 23 (%) | 8 (%) |
| Mild ADRs1 (by subtype) | 16 (69.6) | 8 (100.0) |
| Serum sickness | 1 (4.3) | 1 (25.0) |
| Skin rash (including psoriasis/lupus) | 6 (26.1) | 4 (50.0) |
| Facial flushing | 1 (4.3) | 0 (0.0) |
| Hypoxia | 0 (0.0) | 2 (25.0) |
| Nausea | 2 (8.7) | 1 (12.5) |
| Pruritis | 2 (8.7) | 0 (0.0) |
| Arthralgia | 1 (4.3) | 0 (0.0) |
| Other (unspecified) | 3 (13.0) | 0 (0.0) |
| Severe ADRs1 (by subtype) | 7 (30.4) | 0 (0.0) |
| Anaphylactic (incl. angioedema) | 4 (17.4) | 0 (0.0) |
| Dyspnoea | 1 (4.3) | 0 (0.0) |
| Hypotension | 1 (4.3) | 0 (0.0) |
| Chest pain | 1 (4.3) | 0 (0.0) |
| Other (unspecified) | 0 (0.0) | 0 (0.0) |
| Retrial outcomes2 | ||
| Returned to rapid | NA | 7 |
| Returned to accelerated | NA | 1 |
| Returned to standard | 15 | 0 |
| ADR(s) occurred on retrial | 3 | 0 |
As per Common Toxicity Criteria definitions;
Fastest protocol to which tolerated on ongoing basis (i.e., ≥ 3 infusions). RR: Relative risk; ADR: Adverse drug reaction; NA: Not available.
Moreover, there was a greater likelihood of patients having an infusion reaction (mild or severe) in the standard protocol cohort than patients receiving rapid protocol infusions, RR 3.0, 95%CI (1.2, 7.7), P = 0.02. The median duration from the commencement of infliximab and the first infusion reaction per patient was 17 d (0, 1401) for standard infusions and 637 d (45, 2122) for rapid infusions respectively.
Factors associated with increased risk of infliximab infusion reactions in this cohort
A univariable logistic regression analysis was performed on all patients in the cohort who received three or more infliximab infusions via the rapid infusion protocol. Factors found to be significantly associated (P < 0.05) and/or trending to significance (0.05 ≤ P ≤ 0.15) with the occurrence(s) of infliximab ADR/s were then included into a multivariable logistic regression analysis, as shown in Table 3. In summary, a lower BMI (< 22 kg/m2), history of one or more extra intestinal manifestations, longer disease duration (> 3 years) and previous exposure to another biologic were each independently associated with a higher likelihood of infusion reaction(s) to rapid infusions (each P < 0.05). There was a non-significant trend to a higher risk also with prior episodic and/or a break in maintenance infliximab dosing.
Table 3.
Univariable and multivariable logistic regression analyses depicting factors potentially associated with increased likelihood of infliximab infusion reaction/s with a rapid infusion protocol in this cohort (n = 129)
| Variable | Univariable odds ratio (95%CI) | P value | Multivariable odds ratio (95%CI) | P value |
| Lower BMI (< 22 kg/m2) | 2.0 (0.7, 6.0) | 0.15 | 5.3 (1.3, 21.6) | 0.02 |
| Presence of ≥ 1 extra intestinal manifestation | 4.0 (1.4, 11.8) | 0.01 | 8.8 (2.3, 33.5) | < 0.01 |
| Disease duration ≥ 3 yr | 4.8 (1.1, 20.5) | 0.01 | 6.1 (1.1, 35.1) | 0.04 |
| Previous infliximab exposure (≥ 1 dose) | 5.8 (1.2, 27.2) | 0.02 | ||
| Previous other biologic exposure (any other agent) | 18.6 (1.6, 218.0) | 0.03 | 34.9 (2.1, 576.7) | 0.01 |
| Previous break off infliximab (≥ 3 m) | 5.1 (1.3, 19.4) | 0.03 | 4.8 (0.8, 28.4) | 0.08 |
| Concurrent immunomodulator (any) | 0.1 (0.01, 1.1) | 0.06 | ||
| Concurrent thiopurine | 0.3 (0.1, 1.001) | 0.06 | ||
| Previous adverse drug reaction (any) | 2.2 (0.7, 6.7) | 0.12 | ||
| Known psychiatric comorbidity | 2.4 (0.8, 6.7) | 0.10 | ||
| Pre-medication used | 0.5 (0.3, 1.00) | 0.08 |
Final multivariable model characteristics: Omnibus goodness of fit chi-square = 28.9, P < 0.001, Nagelkerke’s R2 = 0.40. BMI: Body mass index.
Comparison of infusion time/cost and productivity (standard vs rapid infusion cohort)
Median needle to departure time was significantly higher in the standard protocol than the rapid protocol group [108 (70, 253) vs 50 (33, 90) min, P < 0.001] (Figure 2). Based on local costing and consumables, this equated to a median per infusion (per chair) cost of $AUD 49.80 per rapid vs $107.50 per standard protocol infusion (P < 0.001). Hence since inception, rapid infusion protocols resulted in a net cost saving of $AUD 84344 (a net 54% cost reduction) across the whole study centre cohort and follow-up period.
Figure 2.
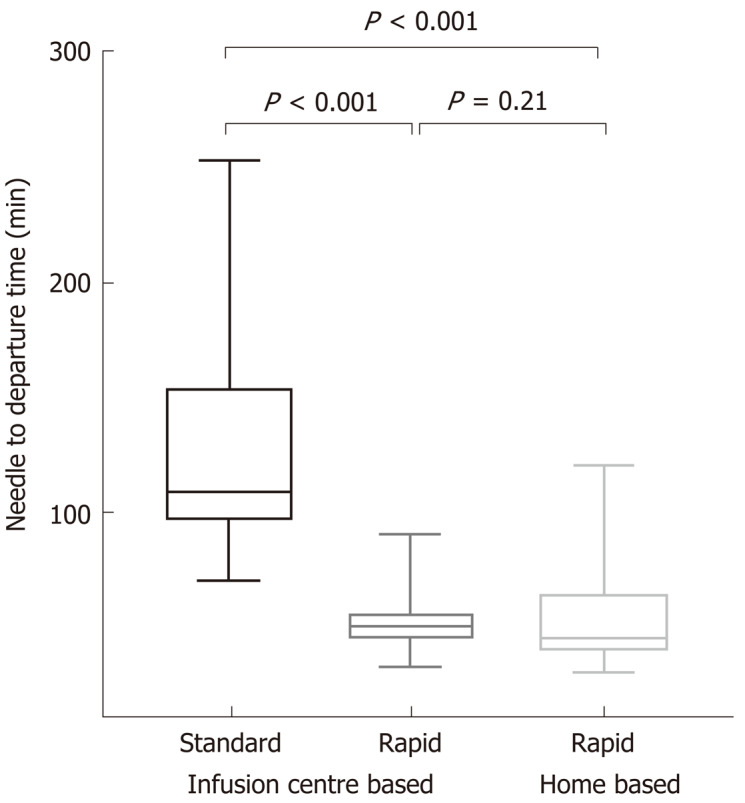
Needle-to-departure times for standard vs rapid (further subdivided by infusion centre and home based) infliximab protocols.
Transition from infusion centre to home-based rapid infusions: Safety and cost analysis
The baseline characteristics of the rapid home and rapid infusion centrecohorts are represented in Table 4. 32 patients were transitioned to home-based infusions having received at least three rapid infusions in hospital infusion centres with no history of infusion reaction/s to rapid infusions. In total, 405 infliximab infusions had hitherto been administered via the home-based service as of the end of the study follow-up period, with no (0%) reported ADRs. Six patients who subsequently withdrew from the home-based infusion service were due to personal choice (n = 3), change to a different biologic (n = 2) or death (n = 1, unrelated to IBD and/or associated therapies).
Table 4.
Characteristics of home-based vs infusion centre-based rapid protocol groups
| Variable | Home based rapid infusion group, n (%) | Infusion centre based rapid infusion group, n (%) | P value |
| Total patients/infusions | 32 / 405 | 97/1067 | - |
| Male (%) | 18 (56.2) | 37 (38.1) | 0.10 |
| Age (median, range) | 36 (18, 79) | 42(16, 86) | 0.05 |
| BMI > 30 | 7 (21.9) | 24 (24.7) | 0.82 |
| Smoker | 6 (18.8) | 20 (20.6) | 1.00 |
| Disease type | |||
| CD | 26 (81.2) | 88 (90.7) | 0.20 |
| UC | 6 (18.8) | 8 (8.2) | 0.11 |
| Indeterminate | 0 (0) | 1 (1.0) | 1.00 |
| Extra-intestinal manifestations | 9 (28.1) | 20 (20.6) | 0.46 |
| Psychiatric co-morbidity | 10 (31.2) | 35 (36.1) | 0.67 |
| ADRs (any severity) | 0 (0.0%) | 8 (8.2) | 0.20 |
BMI: Body mass index; CD: Crohn’s disease; UC: Ulcerative colitis; ADR: Adverse drug reaction.
Moreover, there was no significant difference in needle to departure times between infusion centre and home-based rapid infusions [median 50 (33, 90) vs 45 (31, 90) min respectively, P = 0.21] (Figure 2). In turn, there were similar costs (incorporating staffing and consumables) between these groups (median $49.80 vs $39.20 per infusion, P = 0.20).
Patient satisfaction with home-based infusions: Data from survey
Of the 32 patients who received infliximab via the home-based infusion service, 31 (97% response rate) completed the survey as described. 26 of the 31 were in clinical remission as per CDAI < 150 at the time of survey and the median sIBDQ score in responders was 55 (33, 65) with 57% scoring in the “normal” IBD QoL range as per Swart et al[19].
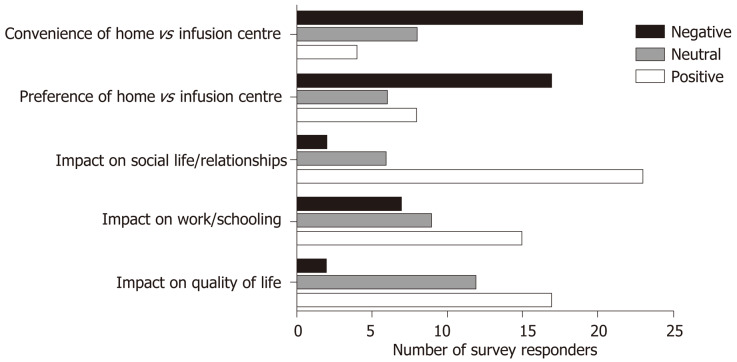
Only 2 patients (6.4%) reported that their infliximab infusions had a negative impact on their quality of life overall. All 31 (100%) patients preferred the rapid infusion vs the standard infusion protocol, with 17 patients (54.8%) specifically stating this was due to enhanced convenience (Figure 3).
Figure 3.
Results of patient satisfaction survey and preferences regarding standard vs rapid and infusion centre vs home-based infusions.
14 of the 31 responders (45.2%) disagreed with or were neutral about a preference for home-based vs infusion centre-based infusions and 19 (61.3%) found home-based infusions to actually be more inconvenient than those administered in an infusion centre (Figure 3).
Qualitatively, reasons provided for this included a lack of coordination and communication of the timing of infusions, unforeseen delays in nurse arrival at their home, lack of nursing staff (e.g., due to illness or extra demand) on certain days forcing patients to attend the parent hospital for their infusion(s), lack of sufficiently skilled nurses resulting in difficulties obtaining intravenous access and/or administering infusion at slower rates than prescribed. Additionally, others reported a reluctance to “medicalise” their home and/or have infusions in presence of family members (especially their children) or work colleagues. Also, some patients missed the familiarity of the same nursing staff and the camaraderie enabled by attending the same infusion centre, as many had done, for several years.
Impact of home-based infusions on work productivity/activity as per survey
Responses from the WPAI-IBD showed that in the week including when infliximab was infused at home, there was a median of 0 h per week missed from paid work (range 0, 36.7 h), with only 4 (12.9%) reporting a total of one or more hours of work missed in the same week. Also, the median overall work impairment (incorporating both percentage of work hours missed and impairment of work performance) was 10.0% (0, 81.0).
DISCUSSION
This study has demonstrated the safety of rapid 30-min infliximab infusions compared to standard 2-h infusions in patients with IBD. Moreover, by applying a rational stepwise protocol as per Figure 1 culminating in almost 1500 rapid infusions, none of these resulted in severe reactions. Furthermore, of the 8 patients who had mild reaction, 7 were able undertake a re-trial of infliximab on premedication with 6 successfully transitioning back to 30-min rapid infusions. These safety data are consistent with similar protocolised rapid infliximab studies previously[9,21,22], yet are critical to confidently administering infliximab infusions in home-based settings. Otherwise, without stringent protocols, infliximab infusion reactions have been reported in up to 5%-10% of infusions in IBD[15,23], which in our view does not provide adequate assurance of safety for home-based infliximab. Indeed, in this cohort, none of the home-based infusions experienced an adverse reaction as a result of this study centre’s stepwise protocol introducing rapid infusions prior to transitioning to the home.
In addition, this study is one of the first to elucidate factors associated with a higher risk of adverse reactions with maintenance rapid 30-min infusions, which enables clinicians to further mitigate the risk of adverse reactions on a case-by-case basis. Although infusion reactions to initial infliximab infusions (e.g., with induction or with episodic dosing) are typically attributable to allergic or massive cytokine release pathways, longer term reactions are postulated to be driven primarily by the development of immunogenicity (i.e., antibodies) to infliximab[24]. Accordingly, this study demonstrated that a longer disease duration (> 3 years) was associated with a higher risk of reaction(s), consistent with the known cumulative incidence of antibodies to infliximab in prospective studies[25]. Also, those with prior biologic exposure and/or a break in previous infliximab dosing were found, at least in univariate analysis, to be at higher risk of reaction(s) as has been shown elsewhere[26]. Furthermore, the presence of extraintestinal manifestations, suggestive of a more systemic inflammatory burden and in turn a greater propensity for immunogenicity – for instance due to presence of alternate cytokine-mediated, immune-complex and/or complement mediated processes – appears to be another important risk factor with rapid infusion reactions. Although there was a non-significant trend in univariable analysis to a protective effect of concomitant thiopurine/immunomodulator therapy on infusion reactions, presumably due to their negating effect on antibody development, this did not persist in multivariable analysis. Interestingly, a lower BMI (< 22 kg/m2) was associated with increased risk of rapid infusion reaction(s) despite recent publications showing increased drug clearance and lower anti-TNF drug trough levels seen with higher BMI[27,28]. However, a lower BMI with resultant reduced volume of distribution may in fact result in relatively higher infliximab trough levels and hence greater potential for immunogenicity and thus acute and delayed ADRs. Infliximab drug levels and anti-drug antibodies were not routinely performed in this cohort, so further study into the potential relationship between these factors are needed.
One of the key benefits of transitioning to 30-min infliximab infusions is that of reduced cost and enhanced productivity for an infusion centre. With introduction of rapid infusions, we demonstrated a halving of needle to departure times per infusion in a real-world context, with attendant cost reduction of 54% across the whole cohort. At an institutional level, this enables a potential doubling of throughput with the same staffing costs for an infusion centre. For the patient and at a societal level, there are additional flow-on benefits such as reduced car parking costs, reduced absence from work and/or family commitments through to improved patient productivity.
Given the increasing array of intravenous therapies, including infliximab, for chronic diseases leading to infusion centre capacity concerns in many jurisdictions worldwide, transitioning to home-based infusions is a logical solution. However, to ensure the economic sustainability of home-based infusion service, nurses theoretically need to conduct at least 3-4 infusions at patients’ homes per standard 8-h shift, allowing for travel between homes, preparation of infusions and intravenous access, communication with patients, etc. Hence administering infliximab slower than over 30 min is unlikely to be cost-effective in most cases. This study has demonstrated the safe, sustainable administration of infliximab in a home-based setting, subject to appropriate patient selection. Furthermore, this study is one of the first to assess patient satisfaction which overall was high, with 81% highly satisfied or satisfied with home-based infusions. In addition, survey responders reported minimal impact on work or other activities via the WPAI during a typical week that included a home-based infliximab infusion.
However, the study also revealed previously un-appreciated caveats to im-plementation of home-based infusions from the patients’ perspective. Almost half of the survey responders were neutral or disagreed that home-based infusions were more convenient, anecdotally for reasons including unforeseen delays to nurse arrival, variable availability of staff to administer infusions, impacts on work and social life and medicalising their home with infusions. These are unintended consequences of transitioning to home-based infusions and pose potential challenges to this model of care which therefore warrant further research and evaluation.
This study has several limitations, including the retrospective nature of data collection, however the risk of recall bias is somewhat mitigated given this study was primarily an evaluation of protocol and safety in a relatively consistent, homogeneous single centre population. Although the sample size for rapid and standard infusion cohorts were relatively large, the home-based infusion subgroup was small which may have led to selection bias and larger scale evaluation of the latter is required in future. Furthermore, given the large time period across which data was collected and local contemporary practice, we were unable to evaluate the putative relationship between infliximab trough/antibody levels and occurrence of infusion reactions. There was a difference in the rate of psychiatric disorders between the groups. While this is unlikely to have an impact on the results it does reflect that there may be some differences between the groups that was not captured in our demographic data. Prospective validation of the results is required.
CONCLUSION
In conclusion, this study comprehensively outlines a safe, patient centred approach to transitioning patients receiving infliximab for IBD from initial standard protocols through to rapid home-based infusions. With careful patient selection, we have demonstrated that the risk of ADRs with home-based infusions is negligible and thus these data are reassuring to patients, clinicians and healthcare providers alike. Whilst there are significant logistical challenges in providing a failsafe, home-based infusion service and ongoing evaluation especially in meeting patient’s care needs is warranted in this regard, most patients were satisfied with this service and the convenience it provides. This study has therefore defined a rigorous framework for the safe, potentially cost saving delivery of not only infliximab but potentially other similar medical infusions at home.
ARTICLE HIGHLIGHTS
Research background
Infliximab is one of the most commonly used biologic therapies in the management of inflammatory bowel disease (IBD). With the development of infliximab biosimilars and resulting reduction in medication cost, its availability and usage worldwide has seen exponential growth for all therapeutic indications.
Research motivation
Previous studies have suggested rapid 30 min infliximab infusions to be safe in patients with IBD. Despite this, few centres have moved to deliver infliximab therapy for IBD at a patient’s home and there remains a paucity of literature demonstrating the cost benefit of rapid infliximab infusion compared to standard therapy.
Research objectives
The primary aims in this study were to evaluate the safety of transition from standard hospital-based infliximab infusions to rapid infusions and thence to home-based infusions in a protocolised manner, while also assessing efficiency, cost savings and patient satisfaction with this process.
Research methods
This retrospective study was conducted across two tertiary IBD centres in Australia. Based on pharmacy prescribing records and historic IBD databases, patients who received infliximab over a 12-year period were identified. Data related to infliximab therapy was retrospectively obtained. Patients that were transitioned to at home infliximab were prospectively surveyed for satisfaction and productivity.
Research results
Our study reinforced previous findings in showing that rapid 30 min infliximab was safe in patients IBD with minimal side effects. Furthermore, with careful patient selection patients receiving rapid infusion infliximab were able to be safely transitioned to home based therapy. Rapid infliximab infusions resulted in the halving of time patients spent receiving an infusion and resulted in a 54% reduction in delivery related costs. Patients found the transition to rapid infliximab to be highly satisfying but were neutral about receiving infliximab therapy at home.
Research conclusions
In our study, rapid 30 min infliximab infusions appeared to be safe and in a protocolised manner could be delivered safely at home. This process has the ability to substantially reduce costs related to delivery of therapy and provides a viable alternative to under resourced infusion centres in the delivery of infusion therapy.
Research perspectives
Rapid Infliximab therapy can be delivered safely at home under the supervision of trained health care staff. Our study provides a framework for other centres to selectively transition patients from traditional infusion centres to alternative destinations such as home based. With further safety studies other infusion therapies could potentially be transitioned safety to be delivered at home at the convenience of the patient.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Human Research Ethics Committee at Eastern Health as audit activity and the committee provided a waiver for informed consent. Reference number: LR 64/2017.
Informed consent statement: The study was reviewed and approved by the Human Research Ethics Committee at Eastern Health as audit activity and the committee provided a waiver for informed consent. Reference number: LR 64/2017.
Conflict-of-interest statement: None declared.
Peer-review started: May 28, 2020
First decision: July 25, 2020
Article in press: September 5, 2020
P-Reviewer: Sozutek A, Velikova TV S-Editor: Gong ZM L-Editor: A P-Editor: Zhang YL
Contributor Information
Anuj Bohra, Department of Gastroenterology, Eastern Health, Box Hill 3128, Victoria, Australia; Eastern Health Clinical School, Monash University, Box Hill 3128, Victoria, Australia. anujbohra@hotmail.com.
Qurat-Al-Ain Rizvi, Department of Gastroenterology, Eastern Health, Box Hill 3128, Victoria, Australia.
Charlotte Yuen Yu Keung, Department of Gastroenterology, Eastern Health, Box Hill 3128, Victoria, Australia.
Abhinav Vasudevan, Department of Gastroenterology, Eastern Health, Box Hill 3128, Victoria, Australia; Eastern Health Clinical School, Monash University, Box Hill 3128, Victoria, Australia.
Daniel R van Langenberg, Department of Gastroenterology, Eastern Health, Box Hill 3128, Victoria, Australia; Eastern Health Clinical School, Monash University, Box Hill 3128, Victoria, Australia.
Data sharing statement
No additional data are available.
References
- 1.Rutgeerts PJ. Review article: efficacy of infliximab in Crohn's disease--induction and maintenance of remission. Aliment Pharmacol Ther. 1999;13 Suppl 4:9–15; discussion 38. doi: 10.1046/j.1365-2036.1999.00025.x. [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Emery P. Infliximab: 12 years of experience. Arthritis Res Ther. 2011;13 Suppl 1:S2. doi: 10.1186/1478-6354-13-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendler SA, Cohen BL, Colombel JF, Sands BE, Mayer L, Agarwal S. High-dose infliximab therapy in Crohn's disease: clinical experience, safety, and efficacy. J Crohns Colitis. 2015;9:266–275. doi: 10.1093/ecco-jcc/jju026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson DJ, Heetun ZS, Redmond CE, Nanda KS, Keegan D, Byrne K, Mulcahy HE, Cullen G, Doherty GA. An accelerated infliximab induction regimen reduces the need for early colectomy in patients with acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13:330–335.e1. doi: 10.1016/j.cgh.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 6.Polman CH, O'Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 7.Jones G, Sebba A, Gu J, Lowenstein MB, Calvo A, Gomez-Reino JJ, Siri DA, Tomsic M, Alecock E, Woodworth T, Genovese MC. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96. doi: 10.1136/ard.2008.105197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, Lukas M, Fedorak RN, Lee S, Bressler B, Fox I, Rosario M, Sankoh S, Xu J, Stephens K, Milch C, Parikh A GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2013;369:711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 9.Neef HC, Riebschleger MP, Adler J. Meta-analysis: rapid infliximab infusions are safe. Aliment Pharmacol Ther. 2013;38:365–376. doi: 10.1111/apt.12389. [DOI] [PubMed] [Google Scholar]
- 10.Saro C, da la Coba C, Casado MA, Morales JM, Otero B. Resource use in patients with Crohn's disease treated with infliximab. Aliment Pharmacol Ther. 2007;26:1313–1323. doi: 10.1111/j.1365-2036.2007.03507.x. [DOI] [PubMed] [Google Scholar]
- 11.Clare DF, Alexander FC, Mike S, Dan G, Allan F, Lisa W, Peter HJ. Accelerated infliximab infusions are safe and well tolerated in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2009;21:71–75. doi: 10.1097/MEG.0b013e3283081afe. [DOI] [PubMed] [Google Scholar]
- 12.Kuin S, Stolte SB, van den Brink GR, Ponsioen CY, Fockens P, D'Haens GR, Löwenberg M. Short article: Remicade infusions at home: an alternative setting of infliximab therapy for patients with Crohn's disease. Eur J Gastroenterol Hepatol. 2016;28:222–225. doi: 10.1097/MEG.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 13.Le Masson G, Solé G, Desnuelle C, Delmont E, Gauthier-Darnis M, Puget S, Durand-Zaleski I. Home versus hospital immunoglobulin treatment for autoimmune neuropathies: A cost minimization analysis. Brain Behav. 2018;8:e00923. doi: 10.1002/brb3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 15.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao W, Rutgeerts P ACCENT I Study Group. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 16.Jannsen Australia. Remicade Product Information. Johnson and Johnson, 2020. [Google Scholar]
- 17.European Organisation for Research and Treatement of Cancer. 1999. Common Toxicity Criteria Version 2.0. [Google Scholar]
- 18.Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn's Relapse Prevention Trial. Am J Gastroenterol. 1996;91:1571–1578. [PubMed] [Google Scholar]
- 19.Swart N, Wellsted D, Lithgo K, Price T, Johnson MW. PWE-111 Assessment and Implications of Health-Related Quality of Life in a District General Cohort of Inflammatory Bowel Disease Patients. Gut. 2013;62 Suppl 1:A176. [Google Scholar]
- 20.Reilly MC, Gerlier L, Brabant Y, Brown M. Validity, reliability, and responsiveness of the work productivity and activity impairment questionnaire in Crohn's disease. Clin Ther. 2008;30:393–404. doi: 10.1016/j.clinthera.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Bhat S, Sharma D, Doherty P, Tham TC, Caddy GR. Are accelerated infliximab infusions safe in patients with inflammatory bowel disease? Inflamm Bowel Dis. 2010;16:1922–1925. doi: 10.1002/ibd.21279. [DOI] [PubMed] [Google Scholar]
- 22.Van Assche G, Vermeire S, Noman M, Amant C, Weyts E, Vleminckx A, Vermeyen MJ, Rutgeerts P. Infliximab administered with shortened infusion times in a specialized IBD infusion unit: a prospective cohort study. J Crohns Colitis. 2010;4:329–333. doi: 10.1016/j.crohns.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Cheifetz A, Smedley M, Martin S, Reiter M, Leone G, Mayer L, Plevy S. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol. 2003;98:1315–1324. doi: 10.1111/j.1572-0241.2003.07457.x. [DOI] [PubMed] [Google Scholar]
- 24.Baert F, Noman M, Vermeire S, Van Assche G, D' Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med. 2003;348:601–608. doi: 10.1056/NEJMoa020888. [DOI] [PubMed] [Google Scholar]
- 25.Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, Bewshea CM, Chanchlani N, Walker GJ, Perry MH, McDonald TJ, Lees CW, Cummings JRF, Parkes M, Mansfield JC, Irving PM, Barrett JC, McGovern D, Goodhand JR, Anderson CA, Ahmad T PANTS Consortium. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn's Disease. Gastroenterology. 2020;158:189–199. doi: 10.1053/j.gastro.2019.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Steenholdt C, Svenson M, Bendtzen K, Thomsen OØ, Brynskov J, Ainsworth MA. Severe infusion reactions to infliximab: aetiology, immunogenicity and risk factors in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;34:51–58. doi: 10.1111/j.1365-2036.2011.04682.x. [DOI] [PubMed] [Google Scholar]
- 27.Dotan I, Ron Y, Yanai H, Becker S, Fishman S, Yahav L, Ben Yehoyada M, Mould DR. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014;20:2247–2259. doi: 10.1097/MIB.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 28.Sharma S, Eckert D, Hyams JS, Mensing S, Thakkar RB, Robinson AM, Rosh JR, Ruemmele FM, Awni WM. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn's disease: results from a randomized, multicenter, phase-3 study. Inflamm Bowel Dis. 2015;21:783–792. doi: 10.1097/MIB.0000000000000327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.