Abstract
The generation of cellular energy in the form of ATP occurs mainly in mitochondria by oxidative phosphorylation. Cytochrome c oxidase (CytOx), the oxygen accepting and rate-limiting step of the respiratory chain, regulates the supply of variable ATP demands in cells by “allosteric ATP-inhibition of CytOx.” This mechanism is based on inhibition of oxygen uptake of CytOx at high ATP/ADP ratios and low ferrocytochrome c concentrations in the mitochondrial matrix via cooperative interaction of the two substrate binding sites in dimeric CytOx. The mechanism keeps mitochondrial membrane potential ΔΨm and reactive oxygen species (ROS) formation at low healthy values. Stress signals increase cytosolic calcium leading to Ca2+-dependent dephosphorylation of CytOx subunit I at the cytosolic side accompanied by switching off the allosteric ATP-inhibition and monomerization of CytOx. This is followed by increase of ΔΨm and formation of ROS. A hypothesis is presented suggesting a dynamic change of binding of NDUFA4, originally identified as a subunit of complex I, between monomeric CytOx (active state with high ΔΨm, high ROS and low efficiency) and complex I (resting state with low ΔΨm, low ROS and high efficiency).
Keywords: Cytochrome c oxidase, Regulation of respiration, Allosteric ATP-inhibition, NDUFA4, Reversible phosphorylation, Efficiency of ATP synthesis, Dimerization of cytochrome c oxidase
Core Tip: This article describes the “allosteric ATP-inhibition of cytochrome c oxidase,” which prevents the formation of reactive oxygen species (ROS) under resting conditions in all eukaryotic cells by keeping the mitochondrial membrane potential ΔΨm at low values. Under stress – via increased calcium concentrations – this mechanism is switched off, accompanied by increased rates of ATP-synthesis with decreased efficiency and formation of deleterious ROS. A hypothesis is described in which NDUFA4 changes its position from complex I to cytochrome c oxidase when the metabolic state changes from the rest to excited state under stress.
INTRODUCTION
All expressions of life require energy in the form of ATP, the universal energy currency of living cells. Large variations in ATP turnover with rates up to 100-fold in skeletal muscle[1,2] occur in cells depending on the tissue. ATP is mostly produced in mitochondria by oxidative phosphorylation. In four enzyme complexes of the respiratory chain in the inner membrane of mitochondria, electrons from NADH and FADH2, the reduced equivalents of nutrients, are successively transferred via the final complex IV = cytochrome c oxidase (CytOx) to molecular oxygen (O2), forming water in a strongly exergonic reaction. In three complexes, complex I (NADH dehy-drogenase), complex III (ubiquinol: Cytochrome c oxidoreductase) and complex IV, the energy of this “cold combustion” of nutrients is released in an electrochemical proton gradient ΔμH+ across the membrane. Peter Mitchell described the energy of ΔμH+ as proton motive force Δp = ΔμH+/F (F = Faraday constant), consisting of an electrical (ΔΨm) and a chemical part (ΔpHm): Δp = ΔΨm − 59ΔpHm (mV)[3]. However, most of the proton motive force in mitochondria consists of ΔΨm and reaches values of 180-200 mV in isolated mitochondria at state 4 (controlled state of respiration at limited ADP)[4,5]. The energy of ΔμH+ is used by the ATP synthase (complex V), via backward flow of protons, to drive the endergonic reaction: ADP + phosphate → ATP. In the active state 3 of isolated mitochondria (presence of ADP), ΔΨm is lower (130-140 mV), since it is partly consumed by the ATP synthase.
REACTIVE OXYGEN SPECIES IN MITOCHONDRIA
Health and optimal life are frequently hurt by the consequences of psychosocial stress. The consequences appear in cells as “oxidative stress” caused by the over-production of reactive oxygen species (ROS, mainly O2.- and H2O2) in mitochondria. While low amounts of ROS have in cells signaling functions[6,7], high amounts produced in mitochondria are generally assumed to participate in aging[8-10] and in the generation of numerous diseases including cancer, hypertension, atherosclerosis, ischemia /reperfusion injury, neurodegenerative diseases like Alzheimer's and Parkinson's disease, rheumatoid arthritis, diabetes mellitus, and mitochondrial diseases[11-14].
It was found that ROS are generated in the respiratory chain at increasing ΔΨm-values above 130-140 mV[15-17]. The superoxide radical anion O2-. is mostly produced at complexes I and III, due to the transfer of a single electron to O2[18,19], and is immediately converted into H2O2 by mitochondrial superoxide dismutases[20].
In most situations of animals, e.g., during sleep, the resting state predominates with high ATP-levels, low amounts of ADP, and low consumption of ATP (at least in skeletal muscles). According to the results with isolated mitochondria at these low rates of ATP consumption (state 4, with ΔΨm values of 180-200 mV[4,5]), large amounts of ROS would be produced under resting conditions. Fortunately in resting living cells, mitochondrial ΔΨm values are low, between 100 and 130 mV (for references see[21]). These low ΔΨm values are sufficient for maximal rates of ATP synthesis, since the rate of ATP synthesis by the ATP synthase is saturated and maximal at 100-120 mV[22]. But how are these low ΔΨm values of 100-130 mV achieved to maintain a healthy life?
It was found, however, that under various stress conditions a transient increase of the mitochondrial membrane potential does occur, called “hyperpolarization,” which in some cases is followed by cell apoptosis[23]. Both, the low ΔΨm values of 100-130 mV in resting living cells and the hyperpolarization of ΔΨm under stress are explained below by the ”allosteric ATP-inhibition of CytOx.“
CYTOCHROME C OXIDASE, A CONTROLLING POINT OF OXIDATIVE METABOLISM
CytOx developed early during evolution as the final oxygen accepting enzyme of respiratory chains for the generation of ATP by oxidative phosphorylation[24]. With increasing organismal complexity during evolution the number of protein subunits in the CytOx complex increased from 2-3 in bacteria over 7 in the slime mold Dictiostelium discoideum, and 11 in yeast to 13 in mammals[25]. In eukaryotes the “catalytic” subunits I-III are encoded on mitochondrial DNA and synthesized in mitochondria. The additional “supernumerary” subunits are encoded by the nuclear DNA and synthesized on cytoplasmic ribosomes. A complicated machinery is required for the transport of these subunits into mitochondria[26] and for the assembly into the 13-subunit CytOx complex of vertebrates[27].
In contrast to many other “oxidases”[28], CytOx produces no ROS during reduction of dioxygen, due to its unique binding site for O2 in subunit I, composed of heme a3, CuB and a tyrosyl-group, allowing simultaneous transfer of 4 electrons to O2[29]. The binding site for cytochrome c containing two copper atoms is located in subunit II, and subunit three stabilizes the core subunits. The catalytic center of CytOx, located in subunits I-III, is very similar in bacteria and in eukaryotes, and the basic functions, i.e. reduction of oxygen[30] and generation of an electrochemical potential ΔμH+[31] are the same. Therefore the role of “supernumerary” subunits in the activity of CytOx was ignored. In the fourth edition of their textbook “Bioenergetics4”[32], Nicholls and Ferguson denied the catalytic function of the supernumerary subunits. However, by the use of subunit-specific antibodies for 3 of the ten nuclear encoded subunits a specific function on the activity of CytOx was demonstrated. In subunit IV: The “allosteric ATP-inhibition” via binding of ATP at its matrix domain at high ATP/ADP-ratios[33], and also in subunit IV: The decrease of cytochrome c affinity by binding ATP to the intermembrane domain at high ATP/ADP-ratios[34]. In subunit Va: The abolishment of the “allosteric ATP-inhibition” by binding of 3,5-diiodothyronine[35], and in subunit VIa-heart isoform: The decrease of H+/e--stoichiometry from 1 to 0.5 at high ATP/ADP-ratios[36].
From application of the metabolic control analysis to isolated mitochondria[37-39] a 5- to 7-fold excess of CytOx capacity was found over the amount required to support the endogenous respiration of mitochondria[40-42]. However, later studies with intact cells demonstrated that CytOx represents the rate limiting step of oxidative phos-phorylation in living cells[43,44].
FEEDBACK INHIBITION OF CYTOX BY ATP: THE ”ALLOSTERIC ATP-INHIBITION OF CYTOX“
The ”allosteric ATP-inhibition of CytOx“ based on the exchange of bound ADP by ATP at the matrix domain of CytOx subunit IV-1 at high ATP/ADP ratios originally discovered in 1997[32,45], represents a feedback inhibition of mitochondrial respiration by its final product ATP. We have described this mechanism in more than 20 publications and discussed its implications on human health more recently[46-48].
The bovine heart enzyme contains 10 high-affinity binding sites for ADP seven of which are exchanged by ATP at high ATP/ADP ratios[34]. The exchange of bound ADP by ATP at high ATP/ADP-ratios (half-maximal at ATP/ADP = 28) induces a sigmoidal inhibition curve in the kinetics of oxygen uptake vs ferrocytochrome c concentration (Hill-coefficient = 2[45]). This kinetic behaviour indicates a cooperativity between two binding sites of the substrate ferrocytochrome c. Since the CytOx monomer contains only one binding site for cytochrome C[49], a dimeric CytOx structure is required for the feedback inhibition of CytOx activity by the “allosteric effector” ATP. At lower ATP/ADP-ratios the CytOx kinetics exhibits normal hyperbolic saturation curves. The allosteric ATP-inhibition is independent of ΔΨm[45]. The ATP/ADP ratio in the mitochondrial matrix for half-maximal inhibition of CytOx activity at ATP/ADP = 28[45] corresponds to the high cytosolic ATP/ADP ratio of 100-1000 determined by 31P-NMR measurements in rat heart[50]. Due to ΔΨm the ATP/ADP-ratio in the mitochondrial matrix will be lower (ATP/ADP = 4-40, see[47]).
The first crystal structure of CytOx was a dimer[51]. But the structure of the physiological relevant CytOx dimer must be slightly different because in the crystals 10 molecules of cholate are bound per CytOx monomer[52]. The exchange of cholate by ADP in the cholate-CytOx is a slow process and accompanied by a spectral change[52]. This contrasts with the immediate exchange of bound ATP by ADP in the ADP-CytOx[53] which indicates the non-physiological structure of the cholate-CytOx crystals[51]. In fact, the crystallisation of the native ADP-CytOx or ATP-CytOx appears not possible. Only by using cholate enough CytOx could be obtained for crystallization (Kyoko Shinzawa-Itoh, personal communication). The control of respiration by the allosteric ATP-inhibition of CytOx, also named “second mechanism of respiratory control”[54], is independent of ΔΨm[45], in contrast to the classical “respiratory control” where mitochondrial respiration is limited at high ΔΨm values[5].
The allosteric ATP-inhibition of CytOx keeps ΔΨm at low values (< 130 mV), due to feedback inhibition of CytOx activity by ATP at high ATP/ADP-ratios, preventing further increase of ΔΨm by proton pumping within complexes I, III, and IV of the respiratory chain. The inhibitory effect of ATP on ΔΨm has also been measured directly in isolated rat liver mitochondria using a tetraphenyl phosphonium electrode[55]. The low ROS production in mitochondria of living cells under resting conditions[18] is thus explained by the allosteric ATP-inhibition of CytOx which maintains low ΔΨm values[46]. Therefore, this mechanism contributes to the health and optimal life of higher organisms.
It was suggested that the allosteric ATP-inhibition of CytOx contributes to an optimal efficiency of oxidative phosphorylation and is switched off under stress and excessive work in order to increase the rate of ATP synthesis which is accompanied by lower efficiency[47]. Furthermore, it was assumed that higher efficiency may be achieved by increased H+/e--stoichiometry of proton pumping in CytOx[46]. In general, a constant H+/e- = 1 was assumed for CytOx[56,57]. The Yoshikawa group identified in bovine heart CytOx a third proton channel, the H-channel[58-61] which is absent in bacterial CytOx[62]. We suggested that the allosteric ATP-inhibition which maintains low ΔΨm values could increase the H+/e--stoichiometry of CytOx to 2, based on additional proton pumping through the H-channel which is energetically possible[47]. In fact, a H+/e--stoichiometry of 2 was previously measured for CytOx in isolated rat liver mitochondria[63-66].
In bovine heart mitochondria, most CytOx (> 85%) occurs as free complexes[67] not assembled into supercomplexes like respirasomes[68]. In the respirasome I1III2IV1 CytOx appears as monomer[69,70] where the binding site between the two monomers in the dimeric crystal structure[51,71] is free and allows dimerization of two respirasomes. This holds also for the megacomplex I2III2IV2[72].
The allosteric ATP-inhibition of CytOx is active in most cell types which express subunit IV-1. The isoform subunit IV-2 was found to be expressed in human cell lines under hypoxia[73]. Also in isolated astrocytes and cerebellar granule cells subunit IV-2 is expressed under hypoxic conditions accompanied by an abolition of the allosteric inhibition of CytOx by ATP[74].
STRESS TURNS OFF THE ALLOSTERIC ATP-INHIBITION OF CYTOX VIA CYTOSOLIC CALCIUM
The fact that the feedback inhibition of CytOx by ATP has been ignored for more than 15 years is also based on its unique biochemical properties. It was found to be dependent on phosphorylation of CytOx subunit I at the cytosolic side. After dephosphorylation of this site by a calcium-activated protein phosphatase (PP1) the allosteric ATP-inhibition of CytOx is switched off. Rephosphorylation by a cAMP-dependent protein kinase (PKA) switches it on again. These observations were made with the isolated enzyme which was partly reconstituted in liposomes[75-77]. Recently these properties could also be shown with intact rat heart mitochondria. In this study a very low concentration of calcium (1-10 micromolar) was sufficient to switch off the allosteric ATP-inhibition[1]. Various stress signals, including psychosocial stress, increase the cytosolic calcium concentration and activate a Ca2+-dependent protein phosphatase which is located in the mitochondrial intermembrane space leading to dephosphorylation of CytOx at the cytosolic side of subunit I[76]. This dephosphorylation is accompanied by loss of the allosteric ATP-inhibition, an increase of ΔΨm and ROS formation[48].
In conclusion, the “allosteric ATP-inhibition of CytOx” has four physiological functions: (1) To maintain a constant high ATP/ADP ratio in cells; (2) To inhibit the oxygen consumption of mitochondria when sufficient ATP is available; (3) To prevent the formation of ROS under resting conditions by keeping the mitochondrial membrane potential ΔΨm at low values; and (4) To increase the rate of respiration and ATP synthesis during excessive workload or stress by switching it off. This is accompanied by reduced efficiency and generation of deleterious ROS.
NDUFA4, A RESPIRATORY CHAIN-ASSOCIATED FACTOR
NDUFA4 was identified as a nuclear-encoded subunit of complex I[78-80]. However, together with two other subunits its gene had significantly increased amino acid substitution rates during primate radiation, suggesting that they have been subjected to adaptive selection[81]. Later, NDUFA4 was no longer considered a subunit of complex I[82]. Recently NDUFA4 was claimed to represent the 14th subunit of mammalian CytOx[83-86]. The cryo-EM structure of a NDUFA4-CytOx complex could be determined, where NDUFA4 is bound to the CytOx monomer exactly at the binding site between the two monomers in the dimeric enzyme[87]. If NDUFA4 would represent an essential subunit of CytOx, a dimeric structure, as determined in CytOx crystals by Tsukihara et al[51], would be impossible. We doubted the claim that NDUFA4 represents the 14th subunit of mammalian CytOx. This doubt is based on immuno-precipitation of the 13-subunit CytOx from Triton X-100 dissolved rat liver mitochondria[88]. In addition, the feedback inhibition of CytOx by ATP via cooperativity of two binding sites for cytochrome c in the dimeric enzyme (the allosteric ATP-inhibition of CytOx), would be impossible with the monomeric NDUFA4-CytOx complex. Recently we concluded from studies with intact isolated rat heart mitochondria[1] that cAMP-dependent phosphorylation at the intermembrane side of CytOx subunit I[76] induces a dimeric enzyme with allosteric ATP-inhibition, while calcium-activated dephosphorylation monomerizes CytOx accompanied by abolishment of the allosteric ATP-inhibition and binding of NDUFA4[87]. These results strongly suggest that stress-dependent increase of cytosolic calcium leads to a rise of ΔΨm and ROS formation at lower efficiency due to loss of the allosteric ATP-inhibition of CytOx[47].
HYPOTHESIS
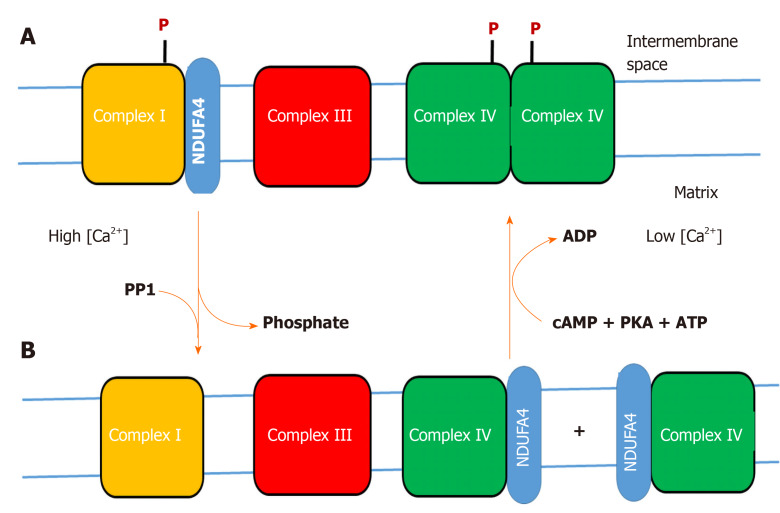
The following hypothesis describes a dynamic change of reversible protein-protein interactions which are not expected from X-ray crystal structures or cryo-EM structures but occur frequently in cells (see e.g.,[89]).
We postulate that NDUFA4 (N-terminal amino acid sequence: MLRQ) changes its binding position between complex I and CytOx, depending on the stress situation and/or energetic (ATP) requirements of cells (Figure 1). We postulate that increased cytosolic calcium concentration (> 1 micromolar), as a consequence of various stress factors, activates a calcium-dependent protein phosphatase in the intermembrane space of mitochondria, which dephosphorylates the CytOx dimer, accompanied by monomerization of CytOx, loss of the allosteric ATP-inhibition, and binding of NDUFA4. Also complex I is postulated to be dephosphorylated by a calcium-dependent protein phosphatase, accompanied by decreased affinity of complex I to NDUFA4 which binds to monomeric COX forming the NDUFA4-CytOx complex[87]. The binding of NDUFA4 to phosphorylated complex I and its dissociation after dephosphorylation could be tested by BN-PAGE with mitochondria either treated with cAMP or with calcium (see[1]), followed by immunodetection with specific antibodies. The physiological function of binding NDUFA4 to (phosphorylated) complex I under relaxed conditions could be to decrease the affinity of complex I to NADH because high NADH/NAD+-ratios were shown to stimulate ROS production in complex I[90-93].
Figure 1.
Hypothesis on the variable binding of NDUFA4 to complex I or cytochrome c oxidase. A: Phosphorylation (P) of complex I and cytochrome c oxidase (CytOx) at low cytosolic calcium (< 1 micromolar) stabilizes binding of NDUFA4 to complex I and the function of the “allosteric ATP-inhibition” of dimeric CytOx (resting state); B: Stress-induced increase of cytosolic calcium (> 1 micromolar) dephosphorylates complex I and CytOx by a calcium-activated PP1, accompanied by monomerization of CytOx and switching off its allosteric ATP-inhibition. NDUFA4 is detached from complex I and binds to monomeric CytOx (excited state). At low cytosolic calcium, a cAMP-dependent protein kinase A phosphorylates complex I and CytOx. This changes the binding position of NDUFA4 from CytOx to complex I, accompanied by dimerization of CytOx and activation of its allosteric ATP-inhibition.
Various stress signals were shown to increase cytosolic calcium concentrations in cells including high glutamate[94] or glucose[95]. In addition, psychosocial stress was shown to increase cytosolic calcium, as shown in cardiomyocytes[96], platelets[97], hippocampal-derived HT22 cells[98], urothelial cells[99], and cardiomyocytes[100]. Under resting conditions the cytosolic calcium concentration is low (about 0.1 micromolar), and a cAMP-dependent PKA rephosphorylates CytOx, accompanied by dimerization and switching on the allosteric ATP-inhibition of CytOx. This was shown in previous in vitro studies with the isolated CytOx by phosphorylation with cAMP-dependent PKA+ATP. In addition it was switched off by dephosphorylation with a calcium-activated PP1[76]. Dimerization of CytOx is possible between single CytOx complexes as well as between respirasomes since the binding domain between monomers in dimeric CytOx[71] is free in supercomplexes[70,72].
The function of binding NDUFA4 to monomeric CytOx is still unclear. The change of dimeric CytOx to monomeric CytOx (NDUFA4-CytOx) is associated with the loss of allosteric ATP-inhibition and increase of the rate of respiration and ATP synthesis at lower efficiency. The dimeric enzyme composed of two phosphorylated 13-subunit monomers represents CytOx in the resting state with higher efficiency (probably with increased H+/e- -stoichiometry = 2)[47]. Our view is different from that of Shinzawa-Itoh et al[101] who described the dimeric CytOx as the physiological standby form in the mitochondrial membrane.
The role of NDUFA4 as 14th subunit of CytOx was suggested by[84] based on mutations in the NDUFA4 gene accompanied by defective CytOx activity in patients with Leigh syndrome. In muscle tissue from patients the NDUFA4 protein was absent while the CytOx complex was still there but without activity. Since the literature is full of papers measuring CytOx activity with the isolated 13-subunit enzyme (without NDUFA4), the physiological function of NDUFA4 remains unknown. We suggest to rename it to “mitochondrial respiratory chain associated factor 1”.
CONCLUSION
A hypothesis is presented suggesting a dynamic change of binding of NDUFA4, originally identified as a subunit of complex I, between monomeric CytOx (active state with high ΔΨm, high ROS and low efficiency) and complex I (resting state with low ΔΨm, low ROS and high efficiency).
Footnotes
Conflict-of-interest statement: No conflict of interests.
Manuscript source: Invited manuscript
Peer-review started: April 16, 2020
First decision: July 25, 2020
Article in press: August 24, 2020
Specialty type: Biochemistry and molecular biology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen GX, Méndez I, Tabaran F, Tang BL S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Ma YJ
References
- 1.Ramzan R, Rhiel A, Weber P, Kadenbach B, Vogt S. Reversible dimerization of cytochrome c oxidase regulates mitochondrial respiration. Mitochondrion. 2019;49:149–155. doi: 10.1016/j.mito.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol. 1997;200:381–386. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 4.Hafner RP, Nobes CD, McGown AD, Brand MD. Altered relationship between protonmotive force and respiration rate in non-phosphorylating liver mitochondria isolated from rats of different thyroid hormone status. Eur J Biochem. 1988;178:511–518. doi: 10.1111/j.1432-1033.1988.tb14477.x. [DOI] [PubMed] [Google Scholar]
- 5.Nicholls DG, Ferguson SJ. 1992. Bioenergetics 2. Academic Press Limited, London; pp. 82–87. [Google Scholar]
- 6.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sreedhar A, Aguilera-Aguirre L, Singh KK. Mitochondria in skin health, aging, and disease. Cell Death Dis. 2020;11:444. doi: 10.1038/s41419-020-2649-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klaus S, Ost M. Mitochondrial uncoupling and longevity - A role for mitokines? Exp Gerontol. 2020;130:110796. doi: 10.1016/j.exger.2019.110796. [DOI] [PubMed] [Google Scholar]
- 10.Yegorov YE, Poznyak AV, Nikiforov NG, Sobenin IA, Orekhov AN. The Link between Chronic Stress and Accelerated Aging. Biomedicines. 2020;8:E198. doi: 10.3390/biomedicines8070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalle-Donne I, Rossi R, Colombo R, Giustarini D, Milzani A. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–623. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal. 2008;10:1343–1374. doi: 10.1089/ars.2007.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles' heel? Nat Rev Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu SS. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci Rep. 1997;17:259–272. doi: 10.1023/a:1027328510931. [DOI] [PubMed] [Google Scholar]
- 16.Korshunov SS, Skulachev VP, Starkov AA. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997;416:15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]
- 17.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–1107. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 18.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rottenberg H, Covian R, Trumpower BL. Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J Biol Chem. 2009;284:19203–19210. doi: 10.1074/jbc.M109.017376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afonso V, Champy R, Mitrovic D, Collin P, Lomri A. Reactive oxygen species and superoxide dismutases: role in joint diseases. Joint Bone Spine. 2007;74:324–329. doi: 10.1016/j.jbspin.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Hüttemann M, Lee I, Pecinova A, Pecina P, Przyklenk K, Doan JW. Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J Bioenerg Biomembr. 2008;40:445–456. doi: 10.1007/s10863-008-9169-3. [DOI] [PubMed] [Google Scholar]
- 22.Kaim G, Dimroth P. ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. EMBO J. 1999;18:4118–4127. doi: 10.1093/emboj/18.15.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadenbach B, Arnold S, Lee I, Hüttemann M. The possible role of cytochrome c oxidase in stress-induced apoptosis and degenerative diseases. Biochim Biophys Acta. 2004;1655:400–408. doi: 10.1016/j.bbabio.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Pierron D, Wildman DE, Hüttemann M, Markondapatnaikuni GC, Aras S, Grossman LI. Cytochrome c oxidase: evolution of control via nuclear subunit addition. Biochim Biophys Acta. 2012;1817:590–597. doi: 10.1016/j.bbabio.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kadenbach B. Structure and evolution of the "Atmungsferment" cytochrome c oxidase. Angew Chem Int Ed Engl. 1983;22:275–282. [Google Scholar]
- 26.Kang Y, Fielden LF, Stojanovski D. Mitochondrial protein transport in health and disease. Semin Cell Dev Biol. 2018;76:142–153. doi: 10.1016/j.semcdb.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Timón-Gómez A, Nývltová E, Abriata LA, Vila AJ, Hosler J, Barrientos A. Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin Cell Dev Biol. 2018;76:163–178. doi: 10.1016/j.semcdb.2017.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Go YM, Chandler JD, Jones DP. The cysteine proteome. Free Radic Biol Med. 2015;84:227–245. doi: 10.1016/j.freeradbiomed.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig B, Bender E, Arnold S, Hüttemann M, Lee I, Kadenbach B. Cytochrome C oxidase and the regulation of oxidative phosphorylation. Chembiochem. 2001;2:392–403. doi: 10.1002/1439-7633(20010601)2:6<392::AID-CBIC392>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 30.Pardhasaradhi K, Ludwig B, Hendler RW. Potentiometric and spectral studies with the two-subunit cytochrome aa3 from Paracoccus denitrificans. Comparison with the 13-subunit beef heart enzyme. Biophys J. 1991;60:408–414. doi: 10.1016/S0006-3495(91)82066-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendler RW, Pardhasaradhi K, Reynafarje B, Ludwig B. Comparison of energy-transducing capabilities of the two- and three-subunit cytochromes aa3 from Paracoccus denitrificans and the 13-subunit beef heart enzyme. Biophys J. 1991;60:415–423. doi: 10.1016/S0006-3495(91)82067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicholls DG, Ferguson SJ. Bioenergetics4. Academic Press Limited, San Diego, 2013. [Google Scholar]
- 33.Arnold S, Kadenbach B. Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur J Biochem. 1997;249:350–354. doi: 10.1111/j.1432-1033.1997.t01-1-00350.x. [DOI] [PubMed] [Google Scholar]
- 34.Napiwotzki J, Kadenbach B. Extramitochondrial ATP/ADP-ratios regulate cytochrome c oxidase activity via binding to the cytosolic domain of subunit IV. Biol Chem. 1998;379:335–339. doi: 10.1515/bchm.1998.379.3.335. [DOI] [PubMed] [Google Scholar]
- 35.Arnold S, Goglia F, Kadenbach B. 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur J Biochem. 1998;252:325–330. doi: 10.1046/j.1432-1327.1998.2520325.x. [DOI] [PubMed] [Google Scholar]
- 36.Frank V, Kadenbach B. Regulation of the H+/e- stoichiometry of cytochrome c oxidase from bovine heart by intramitochondrial ATP/ADP ratios. FEBS Lett. 1996;382:121–124. doi: 10.1016/0014-5793(96)00096-8. [DOI] [PubMed] [Google Scholar]
- 37.Kacser H, Burns JA. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- 38.Heinrich R, Rapoport TA. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974;42:89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 39.Fell D. Understanding the control of metabolism, in Frontiers in Metabolism 1997; 2, Portland Press, London. [Google Scholar]
- 40.Tager JM, Wanders RJA, Groen AK, Kunz W, Bohnensack R, Küster U, Letko G, Böhme G, Duszynski J, Woijtczak L. Control of mitochondrial respiration. FEBS Lett. 1981;151:1–9. doi: 10.1016/0014-5793(83)80330-5. [DOI] [PubMed] [Google Scholar]
- 41.Letellier T, Malgat M, Mazat JP. Control of oxidative phosphorylation in rat muscle mitochondria: implications for mitochondrial myopathies. Biochim Biophys Acta. 1993;1141:58–64. doi: 10.1016/0005-2728(93)90189-m. [DOI] [PubMed] [Google Scholar]
- 42.Letellier T, Heinrich R, Malgat M, Mazat JP. The kinetic basis of threshold effects observed in mitochondrial diseases: a systemic approach. Biochem J. 1994;302(Pt 1):171–174. doi: 10.1042/bj3020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villani G, Attardi G. In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc Natl Acad Sci USA. 1997;94:1166–1171. doi: 10.1073/pnas.94.4.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villani G, Greco M, Papa S, Attardi G. Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J Biol Chem. 1998;273:31829–31836. doi: 10.1074/jbc.273.48.31829. [DOI] [PubMed] [Google Scholar]
- 45.Arnold S, Kadenbach B. The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 1999;443:105–108. doi: 10.1016/s0014-5793(98)01694-9. [DOI] [PubMed] [Google Scholar]
- 46.Kadenbach B, Ramzan R, Wen L, Vogt S. New extension of the Mitchell Theory for oxidative phosphorylation in mitochondria of living organisms. Biochim Biophys Acta. 2010;1800:205–212. doi: 10.1016/j.bbagen.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 47.Kadenbach B, Ramzan R, Vogt S. High efficiency versus maximal performance--the cause of oxidative stress in eukaryotes: a hypothesis. Mitochondrion. 2013;13:1–6. doi: 10.1016/j.mito.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Ramzan R, Vogt S, Kadenbach B. Stress-mediated generation of deleterious ROS in healthy individuals - role of cytochrome c oxidase. J Mol Med (Berl) 2020;98:651–657. doi: 10.1007/s00109-020-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Osuda Y, Shinzawa-Itoh K, Tani K, Maeda S, Yoshikawa S, Tsukihara T, Gerle C. Two-dimensional crystallization of monomeric bovine cytochrome c oxidase with bound cytochrome c in reconstituted lipid membranes. Microscopy (Oxf) 2016;65:263–267. doi: 10.1093/jmicro/dfv381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobson GP, Himmelreich U. Heart design: free ADP scales with absolute mitochondrial and myofibrillar volumes from mouse to human. Biochim Biophys Acta. 2002;1553:261–267. doi: 10.1016/s0005-2728(01)00247-x. [DOI] [PubMed] [Google Scholar]
- 51.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 52.Napiwotzki J, Shinzawa-Itoh K, Yoshikawa S, Kadenbach B. ATP and ADP bind to cytochrome c oxidase and regulate its activity. Biol Chem. 1997;378:1013–1021. doi: 10.1515/bchm.1997.378.9.1013. [DOI] [PubMed] [Google Scholar]
- 53.Napiwotzki J. Bindung von Adeninnukleotiden an die Cytochrom c Oxidase und deren Regulation der Enzymaktivität. 1997; Thesis. Fachbereich Chemie, Philipps-University Marburg, Germany. [Google Scholar]
- 54.Kadenbach B, Arnold S. A second mechanism of respiratory control. FEBS Lett. 1999;447:131–134. doi: 10.1016/s0014-5793(99)00229-x. [DOI] [PubMed] [Google Scholar]
- 55.Ramzan R, Staniek K, Kadenbach B, Vogt S. Mitochondrial respiration and membrane potential are regulated by the allosteric ATP-inhibition of cytochrome c oxidase. Biochim Biophys Acta. 2010;1797:1672–1680. doi: 10.1016/j.bbabio.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 56.Babcock GT, Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992;356:301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- 57.Wikström M. Cytochrome c oxidase: 25 years of the elusive proton pump. Biochim Biophys Acta. 2004;1655:241–247. doi: 10.1016/j.bbabio.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Shimokata K, Katayama Y, Murayama H, Suematsu M, Tsukihara T, Muramoto K, Aoyama H, Yoshikawa S, Shimada H. The proton pumping pathway of bovine heart cytochrome c oxidase. Proc Natl Acad Sci U S A. 2007;104:4200–4205. doi: 10.1073/pnas.0611627104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yoshikawa S. A cytochrome c oxidase proton pumping mechanism that excludes the O2 reduction site. FEBS Lett. 2003;555:8–12. doi: 10.1016/s0014-5793(03)01098-6. [DOI] [PubMed] [Google Scholar]
- 60.Yoshikawa S, Muramoto K, Shinzawa-Itoh K, Aoyama H, Tsukihara T, Shimokata K, Katayama Y, Shimada H. Proton pumping mechanism of bovine heart cytochrome c oxidase. Biochim Biophys Acta. 2006;1757:1110–1116. doi: 10.1016/j.bbabio.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 61.Yoshikawa S, Muramoto K, Shinzawa-Itoh K. The O(2) reduction and proton pumping gate mechanism of bovine heart cytochrome c oxidase. Biochim Biophys Acta. 2011;1807:1279–1286. doi: 10.1016/j.bbabio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 62.Salje J, Ludwig B, Richter OM. Is a third proton-conducting pathway operative in bacterial cytochrome c oxidase? Biochem Soc Trans. 2005;33:829–831. doi: 10.1042/BST0330829. [DOI] [PubMed] [Google Scholar]
- 63.Costa LE, Reynafarje B, Lehninger AL. Stoichiometry of mitochondrial H+ translocation coupled to succinate oxidation at level flow. J Biol Chem. 1984;259:4802–4811. [PubMed] [Google Scholar]
- 64.Reynafarje B, Alexandre A, Davies P, Lehninger AL. Proton translocation stoichiometry of cytochrome oxidase: use of a fast-responding oxygen electrode. Proc Natl Acad Sci USA. 1982;79:7218–7222. doi: 10.1073/pnas.79.23.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reynafarje B, Costa LE, Lehninger AL. Upper and lower limits of the proton stoichiometry of cytochrome c oxidation in rat liver mitoplasts. J Biol Chem. 1986;261:8254–8262. [PubMed] [Google Scholar]
- 66.Setty OH, Shrager RI, Bunow B, Reynafarje B, Lehninger AL, Hendler RW. Direct measurement of the initial proton extrusion to oxygen uptake ratio accompanying succinate oxidation by rat liver mitochondria. Biophys J. 1986;50:391–404. doi: 10.1016/S0006-3495(86)83475-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schägger H, Pfeiffer K. The ratio of oxidative phosphorylation complexes I-V in bovine heart mitochondria and the composition of respiratory chain supercomplexes. J Biol Chem. 2001;276:37861–37867. doi: 10.1074/jbc.M106474200. [DOI] [PubMed] [Google Scholar]
- 68.Schägger H. Respiratory chain supercomplexes of mitochondria and bacteria. Biochim Biophys Acta. 2002;1555:154–159. doi: 10.1016/s0005-2728(02)00271-2. [DOI] [PubMed] [Google Scholar]
- 69.Letts JA, Fiedorczuk K, Sazanov LA. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 70.Letts JA, Sazanov LA. Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat Struct Mol Biol. 2017;24:800–808. doi: 10.1038/nsmb.3460. [DOI] [PubMed] [Google Scholar]
- 71.Lee SJ, Yamashita E, Abe T, Fukumoto Y, Tsukihara T, Shinzawa-Itoh K, Ueda H, Yoshikawa S. Intermonomer interactions in dimer of bovine heart cytochrome c oxidase. Acta Crystallogr D Biol Crystallogr. 2001;57:941–947. doi: 10.1107/s0907444901005625. [DOI] [PubMed] [Google Scholar]
- 72.Guo R, Zong S, Wu M, Gu J, Yang M. Architecture of Human Mitochondrial Respiratory Megacomplex I2III2IV2. Cell. 2017;170:1247–1257.e12. doi: 10.1016/j.cell.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 73.Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 74.Horvat S, Beyer C, Arnold S. Effect of hypoxia on the transcription pattern of subunit isoforms and the kinetics of cytochrome c oxidase in cortical astrocytes and cerebellar neurons. J Neurochem. 2006;99:937–951. doi: 10.1111/j.1471-4159.2006.04134.x. [DOI] [PubMed] [Google Scholar]
- 75.Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 76.Lee I, Bender E, Arnold S, Kadenbach B. New control of mitochondrial membrane potential and ROS formation--a hypothesis. Biol Chem. 2001;382:1629–1636. doi: 10.1515/BC.2001.198. [DOI] [PubMed] [Google Scholar]
- 77.Lee I, Bender E, Kadenbach B. Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol Cell Biochem. 2002;234-235:63–70. [PubMed] [Google Scholar]
- 78.Carroll J, Shannon RJ, Fearnley IM, Walker JE, Hirst J. Definition of the nuclear encoded protein composition of bovine heart mitochondrial complex I. Identification of two new subunits. J Biol Chem. 2002;277:50311–50317. doi: 10.1074/jbc.M209166200. [DOI] [PubMed] [Google Scholar]
- 79.Carroll J, Fearnley IM, Shannon RJ, Hirst J, Walker JE. Analysis of the subunit composition of complex I from bovine heart mitochondria. Mol Cell Proteomics. 2003;2:117–126. doi: 10.1074/mcp.M300014-MCP200. [DOI] [PubMed] [Google Scholar]
- 80.Hirst J, Carroll J, Fearnley IM, Shannon RJ, Walker JE. The nuclear encoded subunits of complex I from bovine heart mitochondria. Biochim Biophys Acta. 2003;1604:135–150. doi: 10.1016/s0005-2728(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 81.Mishmar D, Ruiz-Pesini E, Mondragon-Palomino M, Procaccio V, Gaut B, Wallace DC. Adaptive selection of mitochondrial complex I subunits during primate radiation. Gene. 2006;378:11–18. doi: 10.1016/j.gene.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Zhu J, Vinothkumar KR, Hirst J. Structure of mammalian respiratory complex I. Nature. 2016;536:354–358. doi: 10.1038/nature19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balsa E, Marco R, Perales-Clemente E, Szklarczyk R, Calvo E, Landázuri MO, Enríquez JA. NDUFA4 is a subunit of complex IV of the mammalian electron transport chain. Cell Metab. 2012;16:378–386. doi: 10.1016/j.cmet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 84.Pitceathly RD, Rahman S, Wedatilake Y, Polke JM, Cirak S, Foley AR, Sailer A, Hurles ME, Stalker J, Hargreaves I, Woodward CE, Sweeney MG, Muntoni F, Houlden H, Taanman JW, Hanna MG UK10K Consortium. NDUFA4 mutations underlie dysfunction of a cytochrome c oxidase subunit linked to human neurological disease. Cell Rep. 2013;3:1795–1805. doi: 10.1016/j.celrep.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinkler CA, Kalpage H, Shay J, Lee I, Malek MH, Grossman LI, Hüttemann M. Tissue- and Condition-Specific Isoforms of Mammalian Cytochrome c Oxidase Subunits: From Function to Human Disease. Oxid Med Cell Longev. 2017;2017:1534056. doi: 10.1155/2017/1534056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pitceathly RDS, Taanman JW. NDUFA4 (Renamed COXFA4) Is a Cytochrome-c Oxidase Subunit. Trends Endocrinol Metab. 2018;29:452–454. doi: 10.1016/j.tem.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 87.Zong S, Wu M, Gu J, Liu T, Guo R, Yang M. Structure of the intact 14-subunit human cytochrome c oxidase. Cell Res. 2018;28:1026–1034. doi: 10.1038/s41422-018-0071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kadenbach B. Regulation of Mammalian 13-Subunit Cytochrome c Oxidase and Binding of other Proteins: Role of NDUFA4. Trends Endocrinol Metab. 2017;28:761–770. doi: 10.1016/j.tem.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Minic Z, Dahms TES, Babu M. Chromatographic separation strategies for precision mass spectrometry to study protein-protein interactions and protein phosphorylation. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1102-1103:96–108. doi: 10.1016/j.jchromb.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 90.Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 91.Kussmaul L, Hirst J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A. 2006;103:7607–7612. doi: 10.1073/pnas.0510977103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Adam-Vizi V, Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 93.Hirst J, King MS, Pryde KR. The production of reactive oxygen species by complex I. Biochem Soc Trans. 2008;36:976–980. doi: 10.1042/BST0360976. [DOI] [PubMed] [Google Scholar]
- 94.Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD. Researching glutamate - induced cytotoxicity in different cell lines: a comparative/collective analysis/study. Front Cell Neurosci. 2015;9:91. doi: 10.3389/fncel.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li WA, Moore-Langston S, Chakraborty T, Rafols JA, Conti AC, Ding Y. Hyperglycemia in stroke and possible treatments. Neurol Res. 2013;35:479–491. doi: 10.1179/1743132813Y.0000000209. [DOI] [PubMed] [Google Scholar]
- 96.Turdi S, Yuan M, Leedy GM, Wu Z, Ren J. Chronic social stress induces cardiomyocyte contractile dysfunction and intracellular Ca2+ derangement in rats. Physiol Behav. 2012;105:498–509. doi: 10.1016/j.physbeh.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsuhisa F, Kitamura N, Satoh E. Effects of acute and chronic psychological stress on platelet aggregation in mice. Stress. 2014;17:186–192. doi: 10.3109/10253890.2014.888548. [DOI] [PubMed] [Google Scholar]
- 98.Solanki N, Salvi A, Patki G, Salim S. Modulating Oxidative Stress Relieves Stress-Induced Behavioral and Cognitive Impairments in Rats. Int J Neuropsychopharmacol. 2017;20:550–561. doi: 10.1093/ijnp/pyx017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kullmann FA, McDonnell BM, Wolf-Johnston AS, Kanai AJ, Shiva S, Chelimsky T, Rodriguez L, Birder LA. Stress-induced autonomic dysregulation of mitochondrial function in the rat urothelium. Neurourol Urodyn. 2019;38:572–581. doi: 10.1002/nau.23876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barbiero S, Aimo A, Castiglione V, Giannoni A, Vergaro G, Passino C, Emdin M. Healthy hearts at hectic pace: From daily life stress to abnormal cardiomyocyte function and arrhythmias. Eur J Prev Cardiol. 2018;25:1419–1430. doi: 10.1177/2047487318790614. [DOI] [PubMed] [Google Scholar]
- 101.Shinzawa-Itoh K, Sugimura T, Misaki T, Tadehara Y, Yamamoto S, Hanada M, Yano N, Nakagawa T, Uene S, Yamada T, Aoyama H, Yamashita E, Tsukihara T, Yoshikawa S, Muramoto K. Monomeric structure of an active form of bovine cytochrome c oxidase. Proc Natl Acad Sci USA. 2019;116:19945–19951. doi: 10.1073/pnas.1907183116. [DOI] [PMC free article] [PubMed] [Google Scholar]