Abstract
Lignin is a key secondary cell wall chemical constituent, and is both a barrier to biomass utilization and a potential source of bioproducts. The Arabidopsis transcription factors MYB58 and MYB63 have been shown to upregulate gene expression of the general phenylpropanoid and monolignol biosynthetic pathways. The overexpression of these genes also results in dwarfism. The vascular integrity, soluble phenolic profiles, cell wall lignin, and transcriptomes associated with these MYB‐overexpressing lines were characterized. Plants with high expression of MYB58 and MYB63 had increased ectopic lignin and the xylem vessels were regular and open, suggesting that the stunted growth is not associated with loss of vascular conductivity. MYB58 and MYB63 overexpression lines had characteristic soluble phenolic profiles with large amounts of monolignol glucosides and sinapoyl esters, but decreased flavonoids. Because loss of function lac4 lac17 mutants also accumulate monolignol glucosides, we hypothesized that LACCASE overexpression might decrease monolignol glucoside levels in the MYB‐overexpressing plant lines. When laccases related to lignification (LAC4 or LAC17) were co‐overexpressed with MYB63 or MYB58, the dwarf phenotype was rescued. Moreover, the overexpression of either LAC4 or LAC17 led to wild‐type monolignol glucoside levels, as well as wild‐type lignin levels in the rescued plants. Transcriptomes of the rescued double MYB63‐OX/LAC17‐OX overexpression lines showed elevated, but attenuated, expression of the MYB63 gene itself and the direct transcriptional targets of MYB63. Contrasting the dwarfism from overabundant monolignol production with dwarfism from lignin mutants provides insight into some of the proposed mechanisms of lignin modification‐induced dwarfism.
1. INTRODUCTION
As society increasingly seeks to transition toward renewable resources, plant biomass is increasingly viewed as a potential source of commodity chemicals and novel bioproducts (Ralph, Lapierre, & Boerjan, 2019). Lignin is a key chemical component of plant biomass, conferring strength to cell walls, and resistance to degradation. For uses of plant biomass in forage and potential bioenergy applications, efforts have largely focused on reducing or altering lignin content, as it generally impedes accessibility to the carbohydrate cell wall fractions (DeMeester, de Vries, & Özparpucu, 2018). More recently, lignin is viewed positively as a valuable potential source of chemical feedstocks, and engineering lignin modification has been a major target (Mahon & Mansfield, 2019; Mottiar, Vanholme, Boerjan, Ralph, & Mansfield, 2016; Umezawa, 2018). In studies where lignin has been modified with the aim of biotechnological applications, and in mutant studies where monolignol biosynthesis has been perturbed, unexpected stunted growth phenotypes, termed ligninmodification‐induced dwarfism (LMID), have been observed (reviewed by (Muro‐Villanueva, Mao, & Chapple, 2019). Understanding why modifications in lignin content affect plant growth represents an important challenge.
Lignin is a polymer primarily made up of hydroxycinnamyl alcohols. Lignin also has a remarkable capacity to incorporate a wide range of other components including ferulic acid, tricin, and coniferyaldehyde (Mottiar et al., 2016). Hydroxycinnamyl alcohols are derived from the core phenylpropanoid pathway, commencing with the amino acid phenylalanine and resulting in the production of lignin monomers, or monolignols (Ralph et al., 2019). p‐coumaroyl‐CoA represents a branch point between lignin biosynthesis and the biosynthesis of other phenolic compounds such as flavonoids (Dixon & Barros, 2019; Liu, Luo, & Zheng, 2018; Vanholme, De Meester, Ralph, & Boerjan, 2019). From p‐coumaroyl‐CoA, a series of subsequent hydroxylations, methylations, and reductions results in the formation of the three canonical monolignols (p‐coumaryl‐, coniferyl‐, and sinapyl alcohols). The enzymes catalyzing this series of reactions have been biochemically characterized, and their functions inferred through mutant analysis in Arabidopsis thaliana (Arabidopsis; Van‐Acker et al., 2013; Vanholme, Storme, & Vanholme, 2012) and gene knockdowns in Medicago sativa (alfalfa; Chen & Dixon, 2007) and poplar (Coleman, Park, Nair, Chapple, & Mansfield, 2008a. In addition to elucidating the biosynthetic pathways, the regulation of lignin production has also been studied in Arabidopsis. Both the general phenylpropanoid pathway and the monolignol‐specific pathway are positively regulated by two MYB transcription factors, MYB58 and MYB63 (Zhou, Lee, Zhong, & Ye, 2009). These transcription factors are themselves controlled within the context of vessel and fiber development by upstream master transcription factors (Ohtani & Demura, 2019).
When lignin deposition is triggered, monolignols are synthesized in the cytoplasm of both the lignifying, and in some cases, neighboring cells (Blokhina, Laitinen, & Hatakeyama, 2019; Smith et al., 2013). It remains uncertain exactly how monomers move from their site of synthesis to the wall where they are polymerized. Several possible mechanisms have been proposed, but there is no genetic evidence for transporter‐mediated export of the major monolignols, coniferyl alcohol and sinapyl alcohol (Perkins, Smith, & Samuels, 2019). This may reflect a non‐transporter‐mediated mechanism, such as diffusion (Vermaas et al., 2019). Alternatively, active transport of monolignols by ABC transporters (Alejandro, Lee, & Tohge, 2012; Miao & Liu, 2010) or transport of monolignol glucosides have also been proposed (Tsuyama et al., 2013).
Once in the wall, the monomers are oxidized by secreted laccase and/or peroxidase to form monolignol radicals that ultimately polymerize into lignin. Simultaneous mutation of the two most highly expressed laccases in Arabidopsis inflorescence stems, LAC4 and LAC17, resulted in irregular xylem and reduced Klason lignin phenotypes (Berthet, Demont‐Caulet, & Pollet, 2011). The mutation of a third laccase, lac11, in addition to lac4 and lac17 resulted in severe dwarfism and nearly absent lignin, suggesting these laccases play a major role in lignification (Zhao, Nakashima, & Chen, 2013). In the absence of polymerization machinery in the laccase triple mutant, excess monomers led to the formation of monolignol glucosides (Zhao et al., 2013), likely as a detoxification product (Väisänen et al., 2015). These monolignol glucosides are thought to be sequestered in plant vacuoles. Both nonspecific monolignol export and activity of oxidative enzymes in the cell wall confer flexibility in a plants’ ability to incorporate non‐canonical monomers into lignin (Mottiar et al., 2016). These factors suggest that manipulation of lignin content should be possible, but the deleterious effects on plant growth due to LMID remain an issue.
The mechanisms underlying LMID are currently the topic of intense interest (DeMeester et al., 2018; Panda, Li, Wager, Chen, & Li, 2020). Some mechanisms underlying LMID have been proposed, and then challenged. For example, HCTRNAi (HYDROXYCINNAMOYL COENZYMEA:SHIKIMATE HYDROXYCINNAMOYL TRANSFERASE) mutants (Hoffmann et al., 2005) were proposed to be dwarf due to flavonoid‐induced inhibition of growth and auxin transport (Besseau et al., 2007). This model was not supported by the observation that loss of flavonoids in chalcone synthase mutants did not result in a dwarf phenotype (Li, Bonawitz, Weng, & Chapple, 2010). In CINNAMOYL‐COENZYME A REDUCTASE1 (ccr1‐4) mutants, dwarf growth was reported to be due to ferulic acid accumulation creating disruption of the cell cycle during leaf development (Xue et al., 2015). This model is not supported by the observation that wild‐type growth is possible in ccr1 mutants when a wild‐type copy of the CCR1 gene is expressed exclusively in xylem cells (DeMeester et al., 2018). Other possible mechanisms leading to LMID include loss of vascular integrity, accumulation of pathway intermediates or derivatives, or triggering cell wall integrity sensing (Bonawitz & Chapple, 2013; Gallego‐Giraldo, Liu, & Pose‐Albacete, 2020; Muro‐Villanueva et al., 2019). Forward genetic screens have identified that LMID requires subunits of the transcriptional coregulator Mediator (Bonawitz et al., 2014), as well as an importin‐beta protein required to bring the MYB4 transcriptional repressor into the nucleus (Panda et al., 2020). These results highlight components of pathways that regulate the interaction between lignin defects and associated phenotypes, but leave large gaps in our understanding of the interaction between the pathways and the majority of the other factors involved.
Most studies examining LMID concern mutants with perturbations in the monolignol biosynthesis pathway, leading to phenotypes such as irregular xylem (DeMeester et al., 2018) or lower lignin levels (Van‐Acker et al., 2013). Paradoxically, overexpression of the regulatory MYB58 and MYB63 transcription factors that led to increased gene expression in the phenylpropanoid and monolignol biosynthetic pathways also led to impaired plant growth (Zhou et al., 2009). These plants with overabundant monolignol production and impaired growth provide opportunities to examine some of the proposed mechanisms of LMID. The first objective of this study was to examine the phenotypes of Pro35S::MYB58 (MYB58‐OX) and Pro35S::MYB63 (MYB63‐OX) plants that are relevant to LMID, such as vascular integrity, soluble phenolic profiles, and transcriptomes. One striking result was the high monolignol glucoside levels in these plants. As high monolignol glucosides are also found in loss of function lac4 lac11 lac17 triple mutants (Zhao et al., 2013), we hypothesized that additional copies of the lignin‐related genes LAC4 or LAC17 might rescue the LMID in MYB58‐OX or MYB63‐OX lines. Co‐expression of LAC4 or LAC17 was able to rescue the LMID growth phenotypes of MYB58‐OX or MYB63‐OX, in addition to reducing monolignol glucoside levels and transcriptome changes away from “stress‐related” genes. These data have interesting implications for understanding potential causes of LMID (Bonawitz & Chapple, 2013), arguing against dwarfism from the loss of a monolignol‐derived growth‐promoting molecules or the loss of lignin in vascular bundles, because MYB58‐OX/MYB63‐OX lines have high levels of monolignols and open xylem vessels. Comparing these plants with monolignol biosynthetic mutants (Bonawitz & Chapple, 2013; Vanholme et al., 2012) and engineered plants (DeMeester et al., 2018; Yang, Mitra, & Zhang, 2013) provides further insights into how modification of lignin can produce modified growth phenotypes.
2. MATERIALS AND METHODS
2.1. Plant growth
Arabidopsis seeds were sown on ½ MS agar plates, were vernalized at 4°C for 2–3 days, and were transferred to soil after 7 days of growth together with WT seedlings. Growth conditions for all plants were set to 21°C, 16 hr light/8 hr dark, and 210 μmol m−2 s−1 light intensity.
2.2. Microscopy
A Leica DMR epifluorescence microscope using 350/50 excitation and 455 nm longpass emission filter sets was used to document ultraviolet autofluorescence of Arabidopsis leaves and stems. A Perkin‐Elmer UltraView VoX spinning disk confocal mounted on a Leica DMI6000 inverted microscope and a Hamamatsu 9100‐02 CCD camera were used to image fluorescent proteins in living plant cells using the following excitation and emission filters: GFP (488 and 525), YFP (514 and 540), and RFP/m‐Cherry (561 and 595). To study cell wall localization of LAC4, surface sterilized seeds were plated on GM media (MS media supplemented with 1% Sucrose and 1x Gamborg's Vitamin mix; Phytotechnology labs), vernalized at 4ºC for 2–3 days before being transferred to growth chambers. Seeds were induced to germinate in 8 hr of light, then wrapped in foil and kept in darkness for 7 days. 7‐day‐old etiolated cotyledons were plasmolyzed in 0.4M D‐mannitol (Sigma‐Aldrich) for 1 hr. Three seedlings were imaged as above for three independent lines of each construct for two independent experiments. Hand sections of Arabidopsis stem were Mäule stained (Mitra and Loqué, 2014). To assess cell wall localisation of Lac4‐mCherry, 7 day old etiolated hypocotyls were plasmolyzed in 0.4M mannitol for 1 hour. A line of prUBQ10‐sec‐mCherry (Chou et al 2018) was used as a control for cell wall localized signal.
2.3. Molecular biology
Genomic sequences containing the MYB58 or MYB63 coding sequences were amplified using Phusion® High‐Fidelity DNA Polymerase (New England Biolabs) using the primers listed in Table S4a. These PCR fragments were cloned using gateway cloning methodology (Invitrogen) using the pDONR221 vector as an entry clone and subsequently shuttled into the pK2GW7 binary vector (Karimi, Inzé, & Depicker, 2002).
2.4. Real‐time quantitative PCR
Gene‐specific primers amplifying 190‐240bp amplicons of were designed using primer 3 software (Koressaar & Remm, 2007) and are listed in Table S4b. The APT1 (AT1G27450) gene was used as a reference gene as described in Guénin et al. (2009). Real‐time PCR was performed using iQ SYBR Green supermix (Biorad) and CFX connect real‐time PCR detection system (Biorad). Efficiencies of PCR amplification and quantifications were performed according to the manufacturer's specification, as described in Schmittgen and Livak (2008). Total RNA was isolated from 5‐week‐old plants using TRIzol reagent (Invitrogen) and cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions.
2.5. Soluble phenolics extraction and analysis
Three‐ to four‐week‐old plants were harvested into liquid nitrogen and ground using a mortar and pestle. Fifty to 100 milligrams of ground tissue was combined with 1 ml of “methanol water” solution (49.5% methanol:1% acetic acid in water) and incubated 45°C for 4 hr to extract soluble phenolic compounds. The samples were centrifuged at 15 000 rpm for 15 min and the supernatant was transferred to glass vials. Phase partitioning with 1 ml ethyl ether was performed three times, with the upper layer transferred to new glass vials. The lower phase water‐soluble phase was also transferred to separate glass vials. Samples were allowed to dry out overnight before resuspension in 50% methanol, sonication for 15–20 min and incubation at 35°C for 1 hr. Samples were filter‐sterilized into HPLC vials and soluble phenolic compounds were separated by running approximately 10 μL of sample on an LC30 Chromatography Oven HPLC fitted with a Symmetry C14 column and PDA‐100 Photodiode Array Detector (Dionex). The samples were examined at the wavelengths 280, 320, and 510 nm. Methanol extracts were eluted from the column over a gradient from 95% A (100% water:0.1% trifluoroacetic acid (TFA)) to 45% B (75% acetonitrile:25% methanol: 0.1% TFA) over 50 min, followed by a 10 min wash with 75% B and re‐acclimation of the column with 95% A for 10 min. The flow rate was 1 ml/minute; the column temperature was set to 40°C. Coniferin and syringin HPLC standards were prepared at a concentration of 0.05 mg/ml methanol and run on the HPLC using the conditions listed above.
To analyze the soluble phenolic phase by liquid chromatography/mass spectroscopy, 10 μL of the same samples was run through an Agilent Zorbax Eclipse XDB C18 column (4.6 × 70 mm, particle size 1.8 μm) with a flow rate of 0.7 ml/min at 30°C. The samples were eluted with an increasing concentration of acetonitrile in 5% formic acid from 10% to 25% over 16 min, and from 25% to 100% over 9 min. The detection and analysis of metabolites was performed using a Bruker maXis Impact Ultra‐High Resolution tandem TOF (UHR‐Qq‐TOF) mass spectrometer in positive electrospray ionization mode, temperature 220°C, drying gas flow rate 10 L/min, nebulizer pressure 4 bars, capillary voltage 3800 V, and using sodium formate as a calibrant.
2.6. Structural chemistry analysis
Arabidopsis stems from 8‐ to 10‐week‐old plants were used to determine lignin and carbohydrate content following a modified Klason method (Cullis, Saddler, & Mansfield, 2004). Samples were ground in a Wiley mill to pass a 40 mesh screen, treated with acetone overnight using a Soxhlet, and then dried for 48 hr at 50°C. Approximately 150 mg of dried extractive‐free tissue was treated with 72% sulfuric acid for 2 hr, diluted to ~3% with 112 ml DI water and autoclaved at 121°C for 60 min. The mixture was filtered through a medium coarseness crucible and the retentate dried at 105°C. The acid‐insoluble lignin was determined by weighing the retentate, while the acid‐soluble lignin was measured from an aliquot of the filtrate using an UV spectrophotometer at 205 nm. Carbohydrate contents were determined by HPLC analysis of the filtrate. Glucose, xylose, mannose, galactose, arabinose, and rhamnose were analyzed using a Dx‐600 anion‐exchange HPLC (Dionex) fitted with a CarboPac PA1 column (Dionex) at 1 ml/min and post column detection (100 mM NaOH min−1). Sugar concentrations were calculated from standard curves created from external standards.
2.7. RNA extraction, RNAseq analysis, and defining MYB63 target genes
Total RNA was isolated from three replicated samples of several pooled leaves each of 4‐week‐old plants using TRIzol reagent (Life technologies). RNAseq was performed using an Ion AmpliSeq Transcriptome Gene Expression Kit on an ion torrent (Life Technologies) sequencing platform at the Next Generation Sequencing Centre at the University of British Columbia (http://ngs.med.ubc.ca/). Trimming and filtering of raw FASTQ reads were performed with trimmomatic v0.35 (Bolger, Lohse, & Usadel, 2014) using the following parameters; LEADING:20 TRAILING:20 AVGQUAL:20 MINLEN:40. Other parameters were kept at default. Surviving reads were aligned against the A. thaliana reference genome (TAIR10 genome release; Swarbreck, Wilks, & Lamesch, 2008) with bowtie2 v2.2.7 using the very sensitive‐ local parameter (Langmead & Salzberg, 2012). Count matrices were obtained with htseq‐count v0.6.1 with default parameters (Anders, Pyl, & Huber, 2015). Differential analysis and normalization of count data were performed with DESeq2 (Love, Huber, & Anders, 2014). False discovery rate (FDR) < 0.05 and an absolute log2 fold change >1.5 define differentially expressed (DE) genes between comparisons (Pro35S:MYB63/WT and Pro35S:MYB63xPro35S:LAC17/WT). Gene Ontology (GO) enrichment analysis was performed with agriGO with default settings (Du, Zhou, Ling, Zhang, & Su, 2010). GO terms having FDR < 0.05 were considered significantly enriched. DNA affinity purification sequencing motif peaks (fraction of reads in peaks, FRiP ≥5%) for MYB63 were obtained from O’Malley et al. (2016). Differentially expressed genes whose promoter region (1.5 kb upstream of transcription start site) contains MYB63 motif peaks are defined as high‐confidence MYB63 target genes in this study. The raw sequence reads were deposited in NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra).
3. RESULTS
3.1. Overexpression of MYB58 and MYB63 produces dwarf plants with intact xylem
Wild‐type Columbia‐0 Arabidopsis plants (WT) were transformed with overexpression constructs consisting of the cauliflower mosaic virus 35S promoter driving MYB58 (At1g16490) or MYB63 (At1g79180). Several lines with high levels of MYB gene expression were identified and quantitative real‐time PCR of these lines quantified the specific overexpression of either MYB58 or MYB63 in each respective line (Figure 1a). The overexpression of MYB58 did not affect the levels of MYB63, and conversely overexpression of MYB63 did not affect MYB58 levels (Figure 1a). MYB58‐OX and MYB63‐OX lines were severely dwarfed during all stages of development (Figure 1b–f). The leaves of young seedlings that germinated on plates were pale and chlorotic, compared to WT, but pigmentation recovered after continued development on soil for several weeks (Figure 1c–e). At maturity, the MYB58‐OX and MYB63‐OX lines produced inflorescence stems that were shorter than the WT (Figure 1f). These data are consistent with the dramatic changes in growth and development, as well as ectopic deposition of lignin reported by Zhou et al. (2009), who discovered these monolignol‐associated transcription factors.
FIGURE 1.
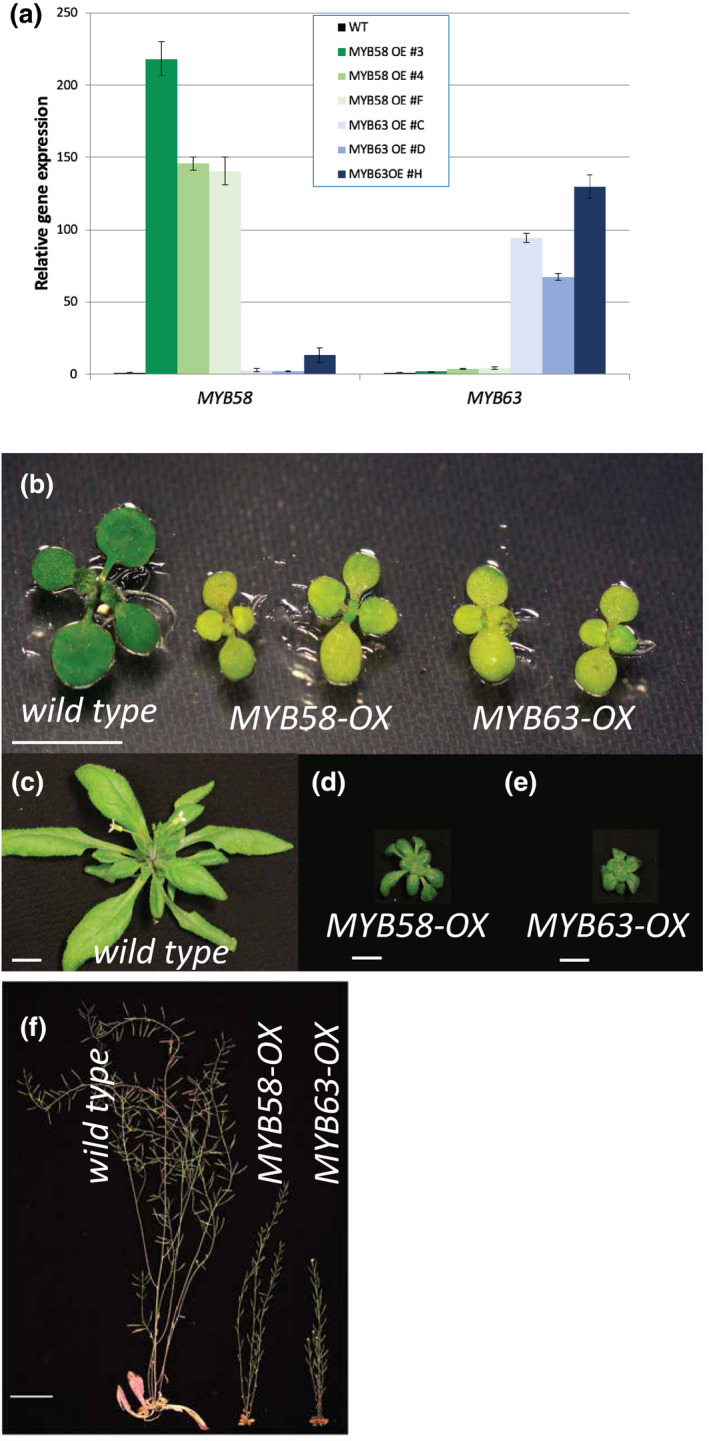
Pro35S::MYB58 (MYB58‐OX) and Pro35S::MYB63 (MYB63‐OX) plants display growth and development defects. (a) Real‐time quantitative PCR of the MYB58 and MYB63 genes from WT, MYB58‐OX, and MYB63‐OX genotypes. Error bars represent the standard error of three technical replicates. (b) 10‐day‐old seedlings of indicated genotypes. (c) 5‐week‐old wild‐type, (d) MYB58‐OX and (e) MYB63‐OX lines (d). (f) Mature, 10‐week‐old wild‐type, MYB58‐OX, and MYB63‐OX lines. Scale bars are 1 cm in (b–e), and 5 cm in F.
In studies examining dwarfism associated with downregulation of lignin, one of the proposed mechanisms leading to decreased growth was insufficient reinforcement of the xylem vessels (Bonawitz & Chapple, 2013; DeMeester et al., 2018). In this study, the mature stems of WT, MYB58‐OX, and MYB63‐OX plants were examined using the intrinsic fluorescence of the lignin (Figure 2) or using Mäule staining (Figure S1). Ectopic lignin in the epidermis, cortex, and pith was observed in the MYB58‐OX and MYB63‐OX lines using autofluorescence (Figure 2a). Some lignification in the cortex of Mäule‐stained sections of MYB63‐OX was also observed (Figure S1). In all cases, the xylem vessels were regular, with open, round profiles (Figure 2b,c), unlike the irregular xylem of lignin‐deficient mutants (Bonawitz et al., 2014; Li et al., 2010). This suggests that the stunted growth of MYB58‐OX and MYB63‐OX is due to factors other than irregular xylem.
FIGURE 2.

Pro35S::MYB58 (MYB58‐OX) and Pro35S::MYB63 (MYB63‐OX) inflorescence stems have regular xylem vessels and ectopic lignin. (a) UV autofluorescence of sections from the base of WT, MYB58‐OX, and MYB63‐OX inflorescence stems. Autofluorescence in wild‐type stems was observed in xylem, interfascicular fibers, and phloem fibers, while the autofluorescence in MYB58‐OX and MYB63‐OX spreads to the cortex (c), epidermis, and pith. (b) Vascular bundle of MYB58‐OX stem showing open xylem vessels. (c) MYB63‐OX stem showing open xylem vessels. V indicates xylem vessels. Scale bars are 50 µm.
3.2. Increased monolignol glucosides and sinapoyl esters, but decreased flavonoids, in MYB58‐OX and MYB63‐OX lines
The consequences of MYB58 or MYB63 overexpression on the soluble phenolic metabolites were not previously examined. Based on their ectopic lignification, we hypothesized that these lines also have elevated levels of monolignols, which could manifest in high monolignol glucoside levels (LeRoy, Huss, Creach, Hawkins, & Neutelings, 2016). The appearance of monolignol glucosides is proposed to be a homeostatic mechanism used by plant cells to balance monolignol metabolism (Lin et al., 2016), analogous to hexosylation of xenobiotics (Vanholme et al., 2012). HPLC‐MS of extracted soluble phenolics from MYB63‐OX leaves was used to examine the major peaks of soluble phenolics (Figure 3a) and identify them based on mass spectroscopy profiles (Figure 3b). In addition to altered phenolic profiles, the leaves of MYB63‐OX exhibit bright blue vacuolar autofluorescence (Figure 3c). Qualitatively, it was clear that there were increased accumulations of monolignol glucosides (Figure 3a), and this was confirmed quantitatively for both MYB58‐OX and MYB63‐OX (Figure 4a). Other phenylpropanoid‐related pathways were also upregulated, with corresponding increases in soluble hydroxycinnamate esters such as sinapoyl glucose and sinapoyl malate (Figures 3a and 4b). As predicted by the upregulation of genes of the phenylpropanoid and monolignol biosynthetic pathways, monolignol glucosides and sinapoyl esters were strongly upregulated in the accumulation in the MYB58‐OX and MYB63‐OX lines.
FIGURE 3.
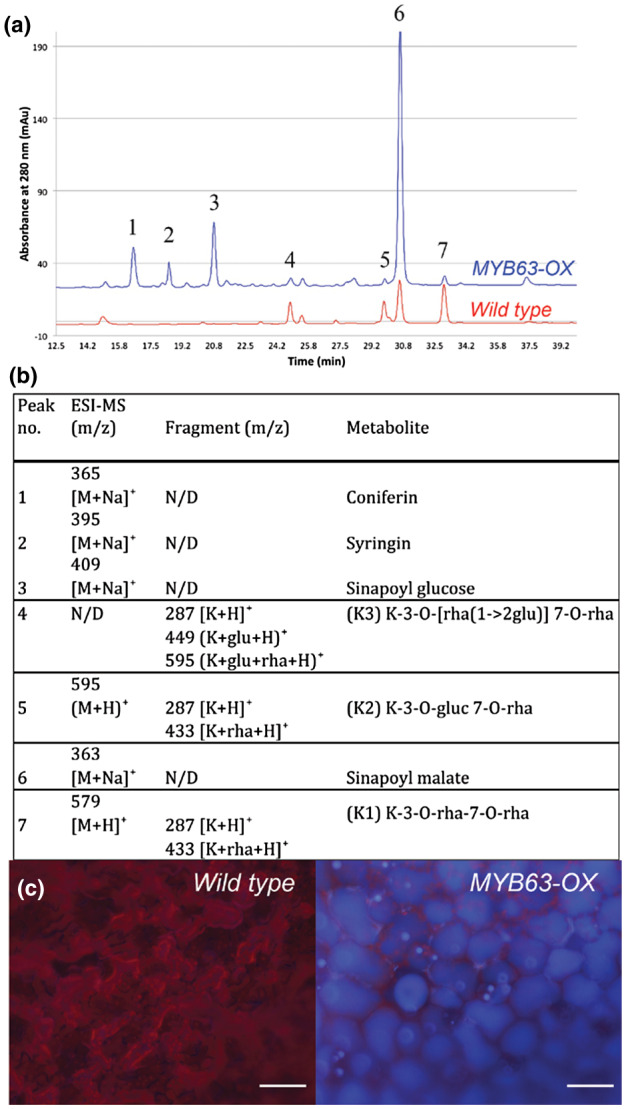
Pro35S::MYB63 (MYB63‐OX) plants accumulate increased monolignol glucosides and sinapoyl esters, in blue autofluorescent vacuoles. (a) HPLC chromatograph of methanol leaf extracts at 280 nm indicating the major phenolic metabolites accumulating in the MYB63‐OX overexpression lines. (b) LC‐MS identification of the soluble phenolic peak shown in A. K1, K2, and K3 indicate Kaempferol glycosides. (c) UV autofluorescence of chlorophyll wild‐type leaf mesophyll is red. UV autofluorescence of MYB63‐OX leaf mesophyll with blue vacuoles. Scale bars: 50 μm in (c).
FIGURE 4.

Pro35S::MYB68 (MYB58‐OX) and Pro35S::MYB63 (MYB63‐OX) plants disrupted soluble phenolic phenotype reversed with LAC17 co‐overexpression. Quantification of the soluble phenolics from Arabidopsis leaves of MYB58‐OX and MYB63‐OX overexpression lines, as well as MYB58‐OX or MYB63‐OX that co‐overexpress Pro35S::LAC17 (LAC17‐OX). (a) monolignol glucosides, coniferin and syringin; (b) hydroxycinnamate esters (HCE), sinapoyl glucose and sinapoyl malate; and (c) kaempferol glycosides. Bars indicate standard deviation. Three replicate experiments. n = 3–6 pooled samples of five leaves from five plants. Means with different letters represent statistically significant differences of total monolignol glucosides, HCEs, or flavonols (Tukey's pairwise comparison, p < .01).
While monolignol and hydroxycinnamoyl esters increased, kaempferol glucosides decreased in MYB58‐OX and MYB63‐OX lines, as might be predicted for a competing metabolic pathway for flavonol biosynthesis (Figures 3a and 4c). Decreased flavonoid production is consistent with the observation that MYB58‐OX and MYB63‐OX lines did not produce anthocyanin pigments under light stress (Figure S2a). In order to quantify anthocyanins, we used photometric quantification of anthocyanin extracts from 3‐week‐old seedlings, as previously described (Mehrtens, Kranz, Bednarek, & Weisshaar, 2005). Anthocyanin quantification of methanol extracts from these plants showed a 50% reduction of anthocyanin content in MYB58‐OX and MYB63‐OX lines compared to WT, and extremely high anthocyanin content in MYB75PAP1D plants (Figure S2b–d). The MYB75PAP1D mutants were a positive control for the accumulation of anthocyanins (Borevitz, Xia, Blount, Dixon, & Lamb, 2000). Overall, there were decreased flavonol levels in both MYB58‐OX and MYB63‐OX lines.
In summary, the soluble phenolic changes detected in the MYB58‐OX and MYB63‐OX lines were consistent with strong, specific upregulation of the general phenylpropanoid and monolignol biosynthetic pathways, leading to monolignol glucoside and hydroxycinnamate ester accumulation in the vacuoles, accompanied by a concomitant decrease in flavonoids.
3.3. Co‐overexpression of lignin‐related LACCASES in MYB58‐OX and MYB63‐OX lines makes soluble phenolic pools similar to wild type
Accumulations of monolignol glucosides, seen here in the MYB58‐OX and MYB63‐OX lines, were also observed in lac11 lac4 lac17 triple mutants (Zhao et al., 2013), which were severely impaired in lignification and dwarfed. We hypothesized that overexpression of lignin‐related laccases such as LAC4 or LAC17 could lead to reduction of monolignol glucosides in the MYB58‐OX and MYB63‐OX lines. Plants were transformed with a construct containing the 35S promoter driving overexpression of either LAC17 (Pro35S::LAC17, LAC17‐OX) or LAC4 (Pro35S::LAC4, LAC4‐OX), with overexpression confirmed by RT‐PCR (data not shown). These plants were crossed with the MYB63‐OX lines, and RT‐PCR identified plants that co‐overexpressed both the laccase and the transcription factor, for example, Pro35S::MYB58 (MYB58‐OX/LAC17‐OX) and Pro35S::MYB63 Pro35S:LAC17 (MYB63‐OX/LAC17‐OX). Quantification of the soluble phenolics by HPLC‐MS indicated that the monolignol glucosides were reduced to WT levels in the double overexpression lines compared to the single overexpression lines (Figure 4a). In both MYB58‐OX/LAC17‐OX and MYB63‐OX/LAC17‐OX lines, the sinapoyl ester levels were also reduced compared to the single overexpression lines (Figure 4b). Interestingly, in the MYB63‐OX background, sinapoyl ester levels were elevated above WT levels, indicating continued increased flux through the phenylpropanoid pathway. The suppression of the kaempferol glycosides observed in the MYB58‐OX and MYB63‐OX lines was also reversed in the MYB58‐OX/LAC17‐OX and MYB63‐OX/LAC17‐OX lines (Figure 4c). Identical results were observed when LAC4 or LAC17 was co‐overexpressed with MYB63 (Figure S3). Clearly, the co‐overexpression of either lignin‐related LAC4 or LAC17 in the MYB58‐OX and MYB63‐OX lines had a strong impact on the soluble phenolics, shifting them toward a WT profile.
3.4. Cell wall composition is restored by co‐overexpression of LAC17 in MYB58‐OX and MYB63‐OX backgrounds
In addition to reverting the phenolic profiles toward WT levels, ectopic lignin (detected by Mäule staining) found in dwarf MYB63‐OX line disappeared in the double MYB63‐OX/LAC17‐OX overexpression lines (Figure S1). This was consistent with the cell wall lignin content, as MYB63‐OX and MYB58‐OX lines had higher total lignin levels than WT, but the double MYB63‐OX/LAC17‐OX and MYB58‐OX/LAC17‐OX overexpression lines were similar to WT levels (Figure 5). This result seems counterintuitive, as the upregulation of monolignol biosynthesis, paired with increased oxidative enzymes ectopically expressed throughout the plant, would be predicted to increase, not decrease, total cell wall lignin levels. The wild‐type cell wall phenotype in the rescued lines, compared to the dwarf MYB63‐OX lines, was also observed in the structural cell wall polysaccharides (Figure S4).The severely dwarfed MYB63‐OX line had the largest deviations in carbohydrate monomer composition, compared to WT, with significantly elevated arabinose and galactose and reduced mannose, glucose, and xylose. The LAC17‐OX, MYB58‐OX, MYB58‐OX/LAC17‐OX, and MYB63‐OX/LAC17‐OX were similar or indistinguishable from WT (Figure S4). Thus, for both cell wall lignin and carbohydrates, co‐overexpression of LAC17 in MYB63‐OX lines led to cell wall composition similar to wild type.
FIGURE 5.
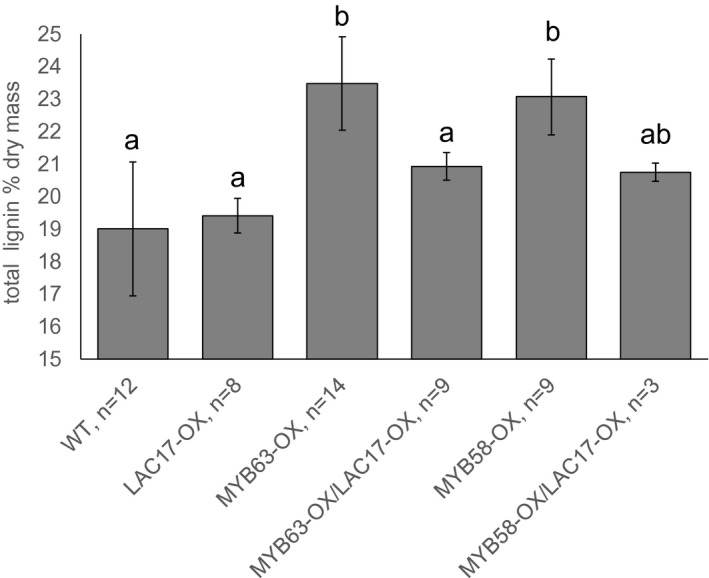
Total lignin levels are high in Pro35S::MYB58 (MYB58‐OX) and Pro35S::MYB63 (MYB63‐OX) stems, but similar to wild type when LAC17 is co‐overexpressed. Stem lignin was determined from the following overexpression lines: MYB58‐OX, MYB63‐OX, LAC17‐OX; and the co‐overexpression lines: MYB63‐OX/LAC17‐OX and MYB58‐OX/LAC17‐OX. Bars indicate standard deviation. Means with different letters represent statistically significant differences. One to three batches of ~90 plants were split into two to four technical replicates each for total n = 3–14 (Dunn's pairwise comparison, p < .05).
3.5. Co‐overexpression of lignin‐related LACCASES rescues growth in MYB58‐OX and MYB63‐OX lines
In addition to restoring soluble phenolic and cell wall composition toward the WT levels, growth was dramatically altered when LAC4 or LAC17 was co‐overexpressed in the MYB58‐OX and MYB63‐OX lines. MYB63‐OX/LAC17‐OX and MYB58‐OX/LAC17‐OX double overexpression lines displayed rosette diameters that were statistically significantly larger than the single overexpression lines (Figure 6a,b). When the MYB63‐OX/LAC17‐OX double overexpression plants were bolting and setting seed, the dwarf MYB63‐OX lines were not yet forming inflorescence stems (Figure 6c). As a control for the possibility that the introduction of an additional 35S expression construct interfered with the MYB63‐induced dwarfism, MYB63‐OX lines were also transformed with Pro35S::GFP‐HDEL, which localizes GFP to the endomembrane system, and the LMID of plants was not rescued (Figure 6b). In addition to rescue by LAC17 overexpression, when LAC4 was co‐overexpressed (Pro35S::LAC4) in the MYB63‐OX lines, growth was significantly increased compared to the dwarf MYB63‐OX lines (Figure 7). This was also the case when LAC4, tagged with a red fluorescent protein (ProUbiquitin10::LAC4‐mCherry), was co‐overexpressed in the MYB63‐OX lines, where it rescued the growth back to WT (Figure 7e–g). In this line, LAC4‐mCherry was localized via spinning disk confocal microscopy to the cell walls of leaf epidermal cells and seedlings (Figure 8), demonstrating that the laccase protein was produced and targeted to its correct cell wall location (Figure 8d). The cell wall localization was confirmed with plasmolysis: treatment with 0.4 M mannitol led to shrinkage of the protoplast from the cell wall, yet the LAC4‐mCherry signal remained associated with the wall (Figure S5). As a control, the plasma membrane marker ABCB11‐GFP (Pro35S::ABCB11‐GFP; Figure 8e–h) was introduced into the MYB63‐OX background. ABCB11‐GFP expression and localization to the plasma membrane were confirmed using spinning disk confocal microscopy, and the resulting co‐overexpression lines remained severely dwarfed as predicted (Figure 8g‐h). Using the ABCB11‐GFP to outline the cell diameter, the difference in cell sizes in the dwarf co‐overexpression lines is apparent (Figure 8h). The impaired growth phenotype associated with upregulation of the phenylpropanoid and monolignol pathways was rescued exclusively by extracellular laccases, suggesting that by the presence of the oxidative cell wall enzyme changed intracellular conditions associated with the observed impaired plant growth.
FIGURE 6.
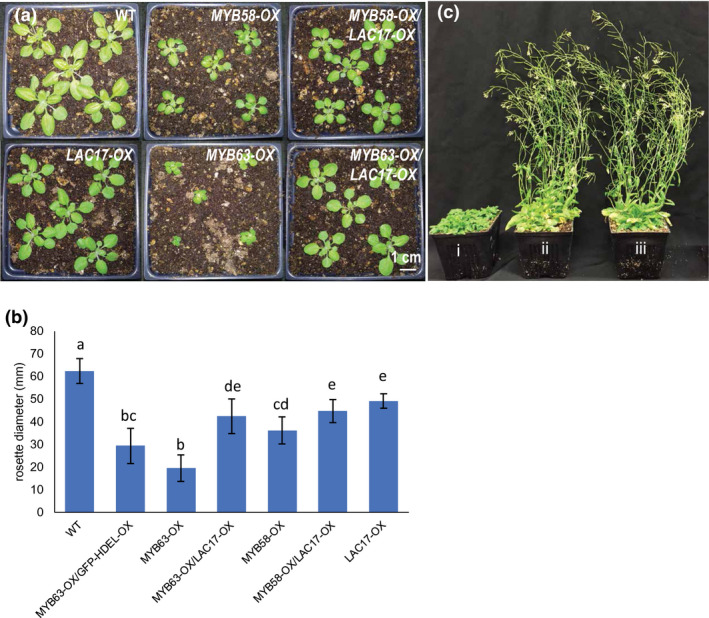
Pro35S::MYB58 (MYB58‐OX) and Pro35S::MYB63 (MYB63‐OX) dwarfism can be rescued by concurrent ectopic LAC17 overexpression. (a) Rosettes of wild type, MYB58‐OX, MYB63‐OX, MYB58‐OX/LAC17‐OX, and MYB63‐OX/LAC17‐OX Arabidopsis lines at 17 days old. (b) Quantification of rosette diameters at 17 days old (n = 9 plants except MYB63‐OX GFP‐HDEL‐OX n = 6 plants). Bars indicate standard deviation. Means with different letters represent statistically significant differences (Tukey's pairwise comparison, p < .01) 3 replicated experiments. (c) After 5 weeks of growth, while MYB63‐OX are at rosette stage (i), MYB63‐OX/LAC17‐OX double overexpression lines (ii) and wild type (iii) are flowering.
FIGURE 7.

LAC4 overexpression rescues Pro35S::MYB63 (MYB63‐OX) growth phenotype. (a,c,e) Wild‐type and MYB63‐OX control Arabidopsis rosettes have similar growth to single overexpression Pro35S::LAC4 (LAC4‐OX) and ProUBQ10::LAC4‐mCherry (LAC4‐mCherry‐OX). MYB63‐OX dwarfism (B) was rescued in (d) double overexpression lines LAC4‐OX/MYB63‐OX and (e) LAC4‐mCherry‐OX/MYB63‐OX (scale bar is 1 cm). (g) Quantified rosette diameters of the above lines. Bars indicate standard deviation. Means with different letters represent statistically significant differences (n = 12 rosettes, Tukey's pairwise comparison, p < .05).
FIGURE 8.

LAC4‐mCherry localized in the cell wall in rescued Pro35S::MYB63 (MYB63‐OX) lines. (a) Wild‐type Arabidopsis rosette. (b) Dwarf MYB63‐OX Arabidopsis rosette. (c) Wild‐type growth phenotype in double overexpression lines expressing ProUBQ10::LAC4‐mCherry (LAC4‐mCherry‐OX) in the MYB63‐OX background. (d) LAC4‐mCherry localized to the cell walls in LAC4‐mCherry‐OX MYB63‐OX lines. (e) Wild‐type growth phenotype in Pro35S::ABCB11‐GFP (ABCB11‐GFP‐OX) expressed in Col‐0 background. (f) Plasma membrane localization of ABCB11‐GFP. Arrows indicate plasma membrane of two cells separated by cell wall. (g) Dwarf MYB63‐OX/ABCB11‐GFP‐OX lines. (h) Plasma membrane localization of ABCB11‐GFP. Scale bars are 1 cm (a,b,c,e,g), 30 µm (d,f,h).
Other hypotheses related to LMID plants from previous studies were tested using the more severe dwarf growth phenotype of MYB63‐OX lines. To test the potential contribution of flavonoids to the reduced growth of MYB63‐OX lines, flavonoid biosynthetic mutant transparent testa4, tt4‐2, deficient in chalcone synthase (Burbulis, Lacobucci, & Shirley, 1996), were transformed with the 35S::MYB63 overexpression construct. These plants showed the same dwarf growth habit and blue fluorescent vacuoles as the 35S::MYB63 in the Col‐0 WT background (Figure S6a). These data provide an independent line of evidence that the decreased growth in Arabidopsis associated with altered phenolics is independent of flavonoids, as demonstrated for HCT‐RNAi and c3’h mutants by Li et al. (2010). To test if the Mediator complex subunits 5A and 5B are required for the dwarf phenotype (Bonawitz et al., 2014), med5a/5b mutants were transformed with the 35S::MYB63 overexpression construct. The resulting transformants were identical to dwarf MYB63‐OX lines (Figure S6b). These data demonstrate that the dwarf growth of MYB63‐OX plants is independent of flavonoid‐mediated growth modulation proposed for loss of function hct lines, and the Mediator 5a5b‐dependent mechanisms that rescue c3’h mutants.
The rescue of the growth phenotype was not due to co‐suppression of MYB63, as transcript levels of MYB63 remain high in the double overexpression lines. When MYB63 expression was measured with quantitative RT‐PCR, both MYB63‐OX/LAC4‐OX and MYB63‐OX/LAC17‐OX lines had MYB63 gene expression ranging from 21‐ to 1683‐fold greater than WT levels (gray boxes in Figure S7). While there was a clear correlation between rosette size and MYB63 gene expression in the single MYB63‐OX lines (yellow triangle in Figure S7) and WT (yellow circles in Figure S7), there was no correlation between rosette size and MYB63 transcript levels in the MYB63‐OX/LAC17‐OX or MYB63‐OX/LAC4‐OX double overexpression plants (gray and black squares in Figure S7).
Several ATP‐binding cassette (ABC) transporters have been correlated with monolignol production (Kaneda et al., 2011; Takeuchi, Kegasa, Watanabe, Tamura, & Tsutsumi, 2018), and ABCG29 was reported to transport p‐coumaryl alcohol (Alejandro et al., 2012). To test if putative monolignol exporters were able to rescue the growth phenotype of MYB63‐OX lines, we co‐overexpressed ABCB11 and ABCG29 with MYB63. Due to difficulty recovering ABCG29 transporter/MYB63 double overexpression lines, plants carrying an inducible MYB63 overexpression construct were also generated by exchanging the VND7 coding sequence with the MYB63 coding sequence in the Pro35::VND7‐VP16‐GR vector described in Yamaguchi et al. (2010; MYB63‐VP16‐GR). When young seedlings were sprayed with dexamethasone to activate the inducible system, a dwarf phenotype, and accumulation of monolignol glucosides, which were identical to those found in Pro35S::MYB63 lines, was observed (Figure 9a–b). When the inducible MYB63‐VP16‐GR construct was introduced into plants overexpressing Pro35S::GFP‐ABCG29 (Figure 9c), there was no rescue of the dwarf growth phenotype induced by the dexamethasone treatment, although the ABC transporters were properly localized to the plasma membrane (Figure 9c). Overexpression of the lipid‐transport ABC transporter, ABCG11 (Pro35S::YFP‐ABCG11; (Bird, Beisson, & Brigham, 2007), was similarly unable to rescue the MYB63‐OX dwarfism in the inducible lines (Figure 9d).
FIGURE 9.

Co‐overexpression of ABC transporters does not suppress the dwarf growth phenotype of Pro35S::MYB63 (MYB63‐OX) lines. (a) Control wild‐type and dwarf MYB63‐OX Arabidopsis rosettes (top) compared to plants overexpressing an inducible MYB63 construct, where MYB63 is fused to the VP16 activation domain and glucocorticoid receptor (Pro35S::MYB63‐VP16‐GR). Uninduced plants, MYB63‐GR‐OX without dexamethasone (−DEX) resemble wild type; plants with MYB63 activity induced by spraying dexamethasone (+DEX) have reduced growth similar to MYB63‐OX. After 5 days of growth on soil dexamethasone at a concentration of 10 μM in water was sprayed every 3–4 days. (b) Representative HPLC of soluble phenolic profiles show a wild‐type pattern for the uninduced MYB63‐GR‐OX and the characteristic high monolignol glucoside pattern from MYB63‐OX and the induced MYB63‐GR + DEX plants. (c) When ABCG29 (Pro35S::GFP‐ABCG29) is co‐overexpressed with MYB63 by crossing it with the inducible MYB63‐GR‐OX lines, growth is wild type in ‐DEX controls and dwarf in + DEX‐induced plants. Spinning disk confocal of these lines demonstrates the GFP‐ABCG29 in the plasma membrane of leaf cells. (d) When the YFP‐ABCG11 (Pro35S::YFP‐ABCG11) is crossed into the inducible MYB63‐GR‐OX lines, growth is wild type in ‐DEX controls and dwarf in + DEX‐induced plants. Spinning disk confocal of plasma membrane localization of GFP‐ABCG11. Scale bars for rosette images are 1 cm (a,c,d), and 30 µm for confocal images (c,d).
Thus, of all the ectopic proteins that we co‐overexpressed in MYB63‐OX lines, only LAC4 and LAC17 (with or without fluorescent tags) were capable of reversing the dwarf phenotype of MYB63‐OX. Conversely, loss of function of LAC4 and LAC17 did not modify the MYB63‐OX phenotype. When the laccase double lac4 lac17 mutant (Berthet et al., 2011) was transformed with the 35S::MYB63 overexpression construct, the transformants also showed dwarf growth similar to MYB63‐OX lines (Figure S6c).
3.6. Co‐overexpression of lignin‐related LACCASES in MYB58‐OX and MYB63‐OX lines shifts the transcriptome from stressed to wild‐type
Transcriptomic analysis was used to obtain a global view of the changes in gene expression underlying the dwarf phenotype in MYB63‐OX lines compared to wild type. The overexpression of the MYB63 in both the MYB63‐OX overexpression and the MYB63‐OX/LAC17‐OX co‐overexpression lines was verified in the RNA‐seq data (Figure S8a). Over 600 genes were upregulated and almost 1,600 downregulated in the dwarf MYB63‐OX lines compared to WT plants (Figure S8b, Table S2). Overall, the transcriptomic analysis highlights a significant upregulation of gene ontology terms relating to phenylpropanoid metabolic processes, including aromatic amino acid biosynthesis (p < 5E‐12; Figure 10, Table S3). There were also increases in expression of genes in GO categories “response to stress” and “response to abiotic stimulus” in the dwarf MYB63‐OX line compared to the rescued double MYB63‐OX/LAC17‐OX lines (Figure 10, Table S2). The ability of purified MYB63 to bind to sites in the Arabidopsis genome was previously mapped by O’Malley et al. (2016) using in vitro DNA affinity purification sequencing. There is strong overlap between that MYB63 target dataset and the genes modulated in the MYB63‐OX line in this study (see starred genes in Figure 11; column A in Table S1). Transcriptomes of the rescued double MYB63‐OX/LAC17‐OX overexpression lines against WT showed that all of the direct target genes for MYB63 had significantly higher expression than WT (Figure 11, Table S3). This analysis confirmed MYB63 target genes continued to be upregulated, within the context of dramatic shifts in the transcriptome away from stress‐responsive genes. The ability of additional laccase outside the cell membrane to trigger dramatic transcriptional shifts suggests monolignol oxidation outside the membrane changes the phenolic pools inside the cells.
FIGURE 10.

Gene Ontology (GO) enrichment analysis in the transcriptome of MYB63‐OX compared to wild‐type Arabidopsis rosette leaves or MYB63‐OX/LAC17‐OX double overexpression lines compared to wild type. Gene Ontology (GO) enrichment analysis, with GO SLIM terms having FDR < 0.05 considered significantly enriched. Dwarf MYB63‐OX (left) have more up‐ and downregulated GO terms than rescued MYB63‐OX/LAC17‐OX (right) lines, details in Table S2. Response to stress indicated by a star.
FIGURE 11.

Transcriptomic analysis of key monolignol biosynthetic genes in MYB63‐OX and MYB63‐ OX/LAC17‐OX leaves compared to wild type. Expression of genes encoding the Arabidopsis monolignol biosynthetic pathway in MYB63‐OX (light gray) and in double overexpression, MYB63‐OX/LAC17‐OX (dark gray), lines. Outlined bars indicate transcript levels significantly different from wild type (p < .05). Genes that are direct MYB63 targets according to O’Malley et al., 2016 are demarcated by a star.
4. DISCUSSION
This study demonstrates that when plants have constitutive expression of phenylpropanoid and monolignol pathway genes due to MYB58 or MYB63 overexpression, there is a strong effect on the soluble phenolic metabolites and stress‐related gene expression in the transcriptome. The plants were dwarfed (Figure 1), with ectopic lignification (Figure 2), as described by Zhao et al. (2013), who identified these monolignol pathway regulatory transcription factors. In this work, MYB58‐OX and MYB63‐OX lines are used as a tool to probe the consequences of upregulation of monolignol biosynthesis. In MYB63‐OX and MYB58‐OX lines, bright blue autofluorescence in the vacuoles (Figure 3) was correlated with increased soluble phenolic profiles, with a characteristic upregulation of monolignol glucosides (Figure 4). Given that increased monolignol glucoside levels are correlated with increases in the monolignol pools (LeRoy et al., 2016; Väisänen et al., 2015), we hypothesized that consumption of monolignols by lignin‐related laccases would lead to decrease monolignol glucoside levels in these plants. When either LAC4 or LAC17 were overexpressed in the high monolignol‐containing lines (MYB58‐OX or MYB63‐OX), not only were monolignol glucoside levels decreased (Figure 4) but also the impaired plant growth was rescued (Figures 6 and 7). Using RNA‐seq, we captured a more complete picture of the complex transcriptional changes elicited by MYB63 overexpression and the co‐expression of MYB63 and LAC17. While there were many groups of differentially expressed genes, the stress‐associated genes were strongly differentially expressed in dwarfed lines compared to rescued lines (Figure 10). The addition of LAC17 seems to broadly alleviate stress and dwarfism, and also alleviates the overaccumulation of soluble phenolics. It is difficult, however, to disentangle the possible effect(s) of the various stress‐induced signaling pathways from those of the direct effects of LAC17 in the cell wall. Taken together, the transcriptional landscape of both lines suggests the potential involvement of many pathways, whose connection to lignin‐associated dwarfism is yet to be elucidated fully. How the overexpression of a cell wall oxidative enzyme changes the cellular phenolic profiles to have an impact on LMID is unclear, but it does permit us to critically evaluate some of the hypotheses proposed to explain LMID.
It has been suggested that one factor contributing to LIMD could be the disruption of the normal geometry and physiological functioning of xylem vessels (Bonawitz & Chapple, 2013; DeMeester et al., 2018). However, MYB58 and MYB63 overexpression lines do not have collapsed or irregularly shaped xylem vessels (Figure 2). As with some severe monolignol biosynthetic mutants (Panda et al., 2020), very young seedlings of MYB63 and MYB58 overexpressing plants are dwarfed even when grown inside highly moist sterile petri dishes where water should be readily available to all cells of the plant (Figure 1b), which also supports the conclusion that dwarfism in this case is due to other factors. In poplar, downregulation of C3’H resulted in irregular xylem, but measurement of physiological parameters such as xylem pressure potential and water‐use efficiency was not consistent with a simple explanation of water stress (Coleman, Samuels, et al., 2008b). As the obvious explanation of water stress is not sufficient, we considered a constellation of other correlated factors among dwarfed and rescued lines.
In our dwarfed and rescued lines, plant size and monolignol glucosides are inversely proportional, with soluble phenolic analyses showing the highest coniferin and syringin in the most dwarf MYB63 overexpression lines. The more modestly dwarfed MYB58 overexpression lines also have modestly elevated coniferin and syringin levels (Figure 4). In the double MYB and LAC17 overexpression lines, where growth was near WT (Figure 6), so too were the monolignol glucoside levels (Figure 4). Monolignol glucosides can accumulate as a method of detoxifying cytoplasmic accumulations of monolignols (Väisänen et al., 2015). When exogenous coniferyl alcohol was applied to BY‐2 cell cultures, levels of coniferin and other related phenolics were elevated (Väisänen et al., 2015). Considering that, and the generally very low levels of free monolignols in cells (Jaini, Wang, Dudareva, Chapple, & Morgan, 2017), one interpretation is that monolignols glucosides in the MYB63‐OX and MYB58‐OX lines are a reflection of elevated intracellular monolignols due to the upregulation of the lignin biosynthesis pathway. If abundant coniferin in the dwarf plants reflects high coniferyl alcohol levels in the cytoplasm, then lines with most severe dwarfism have the most abundant accumulations of G‐type soluble phenolics. This does not support the hypothesis that coniferyl alcohol‐derived growth‐promoting substances, such as dehydro‐diconiferyl glucosides (Binns, Chen, Wood, & Lynn, 1987; Lynn, Chen, Manning, & Wood, 1987), are playing a role in dwarfism in this case. Alternatively, it could be the case that the increase in glucosylation of monolignols limits the monolignols that could be oxidized and undergo combinatorial coupling to form dimers or oligolignols. Feeding experiments in Arabidopsis leaves have demonstrated the cytoplasmic oxidation and coupling of monolignols into oligomers in the cytoplasm (Dima et al., 2015). In the dwarf plants, tying up monolignols in the glucosylated form could occur at the expense of unknown dimers or oligolignols with growth‐promoting properties.
Transcriptional analysis points to monolignols and monolignol glucosides as key factors involved in dwarfism. MYB58 and MYB63 regulate a suite of genes responsible for monolignol biosynthesis, and our RNAseq transcriptomic data are consistent with earlier RT‐PCR data (Zhou et al., 2009). The RNAseq data also validate the in vitro DAP‐seq analysis of MYB63 transcription factor binding to gene promoters (O’Malley et al., 2016), which identified the core monolignol biosynthetic genes as direct binding targets of MYB63 (Figure 11). We see a significant upregulation of many of the core components of the monolignol biosynthetic pathway, as well as a glucosyltransferase (UGT72E2) implicated in the formation of coniferin (Lanot et al., 2006). Although they did not accumulate monolignol glucosides, there was a similar upregulation of the monolignol biosynthetic pathway and UGT72E2 in the double MYB63‐OX LAC17‐OX overexpression lines. This demonstrates that the upregulation of these genes by MYB63 was not abolished by LAC17 co‐overexpression and that the production of monolignols could continue at a level elevated above WT. This level was not high enough to trigger either dwarf growth or monolignol glucoside accumulation, suggesting the presence of the laccase led to lower cytoplasmic monolignol levels.
The drop in monolignol glucoside accumulation in LAC17‐OX MYB63‐OX lines is consistent with the established relationship between increased monolignol glucoside accumulation when laccases are knocked out (Zhao et al., 2013). However, the impact of an extracellular oxidative enzyme on the intracellular phenolics in the overexpression plants implies export of the cytoplasmic monolignols to the wall where the laccases are found (Figure 8). Given the recent in silico modeling indicating that diffusion of monolignols across lipid bilayers is a chemically and energetically plausible mechanism of transport (Vermaas et al., 2019), one mechanism explaining the drop of monolignols glucoside levels in the double overexpression plants would be the consumption of monolignols by laccases in the cell wall, creating a concentration gradients across the cell membrane followed by passive diffusion (Perkins et al., 2019). The elevated monolignols in the single MYB63‐OX lines overpowers the ability of cell wall‐localized oxidative enzymes to consume them, resulting in a buildup, glucosylation by UGT72E2, and their storage as glucosides to mitigate their toxicity. In the MYB63‐OX LAC17‐OX double overexpression lines, the additional copies of laccase may increase the strength of the extracellular sink shifting the balance of monolignols to primarily flowing out of the cell, alleviating the cytoplasmic toxicity. An alternative to this sink‐driven diffusion model is that monolignol transporters compete with the UGT72E2 for monolignol substrate in the LAC17‐OX MYB63‐OX lines, but no candidate transporters were identified in our transcriptome, and overexpression of several different ABC transporters did not rescue the MYB63‐OX dwarfism (Figure 9).
Alterations to the monolignol levels did not happen in isolation with respect to other soluble phenolics. In lines where monolignol glucosides were most elevated relative to WT, the levels of flavonols were depleted (Figure 4). Flavonol biosynthesis branches from the core monolignol biosynthesis pathway at p‐coumaroyl‐CoA (Fraser & Chapple, 2011). The inverse relationship between the two suggests that when monolignol production is favored by upregulation of the downstream monolignol pathway, the flux of p‐coumaroyl‐CoA is preferentially directed toward monolignols at the expense of flavonols. The intermediate levels of flavonols in the MYB58‐OX LAC17‐OX or MYB63‐OX LAC17‐OX lines compared to the single MYB58‐OX or MYB63‐OX lines mirror the other intermediate phenotypes in the double overexpressing lines. It appears that the hydroxycinnamate esters, sinapoyl malate and sinapoyl glucose, move in concert with the monolignol glucosides. Interestingly, this was also observed in lac4 lac11 lac17 triple mutants, where accumulation of monolignol glucosides was associated with elevated hydroxycinnamoyl esters (Zhao et al., 2013). These consistent patterns support the view that complex cross‐talk exists in the phenylpropanoid and monolignol biosynthetic pathways as well as lignin polymerization mechanisms (Zhou et al., 2009).
The complexity of the soluble phenylpropanoid response was reflected in the unexpected result that lignin levels in the rescued double MYB58‐OX LAC17‐OX or MYB63‐OX LAC17‐OX lines were similar to wild type, not higher than the single overexpression lines (Figure 5). Given the presence of ectopic lignin (Figure 2, Figure S1), the MYB63 and MYB58 overexpressing lines were predicted to have increased levels of lignin above wild type. We observed greater ectopic lignin in the cortex, pith, and epidermis using intrinsic autofluorescence compared with Mäule stained sections. We ascribe this to the greater sensitivity of the intrinsic fluorescence that we observe, particularly with respect to lignified primary walls. The hypothesis that increased laccase in the wall increases the consumption of monolignols, thus normalizing the intracellular soluble phenolic profile, leads to the prediction that there would be a corresponding increase in lignin. The lignin levels found in the double overexpressing lines were indistinguishable from wild type. The combination of transcriptional changes and the lignin levels suggests that the presence of additional laccase puts the system into some kind of equilibrium that results in wild‐type lignin levels in spite of changes to the transcriptome, most notably the sustained upregulation of the lignin biosynthetic pathway. This is similar to the case of the c3’h mutants with decreased lignin levels, which could be rescued by the disruption of Mediator components MED5a and MED5b (Bonawitz et al., 2014). The quantity of lignin was restored to wild‐type levels, even though the composition of the lignin remained altered, consisting almost exclusively of H subunits (Bonawitz et al., 2014). This demonstrated that the plant was chemically and physiologically capable of tolerating the C3’H mutation, but that the severe phenotype was due to other signals dependent on MED5a/b. It may be that, in the case of MYB58‐OX and MYB63‐OX, the cause and alleviation of severe dwarf phenotypes may be similarly dependent on intracellular or cell wall homeostatic mechanisms, and independent from the bulk lignification effects of laccase.
Our study of LMID in the context of MYB63, MYB58, and MYB/LAC co‐overexpressing lines has directly answered some questions about dwarfism related to mis‐regulation of the lignin pathway, and raised the possibility of connections to other pathways and mechanisms worthy of further inquiry. We have ruled out the possibility that collapsed xylem is the cause of dwarfism in these lines. MYB/LAC4 or LAC17 co‐overexpression was able to normalize the hyperaccumulation of monolignol glucosides and restore growth, which is not consistent with dwarfism being due to loss of a monolignol‐derived growth factor. Transcriptional analysis suggests that broad transcriptional changes are elicited by both the overexpression of MYB63 and the co‐overexpression of MYB63 and LAC17, with the most striking difference between the two being stress‐associated transcripts associated with dwarfism. We have not fully determined the mechanisms and signaling pathways responsible for the dramatic changes in phenotype that we observe. It is likely that other factors are involved, which may overlap with unknown factors implicated in other related studies of LMID‐associated phenotypic rescues (Bonawitz et al., 2014; Gallego‐Giraldo et al., 2020; Panda et al., 2020).
AUTHOR CONTRIBUTIONS
M.L.P, M.S, and L.S. designed the research. M.L.P, M.S., F.U., R.A.S., R.S., and N.J.H. conducted experiments and analyzed the data. D.C.J.W. performed bioinformatic analysis. S.D.C, S.D.M, and L.S. supervised experiments. M.L.P. and L.S. wrote the article with contributions from all authors.
Accession Numbers
Sequence data from this article can be found in the GenBank data library under accession numbers MYB63, AT1G79180; MYB58, AT1G16490; LAC4, AT2G38080; LAC17, AT5G60020. The BioProject and SRA accession for the RNAseq data are PRJNA345391 and SRP090868, respectively.
Supporting information
Fig S1‐S8
Table S1‐S4
Supplementary Material
ACKNOWLEDGMENTS
Technical assistance from UBC Bioimaging Facility is gratefully acknowledged.
Lane Perkins M, Schuetz M, Unda F, et al. Dwarfism of high‐monolignol Arabidopsis plants is rescued by ectopic LACCASE overexpression. Plant Direct. 2020;4:1–6. 10.1002/pld3.265
Mendel Lane Perkins and Mathias Schuetz are contributed equally to this study.
Funding information
Funding for this study came from Canadian Natural Sciences and Engineering Research Council (NSERC) Discovery Grants to A.L.S., S.D.M., and S.D.C.; NSERC Postgraduate Scholarship—Doctoral award to M.L.P.; the Agreenskills program (European Union's 7th Framework Programme for research, technological development and demonstration under grant agreement numbers FP7‐267196 and FP7‐609398, and INRA) to R.S.
REFERENCES
- Alejandro, S. , Lee, Y. , Tohge, T. et al (2012). AtABCG29 is a monolignol transporter involved in lignin biosynthesis. Current Biology, 22, 1207–1212. [DOI] [PubMed] [Google Scholar]
- Anders, S. , Pyl, P. T. , & Huber, W. (2015). HTSeq–a Python framework to work with high‐throughput sequencing data. Bioinformatics, 31, 166–169. 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthet, S. , Demont‐Caulet, N. , Pollet, B. et al (2011). Disruption of LACCASE4 and 17 results in tissue‐specific alterations to lignification of Arabidopsis thaliana stems. The Plant Cell, 23, 1124–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besseau, S. , Hoffmann, L. , Geoffroy, P. , Lapierre, C. , Pollet, B. , & Legrand, M. (2007). Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. The Plant Cell, 19, 148–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns, A. N. , Chen, R. H. , Wood, H. N. , & Lynn, D. G. (1987). Cell division promoting activity of naturally occurring dehydrodiconiferyl glucosides: Do cell wall components control cell division? Proceedings of the National Academy of Sciences of the United Science of America, 84, 980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, D. , Beisson, F. , Brigham, A. et al (2007). Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. The Plant Journal, 52, 485–498. [DOI] [PubMed] [Google Scholar]
- Blokhina, O. , Laitinen, T. , Hatakeyama, Y. et al (2019). Ray parenchymal cells contribute to lignification of tracheids in developing xylem of Norway SpRuce. Plant Physiology, 181, 1552–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger, A. M. , Lohse, M. , & Usadel, B. (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30, 2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonawitz, N. D. , & Chapple, C. (2013). Can genetic engineering of lignin deposition be accomplished without an unacceptable yield penalty? Current Opinion in Biotechnology, 24, 336–343. [DOI] [PubMed] [Google Scholar]
- Bonawitz, N. D. , Kim, J. I. , Tobimatsu, Y. , Ciesielski, P. N. , Anderson, N. A. , Ximenes, E. , … Chapple, C. (2014). Disruption of Mediator rescues the stunted growth of a lignin‐deficient Arabidopsis mutant. Nature, 509, 376–380. 10.1038/nature13084 [DOI] [PubMed] [Google Scholar]
- Borevitz, J. O. , Xia, Y. , Blount, J. , Dixon, R. A. , & Lamb, C. (2000). Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. The Plant Cell, 12, 2383–2393. 10.1105/tpc.12.12.2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulis, L. E. , Lacobucci, M. , & Shirley, B. W. (1996). A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. The Plant Cell, 8, 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, F. , & Dixon, R. A. (2007). Lignin modification improves fermentable sugar yields for biofuel production. Nature Biotechnology, 25, 759–761. [DOI] [PubMed] [Google Scholar]
- Chou, E. , Schuetz, M. , Hoffmann, N. et al (2018). Distribution, Mobility and Anchoring of Lignin‐Related Oxidative Enzymes in Arabidopsis Secondary Cell Walls. Journal of Experimental Botany, 69, 1849–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, H. D. , Park, J. Y. , Nair, R. , Chapple, C. , & Mansfield, S. D. (2008a). RNAi‐mediated suppression of p‐coumaroyl‐CoA 3′‐hydroxylase in hybrid poplar impacts lignin deposition and soluble secondary metabolism. Proceedings of the National Academy of Sciences of the United States of America, 105, 4501–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman, H. D. , Samuels, A. L. , Guy, R. D. , & Mansfield, S. D. (2008b). Perturbed lignification impacts tree growth in hybrid poplar ‐ A function of sink strength, vascular integrity, and photosynthetic assimilation. Plant Physiology, 148, 1229–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullis, I. F. , Saddler, J. N. , & Mansfield, S. D. (2004). Effect of initial moisture content and chip size on the bioconversion efficiency of softwood lignocellulosics. Biotechnology and Bioengineering, 85, 413–421. [DOI] [PubMed] [Google Scholar]
- De Meester, B. , de Vries, L. , Özparpucu, M. et al (2018). Vessel‐specific reintroduction of CINNAMOYL‐COA REDUCTASE1 (CCR1) in Dwarfed ccr1 mutants restores vessel and xylary fiber integrity and increases biomass. Plant Physiology, 176, 611–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima, O. , Ralph, J. , Vanholme, B. , Boerjan, W. , Kim, H. , & Morreel, K. (2015). Small glycosylated lignin oligomers are stored in arabidopsis leaf vacuoles. The Plant Cell, 27, 695–710. 10.1105/tpc.114.134643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, R. A. , & Barros, J. (2019). Lignin biosynthesis: Old roads revisited and new roads explored. Open Biology, 9, 190215 10.1098/rsob.190215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du, Z. , Zhou, X. , Ling, Y. , Zhang, Z. , & Su, Z. (2010). agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Research, 38, W64–W70. 10.1093/nar/gkq310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser, C. M. , & Chapple, C. (2011). The phenylpropanoid pathway in Arabidopsis. The Arabidopsis Book, 9, e0152 10.1199/tab.0152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego‐Giraldo, L. , Liu, C. , Pose‐Albacete, S. et al (2020). ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE 1 (ADPG1) releases latent defense signals in stems with reduced lignin content. Proceedings of the National Academy of Sciences of the United States of America, 117, 3281–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guénin, S. , Mauriat, M. , Pelloux, J. , Wuytswinkel, O. V. , Bellini, C. , & Gutierrez, L. (2009). Normalization of qRT‐PCR data: The necessity of adopting a systematic, experimental conditions‐specific, validation of references. Journal of Experimental Botany, 60, 487–493. 10.1093/jxb/ern305 [DOI] [PubMed] [Google Scholar]
- Hoffmann, L. , Besseau, S. , Geoffroy, P. , Ritzenthaler, C. , Meyer, D. , Lapierre, C. , … Legrand, M. (2005). Acyltransferase‐catalysed p‐coumarate ester formation is a committed step of lignin biosynthesis. Plant Biosystems, 139, 50–53. [Google Scholar]
- Jaini, R. , Wang, P. , Dudareva, N. , Chapple, C. , & Morgan, J. A. (2017). Targeted metabolomics of the phenylpropanoid pathway in arabidopsis thaliana using reversed phase liquid chromatography coupled with tandem mass spectrometry. Phytochemical Analysis, 28, 267–276. [DOI] [PubMed] [Google Scholar]
- Kaneda, M. , Schuetz, M. , Lin, B. S. P. , Chanis, C. , Hamberger, B. , Western, T. L. , … Samuels, A. L. (2011). ABC transporters coordinately expressed during lignification of Arabidopsis stems include a set of ABCBs associated with auxin transport. Journal of Experimental Botany, 62, 2063–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi, M. , Inzé, D. , & Depicker, A. (2002). GATEWAYTM vectors for Agrobacterium‐mediated plant transformation. Trends in Plant Science, 7, 193–195. [DOI] [PubMed] [Google Scholar]
- Koressaar, T. , & Remm, M. (2007). Enhancements and modifications of primer design program Primer3. Bioinformatics, 23, 1289–1291. 10.1093/bioinformatics/btm091 [DOI] [PubMed] [Google Scholar]
- Langmead, B. , & Salzberg, S. L. (2012). Fast gapped‐read alignment with Bowtie 2. Nature Methods, 9, 357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanot, A. , Hodge, D. , Jackson, R. G. , George, G. L. , Elias, L. , Lim, E. K. , … Bowles, D. J. (2006). The glucosyltransferase UGT72E2 is responsible for monolignol 4‐O‐glucoside production in Arabidopsis thaliana. The Plant Journal, 48, 286–295. [DOI] [PubMed] [Google Scholar]
- LeRoy, J. , Huss, B. , Creach, A. , Hawkins, S. , & Neutelings, G. (2016). Glycosylation Is a major regulator of phenylpropanoid availability and biological activity in plants. Frontiers in Plant Science, 7, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Bonawitz, N. D. , Weng, J.‐K.‐J.‐K. , & Chapple, C. (2010). The growth reduction associated with repressed lignin biosynthesis in arabidopsis thaliana is independent of flavonoids. The Plant Cell, 22, 1620–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, J. S. , Huang, X. X. , Li, Q. , Cao, Y. , Bao, Y. , Meng, X. F. , … Hou, B. K. (2016). UDP‐glycosyltransferase 72B1 catalyzes the glucose conjugation of monolignols and is essential for the normal cell wall lignification in Arabidopsis thaliana. The Plant Journal, 88, 26–42. [DOI] [PubMed] [Google Scholar]
- Liu, Q. , Luo, L. , & Zheng, L. (2018). Lignins: Biosynthesis and Biological Functions in Plants. International Journal of Molecular Sciences, 19, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, M. I. , Huber, W. , Anders, S. et al (2014). Moderated estimation of fold change and dispersion for RNA‐seq data with DESeq2. Genome Biology, 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn, D. G. , Chen, R. H. , Manning, K. S. , & Wood, H. N. (1987). The structural characterization of endogenous factors from Vinca rosea crown gall tumors that promote cell division of tobacco cells. Proceedings of the National Academy of Sciences of the United States of America, 84, 615–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon, E. L. , & Mansfield, S. D. (2019). Tailor‐made trees: Engineering lignin for ease of processing and tomorrow’s bioeconomy. Current Opinion in Biotechnology, 56, 147–155. [DOI] [PubMed] [Google Scholar]
- Mehrtens, F. , Kranz, H. , Bednarek, P. , & Weisshaar, B. (2005). The Arabidopsis transcription factor MYB12 is a flavonol‐specific regulator of phenylpropanoid biosynthesis. Plant Physiology, 138, 1083–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao, Y.‐C. , & Liu, C.‐J. (2010). ATP‐binding cassette‐like transporters are involved in the transport of lignin precursors across plasma and vacuolar membranes. Proceedings of the National Academy of Sciences of the United States of America, 107, 22728–22733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra, P. P. , & Loqué, D. (2014). Histochemical staining of arabidopsis thaliana secondary cell wall elements. Journal of Visualized Experiments: Jove, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottiar, Y. , Vanholme, R. , Boerjan, W. , Ralph, J. , & Mansfield, S. D. (2016). Designer lignins: Harnessing the plasticity of lignification. Current Opinion in Biotechnology, 37, 190–200. [DOI] [PubMed] [Google Scholar]
- Muro‐Villanueva, F. , Mao, X. , & Chapple, C. (2019). Linking phenylpropanoid metabolism, lignin deposition, and plant growth inhibition. Current Opinion in Biotechnology, 56, 202–208. [DOI] [PubMed] [Google Scholar]
- O’Malley, R. C. , Huang, S. C. , Song, L. , Lewsey, M. G. , Bartlett, A. , Nery, J. R. , … Ecker, J. R. (2016). Cistrome and Epicistrome features shape the regulatory DNA landscape. Cell, 166, 1598 10.1016/j.cell.2016.08.063 [DOI] [PubMed] [Google Scholar]
- Ohtani, M. , & Demura, T. (2019). The quest for transcriptional hubs of lignin biosynthesis: Beyond the NAC‐MYB‐gene regulatory network model. Current Opinion in Biotechnology, 56, 82–87. [DOI] [PubMed] [Google Scholar]
- Panda, C. , Li, X. , Wager, A. , Chen, H. , & Li, X. U. (2020). An importin‐beta‐like protein mediates lignin‐modification‐induced dwarfism in Arabidopsis. The Plant Journal, 102(6), 1281–1293. 10.1111/tpj.14701 [DOI] [PubMed] [Google Scholar]
- Perkins, M. , Smith, R. A. , & Samuels, L. (2019). The transport of monomers during lignification in plants: Anything goes but how? Current Opinion in Biotechnology, 56, 69–74. [DOI] [PubMed] [Google Scholar]
- Ralph, J. , Lapierre, C. , & Boerjan, W. (2019). Lignin structure and its engineering. Current Opinion in Biotechnology, 56, 240–249. [DOI] [PubMed] [Google Scholar]
- Schmittgen, T. D. , & Livak, K. J. (2008). Analyzing real‐time PCR data by the comparative CT method. Nature Protocols, 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Smith, R. A. , Schuetz, M. , Roach, M. , Mansfield, S. D. , Ellis, B. , & Samuels, L. (2013). Neighboring parenchyma cells contribute to arabidopsis xylem lignification, while lignification of interfascicular fibers is cell autonomous. The Plant Cell, 25, 3988–3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarbreck, D. , Wilks, C. , Lamesch, P. et al (2008). The Arabidopsis Information Resource (TAIR): Gene structure and function annotation. Nucleic Acids Research, 36, D1009–D1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, M. , Kegasa, T. , Watanabe, A. , Tamura, M. , & Tsutsumi, Y. (2018). Expression analysis of transporter genes for screening candidate monolignol transporters using Arabidopsis thaliana cell suspensions during tracheary element differentiation. Journal of Plant Research, 131, 297–305. 10.1007/s10265-017-0979-4 [DOI] [PubMed] [Google Scholar]
- Tsuyama, T. , Kawai, R. , Shitan, N. , Matoh, T. , Sugiyama, J. , Yoshinaga, A. , … Yazaki, K. (2013). Proton‐dependent coniferin transport, a common major transport event in differentiating xylem tissue of woody plants. Plant Physiology, 162, 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa, T. (2018). Lignin modification in planta for valorization. Phytochemistry Reviews, 17, 1305–1327. [Google Scholar]
- Väisänen, E. E. , Smeds, A. I. , Fagerstedt, K. V. , Teeri, T. H. , Willför, S. M. , & Kärkönen, A. (2015). Coniferyl alcohol hinders the growth of tobacco BY‐2 cells and Nicotiana benthamiana seedlings. Planta, 242, 747–760. 10.1007/s00425-015-2348-7 [DOI] [PubMed] [Google Scholar]
- Van‐Acker, R. , Vanholme, R. , Storme, V. , Mortimer, J. C. , Dupree, P. , & Boerjan, W. (2013). Lignin biosynthesis perturbations affect secondary cell wall composition and saccharification yield in Arabidopsis thaliana. Biotechnology for Biofuels, 6, 46 10.1186/1754-6834-6-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme, R. , De Meester, B. , Ralph, J. , & Boerjan, W. (2019). Lignin biosynthesis and its integration into metabolism. Current Opinion in Biotechnology, 56, 230–239. [DOI] [PubMed] [Google Scholar]
- Vanholme, R. , Storme, V. , Vanholme, B. et al (2012). A systems biology view of responses to lignin biosynthesis perturbations in arabidopsis. The Plant Cell, 24, 3506–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermaas, J. V. , Dixon, R. A. , Chen, F. , Mansfield, S. D. , Boerjan, W. , Ralph, J. , … Beckham, G. T. (2019). Passive membrane transport of lignin‐related compounds. Proceedings of the National Academy of Sciences of the United States of America, 116, 23117–23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, J. , Luo, D. , Xu, D. , Zeng, M. , Cui, X. , Li, L. , & Huang, H. (2015). CCR1, an enzyme required for lignin biosynthesis in Arabidopsis, mediates cell proliferation exit for leaf development. The Plant Journal, 83, 375–387. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M. , Goué, N. , Igarashi, H. , Ohtani, M. , Nakano, Y. , Mortimer, J. C. , … Demura, T. (2010). VASCULAR‐RELATED NAC‐DOMAIN6 and VASCULAR‐RELATED NAC‐DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiology, 153, 906–914. 10.1104/pp.110.154013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, F. , Mitra, P. , Zhang, L. et al (2013). Engineering secondary cell wall deposition in plants. Plant Biotechnology Journal, 11, 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Q. , Nakashima, J. , Chen, F. et al (2013). LACCASE Is Necessary and Nonredundant with PEROXIDASE for Lignin Polymerization during Vascular Development in Arabidopsis. The Plant Cell, 25, 3976–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Lee, C. , Zhong, R. , & Ye, Z.‐H. (2009). MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in arabidopsis. Plant Cell Online, 21, 248–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1‐S8
Table S1‐S4
Supplementary Material


