Abstract
In hypertrophic cardiomyopathy, multimodality imaging is crucial to confirm diagnosis, assess for presence and mechanism of left ventricular outflow tract obstruction, and risk stratification for sudden cardiac death. This review will focus on the application of imaging to assess established and emerging factors to be considered in sudden cardiac death risk stratification.
Keywords: cardiomyopathies, death, diagnosis, heart failure, risk
Over the past decades, significant strides have been made regarding our understanding of hypertrophic cardiomyopathy (HCM), including diagnosis, treatment, and prevention of sudden cardiac death (SCD).1,2 The estimated prevalence of HCM in the general population is 1 in 200 to 500 and patients can be asymptomatic, symptomatic with heart failure, atrial and ventricular arrhythmias and SCD.1,2 HCM is diagnosed in the setting of left ventricular (LV) wall thickness ≥15 or ≥13 mm with a family history of HCM after exclusion of other causes using a combination of clinical evaluation and imaging.1,2 Once a patient is diagnosed with HCM, it is imperative to assess for left ventricular outflow tract (LVOT) obstruction and risk factors for SCD.1,2 In this review, we focus on the use of imaging in risk stratification for SCD in HCM (Table).
Table.
Sudden Cardiac Death Risk and Imaging
| 2011 ACC/AHA Risk Factors | Echocardiography | Cardiac Magnetic Resonance |
|---|---|---|
| Major | ||
| LV wall thickness ≥30 mm | + | ++ |
| Mitigating | ||
| Extensive LGE | − | ++ |
| LVOT obstruction | ++ | − |
| LV apical aneurysm | + | ++ |
| ESC HCM risk SCD calculator | ||
| LV wall thickness | + | ++ |
| LA diameter | + | ++ |
| LVOT gradient, rest or Valsalva | ++ | − |
ACC indicates American College of Cardiology; AHA, American Heart Association; ESC, European Society of Cardiology; HCM, hypertrophic cardiomyopathy; LA, left atrial; LGE, late gadolinium enhancement; LV, left ventricle; LVOT, left ventricular outflow tract; and SCD, sudden cardiac death.
RISK STRATIFICATION FOR SCD
Overall risk of SCD in patients with HCM is low and estimated ≈0.5%/y.1,2 Current recommendations for SCD risk stratification from the 2011 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines include assessment for personal history of ventricular fibrillation or tachycardia, prior resuscitation for SCD, family history of SCD, unexplained syncope, and maximal LV wall thickness (MWT) ≥30 mm all of which are indications for implantable cardioverter defibrillator (ICD) implantation.1 If a patient has documented nonsustained ventricular tachycardia on ambulatory electrocardiography monitoring or an abnormal blood pressure response with exercise stress testing and other SCD risk modifiers, such as late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR), an apical aneurysm, an ICD may be considered.1 The 2014 European Society of Cardiology guidelines recommend HCM risk SCD calculator to assess the 5-year risk of SCD and that an ICD be placed with a 5-year risk score ≥6% (high risk) and potentially those with a 5-year risk ≥4% and <6% (intermediate risk).2,3 The HCM risk SCD calculator incorporates MWT, left atrial (LA) diameter, maximal LVOT gradient (rest/Valsalva provoked), family history of SCD, non-sustained ventricular tachycardia, unexplained syncope, and age.3 Notably, the HCM risk SCD calculator was not validated for use after septal reduction therapy (SRT) and does not allow for the incorporation of additional risk factors.2,3
In an external validation study of 3703 patients, the HCM risk SCD calculator worked reasonably well; the observed 5-year incidence of SCD was 1.4% for the low-risk group and 8.9% for the high-risk group.4 However, other studies comparing predicted risk with observed incidence of SCD have not shown the HCM risk SCD calculator to be capable of discerning between high- and low-risk groups, especially if patients undergoing SRT are included.5 The most recent comparison by Maron et al6 included 2094 patients with HCM with an ICD placed for primary prevention according to the ACC/AHA guideline risk factors, including extensive (>15% of LV mass if quantified) LGE and apical aneurysm. In the overall cohort, 73 patients (3.5%) had an apical aneurysm, and of the 1128 patients who had a CMR, 58 patients (5.1%) had LGE≥15%. The sensitivity and number needed to prevent 1 SCD were 95% and 6.6 for enhanced ACC/AHA criteria versus 58 % and 10.3 for the European Society of Cardiology criteria, respectively. Also, the C-statistics for discriminating patients with and without SCD was better with the ACC/AHA guidelines risk factors than the HCM risk SCD calculator (0.81 versus 0.74). This could be because the enhanced ACC/AHA guideline risk factors incorporates LV apical aneurysm and LGE≥15%.6
We advocate for the assessment of risk for SCD using both methods and for shared decision making when the assessment of risk differs acknowledging the differences in how risk is assessed based on the 2 current methods.
LV Wall Thickness
Initial evaluation by echocardiography includes the careful assessment for the presence, location and extent of LV hypertrophy, resting/provocable LVOT obstruction, severity and cause of mitral regurgitation, papillary muscle abnormalities, apical hypertrophy, or aneurysm; although in cases where echocardiography is not definitive evaluation with CMR should be under-taken.1,2 Wall thickness should be measured in parasternal long- or short-axis at end-diastole perpendicular to the LV cavity with special consideration to avoiding the inclusion of LV or right ventricular trabeculations; however, measurements may be difficult because of subop-timal acoustic windows and discrepancies exist when compared with CMR cases.7–9 A systematic bias is present with higher measurements by echocardiography when measuring the anteroseptum, which is related to erroneous inclusion of right ventricular myocardium, LV trabeculations, or papillary muscles.8,9 CMR has proven particularly useful in identifying maximal hypertrophy in a location other than the basal anteroseptal wall segment because of user-defined imaging planes without dependence on acoustic windows.7–9 Additionally, and perhaps most pertinent to clinical practice, major discrepancies in MWT occur in a significant proportion of cases at either clinical (15 mm) or prognostic (30 mm) cutoffs.7,8 It is important that clinicians review and attempt to resolve differences in MWT between imaging modalities to determine MWT for the individual patient to be used when assessing risk for SCD. The HCM risk SCD calculator treats MWT (measured on echocardiography) as a continuous variable based on evidence that increased wall thickness is directly related to increased risk for SCD even below the ACC/AHA cutoff of 30 mm.10,11 We recommend CMR for (1) patients with suspected HCM and borderline measurements or technically difficult images by echocardiography to aid in diagnosis and (2) in patients with confirmed HCM to ensure the region of MWT is identified along with other prognostic information like LGE.
LV Ejection Fraction
Patients with HCM and LV systolic dysfunction (LV ejection fraction ≤50%) have been shown to have an increased risk of adverse cardiac events.12 These patients were not included in clinical trials assessing the benefit of a primary prevention ICD in heart failure; however, ACC/AHA guidelines note ICD implantation may be considered for these patients,1 but a corresponding statement is not included in the European Society of Cardiology guidelines.2 Current assessment of LV ejection fraction by echocardiography is recommended using the biplane method of discs, however, in situations where it is difficult to assess LV ejection fraction by echocardiography, for instance, due to difficult imaging windows or poor definition of the endocardial border, CMR may be used.
LA Diameter
LA diameter was included as a continuous variable in the HCM risk SCD calculator based on one study demonstrating increased risk for SCD with increasing LA diameter.3,13 Other studies have been conflicting in whether LA diameter is associated with increased risk of SCD or heart failure admissions.14–16 LA volume is considered a more accurate assessment of LA size, although only a few small studies have examined its association with outcomes.17,18 Inclusion of LA diameter in the HCM risk SCD calculator remains contro-versial because it is unclear whether LA enlargement is an independent predictor, or a surrogate of increased wall thickness, diastolic dysfunction, LVOT obstruction, mitral regurgitation, or atrial arrhythmia.
LVOT Obstruction
Although LV hypertrophy is required for diagnosis, presence of LVOT obstruction is what leads to symptoms of heart failure and has become a defining feature of HCM.1,2,19 LVOT obstruction is defined as a resting or provocable peak gradient ≥30 mm Hg, although typically a gradient ≥50 mm Hg is necessary for consideration of SRT.1,2 LVOT obstruction is typically because of basal anteroseptal hypertrophy and systolic anterior motion of the anterior mitral leaflet, although papillary muscle abnormalities have also been implicated20 (Figure 1). LVOT obstruction is present in about a third of patients at rest and ≈70% with provocation.19 Provocative maneuvers used during echocardiography include Valsalva maneuver, inhalation of amyl nitrate, and exercise; dobutamine should not be used because it may provoke LVOT obstruction in healthy subjects. Studies comparing the peak LVOT gradient produced by these 3 methods have shown that an inducible gradient may be produced by only amyl nitrate, Valsalva, or exercise.21 For symptomatic patients without resting LVOT obstruction, exercise stress testing is considered the most physiological method to detect latent obstruction. Care should be taken to evaluate for LVOT gradients before wall motion abnormalities at peak exercise to optimize detection of LVOT obstruction. LVOT peak gradients can also vary significantly during the day making it difficult to ascertain an individual’s true peak LVOT gradient, further underscoring the importance of careful examination.
Figure 1. Echocardiography of hypertrophic cardiomyopathy with left ventricular outflow tract (LVOT) obstruction.
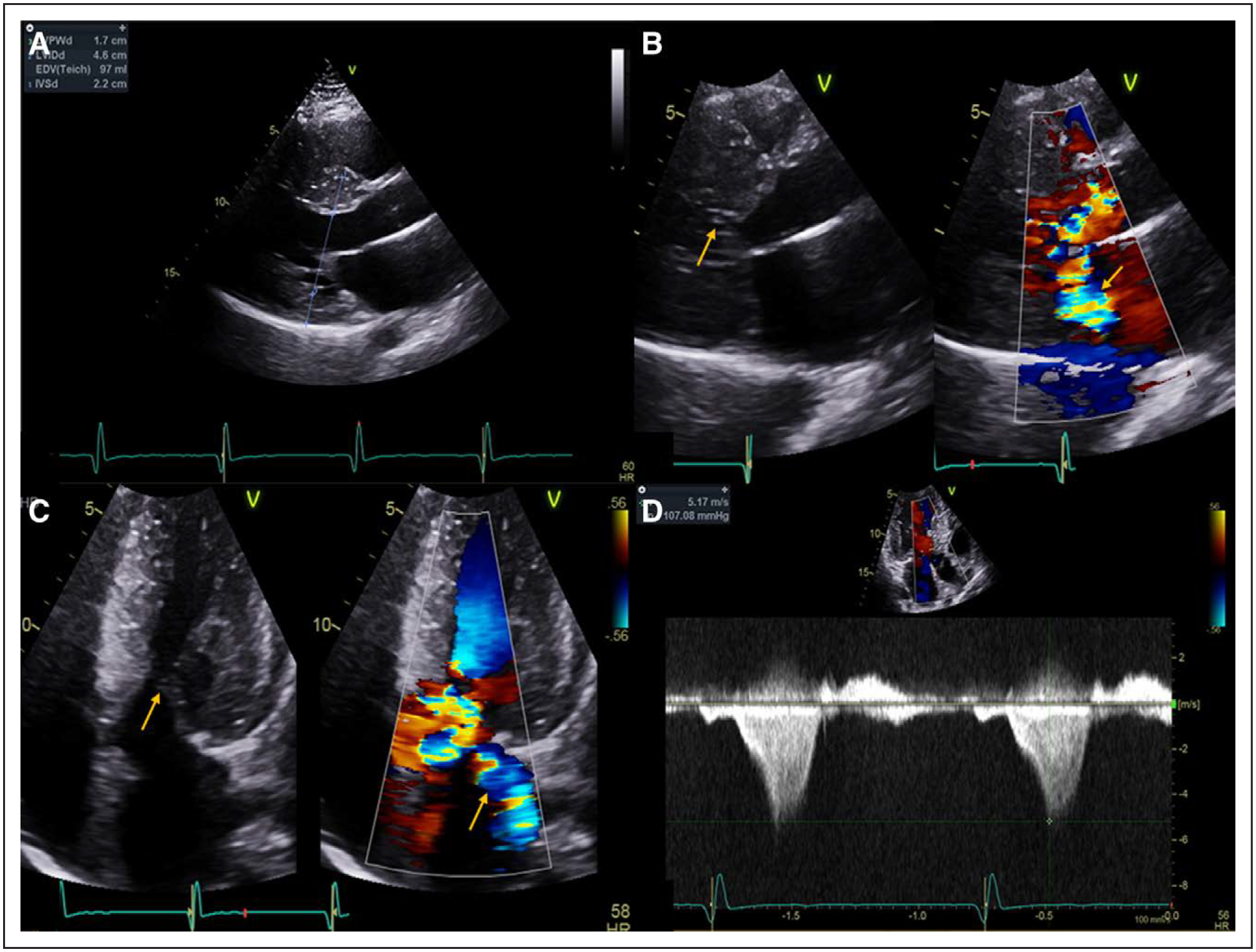
A, Parasternal long-axis view at end-diastole demonstrating an interventricular septal wall thickness of 2.2 cm. B and C, Parasternal long axis and off-axis apical 5-chamber views, respectively, with and without color Doppler demonstrating systolic anterior motion of the mitral valve (arrow, left) with turbulent flow within the LVOT and posteriorly directed mitral regurgitation (arrow, right). D, Continuous wave Doppler through the LVOT demonstrating a peak gradient of 107 mm Hg.
Several studies have demonstrated an association between LVOT obstruction and SCD.22–24 The association between latent obstruction and SCD is not well described; however, a recent study found that in patients without resting LVOT obstruction those with a provoked LVOT gradient ≥90 mm Hg, by Valsalva, amyl nitrate, or exercise, had worse outcomes than those with a provoked gradient 30 to 89 mm Hg, although patients with resting LVOT obstruction had the worst outcomes.25 A recent study also demonstrated that, in obstructed HCM patients, SRT was associated with better longer-term outcomes (including SCD) versus watchful waiting.26
The HCM risk SCD calculator includes maximal peak LVOT gradient at rest or with Valsalva regardless of concurrent medical therapy. Criticisms of this include the paucity of data regarding the association of provocable gradients with SCD, whether other methods of provocation should have been included and the dynamic nature of LVOT obstruction.
Apical Aneurysm
Apical aneurysms, defined as thin-walled akinetic or dyskinetic wall segments with a relatively wide connection to the left ventricular cavity at the apex, have been described in the setting of both midventricular and apical hypertrophy.27 Apical HCM is defined as apical wall thickness ≥15 mm at end-diastole, or maximal apical to basal wall thickness ratio of ≥1.3.28 This results in increased apical cavity pressure, wall stress, and ischemia/infarction which may lead to aneurysm formation. The prevalence of apical aneurysms in HCM was reported to be 1.3% and 4.8% in 2 different cohorts.29,30 Often the region of hypertrophy and presence of an apical aneurysm may be identified with echocardiography, although identification, including of thrombus, can be improved by contrast enhancement and avoiding foreshortening the LV apex31,32 (Figure 2). These maneuvers may aid in differentiating between apical hypertrophy or the presence of an apically displaced papillary muscle creating the appearance of apical hypertrophy (seen in up to 20% of cases)31,32 (Figure 3; Movies I to IV in the Data Supplement). We advocate the use of contrast in situations where the apical endocardium is not well defined and to further define anatomy. CMR may still be necessary to distinguish these features, in particular, the presence of an apical aneurysm which may be missed in ≈40% of cases examined by echocardiography alone.27,32,33 LGE imaging has also demonstrated transmural myocardial fibrosis and scar27 (Figure 4) and may be additionally useful for identifying an apical thrombus.
Figure 2. Echocardiography of apical hypertrophic cardiomyopathy with an aneurysm.
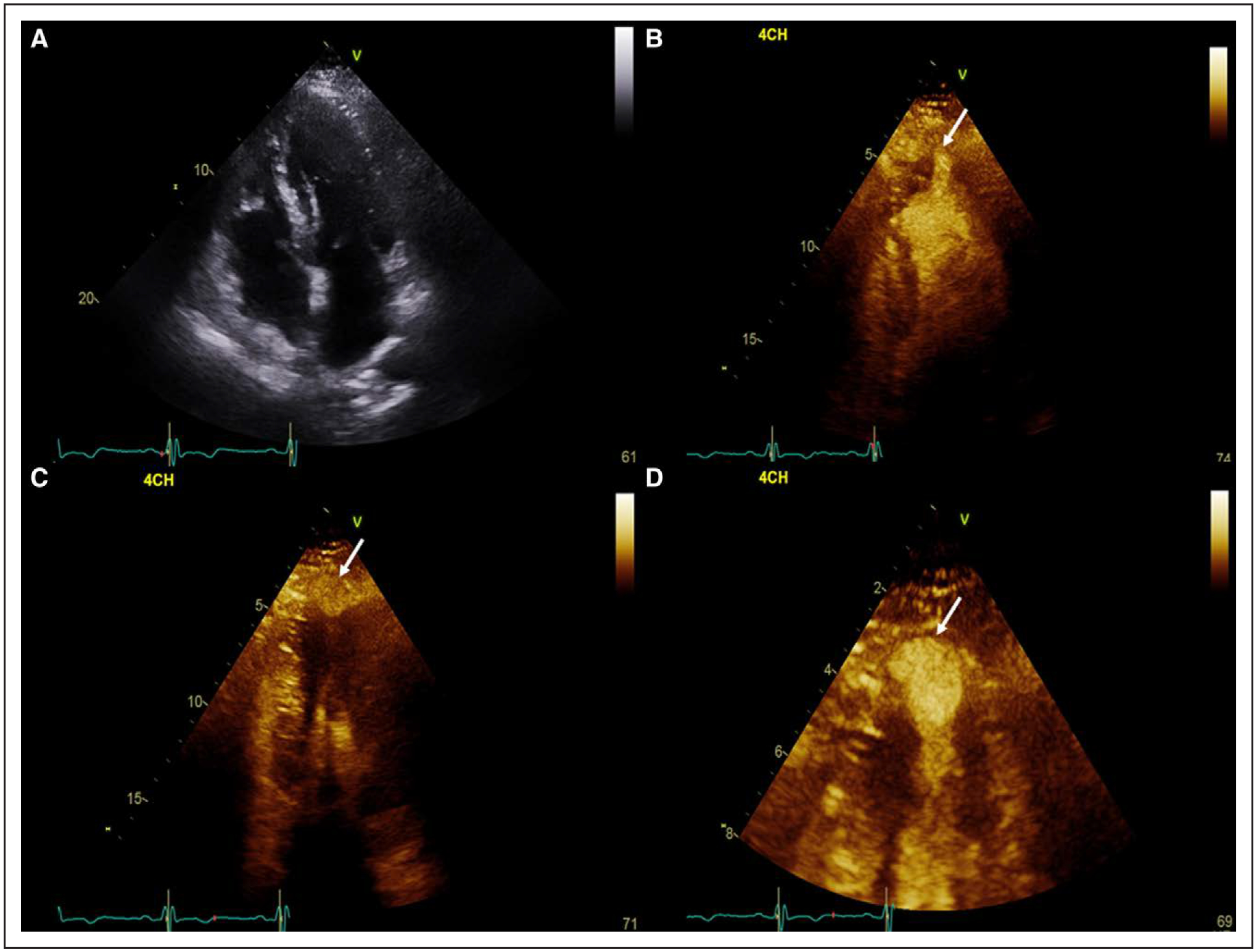
A, Apical 4-chamber view at end diastole with increased apical wall thickness. B, Apical 4-chamber view following administration of contrast in systole demonstrating a possible apical outpouching (arrow). C, Off-axis apical 4-chamber view of the apical outpouching at end systole (arrow). D, Zoomed in view of the apical aneurysm to clearly identify the wall (arrow; Movie I in the Data Supplement).
Figure 3. Apically displaced papillary muscle mistaken for apical hypertrophy.
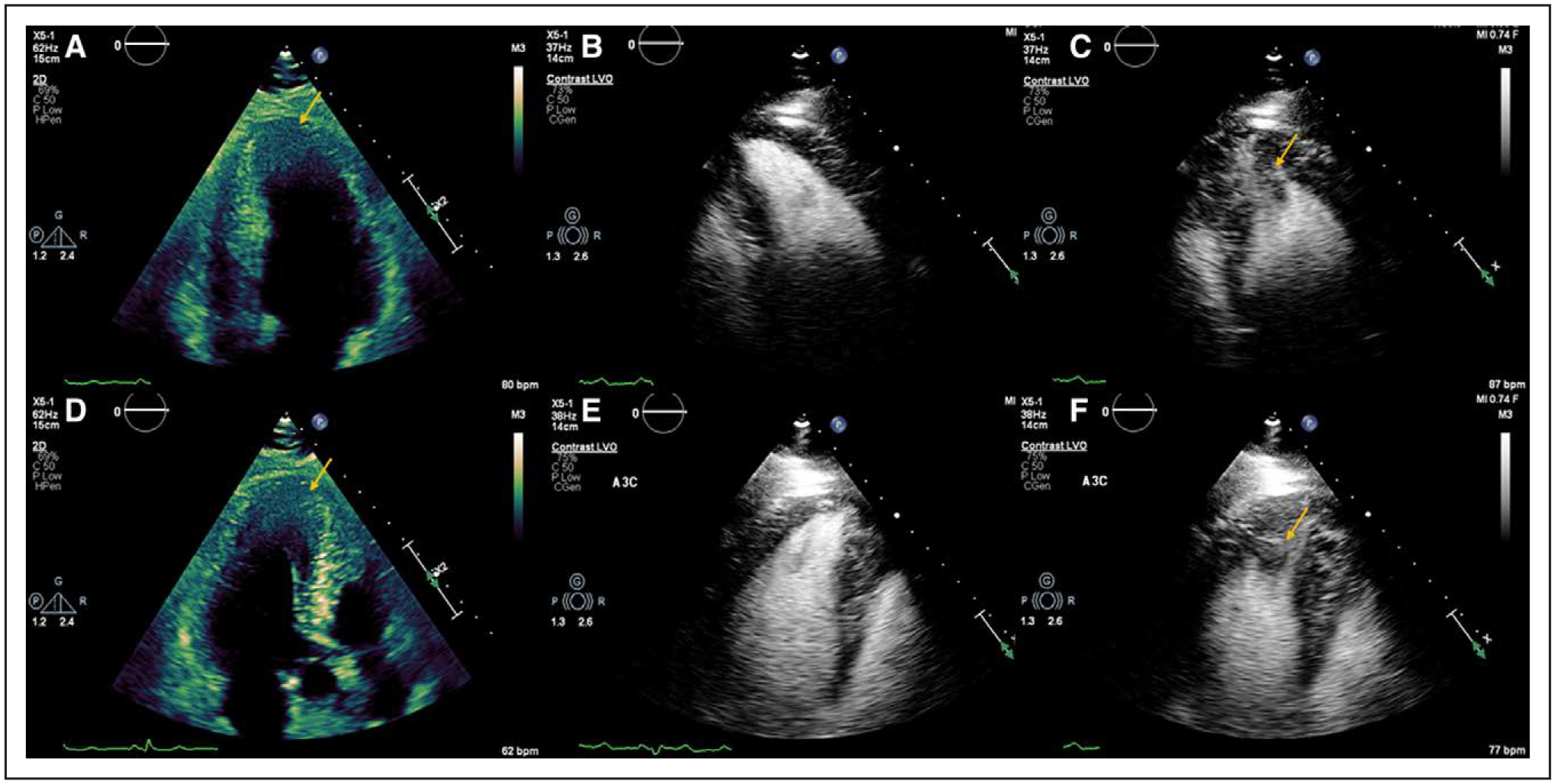
A and D, Apical 4- and 3-chamber views, respectively, with apparent increased wall thickness at the apex (arrow). B and E, Apical 4- and 3-chamber views, respectively, during diastole with administration of contrast agent demonstrating normal wall thickness. C and F, Apical 4- and 3-chamber views, respectively, during systole with administration of contrast agent demonstrating an apically displaced papillary muscle (arrow). LVO indicates left ventricular opacification.
Figure 4. Cardiac Magnetic Resonance of apical hypertrophic cardiomyopathy with a small apical aneurysm.
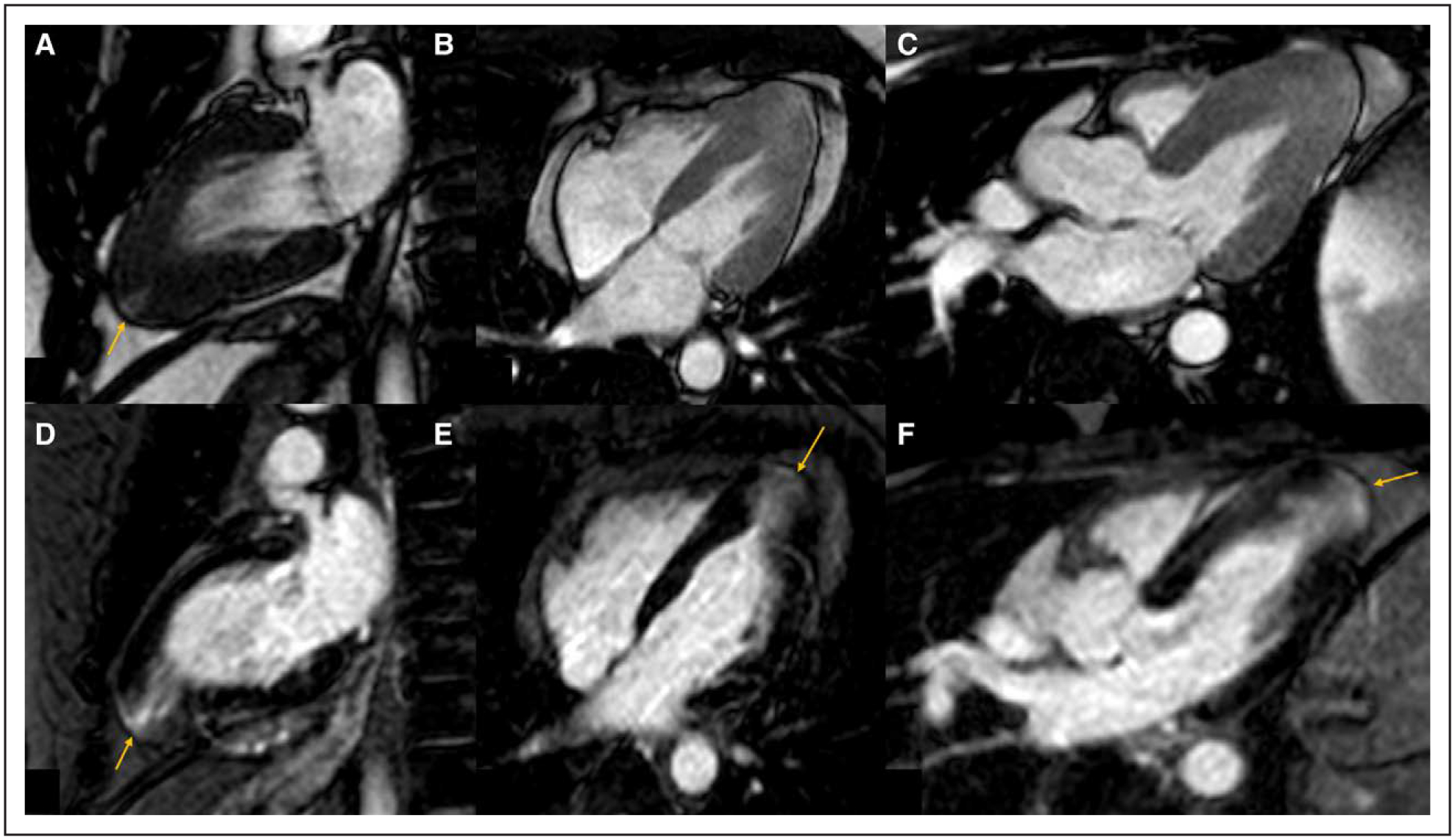
A–C, Balanced steady-state free precession cine imaging in the vertical long axis, 4-chamber and left ventricular outflow tract (LVOT) view at end systole demonstrating increased apical wall thickness with a small apical aneurysm (arrow). D–F, Phase-sensitive inversion recovery imaging in the vertical long axis, 4-chamber and LVOT view demonstrating transmural late gadolinium enhancement of the apical aneurysm (arrow) and subendocardial to mid-myocardial late gadolinium enhancement of the apical segments.
Although initial studies reported a low incidence of serious adverse events, recent reports have suggested otherwise. A study of 247 patients from Japan with apical HCM, of whom 8.5% had an apical aneurysm, found an event rate of 10.1%/y of ICD placement in the subset with an apical aneurysm.34 Similarly, Hanneman et al35 reported increased risk of major adverse events in 93 patients with apical HCM and an apical aneurysm (odds ratio, 4.6) with an event rate of 14.7%/y. Maron et al27 initially reported an annual adverse event rate of 10.5%/y for patients with an apical aneurysm. These results prompted the addition of apical aneurysm to the 2011 ACC/AHA guidelines as a risk modifier (however, size is not a criteria).1 Further analysis of an expanded patient cohort of 1940 patients with HCM demonstrated an increased annual event rate (SCD, appropriate ICD discharge, or resuscitated cardiac arrest) for patients with an apical aneurysm (4.7% versus 2%/y).29 An increased incidence of thromboembolic stroke has also been noticed in the setting of HCM with an apical aneurysm27,29; however, because of paucity of data, there are no specific recommendations in the guidelines and initiation of anticoagulation should be discussed on an individual basis for patients with an apical aneurysm without an apical thrombus.1,2
Late Gadolinium Enhancement by CMR
In addition to clarifying LV anatomy, LGE by CMR can both clarify the diagnosis and aid in risk stratification.1,2 Following their administration, gadolinium contrast agents redistribute from the intravascular space to the extracellular space with accumulation in areas with expansion of extracellular space due to either edema or fibrosis resulting in increased signal intensity on T1 weighted imaging.36 LGE is noted in approximately half of patients with HCM and is commonly described as patchy and mid-myocardial within segments of maximal hypertrophy37–39 (Figure 5).
Figure 5. Variations in patterns of late gadolinium enhancement (LGE) and hypertrophy in patients with hypertrophic cardiomyopathy.
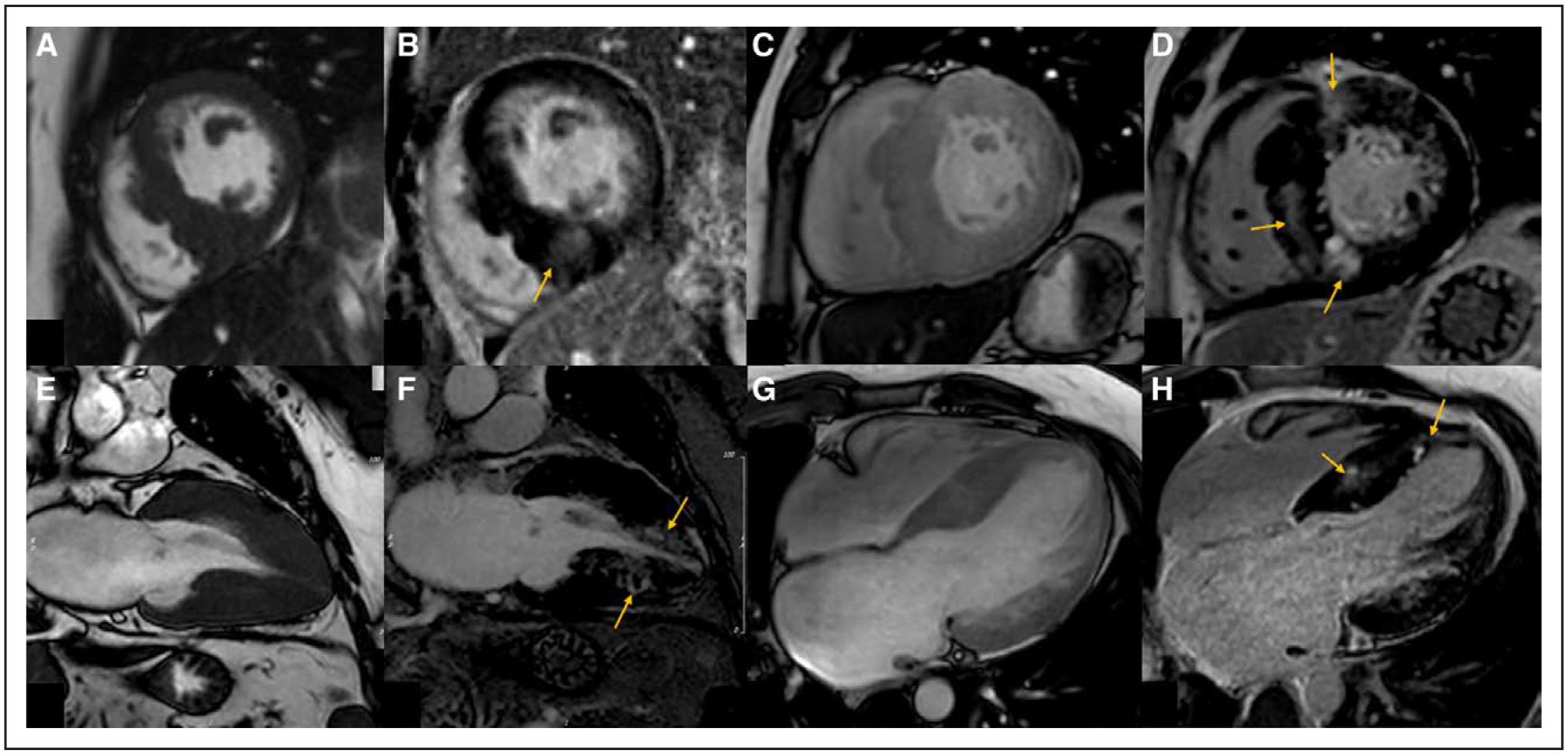
Patient 1: A, Balanced steady-state free precession (B-SSFP) cine imaging demonstrating asymmetrical septal hypertrophy predominantly affecting the mid inferoseptum. B, Phase-sensitive inversion recovery (PSIR) imaging in the short axis with mid-myocardial LGE in an area of maximal hypertrophy (arrow). Patient 2: C, B-SSFP cine imaging demonstrating asymmetrical septal hypertrophy. D, PSIR imaging with more extensive mid-myocardial LGE at the superior and inferior right ventricular insertion points and the mid septum (arrows). Patient 3: E, B-SSFP cine imaging in the vertical long-axis view demonstrating mid and apical hypertrophy. F, PSIR imaging with mid-myocardial LGE in the mid and apical segments (arrows). G, B-SSFP cine imaging in the 4-chamber view demonstrating mid to apical inferoseptal hypertrophy. H, PSIR imaging with mid-myocardial LGE in the mid to apical inferoseptum (arrows).
The presence of LGE is associated with an increased risk of adverse outcomes for patients with HCM. Several cross-sectional studies have demonstrated increased burden of ventricular arrhythmias, SCD, and all-cause mortality in patients who have LGE on CMR.37–40 LGE is also associated with increased heart failure symptoms and admissions and reduced LV ejection fraction in patients with HCM.40,41 A recent meta-analysis including 2993 patients confirmed that the presence of LGE is associated with an increased risk of adverse outcomes including SCD and death and that risk was proportional to the extent of LGE.42 Isolated LGE at the right ventricular insertion points does not appear to be associated with increased risk.37
Although LGE is present in >50% patients with HCM, the overall prevalence of SCD in patients with HCM is far lower1,2; thus, recent studies have focused on identifying a cut point for the extent of LGE beyond which there would be a benefit from an ICD. Although multiple studies have been conducted, the heterogeneity of the imaging sequences, postprocessing techniques has been problematic for the implementation of a precise LGE cutoff.1,2,43 LGE quantification techniques include a visual assessment or software packages that compare the signal intensity of affected areas to those selected as normal myocardium by visual assessment with cutoffs at 2 to 6 SD or full width half maximum.44 Although currently no specific technique is recommended by the Society for Cardiovascular Magnetic Resonance,44 prior studies comparing the various techniques have found that either 6 SD or full width half maximum techniques to potentially be the best in HCM.45,46
Two large studies have used a cut point of 15% LGE of the LV mass using the 6-SD technique. Chan et al37 studied 1293 patients at 7 centers and found that %LGE≥15% was associated with almost a 2-fold increase in the risk of SCD (hazard ratio 2.14) and aided in further risk discrimination over the traditional ACC/AHA guideline risk factors.37 Similarly, Mentias et al39 examined a cohort of 1423 patients deemed to be at low- or intermediate risk for SCD based on the HCM risk SCD calculator and found that %LGE≥15% using the 6-SD method was associated with an increased risk of a composite outcome of SCD and appropriate ICD discharge (odds ratio, 3.04; Figure 6). Currently, there is no specific recommendation regarding the appro-priateness of a follow-up CMR study to examine for progression of LGE,1,2 although this is an area of active investigation and one study demonstrated that progression of LGE on follow-up imaging at 5 to 6 years is associated with adverse outcomes.47
Figure 6. Survival free from events in patients with obstructive hypertrophic cardiomyopathy separated into 4 groups based on late gadolinium enhancement (LGE) <15% or LGE≥15%, and myectomy or no myectomy (left) and for the patients who underwent myectomy by LGE<15% or LGE≥15% (right).
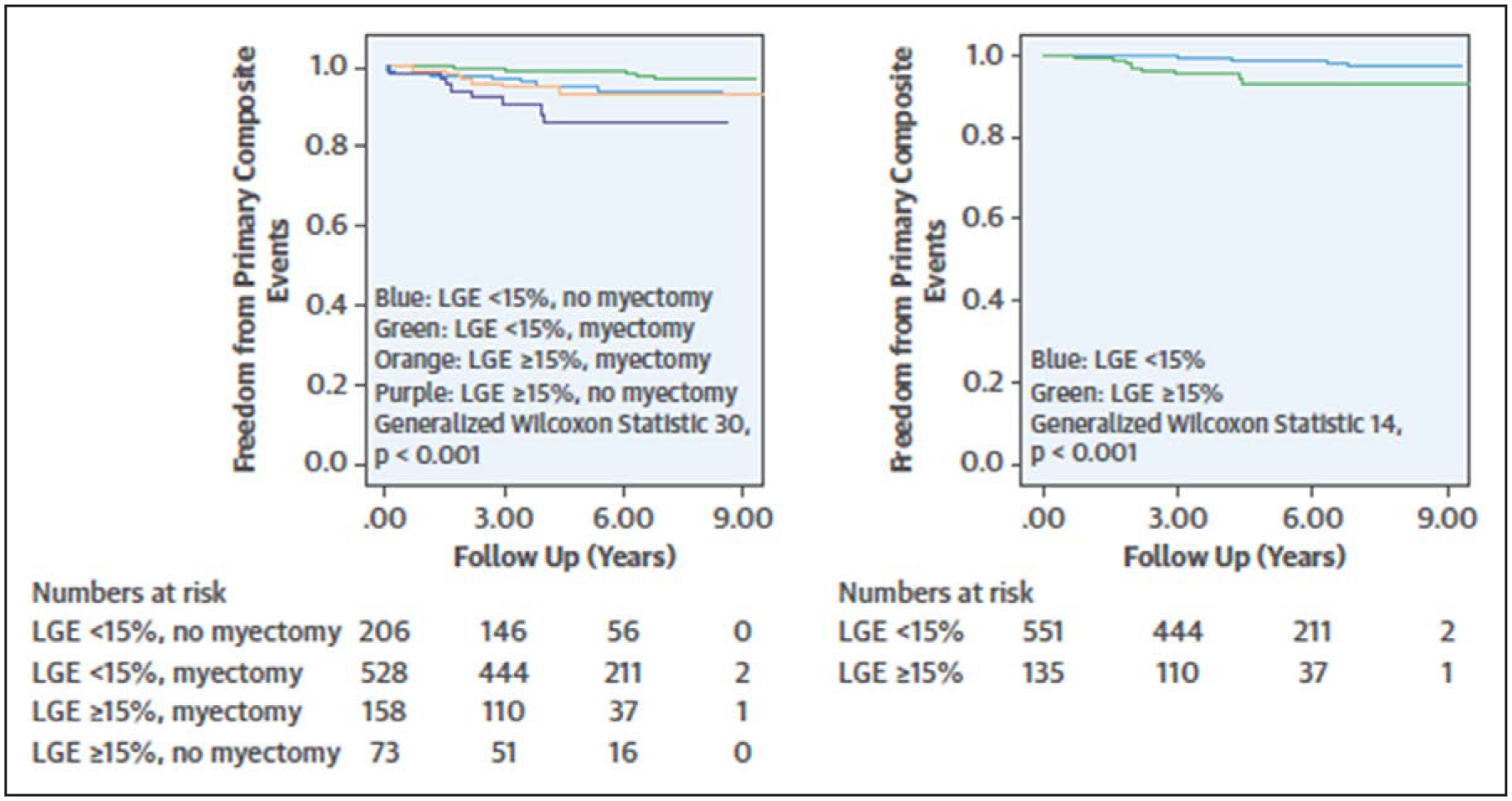
Reprinted from Mentias et al39 with permission. Copyright ©2018, Elsevier.
We advocate for the incorporation of LGE into the risk assessment for SCD for patients in the gray zone (without major ACC/AHA risk factors who have other mitigating risk factors, or low to intermediate risk by the HCM risk SCD calculator) to aid in shared decision making since the presence of extensive LGE (≥15%) is associated with an increased risk of SCD compared with patients with minimal LGE.
POTENTIAL NOVEL TECHNIQUES FOR RISK ASSESSMENT
T1 and T2 Mapping, and Extracellular Volume Fraction by CMR
During T1 mapping, the T1 relaxation time is measured for each individual pixel of the myocardium, and extracellular volume fraction is calculated based on the comparison of native and postgadolinium-chelate contrast agents, allowing for further tissue characterization and the identification of diffuse interstitial fibrosis.36 Regional increased native T1 times have been shown to correlate with areas of increased wall thickness and LGE in HCM.48 Although limited data suggest that increased native T1 times and extracellular volume are associated with adverse outcomes,49 there is more data regarding distinguishing HCM from hypertensive heart disease, Fabry disease, or cardiac amyloidosis.50,51 Increased signal intensity on T2 imaging is associated with edema/inflammation and potentially associated with increased risk for SCD.52 The HCMR study (Hypertrophic Cardiomyopathy Registry) is currently ongoing and seeks to further refine risk stratification in HCM using information gleaned from CMR including LGE and T1/2 mapping.53
Strain Imaging by Echocardiography
LV global longitudinal strain (LV-GLS) has emerged as a sensitive marker of LV function, although its use in clinical decision-making is currently limited both by variations in normal values and lack of standardization between various platforms. Despite this, there are multiple studies investigating potential uses of LV-GLS in the setting of increased LV wall thickness and for risk stratification. In the setting of increased wall thickness, LV-GLS appears to be best used to discern patients with possible cardiac amyloidosis from patients with other etiologies based on apical sparing pattern.54,55 A recent systematic review of studies including over 3000 patients with HCM found that abnormal LV-GLS in patients with HCM is associated with adverse cardiovascular outcomes, although significant heterogeneity in study design including software, LV-GLS cutoffs, and the outcomes chosen, made it difficult to come to a more precise conclusion.56 The use of LV-GLS is not currently recommended in the assessment of HCM patients, although additional studies may be able to shed new light on its potential use for clinical decision making and timing regarding either ICD placement or SRT.1,2,56
SRT as a Mitigating Factor?
Although SRT, either alcohol septal ablation or septal myectomy, is currently indicated for patients with LVOT obstruction and symptoms despite maximal medical therapy, it also potentially affects multiple clinical risk factors for SCD, including MWT, LA size, and peak LVOT gradient. As previously mentioned, the HCM risk SCD calculator should not be used in patients following SRT,3 although theoretically an individual patient’s calculated risk would change following SRT based on alterations in MWT, LA size, and peak LVOT gradient. Multiple observational studies have noted improved survival, including from SCD and appropriate ICD discharge, following SRT.5,57 Inter-estingly in the Mentias et al39 cohort of patients the addition of myectomy was able to further risk stratify patients with %LGE≥15% and conveyed a lower risk of SCD (Figure 6). In keeping with other research examining the effect of myectomy on risk stratification, one study noted that though abnormal LV-GLS worse than the median was associated with cardiac death and appropriate ICD discharge, that this risk appeared to be mitigated by myectomy.58
CONCLUSIONS
In HCM, a careful assessment with echocardiography and CMR is crucial to first confirm the diagnosis, and then to fully evaluate for the presence of LVOT obstruction and features associated with risk of SCD (Figure 7). Proper identification of patients at risk is of the utmost importance, and the identification and continued study of emerging risk factors will continue to improve the identification of patients at risk for SCD.
Figure 7. Multimodality imaging in sudden cardiac death (SCD) risk stratification in hypertrophic cardiomyopathy (HCM).
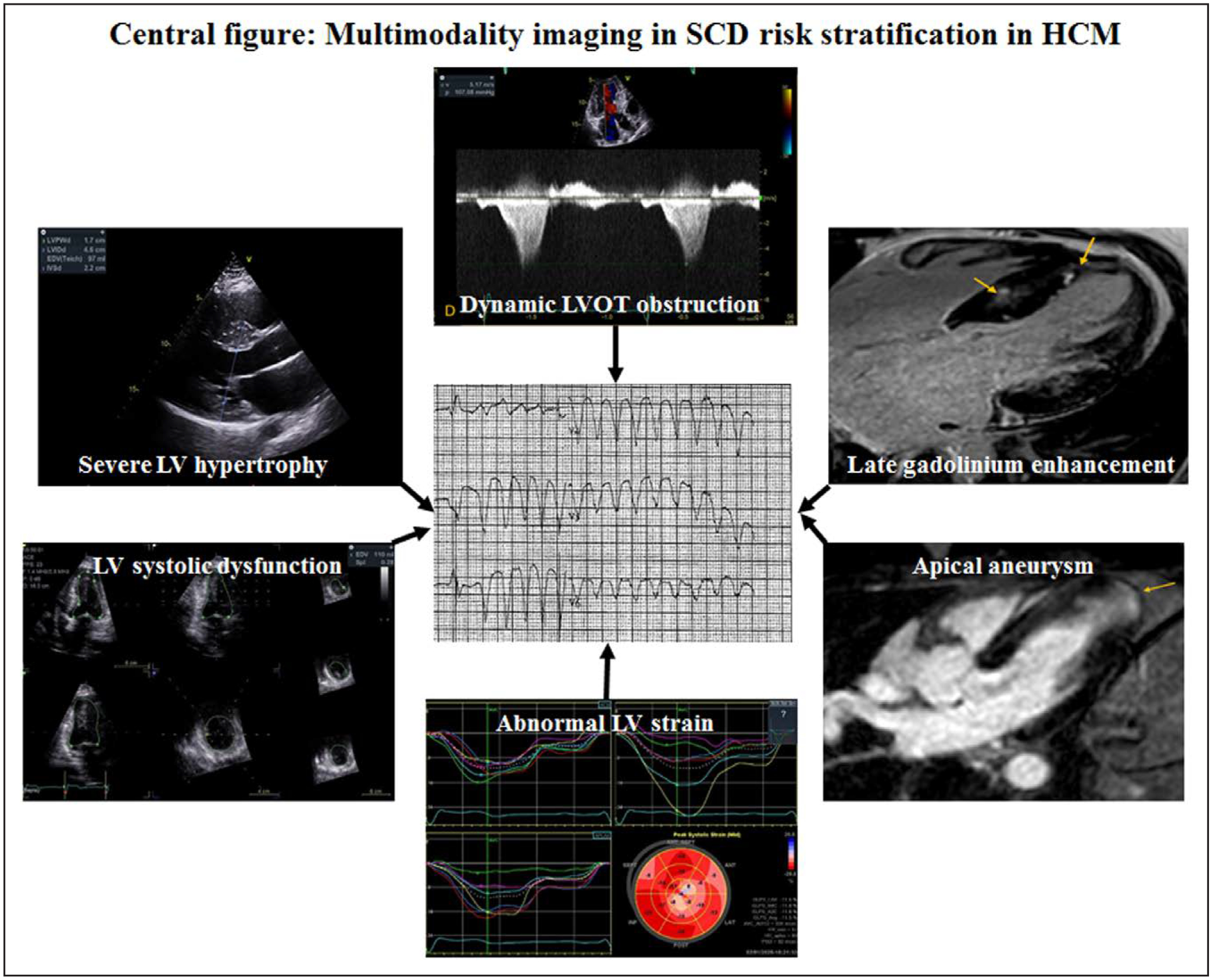
LV indicates left ventricular; and LVOT, left ventricular outflow tract.
Supplementary Material
Disclosures
Dr Desai is supported by the Haslam Family endowed chair in cardiovascular medicine. Drs Desai, Kramer, and Neubauer are supported in part by the NHLBI U01HL117006-01A1. The other authors report no conflicts.
Footnotes
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCIMAGING.119.009026.
REFERENCES
- 1.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Failure Society of America; Heart Rhythm Society; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2011;124:e783–e831. doi: 10.1161/CIR.0b013e318223e2bd [DOI] [PubMed] [Google Scholar]
- 2.Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. ; Authors/Task Force m. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–79. doi: 10.1093/eurheartj/ehu284 [DOI] [PubMed] [Google Scholar]
- 3.O’Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, et al. ; Hypertrophic Cardiomyopathy Outcomes Investigators. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. 2014;35:2010–2020. doi: 10.1093/eurheartj/eht439 [DOI] [PubMed] [Google Scholar]
- 4.O’Mahony C, Jichi F, Ommen SR, Christiaans I, Arbustini E, Garcia-Pavia P, Cecchi F, Olivotto I, Kitaoka H, Gotsman I, et al. International External Validation Study of the 2014 European Society of cardiology guidelines on sudden cardiac death prevention in hypertrophic cardiomyopathy (EVIDENCE-HCM). Circulation. 2018;137:1015–1023. doi: 10.1161/CIRCULATIONAHA.117.030437 [DOI] [PubMed] [Google Scholar]
- 5.Desai MY, Smedira NG, Dhillon A, Masri A, Wazni O, Kanj M, Sato K, Thamilarasan M, Popovic ZB, Lever HM. Prediction of sudden death risk in obstructive hypertrophic cardiomyopathy: potential for refinement of current criteria. J Thorac Cardiovasc Surg. 2018;156:750–759.e3. doi: 10.1016/j.jtcvs.2018.03.150 [DOI] [PubMed] [Google Scholar]
- 6.Maron MS, Rowin EJ, Wessler BS, Mooney PJ, Fatima A, Patel P, Koethe BC, Romashko M, Link MS, Maron BJ. Enhanced American college of cardiology/American Heart Association strategy for prevention of sudden cardiac death in high-risk patients with hypertrophic cardiomyopathy. JAMA Cardiol. 2019;4:644–657. doi: 10.1001/jamacardio.2019.1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bois JP, Geske JB, Foley TA, Ommen SR, Pellikka PA. Comparison of maximal wall thickness in hypertrophic cardiomyopathy differs between magnetic resonance imaging and transthoracic echocardiography. Am J Cardiol. 2017;119:643–650. doi: 10.1016/j.amjcard.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 8.Hindieh W, Weissler-Snir A, Hammer H, Adler A, Rakowski H, Chan RH. Discrepant measurements of maximal left ventricular wall thickness between cardiac magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10:e006309. doi: 10.1161/CIRCIMAGING.117.006309 [DOI] [PubMed] [Google Scholar]
- 9.Phelan D, Sperry BW, Thavendiranathan P, Collier P, Popović ZB, Lever HM, Smedira NG, Desai MY. Comparison of ventricular septal measurements in hypertrophic cardiomyopathy patients who underwent surgical myectomy using multimodality imaging and implications for diagnosis and management. Am J Cardiol. 2017;119:1656–1662. doi: 10.1016/j.amjcard.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 10.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000;342:1778–1785. doi: 10.1056/NEJM200006153422403 [DOI] [PubMed] [Google Scholar]
- 11.Elliott PM, Gimeno Blanes JR, Mahon NG, Poloniecki JD, McKenna WJ. Relation between severity of left-ventricular hypertrophy and prognosis in patients with hypertrophic cardiomyopathy. Lancet. 2001;357:420–424. doi: 10.1016/S0140-6736(00)04005-8 [DOI] [PubMed] [Google Scholar]
- 12.Harris KM, Spirito P, Maron MS, Zenovich AG, Formisano F, Lesser JR, Mackey-Bojack S, Manning WJ, Udelson JE, Maron BJ. Prevalence, clinical profile, and significance of left ventricular remodeling in the end-stage phase of hypertrophic cardiomyopathy. Circulation. 2006;114:216–225. doi: 10.1161/CIRCULATIONAHA.105.583500 [DOI] [PubMed] [Google Scholar]
- 13.Spirito P, Autore C, Rapezzi C, Bernabò P, Badagliacca R, Maron MS, Bongioanni S, Coccolo F, Estes NA, Barillà CS, et al. Syncope and risk of sudden death in hypertrophic cardiomyopathy. Circulation. 2009;119:1703–1710. doi: 10.1161/CIRCULATIONAHA.108.798314 [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Woo A, Monakier D, Jamorski M, Fedwick K, Wigle ED, Rakowski H. Enlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr. 2005;18:1074–1082. doi: 10.1016/j.echo.2005.06.011 [DOI] [PubMed] [Google Scholar]
- 15.Rowin EJ, Hausvater A, Link MS, Abt P, Gionfriddo W, Wang W, Rastegar H, Estes NAM, Maron MS, Maron BJ. Clinical profile and conse-quences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. 2017;136:2420–2436. doi: 10.1161/CIRCULATIONAHA.117.029267 [DOI] [PubMed] [Google Scholar]
- 16.Nistri S, Olivotto I, Betocchi S, Losi MA, Valsecchi G, Pinamonti B, Conte MR, Casazza F, Galderisi M, Maron BJ, et al. Prognostic significance of left atrial size in patients with hypertrophic cardiomyopathy (from the Italian registry for hypertrophic cardiomyopathy). Am J Cardiol. 2006;98:960–965. doi: 10.1016/j.amjcard.2006.05.013 [DOI] [PubMed] [Google Scholar]
- 17.Yang WI, Shim CY, Kim YJ, Kim SA, Rhee SJ, Choi EY, Choi D, Jang Y, Chung N, Cho SY, et al. Left atrial volume index: a predictor of adverse outcome in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2009;22:1338–1343. doi: 10.1016/j.echo.2009.09.016 [DOI] [PubMed] [Google Scholar]
- 18.Hiemstra YL, Debonnaire P, Bootsma M, van Zwet EW, Delgado V, Schalij MJ, Atsma DE, Bax JJ, Marsan NA. Global longitudinal strain and left atrial volume index provide incremental prognostic value in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10:e005706. doi: 10.1161/CIRCIMAGING.116.005706 [DOI] [PubMed] [Google Scholar]
- 19.Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. doi: 10.1161/CIRCULATIONAHA.106.644682 [DOI] [PubMed] [Google Scholar]
- 20.Patel P, Dhillon A, Popovic ZB, Smedira NG, Rizzo J, Thamilarasan M, Agler D, Lytle BW, Lever HM, Desai MY. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging. 2015;8:e003132. doi: 10.1161/CIRCIMAGING.115.003132 [DOI] [PubMed] [Google Scholar]
- 21.Ayoub C, Geske JB, Larsen CM, Scott CG, Klarich KW, Pellikka PA. Comparison of valsalva maneuver, amyl nitrite, and exercise echocardiography to demonstrate latent left ventricular outflow obstruction in hypertrophic cardiomyopathy. Am J Cardiol. 2017;120:2265–2271. doi: 10.1016/j.amjcard.2017.08.047 [DOI] [PubMed] [Google Scholar]
- 22.Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. doi: 10.1056/NEJMoa021332 [DOI] [PubMed] [Google Scholar]
- 23.Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933–1941. doi: 10.1093/eurheartj/ehl041 [DOI] [PubMed] [Google Scholar]
- 24.Gimeno JR, Tomé-Esteban M, Lofiego C, Hurtado J, Pantazis A, Mist B, Lambiase P, McKenna WJ, Elliott PM. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur Heart J. 2009;30:2599–2605. doi: 10.1093/eurheartj/ehp327 [DOI] [PubMed] [Google Scholar]
- 25.Lu DY, Hailesealassie B, Ventoulis I, Liu H, Liang HY, Nowbar A, Pozios I, Canepa M, Cresswell K, Luo HC, et al. Impact of peak provoked left ventricular outflow tract gradients on clinical outcomes in hypertrophic cardiomyopathy. Int J Cardiol. 2017;243:290–295. doi: 10.1016/j.ijcard.2017.04.039 [DOI] [PubMed] [Google Scholar]
- 26.Desai MY, Smedira NG, Bhonsale A, Thamilarasan M, Lytle BW, Lever HM. Symptom assessment and exercise impairment in surgical decision making in hypertrophic obstructive cardiomyopathy: relationship to outcomes. J Thorac Cardiovasc Surg. 2015;150:928–35.e1. doi: 10.1016/j.jtcvs.2015.07.063 [DOI] [PubMed] [Google Scholar]
- 27.Maron MS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, Lesser JR, Udelson JE, Ackerman MJ, Maron BJ. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. 2008;118:1541–1549. doi: 10.1161/CIRCULATIONAHA.108.781401 [DOI] [PubMed] [Google Scholar]
- 28.Suzuki J, Shimamoto R, Nishikawa J, Yamazaki T, Tsuji T, Nakamura F, Shin WS, Nakajima T, Toyo-Oka T, Ohotomo K. Morphological onset and early diagnosis in apical hypertrophic cardiomyopathy: a long term analysis with nuclear magnetic resonance imaging. J Am Coll Cardiol. 1999;33:146–151. doi: 10.1016/s0735-1097(98)00527-0 [DOI] [PubMed] [Google Scholar]
- 29.Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, Maron MS. Hypertrophic cardiomyopathy with left ventricular apical aneurysm: implications for risk stratification and management. J Am Coll Cardiol. 2017;69:761–773. doi: 10.1016/j.jacc.2016.11.063 [DOI] [PubMed] [Google Scholar]
- 30.Xiao Y, Wang LP, Yang YK, Tian T, Yang KQ, Sun X, Jiang Y, Liu YX, Zhou XL, Li JJ. Clinical profile and prognosis of left ventricular apical aneurysm in hypertrophic cardiomyopathy. Am J Med Sci. 2016;351:101–110. doi: 10.1016/j.amjms.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 31.To AC, Lever HM, Desai MY. Hypertrophied papillary muscles as a masquerade of apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2012;59:1197. doi: 10.1016/j.jacc.2011.08.083 [DOI] [PubMed] [Google Scholar]
- 32.Lee SP, Park K, Kim HK, Kim YJ, Sohn DW. Apically displaced papillary muscles mimicking apical hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2013;14:128–134. doi: 10.1093/ehjci/jes113 [DOI] [PubMed] [Google Scholar]
- 33.To AC, Dhillon A, Desai MY. Cardiac magnetic resonance in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2011;4:1123–1137. doi: 10.1016/j.jcmg.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 34.Ichida M, Nishimura Y, Kario K. Clinical significance of left ventricular apical aneurysms in hypertrophic cardiomyopathy patients: the role of diagnostic electrocardiography. J Cardiol. 2014;64:265–272. doi: 10.1016/j.jjcc.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 35.Hanneman K, Crean AM, Williams L, Moshonov H, James S, Jiménez-Juan L, Gruner C, Sparrow P, Rakowski H, Nguyen ET. Cardiac magnetic resonance imaging findings predict major adverse events in apical hypertrophic cardiomyopathy. J Thorac Imaging. 2014;29:331–339. doi: 10.1097/RTI.0000000000000115 [DOI] [PubMed] [Google Scholar]
- 36.Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57:891–903. doi: 10.1016/j.jacc.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. doi: 10.1161/CIRCULATIONAHA.113.007094 [DOI] [PubMed] [Google Scholar]
- 38.Bruder O, Wagner A, Jensen CJ, Schneider S, Ong P, Kispert EM, Nassenstein K, Schlosser T, Sabin GV, Sechtem U, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875–887. doi: 10.1016/j.jacc.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 39.Mentias A, Raeisi-Giglou P, Smedira NG, Feng K, Sato K, Wazni O, Kanj M, Flamm SD, Thamilarasan M, Popovic ZB, et al. Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J Am Coll Cardiol. 2018;72:857–870. doi: 10.1016/j.jacc.2018.05.060 [DOI] [PubMed] [Google Scholar]
- 40.O’Hanlon R, Grasso A, Roughton M, Moon JC, Clark S, Wage R, Webb J, Kulkarni M, Dawson D, Sulaibeekh L, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867–874. doi: 10.1016/j.jacc.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 41.Olivotto I, Maron BJ, Appelbaum E, Harrigan CJ, Salton C, Gibson CM, Udelson JE, O’Donnell C, Lesser JR, Manning WJ, et al. Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2010;106:261–267. doi: 10.1016/j.amjcard.2010.03.020 [DOI] [PubMed] [Google Scholar]
- 42.Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y. Prognostic value of LGE-CMR in HCM: a meta-analysis. JACC Cardiovasc Imaging. 2016;9:1392–1402. doi: 10.1016/j.jcmg.2016.02.031 [DOI] [PubMed] [Google Scholar]
- 43.Maron MS, Rowin EJ, Maron BJ. How to image hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2017;10:e005372. doi: 10.1161/CIRCIMAGING.116.005372 [DOI] [PubMed] [Google Scholar]
- 44.Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 46.Harrigan CJ, Peters DC, Gibson CM, Maron BJ, Manning WJ, Maron MS, Appelbaum E. Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular MR imaging. Radiology. 2011;258:128–133. doi: 10.1148/radiol.10090526 [DOI] [PubMed] [Google Scholar]
- 47.Raman B, Ariga R, Spartera M, Sivalokanathan S, Chan K, Dass S, Petersen SE, Daniels MJ, Francis J, Smillie R, et al. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: mechanisms and clinical implications. Eur Heart J Cardiovasc Imaging. 2019;20:157–167. doi: 10.1093/ehjci/jey135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dass S, Suttie JJ, Piechnik SK, Ferreira VM, Holloway CJ, Banerjee R, Mahmod M, Cochlin L, Karamitsos TD, Robson MD, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:726–733. doi: 10.1161/CIRCIMAGING.112.976738 [DOI] [PubMed] [Google Scholar]
- 49.Avanesov M, Münch J, Weinrich J, Well L, Säring D, Stehning C, Tahir E, Bohnen S, Radunski UK, Muellerleile K, et al. Prediction of the estimated 5-year risk of sudden cardiac death and syncope or non-sustained ventricular tachycardia in patients with hypertrophic cardiomyopathy using late gadolinium enhancement and extracellular volume CMR. Eur Radiol. 2017;27:5136–5145. doi: 10.1007/s00330-017-4869-x [DOI] [PubMed] [Google Scholar]
- 50.Sado DM, White SK, Piechnik SK, Banypersad SM, Treibel T, Captur G, Fontana M, Maestrini V, Flett AS, Robson MD, et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ Cardiovasc Imaging. 2013;6:392–398. doi: 10.1161/CIRCIMAGING.112.000070 [DOI] [PubMed] [Google Scholar]
- 51.Hinojar R, Varma N, Child N, Goodman B, Jabbour A, Yu CY, Gebker R, Doltra A, Kelle S, Khan S, et al. T1 mapping in discrimination of hypertrophic phenotypes: hypertensive heart disease and hypertrophic cardiomyopathy: findings from the international T1 Multicenter Cardiovascular Magnetic Resonance Study. Circ Cardiovasc Imaging. 2015;8:e003285. doi: 10.1161/CIRCIMAGING.115.003285 [DOI] [PubMed] [Google Scholar]
- 52.Gommans DHF, Cramer GE, Bakker J, Dieker HJ, Michels M, Fouraux MA, Marcelis CLM, Verheugt FWA, Timmermans J, Brouwer MA, et al. High T2-weighted signal intensity for risk prediction of sudden cardiac death in hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. 2018;34:113–120. doi: 10.1007/s10554-017-1252-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer CM, Appelbaum E, Desai MY, Desvigne-Nickens P, DiMarco JP, Friedrich MG, Geller N, Heckler S, Ho CY, Jerosch-Herold M, et al. Hypertrophic cardiomyopathy registry: the rationale and design of an international, observational study of hypertrophic cardiomyopathy. Am Heart J. 2015;170:223–230. doi: 10.1016/j.ahj.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu D, Hu K, Niemann M, Herrmann S, Cikes M, Störk S, Gaudron PD, Knop S, Ertl G, Bijnens B, et al. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:1066–1072. doi: 10.1161/CIRCIMAGING.113.000683 [DOI] [PubMed] [Google Scholar]
- 55.Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448. doi: 10.1136/heartjnl-2012-302353 [DOI] [PubMed] [Google Scholar]
- 56.Tower-Rader A, Mohananey D, To A, Lever HM, Popovic ZB, Desai MY. Prognostic value of global longitudinal strain in hypertrophic cardiomyopathy: a systematic review of existing literature. JACC Cardiovasc Imaging. 2019;12:1930–1942. doi: 10.1016/j.jcmg.2018.07.016 [DOI] [PubMed] [Google Scholar]
- 57.Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, et al. Long-term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. doi: 10.1016/j.jacc.2005.02.090 [DOI] [PubMed] [Google Scholar]
- 58.Tower-Rader A, Betancor J, Popovic ZB, Sato K, Thamilarasan M, Smedira NG, Lever HM, Desai MY. Incremental prognostic utility of left ventricular global longitudinal strain in hypertrophic obstructive cardiomyopathy patients and preserved left ventricular ejection fraction. J Am Heart Assoc. 2017;6:e006514. doi: 10.1161/JAHA.117.006514 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


