SUMMARY
CD8+ tissue-resident memory T cells (TRM) persist at sites of previous infection, where they provide rapid local protection against pathogen challenge. CD8+ TRM expressing the α1 chain (CD49a) of integrin VLA-1 have been identified within sites of resolved skin infection and in vitiligo lesions. We demonstrate that CD49a is expressed early following T cell activation in vivo, and TGF-β and IL-12 induce CD49a expression by CD8+ T cells in vitro. Despite this rapid expression, CD49a is not required for the generation of a primary CD8+ T cell response to cutaneous herpes simplex virus (HSV) infection, migration of CD8+ T cells across the epidermal basement membrane, or positioning of TRM within basal epidermis. Rather, CD49a supports CD8+ TRM persistence within skin, regulates epidermal CD8+ TRM dendritic extensions, and increases the frequency of IFN-γ+ CD8+ TRM following local antigen challenge. Our results suggest that CD49a promotes optimal cutaneous CD8+ TRM-mediated immunity.
Graphical Abstract
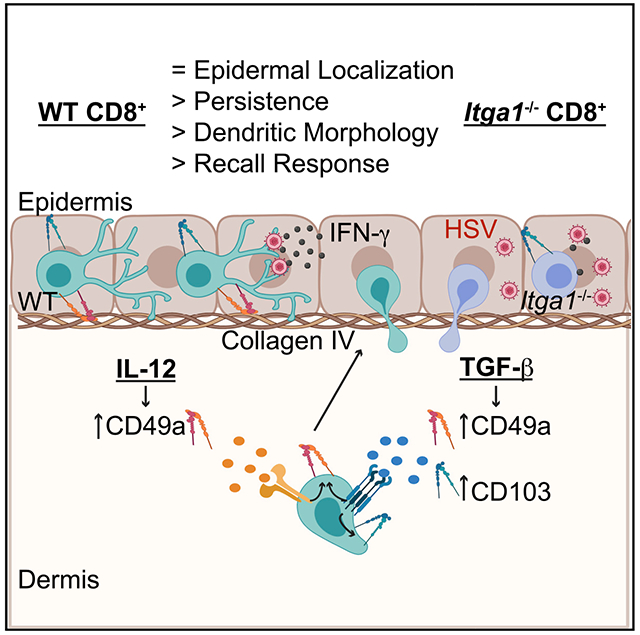
In Brief
Bromley et al. demonstrate that IL-12 or TGF-β can induce CD49a expression by CD8+ T cells. Following herpes simplex virus infection, CD49a is not required for CD8+ T cell entry into or localization within the epidermis. Rather, CD49a promotes skin TRM persistence, dendritic morphology, and optimal response to antigen challenge.
INTRODUCTION
Infection induces the migration of effector T cells into inflamed peripheral tissues. Upon resolution of the immune response, the majority of effectors die by apoptosis. However, a subset of memory T cells persists long-term within peripheral tissues (Gebhardt et al., 2009; Klonowski et al., 2004; Masopust et al., 2010). These tissue-resident memory T cells (TRM) are generated from memory precursor cells within peripheral tissues in response to local cytokine cues (Casey et al., 2012; Mackay et al., 2012) and acquire a phenotype distinct from circulating memory T cells (Mackay et al., 2013, 2016; Masopust et al., 2006; Wakim et al., 2012). Following cutaneous herpes simplex virus (HSV) infection, CD8+ T cells migrate through the dermis and into the epidermis, where they upregulate expression of CD103, the α chain of integrin αEβ7. CD103+ CD8+ TRM persist adjacent to the epidermal basement membrane (Mackay et al., 2013), where they are strategically positioned to provide rapid local memory response against cutaneous pathogen challenge (Gebhardt et al., 2009; Jiang et al., 2012; Teijaro et al., 2011; Wu et al., 2014).
Several tissue-resident cell types, including CD8+ TRM, also express the adhesion receptor CD49a (Chapman and Topham, 2010; Dadi et al., 2016; Gebhardt et al., 2009; Oja et al., 2018; Peng et al., 2013; Ray et al., 2004). Integrin subunit alpha 1 (Itga1) encodes CD49a, the α chain of the α1β1 integrin very late antigen-1 (VLA-1). VLA-1 was named on the basis of the finding that it is expressed on long-term activated T cells in vitro (Hemler et al., 1985). In vivo, CD49a has been detected on TRM recovered from tissues, including skin, late following resolution of infection (Gebhardt et al., 2009; Mackay et al., 2016; Ray et al., 2004). Moreover, a recent study determined that CD49a expression identifies human cutaneous CD8+ TRM poised for IFN-γ secretion and cytotoxic function (Cheuk et al., 2017). Although these expression analyses are suggestive of a possible role for CD49a in the regulation of cutaneous CD8+ TRM immune response, the mechanisms that regulate CD8+ TRM CD49a expression and its role in promoting CD8+ TRM-mediated immunity in vivo are incompletely defined.
CD49a can be expressed rapidly by T cells during inflammation (Andreasen et al., 2003; Haddadi et al., 2017), and so VLA-1 may regulate TRM at multiple stages: TRM precursor recruitment, TRM formation, persistence, and response. VLA-1 binds collagen I, collagen IV, and laminin, major components of the extracellular matrix (Gardner, 2014), and VLA-1 plays an essential role in leukocyte migration within skin in several mouse models of cutaneous inflammation (Andreasen et al., 2003; de Fougerolles et al., 2000). Additionally, a critical role for VLA-1 in disease development was found using a xenotransplantation model of psoriasis. Collagen IV and laminin are components of the epidermal basement membrane, and T cells required VLA-1 for entry into the epidermis and induction of the psoriatic phenotype (Conrad et al., 2007). Given that CD8+ T cell epidermal entry is required for CD8+ TRM CD103 expression and long-term persistence following HSV infection (Mackay et al., 2013), we hypothesized that VLA-1 might be essential for T cell entry into the epidermis for CD103+ TRM formation.
Imaging studies demonstrate that TRM migrate slowly within the skin, preferentially maintaining contact with the epidermal basement membrane. These T cells exhibit a dendritic morphology, extending dendrites laterally along the basement membrane, which is predicted to facilitate their patrol of skin for cognate antigen (Ariotti et al., 2012; Zaid et al., 2014). Within hours after local cognate antigen challenge, CD8+ TRM secrete cytokines that activate innate immune cells and induce expression of chemokines, resulting in recruitment of circulating memory T cells and amplification of the memory response (Ariotti et al., 2014; Schenkel et al., 2013, 2014). CD49a expression has been identified on T cells that are enriched for IFN-γ secretion, suggesting that CD49a may identify T cells with distinct effector function (Cheuk et al., 2017; Goldstein et al., 2003). However, whether CD49a is required for the differentiation of IFN-γ-secreting TRM or their response to local antigen challenge in vivo is unknown.
Here, using a mouse model of cutaneous HSV infection, we examined the requirement for CD49a in the formation, localization, persistence, and function of cutaneous CD8+ TRM. We demonstrated that CD49a was expressed early following T cell activation and could be detected on circulating HSV-specific CD8+ T cells before their entry into HSV-infected skin. Unlike CD103, CD49a was induced not only by TGF-β but also by IL-12. Nonetheless, despite this early CD49a expression by wild-type (WT) CD8+ T cells, Itga1−/− CD8+ T cells differentiated and initially entered into infected skin comparably with WT CD8+ T cells. Moreover, Itga1−/− T cells were able to migrate through the dermis, across the epidermal basement membrane, and into the epidermis, where they expressed CD103 and effectively cleared replicating virus. However, fewer Itga1−/− CD8+ TRM than WT TRM were recovered from skin 35 days following HSV infection. Imaging of Itga1−/− CD8+ skin TRM demonstrated that they were less dendritic than WT CD8+ TRM, and this correlated with a decreased memory IFN-γ response following cognate antigen challenge. Thus, we demonstrate that CD49a was not required for the generation of a primary CD8+ T cell response to cutaneous HSV infection or for the formation of CD8+ TRM. However, CD49a regulated CD8+ TRM persistence and effector function, promoting robust response to local antigen challenge.
RESULTS
CD49a Is Expressed Early on CD8+ T Cells following HSV Infection
To determine the requirements for CD49a in cutaneous CD8+ TRM formation, we used a mouse zosteriform model of HSV infection (Goel et al., 2002; van Lint et al., 2004). We first analyzed CD49a expression by endogenous CD8+ T cells specific for the HSV gB determinant. Mice were infected with HSV-1 KOS (Colgrove et al., 2016) by skin scarification, and after 9–30 days, cells recovered from the skin infection site, contralateral skin, spleen, and draining brachial lymph node (LN) were stained with H2-Kb MHC class I tetramers containing the immunodominant gB(498–505) peptide epitope and with antibody directed against CD49a. In agreement with previous studies (Gebhardt et al., 2009), we found that CD49a was expressed on >95% of cutaneous memory HSV-specific CD8+ T cells recovered from the primary infection site 30 days post-infection (Figure 1A). At this memory time point, CD49a expression was enriched on cutaneous memory H-2Kb-HSV/gB+ CD8+ CD44hi T cells compared with those recovered from the spleen or draining LNs. We also examined CD49a expression by HSV-specific CD8+ T cells recovered from contralateral skin 30 days post-infection. Although most HSV-specific CD8+ T cells isolated from contralateral skin also expressed CD49a at this memory time point (Figure 1A), many more HSV-specific CD8+ T cells were recovered from the ipsilateral flank skin compared with the uninvolved contralateral skin (Figure S1).
Figure 1. Differing Kinetics of CD49a and CD103 Expression by HSV-gB/Kb CD8+ T Cells.
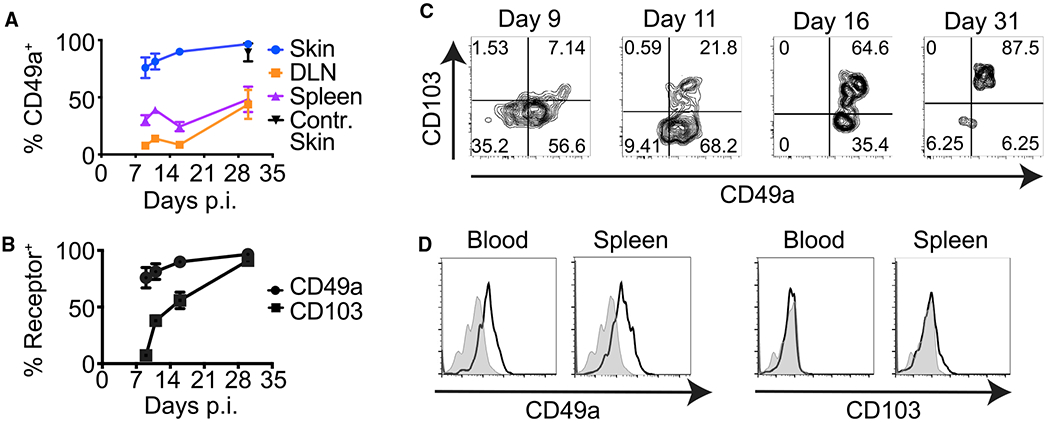
(A) CD49a expression by HSV-gB/Kb CD8+ T cells in the spleen, draining lymph node (DLN), initial skin infection site, and contralateral (Contr.) skin at the indicated times post-HSV KOS infection (p.i.). Error bars denote SEM.
(B) Frequencies of CD49a+ versus CD103+ HSV-gB/Kb CD8+ T cells in the skin at the indicated times post-infection. Error bars denote SEM.
(C) Representative flow cytometry plots demonstrating expression of CD49a and CD103 by cutaneous HSV-gB/Kb CD8+ T cells at the indicated times post-infection.
(D) Representative histograms showing frequencies of CD49a+ HSV-gB/Kb CD8+ T cells versus CD103+ HSV-gB/Kb CD8+ T cells in the DLN, blood, and spleen 7 days post-infection (gray shading, fluorescence minus one [FMO] control; black line, stained cells).
Data are pooled from two experiments with six mice per time point (A and B) or are representative of two experiments with six mice per time point (C and D).
The TRM adhesion receptor CD103 is expressed on cutaneous CD8+ T cells late following HSV infection, and deletion of Itgae (encoding CD103) prevents the persistence of epidermal CD8+ TRM (Mackay et al., 2013). To determine whether CD49a, like CD103, is expressed only late following infection, we examined the kinetics of CD49a expression. Although the majority of CD8+ T cells recovered from the initial skin infection site at a memory time point co-expressed CD49a and CD103, CD49a expression preceded CD103 on cutaneous CD8+ T cells (Figures 1B and 1C). Greater than 70% of CD8+ T cells within the skin expressed CD49a at the peak of the response (day 9 [d9]) post-HSV infection. In contrast, cutaneous CD8+ T cells lacked CD103 expression at this early time point. These results suggest that although CD103 expression is acquired only after CD8+ T cell entry into the skin, CD49a expression by CD8+ T cells may be induced in the lymphoid tissues early after activation, and before their migration into non-lymphoid tissues. Indeed, analysis of HSV-specific CD8+ T cells recovered from secondary lymphoid tissues and the circulation demonstrated they expressed CD49a, but not CD103 early (d7) following infection (Figure 1D).
CD49a and CD103 Expression May Be Differentially Regulated
Given our findings that CD49a is induced earlier on cutaneous CD8+ T cells than CD103, and that CD49a is induced on CD8+ T cells within lymphoid tissues while CD103 is not, we next generated in vitro cultures to examine whether CD49a and CD103 are differentially regulated by cytokines. Previous studies have demonstrated that TGF-β drives CD103 and CD49a expression by CD8+ T cells in vivo (Casey et al., 2012; Mackay et al., 2013; Wang et al., 2004; Zhang and Bevan, 2013). Additionally, in vitro culture of early CD8+ T cell effectors with TGF-β induces their expression of CD103 (Casey et al., 2012). In agreement with these data, we found that culture of d5 in vitro-activated CD8+ T cells with TGF-β induced their expression of both CD49a and CD103. We then tested whether culture with various other cytokines could induce CD8+ TRM receptor expression. Although most cytokines tested had no effect, we found that IL-12 alone induced expression of CD49a, but not CD103. Furthermore, addition of IL-12 along with TGF-β to CD8+ T cell cultures inhibited TGF-β-induced CD103 expression but did not prevent expression of CD49a (Figures 2A and 2B).
Figure 2. Differential Regulation of CD49a and CD103 Expression in CD8+ T Cells by Cytokines.
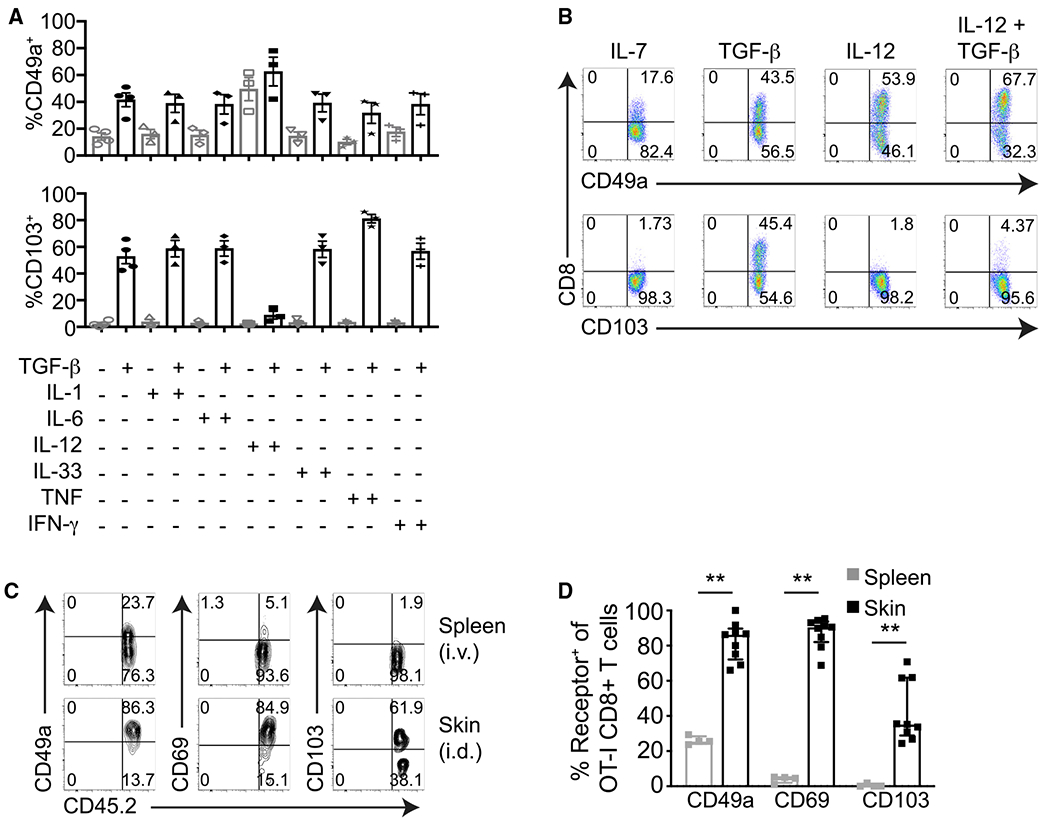
(A and B) Day 5 in vitro anti-CD3- and anti-CD28-activated CD8+ T cells were incubated for 3 days with the indicated cytokines and then examined for their expression of CD103 and CD49a by flow cytometry. (A) Frequencies of CD49a+ or CD103+ CD8+ T cells after in vitro culture with cytokines. Error bars denote SEM. (B) Representative flow cytometry plots demonstrating CD49a or CD103 expression by CD8+ T cells after in vitro culture with cytokines.
(C and D) Day 5 in vitro-activated CD8+ CD45.2+ OT-I T cells were injected intravenously (i.v.) or intradermally (i.d.) into CD45.1+ recipient mice. (C) Flow cytometry plots of CD49a, CD69, and CD103 expression by OT-I T cells recovered from the spleen 9 days after i.v. transfer and from the skin 9 days after i.d. transfer (gated on CD8+ CD45.2+ T cells).
(D) Frequencies of CD49a+, CD103+, or CD69+ CD8+ OT-I T cells recovered from the spleen and skin 9 days post-transfer. **p = 0.0028, two-tailed Mann-Whitney test. Median, IQR.
Data are compiled from at least three experiments (A), are representative of at least three experiments (B), are representative of three experiments (C), or are pooled from three experiments with nine i.d. mice and four i.v. mice total (D).
Differentiation of TRM is thought to be regulated by the local tissue microenvironment. KLRG1− CD8+ early effector T cells adoptively transferred directly into the skin upregulate expression of the TRM adhesion receptor CD103 in situ (Mackay et al., 2013). To determine whether the local microenvironment can also induce expression of CD49a by cutaneous CD8+ T cells, we adoptively transferred d5 in vitro-activated CD8+ T cells either intradermally or intravenously into congenic recipient mice. After 9 days, we recovered the adoptively transferred CD8+ T cells from the skin and spleen, respectively, and analyzed their expression of CD49a and CD103. In agreement with published literature, CD8+ T cells upregulated CD103 expression following their direct intradermal transfer (Mackay et al., 2013). In contrast, CD8+ T cells recovered from secondary lymphoid tissues following intravenous transfer lacked CD103 expression. In parallel, we found that CD8+ T cells recovered from skin following their direct intradermal transfer were enriched for CD49a expression (Figures 2C and 2D). Our experiment did not exclude the possibility that CD8+ T cells exited skin, recirculated through lymphoid tissues, and expressed CD49a before reentering the skin. However, because the geometric mean fluorescence intensity (gMFI) of CD49a staining was higher for CD8+ T cells recovered from skin compared with those recovered from spleen (Figure 2C; Figure S2), our results suggest that the local microenvironment can induce and/or increase CD49a expression by CD8+ T cells after their entry into peripheral tissues.
CD49a Is Not Required for CD8+ T Cell Activation, Tissue Entry, or Viral Clearance
Because CD49a is expressed on a fraction of CD8+ T cells early after HSV infection, we examined whether VLA-1 plays an essential role in initial CD8+ T cell activation or differentiation. Seven days following infection, we isolated the spleen, draining LN, and initial skin infection site and stained recovered leukocytes with H-2Kb-HSV/gB tetramerto identify endogenous HSV-specific CD8+ T cells and with antibodies directed against the T cell differentiation markers CD44, KLRG1, and CD62L. A similar fraction of WT and Itga1−/− H-2Kb-HSV/gB+ CD8+ T cells expressed the T cell activation/memory marker CD44 (Figure 3A). TRM are thought to differentiate from KLRG1− CD8+ precursor cells (Mackay et al., 2013), and flow cytometric analysis demonstrated a comparable fraction of KLRG1− H-2Kb-HSV/gB+ CD8+ T cells within secondary lymphoid tissues and skin of WT and Itga1−/− mice following HSV infection (Figure 3A). Most WT and Itga1−/− H-2Kb-HSV/gB+ CD8+ T cells recovered from skin had downregulated expression of the lymphoid tissue homing receptor CD62L (Figure 3A).
Figure 3. Itga1−/− HSV-gB/Kb CD8+ T Cells Are Activated, Enter into the Skin, and Clear Replicating HSV.
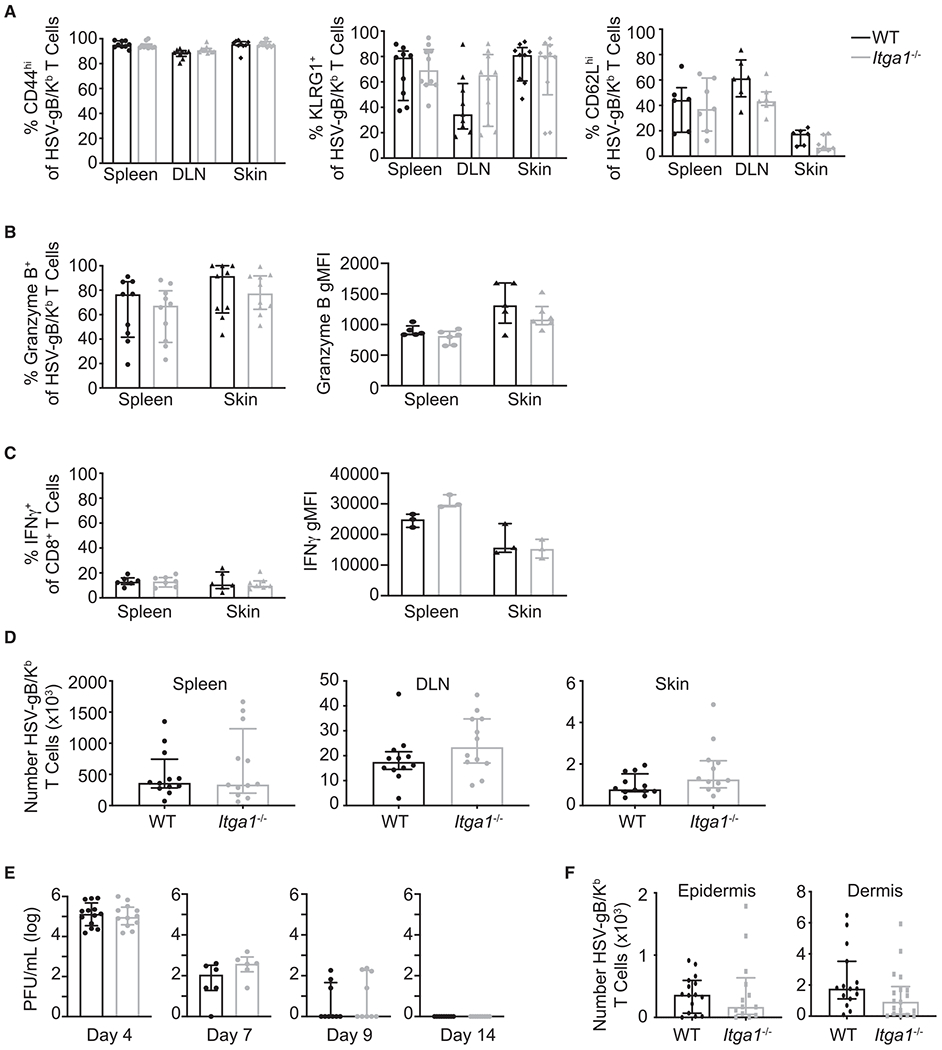
WT and Itga1−/− mice were infected with HSV-KOS.
(A and B) WT and Itga1−/− HSV-gB/Kb CD8+ T cells differentiate comparably following HSV infection. (A) Proportion of HSV-gB/Kb CD8+ T cells expressing CD44, KLRG1, and CD62L in spleen, DLN, and skin 7 days post-infection. Median, IQR. (B) Left panel: frequency of HSV-gB/Kb CD8+ T cells expressing granzyme B+ in spleen and skin 7 days post-infection. Right panel: granzyme B geometric mean fluorescence intensity gated on CD44hi HSV-gB/Kb CD8+ granzyme B+ T cells. Median, IQR.
(C) Left panel: frequency of IFN-γ+ among total CD8+ T cells after in vitro re-stimulation of day 9 skin cells or splenocytes with gB(498–505) peptide. Right panel: IFN-γ geometric mean fluorescence intensity gated on CD44hi HSV-gB/Kb CD8+ IFN-γ+ T cells. Median, IQR.
(D) HSV-gB/Kb CD8+ T cells enter comparably into HSV-infected skin of WT and Itga1−/− mice. Numbers of HSV-gB/Kb T cells recovered from the spleen, DLN, and skin of WT versus Itga1−/− mice 7 days post-infection. Median, IQR.
(E) Both WT and Itga1−/− mice clear replicating HSV. Skin HSV titers 4, 7, 9, and 14 days post-infection. Median, IQR.
(F) HSV-gB/Kb CD8+ T cells migrate into the epidermis of HSV-infected skin of WT and Itga1−/− mice. Numbers of HSV-gB/Kb CD8+ T cells recovered from the epidermis and dermis of WT versus Itga1−/− mice 14 days post-infection. Median, IQR.
Data are compiled from three experiments (A, B left panel, and E) or from two experiments (C left panel, D) or are representative of two experiments (B, C right panel). No significant differences between groups were observed using Mann-Whitney tests.
Previous studies have correlated T cell granzyme B expression and IFN-γ secretion with T cell CD49a expression (Cheuk et al., 2017; Goldstein et al., 2003). Given this link between CD49a expression and CD8+ T cell effector function, we examined whether Itga1 deficiency impaired the differentiation of CD8+ T cells following HSV infection. A similar fraction of CD8+ T cells isolated from both the spleen and initial skin infection site of WT and Itga1−/− mice expressed granzyme B (Figure 3B, left panel), and there was no difference in mean fluorescence intensity (gMFI) of granzyme B staining (Figure 3B, right panel). Similarly, a comparable fraction of recovered WT and Itga1−/− CD8+ T cells generated IFN-γ following in vitro re-stimulation (Figure 3C, left panel), and there was no significant difference in IFN-γ gMFI (Figure 3C, right panel). Together, these results suggest that WT and Itga1−/− CD8+ T cells differentiated comparably. Additionally, Itga1−/− CD8+ T cells appeared to expand normally; equivalent numbers of HSV-specific CD8+ T cells were recovered from the spleen and draining LNs of WT and Itga1−/− mice 7 days post-infection (Figure 3D). Notably, Itga1−/− HSV-specific CD8+ T cells were able to initially enter into the skin, as we recovered H-2Kb-HSV/gB+ CD8+ T cells from the initial skin infection site of both WT and Itga1−/− mice 7 days post-infection (Figure 3D).
Replicating HSV can be cleared from the skin at the primary site of infection in the absence of CD8+ T cells (Manickan and Rouse, 1995; Nash et al., 1987). Nonetheless, as CD49a is expressed on multiple cells types, we next determined whether lack of CD49a affects the ability of mice to clear replicating HSV from the skin. WT and Itga1−/− mice were infected with HSV-1 by skin scarification. Four, 7, 9, and 14 days post-infection, a 1 cm2 piece of skin at the initial skin infection site was isolated, and plaque assays were performed on tissue homogenates. Control of cutaneous infection was similar; replicating virus yield from the skin of WT and Itga1−/− mice was comparable at each time point tested (Figure 3E). These data demonstrate that WT and Itga1−/− CD8+ T cells are activated, differentiate, and migrate comparably into skin to control HSV infection.
CD8+ T Cell Epidermal Entry Is CD49a Independent
CD8+ T cell entry into the epidermis is a critical step in the formation of cutaneous CD103+ TRM following HSV infection (Mackay et al., 2013). CD49a ligands are components of the epidermal basement membrane, so T cell migration from the dermis into the epidermis might depend on CD49a-ligand interactions. To determine whether CD49a expression by HSV-specific CD8+ T cells is required for their entry into the epidermis following HSV infection, the skin at the initial infection site of WT and Itga1−/− mice was isolated 14 days post-infection, a time point when most cutaneous WT H-2Kb-HSV/gB+ CD8+ T cells express CD49a, and ~50% express CD103 (Figure 1B). Then, epidermis was separated from dermis, and numbers of recovered epidermal and dermal H-2Kb-HSV/gB+ CD8+ T cells were quantitated. We identified similar numbers of H-2Kb-HSV/gB+ CD8+ T cells within the epidermis of WT and Itga1−/− mice (Figure 3F), suggesting that CD8+ T cell epidermal entry in this mouse model of HSV infection is CD49a independent.
Fewer Itga1−/− Than WT CD8+ TRM Persist in Previously Infected Skin
Although we found that WT and Itga1−/− CD8+ T cells were able to initially enter into skin and migrate into the epidermis, we hypothesized that CD49a might promote the formation and maintenance of CD8+ memory T cells within the skin. To determine whether CD49a promotes TRM persistence within skin, we isolated the spleen and the skin at the initial infection site from WT and Itga1−/− mice 35 days post-HSV infection. Similar numbers of H-2Kb-HSV/gB CD8+ CD44hi T cells were recovered from the spleen of Itga1−/− and WT mice. However, fewer H-2Kb-HSV/gB CD8+ CD44hi T cells were recovered from the skin of Itga1−/− mice compared with WT mice, suggesting that CD49a expression facilitates cutaneous CD8+ TRM formation and/or persistence following HSV infection (Figure 4A). At this memory time point, most Itga1−/−-HSV-gB/Kb CD8+ CD44hi T cells recovered from the skin expressed CD103, indicating that CD49a is not required for subsequent differentiation to a CD8+ CD103+ TRM phenotype (Figure 4B). However, CD49a is advantageous for the persistence of CD8+ TRM within this barrier tissue.
Figure 4. Decreased Itga1−/− HSV-gB/Kb CD8+ T Cell Persistence.
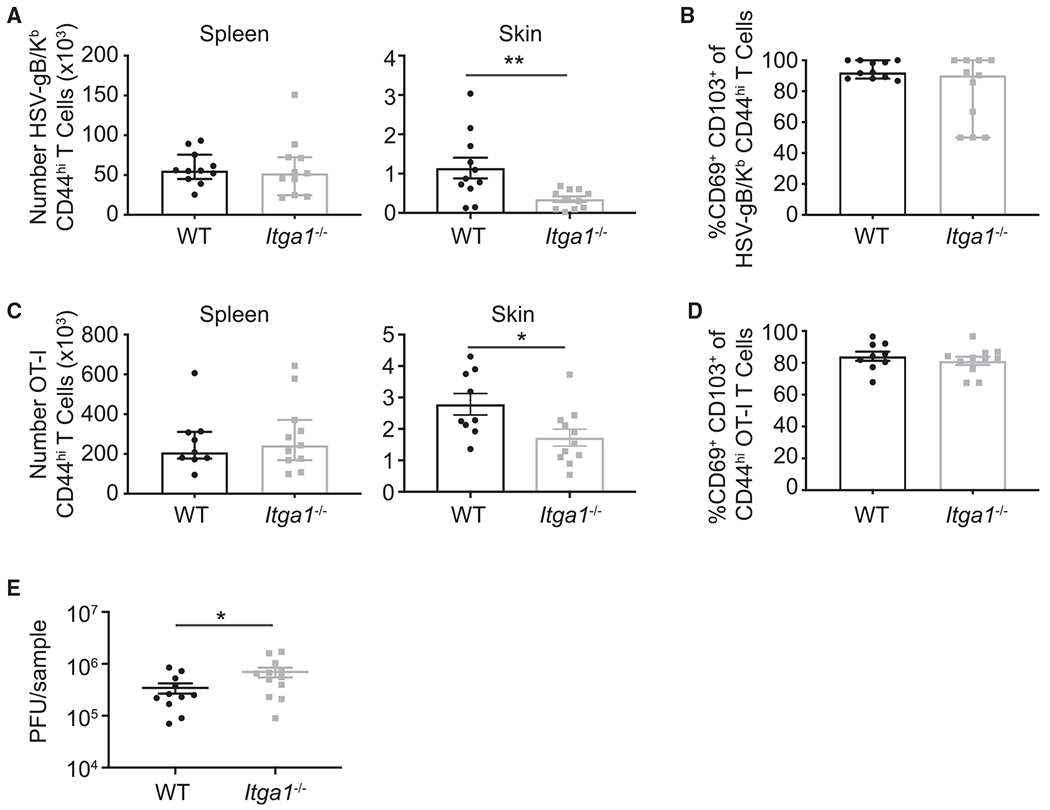
(A) Numbers of HSV-gB/Kb CD44hi CD8+ T cells recovered from the spleen and skin of WT versus Itga1−/− mice 35 days post-HSV-KOS-infection. **p = 0.0090, unpaired two-tailed t test. Error bars denote SEM.
(B) Frequency of CD69+ CD103+ HSV-gB/Kb CD44hi CD8+ T cells in the skin 35 days post-infection. No significant difference was observed using Mann-Whitney test. Median, IQR.
(C) WT OT-I CD8+ T cells (5 × 104) or Itga1−/− OT-I CD8+ T cells (5 × 104) were adoptively transferred into congenic recipient mice. Numbers of WT versus Itga1−/− CD49a-deficient OT-I CD44hi T cells recovered from the spleen and skin 35 days post-HSV-OVA infection. *p = 0.0237, unpaired two-tailed Student’s t test. Error bars denote SEM.
(D) Frequency of CD69+ CD103+ OT-I CD44hi CD8+ T cells in the skin 35 days post-infection. No significant difference was observed using unpaired two-tailed Student’s t test. Error bars denote SEM. No significant difference was observed for spleen using Mann-Whitney tests. Median, IQR (A and C).
(E) WT OT-I CD8+ T cells (5 × 104) or Itga1−/− OT-I CD8+ T cells (5 × 104) were adoptively transferred into recipient mice, which were then injected twice intradermally with 3 μg OVA encoding plasmid. After 30 days, the injection site was infected with 106 plaque-forming units (PFU) HSV-OVA. Skin HSV titers 4 days post-challenge. p = 0.0262, unpaired one-tailed Student’s t test. Error bars denote SEM.
Data are compiled from three experiments (A–D) or two experiments (E).
In addition to CD8+ T cells, other immune cells, including CD4+ T cells and tissue-resident natural killer (NK) cells, express CD49a. To determine whether CD8+ T cells require CD49a expression for their persistence within skin, we adoptively transferred naive WT OT-I CD8+ T cells (which have transgenic expression of a T cell receptor [TCR] specific for ovalbumin) or Itga1−/− CD8+ OT-I T cells intravenously into congenic recipient WT mice. The next day, recipient mice were infected with recombinant HSV expressing ovalbumin (HSV-OVA). After 35 days, the spleen and skin at the initial infection site were isolated, and recovered CD8+ OT-I CD44hi T cells were quantitated. Although similar numbers of WT and Itga1−/− CD44hi CD8+ OT-I T cells were recovered from spleen, fewer Itga1−/− than WT CD44hi CD8+ OT-I TRM were recovered from previously infected skin (Figure 4C). Again, at this memory time point, most Itga1−/− CD44hi OT-I T cells recovered from the skin expressed CD103, indicating that CD49a is not required for subsequent differentiation to a CD8+ CD103+ TRM phenotype (Figure 4D). These data suggest that CD49a expression by CD8+ TRM promotes their persistence within the epidermis.
CD8+ TRM contribute to protection from HSV challenge (Gebhardt et al., 2009; Park et al., 2018). Given that CD49a expression was important for CD8+ TRM persistence within skin, we next compared viral clearance by WT and Itga1−/− CD8+ TRM. To induce cutaneous CD8+ TRM, WT or Itga1−/− CD8+ T cells were adoptively transferred into recipient mice followed by injection with OVA-DNA in the flank skin (Mani et al., 2019). We hypothesized that mice harboring Itga1−/− CD8+ OT-I TRM within flank skin would have higher viral titers than mice containing WT OT-I TRM following challenge with HSV-OVA. In fact, 4 days post-virus challenge, HSV titers in the skin were ~2-fold higher in mice harboring Itga1−/− CD8+ OT-I TRM (Figure 4E), suggesting that reduced numbers of Itga1−/− CD8+ TRM in the skin may contribute to impaired viral clearance.
CD49a Expression Contributes to Epidermal TRM Morphology
To better understand the role for CD49a in TRM function within the skin, we first examined the effect of Itga1 deficiency on TRM positioning within the skin. Quantitation of WT and Itga1−/− CD8+ OT-I TRM in confocal images again suggested fewer Itga1−/− than WT CD8+ OT-I TRM were resident within the skin (Figures 5A and 5B, left panel). However, both WT and Itga1−/− CD8+ OT-I TRM lodged within the epidermis, adjacent to the dermal-epidermal border (Figures 5A and 5B, middle panel). Additionally, similar fractions of WT and Itga1−/− CD8+ OT-I TRM were detected in interfollicular epithelium and in hair follicle epithelium (Figure 5B, right panel), suggesting that Itga1−/− CD8+ TRM localized normally within the epidermis following HSV infection.
Figure 5. Comparison of WT and Itga1−/− Epidermal CD8+ TRM Localization and Morphology.
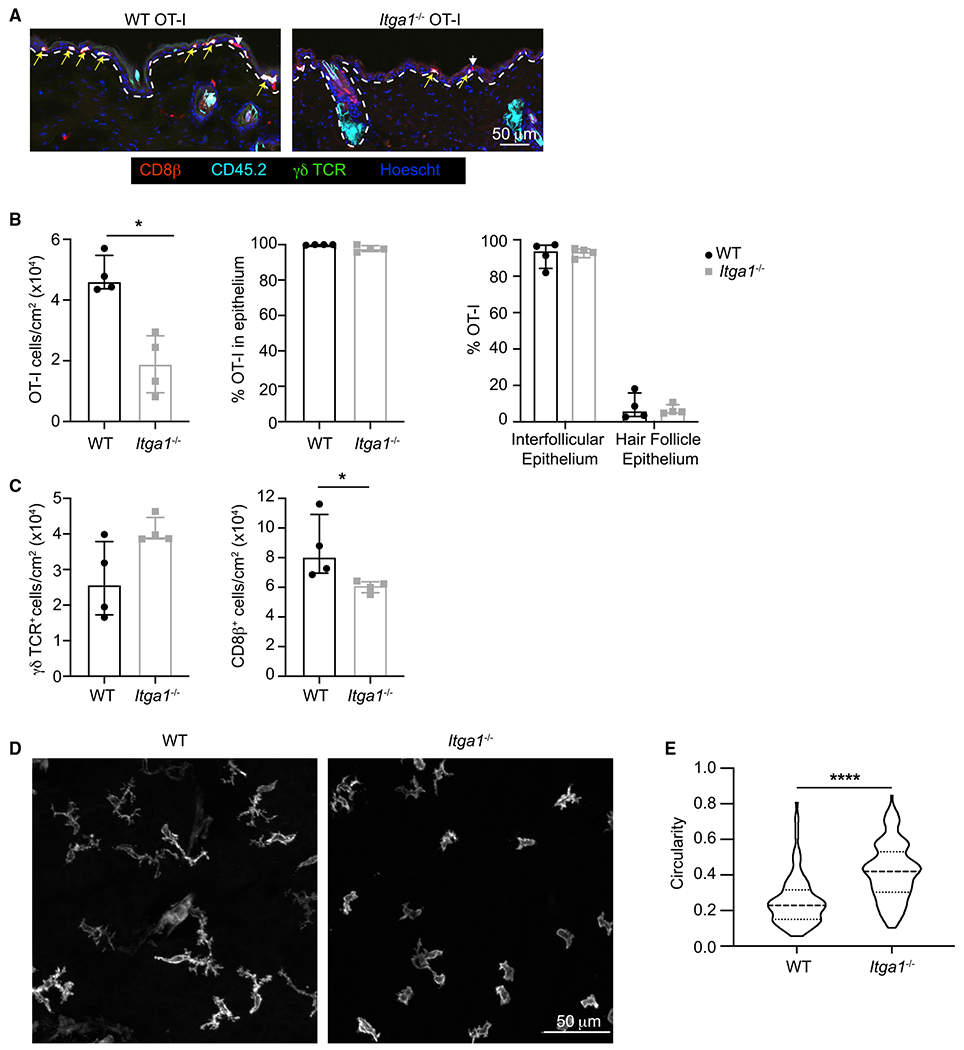
(A) WT CD45.2+ OT-I CD8+ T cells (5 × 104) or Itga1−/− CD45.2+ OT-I CD8+ T cells (5 × 104) were adoptively transferred into CD45.1+ recipient mice. Histological cross-sections of a 1 cm2 area of flank skin surrounding the initial HSV infection sites. Yellow arrows indicate CD45.2+ OT-I T cells; white arrowheads indicate endogenous CD8+ T cells. Dashed lines indicate the dermal-epidermal border. Scale bar represents 50 um.
(B) Densities of WT and Itga1−/− OT-I T cells in the skin (left panel) and comparison of their localization within the epidermis (middle panel) and intrafollicular epithelium versus hair follicle epithelium (right panel). Median, IQR. p = 0.0286, two-tailed Mann-Whitney test.
(C) Densities of γδ T cells (left panel) and CD8b+ T cells (right panel) within the epidermis. Median, IQR. p = 0.03, two-tailed Mann-Whitney test.
(D) Representative images of epidermal CD8b+ T cells in skin of HSV-infected WT or Itga1−/− mice at least 30 days post-infection. Scale bar represents 50 um.
(E) Circularity of epidermal CD8b+ T cells in skin of HSV-infected WT or Itga1−/− mice at least 30 days post-infection. p < 0.0001, two-tailed Mann-Whitney test. Median, IQR.
Data are compiled from two experiments, with a total of six WT and six Itga1−/− mice.
Previous studies proposed that following HSV-1 infection, epithelial CD8+ TRM replace γδ T cells (Zaid et al., 2014), perhaps by competing for space and/or survival signals. Because we recovered decreased numbers of CD8+ OT-I TRM from the skin of Itga1−/− compared with WT mice, we next asked whether increased numbers of γδ T or CD8+ T cells persisted in the skin of Itga1−/− mice. γδ T cells were largely excluded from areas closest to the initial HSV infection site in both WT and Itga1−/− mice, where densities of CD8+ OT-I TRM were highest (Figure 5A). However, γδ T cells were readily observed in areas more distal to the site of initial infection (Figure S3), and the total numbers of γδ T cells within a 1 cm2 area of skin surrounding the site of initial HSV infection did not significantly differ between WT and Itga1−/− mice (Figure 5C, left panel). In contrast, numbers of epithelial CD8+ T cells were decreased in Itga1−/− mice compared with WT mice (Figure 5C, right panel). These results suggest that although CD49a is not required for the persistence of γδ T cells, γδ T cells do not replace Itga1−/− CD8+ TRM within the epithelium.
Previous imaging studies demonstrated that epidermal CD8+ TRM migrate slowly within the epidermis but continuously extend and retract cellular processes (Ariotti et al., 2012; Zaid et al., 2014). To determine whether CD49a regulates epidermal CD8+ TRM interaction with the extracellular matrix, >30 days post-HSV infection, whole mounts of flank skin from WT and Itga1−/− mice were stained with antibodies directed against CD8β. Z stack confocal images were acquired, and epidermis and dermis were distinguished by differences in autofluorescence (Figure S4). Confocal image analysis demonstrated that epidermal WT CD8+ T cells were elongated, with numerous, often multi-branched cell processes extending in multiple directions (Ariotti et al., 2012; Zaid et al., 2014; Figure 5D). Epidermal Itga1−/− CD8+ T cells were also spread, but generally appeared less elongated and less dendritic than WT CD8+ T cells (Figure 5D). Cell circularity measurements of confocal maximum intensity projections, where the circularity of a perfectly round cell measures 1.0, confirmed that Itga1−/− CD8+ T cells were on average more circular than WT CD8+ T cells (Figure 5E). These data suggest that CD49a regulates interactions of epidermal CD8+ TRM with the tissue, influencing their formation of dendritic projections.
CD49a Regulates CD8+ TRM Response to Antigen Challenge
CD8+ TRM extension of dendritic protrusions is proposed to facilitate their interaction with cognate antigen (Ariotti et al., 2012). Given our finding that Itga1−/− CD8+ TRM appeared less dendritic than WT CD8+ TRM, we hypothesized that Itga1 deficiency might impair the ability of cells to respond to local antigen challenge. Previous studies have demonstrated that following antigen challenge, CD8+ TRM secrete IFN-γ, which activates innate cells and induces expression of chemokines and adhesion receptors, facilitating the recruitment of additional cells into the tissue for pathogen control (Ariotti et al., 2014; Schenkel et al., 2013, 2014). Therefore, we examined IFN-γ secretion as a readout for WT and Itga1−/− CD8+ TRM response to cognate antigen challenge. First, WT and Itga1−/− mice were infected with HSV-KOS. Then, in order to ensure that we studied local CD8+ TRM response to antigen challenge, 30 days post-infection, mice were injected intraperitoneally (i.p.) with anti-CD8 antibody to deplete circulating CD8+ T cells. After 4 days, mice were challenged by intradermal injection with 3 μg HSV gB(498–505) cognate peptide or with 3 μg OVA(257–264) control peptide at the initial infection site. Ten hours later, the skin injection site was isolated for gene expression analysis of Ifng and the IFN-γ-induced chemokines Cxcl9 and Cxcl10. qRT-PCR analysis revealed that Itga1−/− mice expressed significantly less Ifng and Cxcl9 than WT mice following challenge with HSV gB(498–505) (Figure 6A). These results suggest that the total expression of Ifng and IFN-γ-induced chemokines is decreased in the skin of Itga1−/− mice following cognate antigen challenge.
Figure 6. Reduced IFN-γ Secretion by Itga1−/− CD8+ TRM.
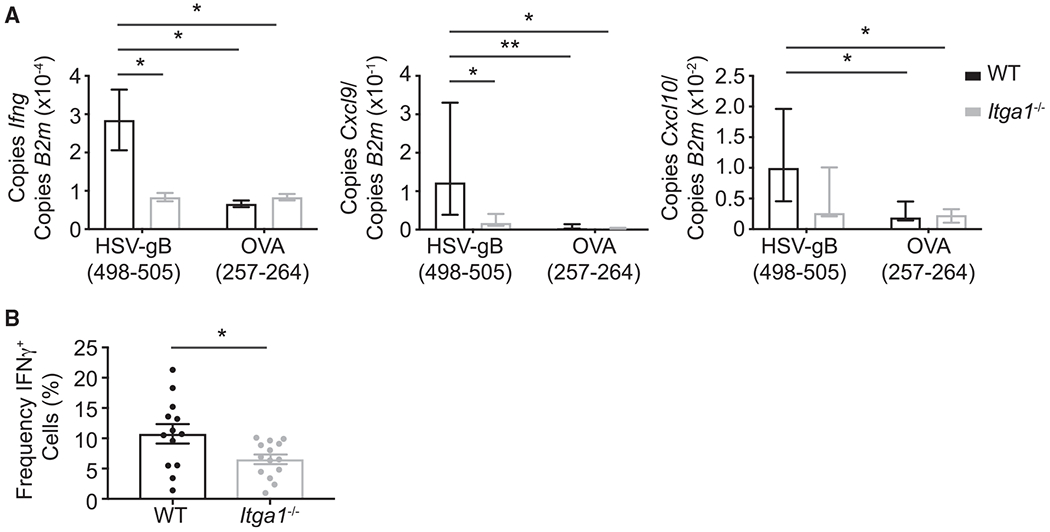
(A) WT or Itga1−/− mice were infected with HSV-KOS. Thirty-five days post-infection, the initial site of infection was injected intradermally with either gB or OVA peptide. Ifng, Cxcl9, and Cxcl10 were measured using qRT-PCR from skin 10 h post-challenge. Ifng: *p = 0.0303, Student’s t test, error bars denote SEM; Cxcl9: **p = 0.0052, Mann-Whitney test, median, IQR; Cxcl10: no significant difference using Mann-Whitney test, median, IQR. Data are compiled from three experiments, with a total of five to eight mice per condition.
(B) WT OT-I CD8+ T cells (5 × 104) or Itga1−/− OT-I CD8+ T cells (5 × 104) were adoptively transferred into congenic recipient mice. Thirty-five days post-HSV-OVA infection, mice were injected locally with OVA peptide. Ten hours post-challenge, mice were injected i.v. with brefeldin A and sacrificed 6 h later. Cells recovered from the initial infection site were stained for IFN-γ. The frequency of IFN-γ+ cells among CD8+ CD44hi CD103+ OT-I T cells is shown. *p = 0.0238, Student’s t test. Error bars denote SEM. Data are compiled from three experiments.
Previous studies demonstrated that culturing CD49a+ CD8+ TRM on collagen-coated wells during activation increases the frequency of IFN-γ-secreting CD8+ T cells compared with CD49a+ CD8+ TRM activated in uncoated wells (Cheuk et al., 2017). Therefore, we examined whether the increased Ifng expression observed in the skin of WT compared with Itga1−/− mice following antigen challenge (Figure 6A) is simply due to the increased numbers of WT TRM versus Itga1−/− TRM present in the skin at a memory time point (Figures 4A and 4C), or whether CD49a increases the frequency of IFN-γ-secreting CD8+ TRM following cognate antigen challenge in vivo. WT or Itga1−/− CD8+ OT-I T cells were transferred into recipient congenic mice, which were then infected with HSV-OVA. Again, after 30 days, mice were injected i.p. with anti-CD8α antibody to deplete circulating CD8+ T cells. Four days later, mice were challenged by intradermal injection of cognate OVA peptide. After 10 h, mice were injected intravenously (i.v.) with brefeldin A in order to prevent cytokine secretion and allow detection of CD8+ T cells actively producing IFN-γ in vivo. Six hours later, the skin injection site was isolated, and the fractions of WT and Itga1−/− CD8+ OT-I T cells secreting IFN-γ were quantitated by flow cytometry. The frequency of IFN-γ+ Itga1−/− OT-I TRM was reduced by ~50% compared with IFN-γ+ WT OT-I TRM (Figure 6B). These data demonstrate that in vivo, CD49a-ligand interactions increase the frequency of IFN-γ+ WT CD8+ TRM compared with IFN-γ+ Itga1−/− CD8+ TRM following local cutaneous antigen challenge.
DISCUSSION
Our results describe the expression and function of the adhesion receptor CD49a in cutaneous CD8+ TRM. Itga1−/− CD8+ T cells were initially activated, differentiated, migrated into HSV-infected skin, and cleared replicating virus comparably with WT CD8+ T cells. Additionally, Itga1−/− CD8+ T cells migrated through the dermis and entered into the epidermis, where they expressed CD103. Despite the formation of Itga1−/− epidermal CD8+ CD103+ TRM, fewer Itga1−/− than WT CD8+ TRM persisted long-term within the skin. Confocal image analysis of whole-mount skin demonstrated that Itga1−/− CD8+ TRM were less dendritic than WT CD8+ TRM. Moreover, although Itga1−/− CD8+ effector T cells differentiated and secreted IFN-γ upon ex vivo stimulation, Itga1−/− CD8+ TRM exhibited decreased effector function in vivo following cognate antigen challenge. Impaired viral clearance in the memory phase might be caused by both reduced Itga1−/− CD8+ TRM persistence as well as effector response. Thus, our study defines a role for CD49a in regulating cutaneous CD8+ TRM persistence and response.
Using a zosteriform model of HSV infection, we demonstrated that CD49a is expressed early following T cell activation and was detected on lymphoid and circulating CD8+ T cells before their entry into HSV-infected skin. TGF-β induces both CD49a and CD103 expression in vivo (Casey et al., 2012; Mackay et al., 2013; Wang et al., 2004; Zhang and Bevan, 2013). However, CD49a was induced more rapidly than CD103 on CD8+ T cells following HSV infection, suggesting different molecular regulation of their expression. Our in vitro culture studies demonstrated that whereas IL-12 upregulated CD49a expression, it inhibited TGF-β-induced CD103 expression. Previous studies revealed that IL-12 inhibits CD103 expression by CD8+ T cells following Yersinia infection, and CD103− CD69+ CD8+ T cells localize in clusters in the intestinal lamina propria of infected mice (Bergsbaken et al., 2017). Expression of CD49a by these CD103− CD8+ TRM was not examined, but IL-12-induced CD49a expression is one possible mechanism contributing to the persistence of CD103− TRM within tissues. Like Itga1−/− CD8+ TRM, Itgae−/− TRM also exhibit a defect in their long-term persistence within skin (Mackay et al., 2013). It will be interesting to determine whether dual deficiency in CD49a and CD103 might have a more profound effect on maintenance of cutaneous TRM than deficiency of either individual receptor.
Despite rapid CD49a expression by WT CD8+ T cells following HSV infection, Itga1−/− CD8+ T cells were activated, differentiated and initially entered into infected skin comparably with WT T cells. Upon entry into the skin, Itga1−/− T cells were able to migrate through the dermis, across the epidermal basement membrane, localize in the basal epidermis, and effectively clear replicating virus. In contrast, in a xenotransplantation model of psoriasis, CD49a blockade prevents the migration of T cells from the dermis into the epidermis (Conrad et al., 2007). The reason for this difference in the requirement for CD49a expression is unclear, but perhaps in the setting of HSV infection, additional adhesion receptors are expressed by CD8+ T cells and/or by the HSV-infected skin that compensate for lack of CD49a expression and allow CD8+ T cell migration into and positioning within the epidermis. Although Itga1−/− CD8+ T cells were able to enter into the epidermis, fewer CD8+ memory T cells were recovered from the skin of Itga1−/− mice compared with WT mice 35 days post-HSV infection. Nonetheless, within the epidermis, Itga1−/− CD8+ T cells expressed CD103, demonstrating that CD49a was not required for CD8+ TRM formation per se, but provided an advantage for TRM persistence. These results parallel a previous study in which numbers of memory CD8+ T cells recovered from the lung late following influenza infection were decreased in Itga1−/− mice compared with WT mice, as well as in WT mice treated with a CD49a-neutralizing antibody (Ray et al., 2004). Our results further demonstrate that the CD49a requirement for cutaneous CD8+ CD103+ TRM formation is T cell intrinsic. Fewer adoptively transferred Itga1−/− than WT OT-I CD8+ CD103+ T cells persisted within the skin following HSV-OVA infection. CD49a is expressed by multiple tissue-resident cell types, including, for example, NKT cells (Mackay et al., 2016) and tissue-resident NK cells (Mackay et al., 2016; Peng et al., 2013). Whether CD49a also regulates the persistence of these innate lymphoid cells within tissues is unknown.
Both their tight confinement within the epidermis and their interaction with the epidermal environment may regulate CD8+ TRM dendritic morphology. Indeed, previous imaging studies measured differences in dendrite projection angles between epidermal CD8+ TRM and γδ T cells, suggesting that cell-specific interactions with their surroundings may regulate cell dendrite extensions (Ariotti et al., 2012; Zaid et al., 2014). Our immunofluorescence confocal imaging demonstrated that Itga1−/− CD8+ TRM were more circular than WT CD8+ TRM within the skin. Although neither WT nor Itga1−/− CD8+ TRM were entirely rounded, WT CD8+ T cells appeared more elongated, with numerous dendritic extensions. However, the requirements for adhesion receptors in regulating TRM behavior might differ depending on the experimental system and/or tissue examined. Differences in integrin and integrin ligand densities may influence cell adhesion, spreading, and migration in different tissues and infection models (Bank et al., 1994; Palecek et al., 1997). For example, intravital imaging suggested that the morphologies of Itgae−/− TRM and WT TRM were equivalent in the skin following HSV infection (Zaid et al., 2017). In contrast, a recent study using a two-dimensional (2D) in vitro system demonstrated that CD8+ T cells on an e-cadherin substrate displayed significantly more cell processes than cells plated on collagen IV. Rather, collagen IV-CD49a interaction promoted CD8+ T cell migration in vitro, as well as in the trachea in vivo (Reilly et al., 2020). Although our data suggest that CD49a was not required for CD8+ T cell migration into the epidermis, CD49a did regulate TRM dendritic extensions in the skin in vivo. Future studies will examine how this affects TRM migration within the epidermis.
Our results also suggest that CD49a augments TRM cytokine secretion. We found that IFN-γ transcripts were increased in the skin of HSV-immune WT mice compared with Itga1−/− mice following local antigen challenge. Here, we demonstrated an increased fraction of WT OT-I compared with Itga1−/− OT-I TRM produced IFN-γ following intradermal antigen challenge, suggesting that CD49a increases TRM IFN-γ secretion in vivo. Whether CD49a acts as a costimulatory receptor or regulates CD8+ TRM migration/dendrite probing to increase antigen encounter in vivo will require additional studies. Previous studies suggest that CD103 restrains T cell motility, while CD49a facilitates migration (Reilly et al., 2020; Zaid et al., 2017). Our results suggest that CD49a and CD103 expression are differentially regulated by IL-12. Future studies will examine whether IL-12 might regulate adhesion receptor expression to coordinate TRM behavior during the response to antigen challenge.
Finally, our data highlight a difference in the requirement of CD49a for CD8+ T cell response during the acute versus memory phases of the immune response to HSV infection. During the primary response, Itga1−/− CD8+ effector T cells accumulated within the skin and secreted IFN-γ upon ex vivo stimulation, suggesting that CD49a is dispensable for effector CD8+ T cell migration and response. Indeed, Itga1−/− CD8+ effector T cells cleared replicating virus comparably to WT CD8+ effector T cells during the primary response to HSV infection. In contrast, fewer Itga1−/− than WT CD8+ TRM persisted within the epidermis at memory time points, and they exhibited decreased effector function in vivo following cognate antigen challenge. Both reduced Itga1−/− CD8+ TRM persistence and decreased effector response to antigen challenge might contribute to impaired viral clearance by Itga1−/− CD8+ TRM in the memory phase.
In summary, CD49a serves multiple functions to promote CD8+ TRM memory response. Our findings suggest that CD49a is not required for the initial CD8+ T cell response to HSV infection. However, CD49a promotes the persistence of TRM within skin and enhances TRM effector function upon cognate antigen challenge. Of note, however, CD49a also defines IFN-γ+ CD8+ TRM within vitiligo lesions (Cheuk et al., 2017); it is possible that CD49a expression by CD8+ TRM may have detrimental effects in local cutaneous diseases.
Therefore, our studies define the functions of CD49a in promoting cutaneous CD8+ TRM persistence and response within a local skin infection site and may have implications for treatment of inflammatory diseases mediated by CD49a+ CD8+ TRM.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Shannon Bromley (sbromley@mgh.harvard.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
This study did not generate/analyze datasets or code.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Itga1−/− mice were obtained from Dr. David Topham (University of Rochester) with permission from Biogen Idec. C57BL/6 mice, OT-I mice, and B6.SJL-PtprcaPep3b/BoyJ (CD45.1) mice were obtained from Jackson Laboratories or Charles River Laboratories. Itga1−/− mice were crossed with OT-I mice to generate Itga1−/− OT-I mice. OT-I mice were crossed with CD45.1 mice to generate OT-I CD45.1 mice. All mice were maintained on the C57BL/6 background. Mice were housed and bred in a specific pathogen-free micro-isolator environment at Massachusetts General Hospital. Adult male and female mice ranging from 6-12 weeks old were used in experiments. Animal studies were approved and carried out according to the standards and guidelines set forth by the Institutional Care and Use Committee at Massachusetts General Hospital.
METHOD DETAILS
HSV Infections
HSV-1 KOS (Colgrove et al., 2016) was obtained from Dr. David Knipe (Harvard University). HSV-OVA (Mackay et al., 2013) was obtained from Dr. Thomas Gebhardt (University of Melbourne). Epicutaneous infection by scarification was done by abrading a small area of skin near the top of the spleen using 150-grit sandpaper attached to the end of a pencil (Goel et al., 2002). An (~5 mm2) area of skin was abraded with 20 strokes of the sandpaper. Then, 106 plaque-forming units (PFU) of HSV-1 KOS or HSV-OVA in 10 μL HBSS was applied to the abraded site, and mice were bandaged for 2 days as described (Goel et al., 2002; van Lint et al., 2004).
Isolation of cells from tissues
Blood (0.5 mL) was collected from mice by cardiac puncture. Red blood cells were then lysed by incubation with ACK lysis buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA). A 1 cm2 piece of skin at the initial infection site was excised, scraped to remove fat, minced, and digested in 5 mL skin digestion buffer which consists of RPMI containing 1% FBS, 10 mM HEPES, 0.4 mg/mL Liberase TM (Roche), 2.5 mg/mL hyaluronidase (Sigma), and 0.4 mg/mL DNase I (Sigma) at 37°C for 45 minutes. Enzymes were quenched by addition of 15 μL 0.5 M EDTA, and digested skin was then dissociated using the Tumor Imp 01 setting on a GentleMacs (Miltenyi). The resulting cell suspension was filtered and washed through a 70 μm nylon filter. For isolation of cells from epidermis and dermis, the epidermis was affixed to a piece of opsite flexigrid tape (Smith and Nephew). The skin was then floated dermis side down on 5 mL HBSS containing 3 mg/mL dispase (GIBCO) at 37°C for 60 minutes, and dermis was physically separated from epidermis. Epidermis and dermis were minced, digested with Liberase TM as described above, but for 35 minutes. Draining brachial LNs and spleen were isolated, mushed through a filter, and red blood cells were lysed by incubation with RBC lysis buffer (Sigma).
Staining for flow cytometry
Isolated leukocytes were surface-stained with antibodies directed against CD3 (145-2C11), CD45 (30-F11), CD45.1 (A20), CD45.2 (104), CD8 (5H10), CD4 (GK1.5), CD44 (IM7), CD49a (Ha31/8), CD103 (2E7), CD69 (H1.2/F3), KLRG1 (2F1/KLRG1), CD62L (MEL-14) all from Biolegend, BD Biosciences, eBioscience, or ThermoFisher. For tetramer staining, leukocytes were incubated with TruStain FcX (Biolegend) and surface stained for CD8. After washing, cells were incubated with H-2Kb-HSV/gB tetramer (NIH tetramer core facility) for 25 minutes at 37°C. Then, cells were washed and stained with antibodies directed against other cell surface markers. Cell viability was determined by staining with fixable viability dye eFluor 506 or eFluor 780 (eBioscience). For granzyme B staining, cells were surface stained, fixed and permeabilized with BD Cytofix/Cytoperm Kit (BD Bioscences), and then stained for granzyme B (GB11). The stained samples were acquired with LSRII, Fortessa X20 (BD), or CytoFlex (Beckman Coulter) flow cytometers and analyzed with FlowJo software (TreeStar). Cell numbers were determined using CountBright Absolute counting beads (Invitrogen) as described (Moon et al., 2009).
Standard intracellular cytokine analysis
Spleen and skin cell populations were cultured in 96-well round-bottom plates at 106 cells per well in complete CR10 media (RPMI, 10% FBS, 10 mM HEPES, 2 mM L-glutamine, 50 μM β-mercaptoethanol, 100 U/mL penicillin, 100 mg/mL streptomycin, and 0.1 mM non-essential amino acids). 106 irradiated splenocytes isolated from naive, congenic C57BL/6 mice were added to the wells with or without 10 μM of the HSV-1 gB498-505 peptide and 1 μg/mL anti-CD28 antibody. 10 μg/mL brefeldin A was added to the wells at the start of culture. After a 4 hour incubation at 37°C, cells were placed on ice, washed and stained with antibodies directed against CD3, CD8, CD45.1, CD45.2, CD44 and fixable viability dye. Cells were fixed and permeabilized using Fix and Perm media A and B (Thermo Fisher) and stained intracellularly with anti-IFN-γ (XMG1.2).
Adoptive transfer of transgenic CD8+ T cells
CD8+ T cells were isolated from Itga1−/− OT-I or WT OT-I transgenic mice by negative selection (Miltenyi). Then, 5 × 104 CD8+ T cells in 200 μL PBS were adoptively transferred i.v. into congenic recipient mice. In vitro-activated CD8+ OT-I T cells were generated by activating splenocytes from OT-I transgenic mice with peptide-pulsed splenocytes. Briefly, 5 × 106 irradiated C57BL/6 splenocytes were pulsed with 1 ug/mL OVA (257-264) for 1 hour at 37°C. Then, 5 × 106 OT-I splenocytes were cultured with the OVA- pulsed splenocytes in 40 mL CR10 media. After 2 days, cultures were split daily with fresh CR10 media containing 7.5 ng/mL recombinant IL-2 (Peprotech). After 5 days of culture, 106 OT-I T cells in PBS were injected intravenously or intradermally into the flank of recipient congenic mice as described (Mackay et al., 2013).
Viral titers
A 1 cm2 piece of skin at the primary infection site was removed from mice, minced and placed in 1 mL of DMEM (Life Technologies). Samples were frozen in a dry ice / ethanol bath and kept at −80°C until use. The presence of infectious virus in tissue samples was determined using p.f.u. assays on confluent Vero cell monolayers in 24-well plates (Blaho et al., 2005). Samples were thawed and homogenized using the GentleMACS homogenizer (Miltenyi) RNA_01 program, and 10-fold serial dilutions were then tested for plaque formation to determine viral titer in the original tissue sample. After two days, cell monolayers were stained with crystal violet staining solution (0.5% crystal violet in 20% ethanol) to visualize plaques.
Confocal immunofluorescence and analysis
To compare the morphology of WT and Itga1−/− CD8+ TRM, a 1 cm2 piece of skin from the initial infection site was harvested, scraped of fat, and fixed at 4°C for 1 hour in PLP buffer (0.2 M NaH2PO4, 0.2M Na2HPO4, 0.2 M I-lysine and 0.1 M sodium periodate in 2% paraformaldehyde). The skin was blocked (PBS, 2% BSA, 0.5% normal serum block (Biolegend), 0.2% TruStain FcX (Biolegend) in PBS) for 2 hours at room temperature. Then, the skin was stained overnight with a 1:100 dilution of CD8β-PE (YTS156.7.7; Biolegend) at 4°C, followed by washing with PBS 0.1% Tween 20. Finally, the skin was counterstained with 0.1 μg/mL Hoescht solution to detect nuclei. Images were acquired with a confocal microscope (Carl Zeiss Microimaging) using a 40x objective. Z stack images through the skin whole-mounts were acquired with 1.2 μm intervals. Epidermis and dermis was identified based on autofluorescence at 633 nm. Image Processing was performed using FIJI software. Circularity measurements were performed in FIJI on maximum intensity projections that were first thresholded, dilated, closed, fill holes and eroded.
For analysis of γδ T cell and CD8+ TRM numbers and localization within the skin, a 1 cm2 piece of skin from the initial infection site was harvested, scraped of fat, embedded in optimum cutting temperature (OCT) compound (Tissue-Tek), frozen on dry ice, 10 μm sections were cut, and slides were dried for 1 hour at 37°C. Sections were fixed in ice-cold 1:1 acetone:methanol for 10 minutes, washed in PBS, and blocked for 1 hour at room temperature. Endogenous biotin was blocked using a biotin/streptavidin blocking kit (Vector laboratories). Sections were then incubated with biotinylated anti-CD45.2 antibody (1:50) for 1 hour at room temperature. Sections were washed with PBS 0.1% tween and then incubated with anti-CD8β-PE (1:100), anti-γδ TCR-AF488 (1:100), and SA-AF647 (1:100) for 1 hour at room temperature. Next, sections were washed with PBS 0.1% tween, counterstained with Hoescht, and mounted using Prolong Diamond. Images along the entire length of the skin sections were acquired with a confocal microscope (Carl Zeiss Microimaging) using a 20x objective and processed using FIJI software. CD8+ T cells and γδ T cells were counted manually, and the density of cells was determined by extrapolating the number of cells imaged in the skin surface section (10 μm thick x measured length of epithelium in the field of view) to the number of cells per cm2 skin as described (Mani et al., 2019). Nonconsecutive sections were analyzed.
In vitro cytokine stimulation assays
Spleens were harvested from C57BL/6 mice, single-cell suspensions were prepared, and T cells were activated in culture with 1 μg/mL anti-CD3 and 1 μg/mL anti-CD28. Cells were split 1:2 with fresh media containing 7.5 ng/mL recombinant IL-2 daily starting 2 days after initial culture. After 5 days, the activated T cells were plated at 2 × 105 cells/well in 200 μL media containing mouse IL-7 at 10 ng/mL in 96-well U-bottomed plates. Where indicated, cultures were supplemented with the following cytokines (all from Peprotech): human TGF-β1 at 10 ng/mL, mouse IL-1 at 100 ng/mL, mouse IL-6 at 100 ng/mL, mouse IL-12 at 10 ng/mL, mouse IL-33 at 100 ng/mL, mouse TNF-α at 125 ng/mL, mouse IFN-γ at 10 ng/mL. Cells were then incubated for 72 hours before staining for flow cytometric analysis.
Antigen-independent generation of CD8+ TRM and recall response
To induce CD8+ TRM cells, 5 × 104 WT- or Itga1−/−− CD8+ OT-I T cells in 200 μL PBS were adoptively transferred i.v. into recipient mice. Recipient mice were then injected intradermally twice (separated by three days) with 3 μg of the plasmid, pODCAGGS (Mani et al., 2019) which encodes full length chicken ovalbumin, kindly provided by T. Mempel. At least 28 d after DNA vaccination, mice were injected i.p. with 500 μg anti-CD4 (GK1.5; Biolegend) and 300 μg anti-CD8 (clone YTS169.4; BioXCell) antibodies to deplete circulating T cells. After 4 d, mice were bled and stained for CD4 and CD8 to confirm T cell depletion. Then, mice were infected with 106 pfu HSV-OVA at the site of DNA vaccination. After 4 d, a 1 cm2 piece of skin surrounding the infection site was isolated, minced and placed in 1 mL of DMEM (Life Technologies) for determination of viral titers by plaque assay.
Depletion of circulating CD8+ T cells and peptide challenge
30 d after HSV-1 KOS infection, mice were injected i.p. with 300 μg anti-CD8 antibody (clone YTS169.4; BioXCell) in PBS. 4 d after anti-CD8 antibody injection, mice were bled, and blood stained for CD8 to ensure depletion of circulating CD8+ T cells. Mice whose circulating CD8+ T cells were not depleted were excluded from the experiment. Then, mice were injected intradermally at the initial infection site with 3 μg HSV gB(498-505) peptide in 50 μL PBS or with 3 μg OVA(257-264) peptide. 10 hours post-challenge, mice were injected intravenously with 500 μL of 0.5 mg/mL brefeldin-A in PBS as described (Liu and Whitton, 2005) for subsequent direct intracellular cytokine staining. After 6 hours, the skin was harvested for direct intracellular cytokine staining.
Direct intracellular cytokine staining
For direct intracellular cytokine staining, spleen and a 1 cm2 piece of skin at the peptide challenge site were harvested and placed on ice. Skin was minced and digested in skin digestion buffer containing 10 μg/mL Brefeldin A as described above. Recovered leukocytes were then stained in buffer containing Brefeldin A with antibodies directed against CD3, CD8, CD45.1, CD45.2, CD44 and fixable viability dye. Cells were fixed and permeabilized using Fix and Perm media A and B (Thermo Fisher) and stained intracellularly with anti-IFN-γ (XMG1.2).
RNA isolation and Reverse transcription (RT)- Quantitative (q) PCR
10 hours after peptide challenge, a 1 cm2 piece of skin at the initial infection site was isolated. Fat was removed, the skin was minced, snap frozen in liquid nitrogen, and stored at −80°C until use. Skin samples were homogenized in TRIzol (ThermoFisher) using the GentleMACS homogenizer (Miltenyi) RNA_02 program. Total RNA was isolated from the skin, and RT-qPCR was performed as described (Means et al., 2003) with the LightCycler 96 quantitative PCR system (Roche). Primer sequences used for qPCR included: B2m F-CCGAACATACTGAACTGCTACGTAA ; B2m R-CCCGTTCTTCAGCATTTGGA ; Cxcl9 F-AATGCACGATGCTCCTGCA; Cxcl9 R-AGGTCTTTGAGGGATTTGTAGTGG; Cxcl10 F-GCCGTCATTTTCTGCCTCA; Cxcl10 R-CGTCCTTGCGAGAGGGATC; Ifng F-AACGCTACACACTGCATCTTGG; Ifng R-GCCGTGGCAGTAACAGCC.
QUANTIFICATION AND STATISTICAL ANALYSIS
Data was assessed for normality using D’Agostino and Pearson test. Student’s t tests or Mann–Whitney tests were used depending on whether data exhibited Gaussian distribution. All statistical analyses were done in Prism v7.0 or v8.0 (Graphpad), and for all analyses p values of less than 0.05 were considered significant as indicated by one or more asterisks (*). All experiments were performed at least two times, with similar results obtained each time.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Ultra-Leaf Purified anti-mouse CD4 (GK1.5) | Biolegend | Cat# 100458; RRID AB_2810319 |
| InVivoMab anti-mouse CD8α (YTS169.4) | BioXCell | Cat# BE0117; RRID AB_10950145 |
| Ultra-Leaf Purified anti-mouse CD28 (37.51) | Biolegend | Cat# 102116; RRID AB_11147170 |
| FITC anti-mouse CD3 (17A2) | Biolegend | Cat# 100204 RRID AB_312661 |
| APC anti-mouse CD3 (17A2) | Biolegend | Cat# 100236 RRID AB_2561456 |
| BD Horizon BUV395 anti-mouse CD3ε (145-2C11) | BD Biosciences | Cat# 563565 RRID AB_2738278 |
| APC anti-mouse CD4 (GK1.5) | Biolegend | Cat# 100412 RRID AB_3126997 |
| Alexa Fluor 700 anti-mouse CD8α (5H10) | ThermoFisher Scientific | Cat# MCD0829 RRID AB_10375174 |
| PE anti-mouse CD8β (YTS156.7.7) | Biolegend | Cat# 126608 RRID AB_961298 |
| PerCP/Cyanine5.5 anti-mouse/human CD44 (IM7) | Biolegend | Cat# 103032 RRID AB_2076204 |
| APC/Cyanine7 anti-mouse/human CD44 (IM7) | Biolegend | Cat# 103028 RRID AB_830785 |
| Brilliant Violet 605 anti-mouse CD45 (30-F11) | Biolegend | Cat# 103140 RRID AB_2562342 |
| PE anti-mouse CD45.1 (A20) | Biolegend | Cat# 110708 RRID AB_313497 |
| Biotin anti-mouse CD45.2 (104) | Biolegend | Cat# 109804 RRID AB_313441 |
| FITC anti-mouse CD45.2 (104) | Biolegend | Cat# 109806 RRID AB_313443 |
| Alexa Fluor 647 anti-rat/mouse CD49a (Ha31/8) | BD Biosciences | Cat# 562113 RRID AB_11153312 |
| APC anti-mouse CD62L (MEL-14) | Biolegend | Cat# 104412 RRID AB_313099 |
| PE/Cyanine7 anti-mouse CD69 (H1.2F3) | Biolegend | Cat# 104512 RRID AB_493564 |
| Pacific Blue anti-mouse CD103 (2E7) | Biolegend | Cat# 121418 RRID AB_2128619 |
| Alexa Fluor 488 anti-mouse TCR γ/δ (GL3) | Biolegend | Cat# 118128 RRID AB_2562771 |
| Pacific Blue anti-human/mouse Granzyme B (GB11) | Biolegend | Cat# 515408 RRID AB_2562196 |
| Brilliant Violet 421 anti-mouse IFN-γ (XMG1.2) | Biolegend | Cat# 505830 RRID AB_2563105 |
| PerCP/Cyanine5.5 anti-mouse/human KLRG1 (MAFA) (2F1/KLRG1) | Biolegend | Cat# 138418 RRID AB_2563015 |
| TruStain FcX (anti-mouse CD16/32) (93) | Biolegend | Cat# 101320 RRID AB_1574975) |
| Bacterial and Virus Strains | ||
| HSV-KOS | Dr. David Knipe Harvard Medical School (Colgrove et al., 2016) | N/A |
| HSV-OVA | Dr. Thomas Gebhardt University of Melbourne (Mackay et al., 2013) | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| OVA peptide (257-264) SIINFEKL | GenScript | Cat# RP10611 |
| HSV-gB2 (498-505) SSIEFARL | GenScript | Cat# RP19992 |
| Recombinant murine IL-1β | Peprotech | Cat# 211-11B |
| Recombinant murine IL-2 | Peprotech | Cat# 212-12 |
| Recombinant murine IL-6 | Peprotech | Cat# 216-16 |
| Recombinant murine IL-12 p70 | Peprotech | Cat# 210-12 |
| Recombinant murine IL-33 | Peprotech | Cat# 210-33 |
| Recombinant murine IFN-γ | Peprotech | Cat# 315-05 |
| Recombinant human TGF-β1 | Peprotech | Cat# 100-21 |
| Recombinant murine TNF-α | Peprotech | Cat# 315-01A |
| eBioscience Fixable viability dye eFluor 506 | ThermoFisher Scientific | Cat# 65-0866-18 |
| eBioscience Fixable viability dye eFluor 780 | ThermoFisher Scientific | Cat# 65-0865-14 |
| BD Cytofix/Cytoperm Fixation/Permeabilization Solution Kit | BD Biosciences | Cat# 554715 |
| PE-labeled H-2Kb-HSV/gB SSIEFARL | NIH tetramer core | N/A |
| Liberase TM Research Grade | Sigma Aldrich | Cat# 5401127001 |
| DNase I grade II, from bovine | Sigma Aldrich | Cat# 10104159001 |
| Brefeldin A | Sigma Aldrich | Cat# B6542 |
| normal serum block | Biolegend | Cat# 927502 |
| Crystal violet | Sigma Aldrich | Cat# C6158 |
| OCT compound | Tissue-Tek | Cat# 4583 |
| Tween20 molecular biology grade | Promega | Cat# H5151 |
| Hoescht 33342, Trihydrochloride, Trihydrate | Thermo Fisher Scientific | Cat# H1399 |
| Alexa Fluor 647 Streptavidin | Biolegend | Cat# 405237 |
| TRIzol Reagent | Thermo Fisher Scientific | Cat# 15596018 |
| FastStart Essential DNA Green Master | Roche | Cat# 06924204001 |
| TaqMan Reverse Transcription Reagents | Thermo Fisher Scientific | Cat# N8080234 |
| Critical Commercial Assays | ||
| CD8+ T cell isolation kit, mouse | Miltenyi Biotec | Cat# 130-104-075 |
| Countbright absolute counting beads | Thermo Fisher Scientific | Cat# C36950 |
| Fix & Perm Cell Permeabilization kit | Thermo Fisher Scientific | Cat# GAS003 |
| Streptavidin/Biotin Blocking kit | Vector Laboratories | Cat# SP-2002 |
| Experimental Models: Cell Lines | ||
| Vero Cells | Dr. Thorsten Mempel Massachusetts General Hospital | N/A |
| Experimental Models: Organisms/Strains | ||
| Mouse: C57BL/6 | The Jackson Laboratory | Cat# 027 |
| Mouse: C57BL/6 | Charles River Laboratoy | Cat# 000664 |
| Mouse: OT-I. C57BL/6-Tg(TcraTcrb)1100Mjb/J | The Jackson Laboratory | Cat# 003831 |
| Mouse: CD45.1. B6.SJL-Ptprca Pepcb/BoyJ CD45.1 | The Jackson Laboratory | Cat# 002014 |
| Mouse: CD45.1. B6-Ly5.1/Cr | Charles River Laboratory | Cat# 564 |
| Mouse: Itga1−/− | (Ray et al., 2004) | N/A |
| Oligonucleotides | ||
| B2m F-CCGAACATACTGAACTGCTACGTAA | This manuscript | N/A |
| B2m R-CCCGTTCTTCAGCATTTGGA | This manuscript | N/A |
| Cxcl9 F-AATGCACGATGCTCCTGCA | This manuscript | N/A |
| Cxcl9 R-AGGTCTTTGAGGGATTTGTAGTGG | This manuscript | N/A |
| Cxcl10 F-GCCGTCATTTTCTGCCTCA | This manuscript | N/A |
| Cxcl10 R-CGTCCTTGCGAGAGGGATC | This manuscript | N/A |
| Ifng F-AACGCTACACACTGCATCTTGG | This manuscript | N/A |
| Ifng R-GCCGTGGCAGTAACAGCC | This manuscript | N/A |
| Software and Algorithms | ||
| ImageJ Fiji v1.0 | (Schindelin et al., 2012) | https://imagej.net/Fiji |
| FlowJo v8, v10 | TreeStar | https://www.flowjo.com/solutions/flowjo/downloads |
| Prism v7, v8 | GraphPad | https://www.graphpad.com/scientific-software/prism/ |
| CytExpert | Beckman Coulter | https://www.beckman.com/flow-cytometry/instruments/cytoflex/software |
| BD FACS DIVA 8 | BD Biosciences | https://www.bdbiosciences.com/en-us/instruments/research-instruments/research-software/flow-cytometry-acquisition/facsdiva-software |
Highlights.
IL-12 or TGF-β can induce CD49a expression by CD8+ T cells
CD49a is not required for CD8+ T cell entry into or localization within the epidermis
CD49a promotes CD8+ TRM persistence within the epidermis
CD49a regulates TRM dendritic morphology and response to antigen challenge
ACKNOWLEDGMENTS
We would like to thank Dr. David Topham for providing Itga1−/− mice, Dr. David Knipe for providing HSV-KOS and advice on working with HSV, and Dr. Thomas Gebhardt for providing HSV-OVA. The following reagent was obtained through the NIH Tetramer Core Facility: H-2K(b) HSV-1 gB 498-505 SSIEFARL. The graphical abstract was produced using BioRender. This work was supported by NIH grant R01 AI121546 (to S.K.B.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2020.108085.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Andreasen SØ, Thomsen AR, Koteliansky VE, Novobrantseva TI, Sprague AG, de Fougerolles AR, and Christensen JP. (2003). Expression and functional importance of collagen-binding integrins, alpha 1 beta 1 and alpha 2 beta 1, on virus-activated T cells. J. Immunol 171, 2804–2811. [DOI] [PubMed] [Google Scholar]
- Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van Rheenen J, Marée AFM, Zal T, et al. (2012). Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl. Acad. Sci. U S A 109, 19739–19744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song J-Y, Jacobs H, Haanen JB, and Schumacher TN (2014). T cell memory. Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346, 101–105. [DOI] [PubMed] [Google Scholar]
- Bank I, Book M, and Ware R (1994). Functional role of VLA-1 (CD49A) in adhesion, cation-dependent spreading, and activation of cultured human T lymphocytes. Cell. Immunol. 156, 424–437. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Bevan MJ, and Fink PJ (2017). Local inflammatory cues regulate differentiation and persistence of CD8+ tissue-resident memory T cells. Cell Rep 19, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaho JA, Morton ER, and Yedowitz JC (2005). Herpes simplex virus: propagation, quantification, and storage. Curr. Protoc. Microbiol Chapter 14, Unit 14E.1. [DOI] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. (2012). Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol 188, 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman TJ, and Topham DJ (2010). Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J. Immunol 184, 3841–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheuk S, Schlums H, Gallais Sérézal I, Martini E, Chiang SC, Marquardt N, Gibbs A, Detlofsson E, Introini A, Forkel M, et al. (2017). CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 46, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgrove RC, Liu X, Griffiths A, Raja P, Deluca NA, Newman RM, Coen DM, and Knipe DM (2016). History and genomic sequence analysis of the herpes simplex virus 1 KOS and KOS1.1 sub-strains. Virology 487, 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, Kotelianski V, Gardner H, and Nestle FO (2007). Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat. Med 13, 836–842. [DOI] [PubMed] [Google Scholar]
- Dadi S, Chhangawala S, Whitlock BM, Franklin RA, Luo CT, Oh SA, Toure A, Pritykin Y, Huse M, Leslie CS, and Li MO (2016). Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 164, 365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fougerolles AR, Sprague AG, Nickerson-Nutter CL, Chi-Rosso G, Rennert PD, Gardner H, Gotwals PJ, Lobb RR, and Koteliansky VE (2000). Regulation of inflammation by collagen-binding integrins alpha1beta1 and alpha2beta1 in models of hypersensitivity and arthritis. J. Clin. Invest 105, 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner H (2014). Integrin α1β1. Adv. Exp. Med. Biol 819, 21–39. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, and Carbone FR (2009). Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol 10, 524–530. [DOI] [PubMed] [Google Scholar]
- Goel N, Docherty JJ, Fu MM, Zimmerman DH, and Rosenthal KS (2002). A modification of the epidermal scarification model of herpes simplex virus infection to achieve a reproducible and uniform progression of disease. J. Virol. Methods 106, 153–158. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Ben-Horin S, Li J, Bank I, Jiang H, and Chess L (2003). Expression of the alpha1beta1 integrin, VLA-1, marks a distinct subset of human CD4+ memory T cells. J. Clin. Invest 112, 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadi S, Thanthrige-Don N, Afkhami S, Khera A, Jeyanathan M, and Xing Z (2017). Expression and role of VLA-1 in resident memory CD8 T cell responses to respiratory mucosal viral-vectored immunization against tuberculosis. Sci. Rep 7, 9525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME, Jacobson JG, Brenner MB, Mann D, and Strominger JL (1985). VLA-1: a T cell surface antigen which defines a novel late stage of human T cell activation. Eur. J. Immunol 15, 502–508. [DOI] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, and Kupper TS (2012). Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 483, 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, and Lefrançois L (2004). Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity 20, 551–562. [DOI] [PubMed] [Google Scholar]
- Liu F, and Whitton JL (2005). Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J. Immunol 174, 5936–5940. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, and Gebhardt T (2012). Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. U S A 109, 7037–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. (2013). The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat. Immunol 14, 1294–1301. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. (2016). Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463. [DOI] [PubMed] [Google Scholar]
- Mani V, Bromley SK, Äijó T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. (2019). Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366, eaav5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickan E, and Rouse BT (1995). Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse-models. J. Virol 69, 8178–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, and Ahmed R (2006). Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J. Immunol 176, 2079–2083. [DOI] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. (2010). Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med 207, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means TK, Hayashi F, Smith KD, Aderem A, and Luster AD (2003). The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J. Immunol 170, 5165–5175. [DOI] [PubMed] [Google Scholar]
- Moon JJ, Chu HH, Hataye J, Pagán AJ, Pepper M, McLachlan JB, Zell T, and Jenkins MK (2009). Tracking epitope-specific T cells. Nat. Protoc 4, 565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash AA, Jayasuriya A, Phelan J, Cobbold SP, Waldmann H, and Prospero T (1987). Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J. Gen. Virol 68, 825–833. [DOI] [PubMed] [Google Scholar]
- Oja AE, Piet B, van der Zwan D, Blaauwgeers H, Mensink M, de Kivit S, Borst J, Nolte MA, van Lier RAW, Stark R, and Hombrink P (2018). Functional heterogeneity of CD4+ tumor-infiltrating lymphocytes with a resident memory phenotype in NSCLC. Front. Immunol 9, 2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, and Horwitz AF (1997). Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature 385, 537–540. [DOI] [PubMed] [Google Scholar]
- Park SL, Zaid A, Hor JL, Christo SN, Prier JE, Davies B, Alexandre YO, Gregory JL, Russell TA, Gebhardt T, et al. (2018). Local proliferation maintains a stable pool of tissue-resident memory T cells after antiviral recall responses. Nat. Immunol 19, 183–191. [DOI] [PubMed] [Google Scholar]
- Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, and Tian Z (2013). Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest 123, 1444–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray SJ, Franki SN, Pierce RH, Dimitrova S, Koteliansky V, Sprague AG, Doherty PC, de Fougerolles AR, and Topham DJ (2004). The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 20, 167–179. [DOI] [PubMed] [Google Scholar]
- Reilly EC, Lambert Emo K, Buckley PM, Reilly NS, Smith I, Chaves FA, Yang H, Oakes PW, and Topham DJ (2020). TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl. Acad. Sci. U S A 117, 12306–12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, and Masopust D (2013). Sensing and alarm function of resident memory CD8+ T cells. Nat. Immunol 14, 509–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, and Masopust D (2014). T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, and Farber DL (2011). Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J. Immunol 187, 5510–5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, and Carbone FR (2004). Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J. Immunol 172, 392–397. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, and Bevan MJ (2012). The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol 189, 3462–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Yuan R, Feng Y, El-Asady R, Farber DL, Gress RE, Lucas PJ, and Hadley GA (2004). Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J. Immunol 172, 214–221. [DOI] [PubMed] [Google Scholar]
- Wu T, Hu Y, Lee Y-T, Bouchard KR, Benechet A, Khanna K, and Cauley LS (2014). Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J. Leukoc. Biol 95, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, Manton JH, Heath WR, and Mueller SN (2014). Persistence of skin-resident memory T cells within an epidermal niche. Proc. Natl. Acad. Sci. U S A 111,5307–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid A, Hor JL, Christo SN, Groom JR, Heath WR, Mackay LK, and Mueller SN (2017). Chemokine receptor-dependent control of skin tissue-resident memory T cell formation. J. Immunol 199, 2451–2459. [DOI] [PubMed] [Google Scholar]
- Zhang N, and Bevan MJ (2013). Transforming growth factor-β signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity 39, 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate/analyze datasets or code.


