Abstract
ES is a 41-year-old transgender male who presented to medical oncology as a referral from surgical oncology for T2N0M0 right breast cancer. At that time, he was receiving weekly testosterone injections intramuscularly at 0.25 mg, and had been on this regimen for 7 months. He was planning bilateral mastectomies in the spring. That winter, he palpated a mass in his right breast, and imaging revealed a 2.5-cm hypoechoic mass. A biopsy of the mass revealed invasive ductal carcinoma, with a nuclear grade of 3. His tumor was estrogen receptor positive (H-score of 180), progesterone receptor positive (H-score of 90), androgen receptor positive (H-score of 220), and HER-2/neu positive by FISH, with a Ki-67 of 90%.
The clinical experience of ES illustrates challenges common to the sexual and gender minority population. ES presented to the clinic with a female friend. At that time, his name and gender in the electronic medical record (EMR) matched his driver’s license, and not his preferred name or gender. Consequently, when he was called from the waiting room, he was called by his former name.
The staff had not been notified of the appropriate name or pronoun to use prior to ES’s arrival, and consequently was associating ES with a female gender, as indicated in the EMR. When the question about menstrual status was addressed, he stated that he had not had a period in 7 months since beginning testosterone treatment. The medical assistant questioned the use of testosterone, and ES had to explain his gender reassignment journey.
By the time the physician assistant (PA) entered the room to perform the first part of the shared visit, ES was visibly upset, expressing anger with nonverbal cues. During the conversation, the PA did not acknowledge or establish the relationship of ES’s female friend. The PA did not address the issue of gender identity, the use of testosterone, or plans for reassignment surgery. The physician also deferred discussion of gender reassignment during his portion of the visit and, without asking about gender reassignment, recommended cessation of testosterone therapy. Neoadjuvant docetaxel/cyclophosphamide/trastuzumab/pertuzumab was ordered, and ES agreed to treatment, but declined following the suggestion to stop testosterone.
At each treatment visit, ES had to check in at the desk with his former name, resulting in confusion from the staff. With each lab draw, his name and birthdate were confirmed using the former name. With every administration of chemotherapy, double-nurse verification at the chairside was performed by reading his arm band and comparing it to the drug label. For each visit, ES had to use his old name six times. With new staff assigned to him each week, he felt pressured to explain his gender identity to several new people at each visit. At one particular visit, a patient’s family in the next cubicle overheard this conversation, and ES overheard them discussing and laughing about his gender identity. ES dreaded his chemotherapy appointments not just due to the expected toxicity, but also because of the insensitivity toward his chosen gender.
ES tolerated chemotherapy and proceeded to surgery. He underwent bilateral mastectomies by the surgical oncologist, who had discussed his gender reassignment with him and had referred him to plastic surgery for co-management of the surgical intervention. The plastic surgery team planned for reconstruction to include skin and soft-tissue rearrangement to give an incision line along the lower border of the pectoralis for better male cosmetic outcomes. Together, they performed bilateral mastectomies with right sentinel lymph node biopsy, horizontal mastopexy, and nipple-areolar grafting. Ultimately, the pathology revealed a complete response, and ES was pleased with the cosmetic outcome.
ES was then started on tamoxifen. Again it was suggested that he discontinue testosterone therapy. He determined that he was more comfortable with an increased risk of recurrence than he was with feminine physical characteristics and chose to continue testosterone. After 6 weeks of tamoxifen, his menses resumed. He elected to discontinue tamoxifen. He had no more vaginal bleeding after that episode. He was referred for bilateral salpingo-oopherectomy (BSO), with the intent to treat with an aromatase inhibitor. He proceeded with BSO and opted against the aromatase inhibitor, citing concerns about unknown interactions with gender-affirming medication. He completed 1 year of trastuzumab (Herceptin) and continued surveillance visits.
Sexual and gender minorities (SGM) represent a population of individuals who experience disparities in health care that put them at risk for poor health-care outcomes. Sexual and gender minority is an umbrella term that encompasses lesbian, gay, two-spirit, bisexual, and transgender populations, as well as those whose sexual orientation, gender identity and expressions, or reproductive development vary from traditional, societal, cultural, or physiological norms. This includes individuals with disorders or differences of sex development, sometimes known as intersex (National Institutes of Health [NIH], 2017). As our society becomes more inclusive of gender minority individuals, recognizing that a patient’s gender may be nonbinary can help the health-care provider understand new terminology (see Table 1) and the specific stressors encountered by patients in gender transition, with which they may not be familiar.
Table 1. Gender Identity and Sexual Orientation Terms.
| Term | Definition | Associated terms |
|---|---|---|
| Sexual and gender minorities | Sexual orientation, gender identity and expressions, or reproductive development varies from traditional, societal, cultural, or physiological normsa | Includes lesbian, gay, two-spirit, bisexual, and transgender |
| Transgender male | Female genotype, identifies as male | Female-to-male |
| Transgender female | Male genotype, identifies as female | Male-to-female |
| Cis- | Prefix meaning gender identity corresponds with birth sex | Straight |
| Trans- | Prefix meaning gender identity does not correspond with birth sex | |
| Lesbian | Identifies as female and attracted to females | |
| Gay | Identifies as birth gender and attracted to same sex | |
| Two-spirit | Combined male and female traits; considered neither male nor female, but a third gender | |
| Bisexual | Identifies as birth gender and attracted to opposite and same sex |
Note. aNational Institutes of Health (2017).
HEALTH CARE IN SEXUAL AND GENDER MINORITY PATIENTS
In health care overall, SGM populations face issues of access and inclusion, including poor insurance coverage and less access to traditional medical services than the population at large. Sexual and gender minority populations may perceive judgment from health-care providers about their lifestyle and experience estrangement from traditional sources of emotional and financial support, such as their family of origin (Blosnich, Farmer, Lee, Silenzio, & Bowen, 2014; Diamant, Wold, Spritzer, & Gelberg, 2000).
These same issues are theoretically present in access to cancer care. Although disparity may exist across the cancer care continuum, the documented cancer care disparity to date is in cancer screening. Sexual and gender minority populations are less likely to have recommended cancer screening as per national guidelines, which may result in later-stage diagnosis (Quinn et al., 2015).
In the national discussion of SGM patients and cancer care, one specific group that has been increasingly recognized but understudied is the transgender population (Joint, Chen, & Cameron, 2018). A cisgender person refers to a person whose gender identity corresponds with their birth sex, while a transgender person refers to a person whose gender identity is different from that of their birth sex. A transgender woman is a female who was assigned male anatomy at birth, while a transgender male is a male who was assigned female anatomy at birth. It is the purpose of this paper to elucidate the real and potential disparities in the delivery of cancer care by illustrating the experience of one SGM patient receiving breast cancer care.
GENDER TRANSITION
It is important to know that gender transition has many steps. Social transition of gender can start before or after initiation of gender-affirming medical treatment. Patients can present for medical care at any point along this continuum. One may have a gender identity that differs from the sex assigned at one’s birth, but not be in this process of transition because of financial, social, or medical reasons. The steps involved in gender-affirming medical treatment are as follows.
A trained mental health professional verifies that an individual meets diagnostic criteria of gender dysphoria (GD) or gender incongruence
-
Endocrine treatment begins
a. Transgender males: intramuscular or transdermal testosterone
b. Transgender females: intramuscular, oral or transdermal estradiol with either an antiandrogen or gonadotropin-releasing hormone (GnRH) agonist
-
Gender-affirming surgery can be performed after at least 1 year of hormone treatment and after the social role is satisfactorily changed. If hormone therapy is contraindicated or not desired by the individual, surgery can proceed after diagnosis of GD is confirmed, and may entail the following procedures:
-
c. Surgery that directly affects fertility
i. Penectomy and gonadectomy, with creation of neovagina
ii. Oophorectomy, vaginectomy, complete hysterectomy, with or without creation of neopenis
-
d. Surgery that does not directly affect fertility
i. Transgender males: mastectomy (usually after androgen therapy is initiated)
ii. Transgender females: breast augmentation (can be performed after at least 2 years of estrogen therapy)
-
After successful transition, the individual is maintained on endocrine treatment with periodic monitoring of sex steroid hormone levels (Hembree et al., 2017)
BREAST CANCER IN THE TRANSGENDER POPULATION
Breast cancer is the most common cancer among women in the United States. In 2019 alone, there will be an estimated 271,270 new cases of invasive breast cancer diagnosed in cisgender women, with 42,260 expected deaths. In cisgender men, there will be an estimated 2,670 new cases of invasive breast cancer diagnosed, with 500 expected deaths. (American Cancer Society, 2019).
It is estimated that 0.6% of United States adults, or 1.4 million individuals, identify as transgender (Flores, Herman, Gates, & Brown, 2016). While there is insufficient evidence to estimate breast cancer prevalence in the transgender population (Joint et al., 2018), it has been shown that the stage and kind of medical transition undergone by a trans person can influence their risk of developing breast cancer. For example, gender confirmation surgery involving the removal of breast tissue can greatly reduce the risk of developing breast cancer. Exogenous hormones are known to play a role in breast cancer development in the cisgender population, as shown by the Women’s Health Initiative study where hormone replacement therapy demonstrated an increased risk of breast cancer (Writing Group for the Women’s Health Initiative Investigators, 2002). The role of exogenous hormones in breast cancer pathogenesis in the trans population is uncertain. In addition, the impact of gender-affirming hormones on breast cancer risk is unclear. Breast cancer statistics, risk factors, screening recommendations, and treatment considerations for the transgender population require further investigation.
Breast Cancer in Transgender Men
Female-to-male transgender individuals, or transgender men, are defined as those born with a female genotype, but who psychosocially identify as male. Transgender men have lower age-specific rates of breast cancer as compared to cisgender women, which may be accounted for by the high rates of mastectomy and the effects of testosterone therapy (Irwig, 2017). The risk of breast cancer in transgender males is related to their age, completion of mastectomy, and the length of exposure to testosterone therapy, if applicable. The limitations to studying lifetime risk of breast cancer in transgender men is the young age of subjects in many studies, the high rate of mastectomy in some populations, and the loss to follow-up (Quinn et al., 2015).
Many transgender males take exogenous testosterone to induce and maintain masculine secondary sex characteristics. Testosterone therapy in transgender men alters the composition of breast tissue by reducing the amount of glandular tissue and increasing the amount of fibrous connective tissue (Grynberg et al., 2010; Slagter, Gooren, Scorilas, Petraki, & Diamandis, 2006). Testosterone administered to transgender men undergoes aromatization to estradiol, producing serum estradiol levels in transgender men comparable to that of the follicular phase of the menstrual cycle (Gooren & T’Sjoen, 2018). Serum estradiol levels do not decrease substantially as a result of testosterone treatment. Prolonged and unopposed estrogen and androgen stimulation increases the risk for breast cancer.
There are two possible pathways that have been described, suggesting that exogenous testosterone could stimulate the activity of hormone receptors in residual breast tissue. In the first pathway, testosterone is converted by the enzyme aromatase to estradiol. Estradiol stimulates breast cell proliferation by activating estrogen receptors found in breast tissue. Thus, testosterone indirectly stimulates estrogen receptors. In the second pathway, testosterone is converted by the enzyme 5α-reductase to dihydrotestosterone. This directly affects androgen receptors in androgen receptor–positive breast cancers because dihydrotestosterone has a dissociation rate from the androgen receptor that is five-fold slower than that of testosterone (Secreto & Zumoff, 2012; Yager & Davidson, 2006). These pathways are theoretical and transgender males do not have a documented greater incidence of breast cancer than the general population. However, more prospective research is needed regarding the long-term effects of testosterone therapy in transgender men.
Breast cancer risk in transgender men is reduced by the completion of bilateral mastectomy. Although a mastectomy removes much of the breast tissue, a small amount of residual breast tissue is typically left underneath the nipple-areola complex after subcutaneous mastectomy to prevent a depression. As case reports have shown, there is a possibility of this remnant tissue becoming malignant, although the exact risk of breast cancer in residual tissue is unknown (Irwig, 2017). Due to financial constraints, not all transgender men undergo mastectomy during gender reassignment.
It is important that transgender men still undergo breast cancer screenings. Transgender men who have not undergone bilateral mastectomy, or who have only undergone breast reduction surgery, should undergo screening per current guidelines for cisgender women. However, no reliable evidence exists to guide the screening of transgender men who have undergone mastectomy. Some guidelines recommend annual chest wall exams in transgender men after mastectomy. However, this is not based on evidence, and it is contrary to the movement away from clinician exams in general for cisgender women. Clinicians should engage in dialogue with transgender men who have undergone bilateral mastectomy about the unknown risks associated with residual breast tissue, as well as the possible technical limitations of mammography. In the case of any new complaints, diagnostic physical exams are appropriate. The evaluation of a palpable lesion may be difficult since most or nearly all breast tissue is removed, and mammography for the evaluation of a palpable lesion may not be technically feasible. Therefore, alternatives such as ultrasound or MRI may be necessary.
The approach to the treatment of breast cancer in transgender men has several considerations that are not present in cisgender breast cancers. The concomitant use of aromatase inhibitors with testosterone may decrease the effectiveness of both the breast cancer treatment as well as the gender reassignment treatment. Since some breast cancers express androgen receptors, exogenous testosterone treatments may decrease the effectiveness of breast cancer treatment. Health-care providers should consider the unclear link between androgens and breast cancer, as well as the potential for the aromatization of testosterone to estrogens. For breast cancer treatment, there are surgical considerations as well. Unlike cisgender women, who may elect breast-conserving surgery or breast reconstruction after mastectomy, transgender men often desire a masculine chest contour. In this situation, the breast surgery method would include chest reconstruction for transgender men, including skin and soft tissue rearrangement to give an incision line along the lower border of the pectoralis for better male cosmetic outcomes.
Breast Cancer in Transgender Women
Male-to-female transgender individuals, or transgender women, are defined as those born with a male genotype, but who psychosocially identify as female. The incidence of breast cancer in transgender women is conflicting and poorly reported. Transgender women have a reduced risk of breast cancer due to lower lifetime exposure to estrogen and minimal exposure or the absence of exposure to progesterone.
The transition from male to female may require the transgender woman to take exogenous estrogen. There is a risk of breast cancer associated with estradiol use. Several cases of breast cancer have been reported in transgender women (Maglione et al., 2014). Typically, exogenous estrogen treatments are taken in conjunction with an antiandrogen treatment. Antiandrogen treatments include spironolactone, GnRH analogues, and a 5α-reductase inhibitor, finasteride. Progesterone is not typically included in the hormonal treatment of transgender women secondary to concerns of the risk of both cardiovascular disease and venous thromboembolism.
The existing recommendations for cisgender woman vary with respect to the frequency of screening. As with the age of onset, given the likely lower incidence in transgender women, it is recommended that screening mammography be performed every 2 years beginning at the age of 50 and after 5 to 10 years of feminizing hormone use. Providers and patients should discuss the risks of overscreening and perform an assessment of individual risk factors. Screening mammography is the primary recommended modality for breast cancer screening in transgender women. Complicating the issue of self-awareness of breast changes for transgender women is the “new” experience of living with breasts in development. This may be associated with breast pain, tenderness, and nodularity, causing the transgender woman to perform frequent self-examinations with concerns about breast findings. However, as with cisgender women, routine self-breast exams for breast cancer screening are not recommended in transgender women (Center of Excellence for Transgender Health, 2016).
The general treatment approach to breast cancer in transgender women undergoing hormone therapy would include a recommendation to discontinue estrogen hormone therapy. However, since hormone therapy can profoundly improve quality of life and have a strong impact on one’s sense of gender affirmation, discontinuation of estrogen therapy in a transgender woman diagnosed with breast cancer is not straightforward. Providers are advised to engage in informed discussions with their patients, considering such variables as the type and stage of breast cancer, hormone receptor status, and the presence of a deleterious breast cancer gene mutation.
HEALTH CARE SYSTEM FACTORS
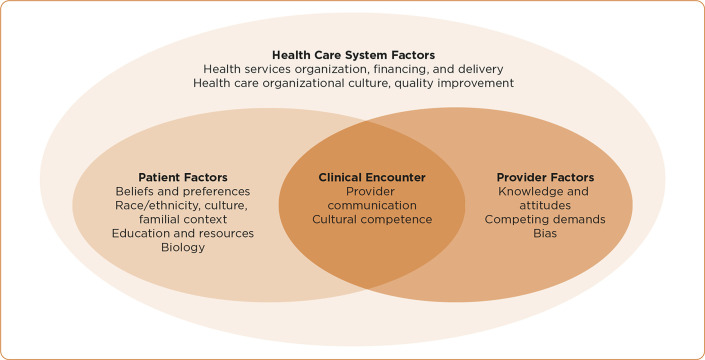
The inflexibility of the traditional health care delivery system to accommodate minority gender identities exposes ES to vulnerability beyond his sexual minority status. The Model of Health-Care Disparity, used to delineate aspects of the US health-care system, can provide an explanatory model for the health-care experience of ES (Figure 1).
Figure 1.
Model of Health-Care Disparity. Adapted from Kilbourne, Switzer, Hyman, Crowley-Matoka & Fine (2006).
Patient Factors
The identification of ES as a gender that differed from that which was specified in the medical record uncovers multiple issues. The infrastructure within health care that identifies patients, protects their privacy, and generates invoices is not adequately flexible to accommodate an individual identifying with a gender that is different from what is on the individual’s government-issued documents, such as birth certificates, driver’s licenses, or US passports. Unfortunately, the process of changing one’s name and gender on official documents requires a complex expenditure of time, money, and effort. According to the 2015 US Transgender Survey, only 11% of respondents had their preferred name and gender on all official identification and documents (James et al., 2016). This process can cost up to $500, and even if affordable, an individual may discontinue the process due to cumbersome requirements or court denial (James et al., 2016).
For example, in Pennsylvania, the state where ES resides, a petition must be filed with the Court of Common Pleas, accompanied by a fingerprint card and a background check. Finally, a court hearing is required, prior to which there must be a notice published in two newspapers as well as a judgment search in each county of residence for the past 5 years. At the hearing, the petition will be granted or denied. If the petition is granted, the process of changing legal documents, including driver’s license, social security card, passport, and birth certificate can begin. On top of these requirements, the applications requesting a gender change must be accompanied by a signed physician statement indicating that the new gender documentation reflects the identified gender of the applicant (Transgender Legal Defense & Education Fund, 2018). Due to the expense and complexity of a formal name and gender identification change, the process of officially changing one’s name and identified gender may be delayed, leading to chronic discrepancy of gender identification during health-care system encounters.
Psychosocial challenges of sexual minority status can include estrangement from family and fear of discrimination. Because of these challenges, individuals with cancer in the SGM community have an even higher risk of stress, anxiety, and depression (James et al., 2016). The American Society of Clinical Oncology (ASCO) position statement on strategies for reducing cancer health disparities among SGM populations recommends that the identities of SGM patients are safely disclosed and that they receive appropriate referrals to support networks (Griggs et al. 2017).
The Clinical Encounter
As illustrated in the case study, ES experienced a multitude of emotions related to breast cancer diagnosis and treatment, which were perhaps further complicated and/or exacerbated by his transgender status. Foremost among these issues is the disclosure of transgender status to health-care providers, which patients describe as awkward or embarrassing (National Institutes of Health, 2017). The ASCO position statement recommends that clinical practice build a capacity for the safe disclosure of sexual and/or gender identities (Griggs et al., 2017). This opportunity for disclosure, perhaps through collection of demographic data and preferred pronouns prior to the visit, could prevent insensitivity in the patient-clinician encounter.
Provider Factors
As illustrated in the case study, the clinical encounter and provider factors are critically important. A key component to the safe and appropriate disclosure of gender identity is cultural competence on the part of all staff. Clinicians need to be aware of the appropriate pronouns to be utilized. ES should have been referred to as “he” in this scenario, determined by the intake questions prior to the clinical encounter.
It is important that staff members have a working knowledge of the process and medications used in gender transition, thus reducing the possibility of hurtful or poorly informed questions being posed to the patient or his/her family. All clinical questions should be posed in a thoughtful and kind manner.
Evidence-based cancer practice for sexual minority patients is limited, due to a lack of specificity in cancer registry analysis (Griggs et al., 2017). Gender identity information is a valuable contribution to the national network of cancer registries. In order to improve the quality of evidence-based cancer care, it is crucial that the traditional variables or data elements capture valuable gender and sexual identity demographics, for both clinical practice and research. One of the recommendations of the ASCO 2017 position statement is to begin to capture this important patient information in cancer registry information and clinical trial enrollment. These changes will document cancer incidence, response to treatment, unique toxicities, and outcomes incorporating gender and sexual orientation demographics (Griggs et al., 2017).
BEYOND THE CLINIC
The American Society of Clinical Oncology recommends that clinicians advocate for policy decisions that ensure health-care equity and ensure full privacy (Griggs et al., 2017). First, the access to culturally appropriate care for SGM individuals can be ensured across the cancer continuum both by expanding and promoting cultural competency training and incorporating SGM training into the curricula of health-care providers’ professional training. Other recommendations for the improved quality of care include the collection and use of SGM-relevant data, with prompt follow-up and continuity of care.
Further research is still needed. There is insufficient knowledge about health-care needs, health outcomes, lived experiences, and effective interventions to improve outcomes for SGM individuals. To promote further research among SGM populations, ASCO recommends the inclusion of SGM status as a required data element in cancer registries and clinical trials. In addition, they recommend the development of a research-training program for current and future scientists that will raise their awareness of SGM health issues.
Footnotes
The authors have no conflicts of interest to disclose.
References
- American Cancer Society. (2019). Cancer Facts and Figures 2019. Atlanta, GA: American Cancer Society; Retrieved from https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html [Google Scholar]
- Blosnich J. R., Farmer G. W., Lee J. G. L., Silenzio V. M. B., & Bowen D. J. (2014). Health inequalities among sexual minority adults: Evidence from ten U.S. states, 2010. American Journal of Preventive Medicine, 46(4), 337–349. 10.1016/j.amepre.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center of Excellence for Transgender Health. (2016). Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people (2nd ed.). Retrieved from http://transhealth.ucsf.edu/protocols [Google Scholar]
- Diamant A. L., Wold C., Spritzer K., & Gelberg L. (2000). Health behaviors, health status, and access to and use of health care: A population-based study of lesbian, bisexual, and heterosexual women. Archives of Family Medicine, 9, 1043–1051. 10.1001/archfami.9.10.1043 [DOI] [PubMed] [Google Scholar]
- Flores A. R., Herman J. L., Gates G. J., & Brown T. N. T. (2016). How many adults identify as transgender in the United States? The Williams Institute at the UCLA School of Law. Retrieved from https://williamsinstitute.law.ucla.edu/research/how-many-adults-identify-as-transgender-in-the-united-states/
- Gooren L. J., & T’Sjoen G.(2018). Endocrine treatment of aging transgender people. Reviews in Endocrine and Metabolic Disorders, 19(3), 253–262. 10.1007/s11154-018-9449-0 [DOI] [PubMed] [Google Scholar]
- Griggs J., Maingi S., Blinder V., Denduluri N., Khorana A. A., Norton L.,…Rowland J. H. (2017). American Society of Clinical Oncology position statement: Strategies for reducing cancer health disparities among sexual and gender minority populations. Journal of Clinical Oncology, 35(19), 2203–2208. 10.1200/JCO.2016.72.0441 [DOI] [PubMed] [Google Scholar]
- Grynberg M., Fanchin R., Dubost G., Colau J. C., Brémont-Weil C., Frydman R., & Ayoubi J. M. (2010). Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reproductive Biomedicine Online, 20(4), 553–558. 10.1016/j.rbmo.2009.12.021 [DOI] [PubMed] [Google Scholar]
- Hembree W. C., Cohen-Kettenis P. T., Gooren L., Hannema S. E., Meyer W. J., Murad M. H.,…T’Sjoen G. G. (2017). Endocrine W735-W740. treatment of gender-dysphoric/gender-incongruent persons: An Endocrine Society * Clinical Practice Guideline. Journal of Clinical Endocrinology & Metabolism, 102(11), 3869–3903. 10.1210/jc.2017-01658 [DOI] [PubMed] [Google Scholar]
- Irwig M. (2017). Clinical dilemmas in the management of transgender men. Current Opinion in Endocrinology & Diabetes and Obesity, 24(3), 233–239. 10.1097/MED.0000000000000337 [DOI] [PubMed] [Google Scholar]
- James S. E., Herman J. L., Rankin S., Keisling M., Mottet L., & Anafi M. (2016). The Report of the 2015 U.S. Transgender Survey. Washington, DC: National Center for Transgender Equality. [Google Scholar]
- Joint R., Chen Z. E., & Cameron S. (2018). Breast and reproductive cancer in the transgender population: A systematic review. British Journal of Obstetrics and Gynecology, 125(12), 1505–1512. 10.1111/1471-0528.15258 [DOI] [PubMed] [Google Scholar]
- Kilbourne A. M., Switzer G., Hyman K., Crowley-Matoka M., & Fine M. J. (2006). Advancing health disparities research within the health care system: A conceptual framework. American Journal of Public Health, 96(12), 2113–2121. https://dx.doi.org/10.2105%2FAJPH.2005.077628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglione K. D., Margolies L., Jaffer S., Szabo J., Schmidt H., Weltz C., & Sonnenblick E. B. (2014). Breast cancer in male-to-female transsexuals: Use of breast imaging for detection. American Journal of Roentgenology, 203(6), W735–W740. 10.2214/AJR.14.12723 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. (2017). Sexual and Gender Minority Research Office Annual Report. Retrieved from https://dpcpsi.nih.gov/sgmro
- Quinn G. P., Sanchez J. A., Sutton S. K., Vadaparampil S. T., Nguyen G. T., Green L.,…Schabath M. B. (2015). Cancer and lesbian, gay, bisexual, transgender/transsexual, and queer/questioning (LGBTQ) populations. CA: A Cancer Journal for Clinicians, 65(5), 384–400. 10.3322/caac.21288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secreto G., & Zumoff B. (2012). Role of androgen excess in the development of estrogen receptor-positive and estrogen receptor-negative breast cancer. Anticancer Research, 32(8), 3223–3228. Retrieved from http://ar.iiarjournals.org/content/32/8/3223.long [PubMed] [Google Scholar]
- Slagter M. H., Gooren L. J., Scorilas A., Petraki C. D., & Diamandis E. P. (2006). Effects of long-term androgen administration on breast tissue of female-to-male transsexuals. Journal of Histochemistry & Cytochemistry, 54(8), 905–910. 10.1369/jhc.6A6928.2006 [DOI] [PubMed] [Google Scholar]
- Transgender Legal Defense & Education Fund (2018). Name Change Project Attorney Handbook for Allegheny County, PA. New York, NY: Dumlao. [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators. (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA, 288(3), 321–333. 10.1001/jama.288.3.321 [DOI] [PubMed] [Google Scholar]
- Yager J. D., & Davidson N. E. (2006). Estrogen carcinogenesis in breast cancer. New England Journal of Medicine, 354, 270–282. 10.1056/NEJMra050776 [DOI] [PubMed] [Google Scholar]