Abstract
Cabozantinib inhibits tyrosine kinase activity at the MET, AXL, and VEGF receptors and is approved for front-line therapy in metastatic clear cell renal cell carcinoma. Little off-protocol data exist on tolerability and response. The objective of this retrospective study was to assess off-protocol tolerability and response rates in patients with metastatic clear cell renal cell carcinoma being treated with cabozantinib. Data on baseline disease characteristics and treatment details were retrospectively gathered for patients with metastatic clear cell renal cell carcinoma treated with cabozantinib at The University of Texas MD Anderson Cancer Center from 2015 to 2017. A blinded radiologist determined the best response according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. Descriptive statistics were utilized. Cabozantinib has a high disease control rate (92%), even as a late line of therapy in metastatic clear cell renal cell carcinoma. However, careful monitoring is warranted, as many patients require treatment breaks and dose reductions for therapy-related toxicity.
Kidney cancer continues to be among the 10 most common cancers in both men and women, with an estimated 73,820 new cases in 2019. Metastatic kidney cancer remains largely incurable, with an estimated 14,770 deaths projected in 2019 (American Cancer Society, 2019). Kidney cancer is largely a disease of the aging; 91% of patients are diagnosed at 45 years or older and 48% are diagnosed at 65 years or older (National Cancer Institute, 2017). Renal cell carcinoma (RCC), the most common form of kidney cancer, consists of a heterogeneous group of cancers arising from the nephron. Clear cell RCC accounts for approximately 70% of all RCCs and is the focus of most treatment-related studies (National Cancer Institute, 2017).
NEW THERAPIES FOR METASTATIC RENAL CELL CARCINOMA
Until recently, there was a paucity of available systemic treatment options for metastatic RCC; however, in the last decade a number of new therapies have been approved, yielding significant progress for this disease. These drugs include therapies targeting vascular endothelial growth factor (VEGF) and its receptors (VEGFR), mammalian target of rapamycin (mTOR) inhibitors, and immune checkpoint inhibitors. VEGF-targeted therapies block VEGFR function and the downstream signaling pathway, resulting in angiogenesis inhibition (Rini & Small, 2005).
There are currently three commonly used options in the first-line setting: pazopanib (Votrient) and sunitinib (Sutent) are both oral anti-VEGF therapies, and the more recently approved cabozantinib (Cabometyx) targets VEGF, mesenchymal-epithelial transition factor (MET), and the anexelekto (AXL) gene (Choueiri et al., 2016, 2017). MET signaling works along with VEGF to promote angiogenesis, tumor growth, and metastasis, while AXL may play a role in developing resistance to VEGFR agents such as pazopanib and sunitinib. By inhibiting both AXL and MET, cabozantinib has been shown to overcome resistance secondary to prolonged sunitinib in preclinical models (Zhou et al., 2016).
Most recently, a combination of nivolumab (Opdivo), a programmed cell death protein 1 (PD-1) immune checkpoint inhibitor antibody, plus ipilimumab (Yervoy), an anticytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) antibody, was approved for first-line therapy in patients with International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) intermediate- and poor-risk disease (Motzer et al., 2018).
Other therapies available in the first-line setting include bevacizumab (Avastin) plus interferon-alpha, high-dose interleukin-2, as well as temsirolimus (Torisel) for patients with IMDC poor-risk disease; these are not as commonly used due to their associated toxicities (National Comprehensive Cancer Network [NCCN], 2018). Furthermore, sunitinib, pazopanib, and cabozantinib have shown activity in poor-risk substrates and are generally preferred over temsirolimus due to their oral administration and efficacy (Choueiri & Motzer, 2017). Moreover, with the approval of combination nivolumab plus ipilimumab for intermediate- and poor-risk disease, temsirolimus will likely be used less and less.
FOCUS ON CABOZANTINIB
Cabozantinib was initially approved in 2016 in the second-line setting based on the results of the METEOR trial, which demonstrated a significant survival advantage over everolimus (Afinitor; Choueiri et al., 2016). METEOR was a randomized phase III trial that compared cabozantinib at 60 mg to everolimus at 10 mg in patients with metastatic clear cell RCC who had previously progressed on at least one prior VEGFR tyrosine kinase inhibitor (TKI). Median overall survival (OS) for those in the cabozantinib group was 21.4 months as compared to 16.5 months in those treated with everolimus (p = .00026). Objective response rate (ORR) in the cabozantinib arm was 17% as opposed to 3% in the everolimus arm (p < .0001), and progression-free survival (PFS) was also improved in the cabozantinib arm (7.4 months) as compared to the everolimus arm (3.9 months; p < .0001; Choueiri et al., 2016).
The CABOSUN trial later demonstrated a survival advantage for cabozantinib over sunitinib in the front-line setting (Choueiri et al., 2017). This was a randomized phase II clinical trial comparing cabozantinib to sunitinib as first-line therapy in patients with metastatic RCC. Cabozantinib improved median PFS as compared to sunitinib (8.2 months vs. 5.6 months) and was associated with a 34% reduction in rate of progression or death. Furthermore, reported ORR in the cabozantinib arm was 46% as opposed to 18% in the sunitinib arm (Choueiri et al., 2017). The results of this trial led to the U.S. Food and Drug Administration approval of cabozantinib for first-line therapy in patients with metastatic renal cell carcinoma in December 2017 (NCCN, 2018). The current recommended dose of cabozantinib is 60 mg without food daily until there is no longer therapeutic benefit or unacceptable toxicities occur.
Both the METEOR and CABOSUN trials reported a high incidence of grade 3 or 4 adverse events: 67% in the CABOSUN trial and 39% in the METEOR trial (Choueiri et al., 2016, 2017). The most common grade 3 or 4 adverse events reported in the CABOSUN trial were diarrhea (10%), fatigue (6%), hypertension (28%), hand-foot syndrome (palmar-plantar erythrodysesthesia; 8%), and hematologic adverse events (3%; Choueiri et al., 2017). The most common grade 3 or 4 adverse events reported in the METEOR trial were hypertension (15%), diarrhea (13%), fatigue (11%), hand-foot syndrome (8%), anemia (6%), hyperglycemia (11%), and hypomagnesemia (5%; Choueiri et al., 2016). As cabozantinib becomes more widely used in patients with RCC, it is important that clinicians understand the toxicity profile and subsequent toxicity management.
OBJECTIVE
The objective of this retrospective study was to assess off-protocol tolerability and response rates in patients with metastatic clear cell renal cell carcinoma being treated with cabozantinib.
METHODS AND VARIABLES
Institutional review board approval at The University of Texas MD Anderson Cancer Center was granted under protocols PA15-0548 and PA16-736. This study was based on 38 patients who had received cabozantinib as second-line therapy and completed baseline imaging studies at the initiation of cabozantinib and at least one subsequent restaging image to assess for response to treatment. Clinical data were collected from the institution’s electronic health records.
The primary endpoints were PFS and overall survival (OS) from the time of therapy initiation to completion. A blinded radiologist determined the best response per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. Secondary endpoints included the frequency of adverse events, neutrophil-lymphocyte ratio (NLR) shift while on treatment, and frequency of dose adjustment. Adverse events were graded according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Categorical data were tabulated with frequency and percentage, and continuous variables were summarized using descriptive statistics. A logrank test was used for variable analysis, and Kaplan-Meier methods were used for survival analysis.
SAMPLE AND SETTING
This was a retrospective chart review of baseline and treatment details of 38 patients with metastatic clear cell renal cell carcinoma treated with cabozantinib at MD Anderson Cancer Center, a large comprehensive cancer center in Houston from 2015 to 2017. Of the 115 patients initially screened, 38 patients had clear cell histology and at least 12 weeks of follow-up to assess the toxicity and efficacy of cabozantinib. The median age at metastatic clear cell renal cell carcinoma diagnosis was 58 years (range, 37–71). Twenty-five patients (66%) were male and 13 patients (34%) were female. Twenty-two (58%) had de novo metastatic disease. Four patients had sarcomatoid dedifferentiation. Thirty-two (84%) had undergone a nephrectomy. This cohort had a median of three sites of metastatic disease, including the following high-risk metastatic sites: bone 22 (57%), liver 9 (23%), and brain 6 (16%). Six patients (16%) received cabozantinib as second-line therapy, 10 (26%) as third-line, and 22 (58%) as fourth-line therapy or later. By IMDC risk score, 7 (18%) were favorable, 22 (58%) were intermediate, and 9 (24%) were poor risk. All patients had received at least one prior VEGFR-TKI, and 24 (63%) had received a prior immune checkpoint inhibitor (Table 1).
Table 1. Patient Characteristics.
| Variable | N (%) |
|---|---|
| Gender | |
| Male | 25 (66%) |
| Female | 13 (34%) |
| Median age at time of metastatic diagnosis (range) | 57.9 years (36.8–71.0 years) |
| Up-front extent of disease | |
| Localized | 16 (42%) |
| Metastatic | 22 (58%) |
| ECOG performance status | |
| 0 | 2 (5%) |
| 1 | 21 (55%) |
| 2 | 15 (40%) |
| 3 | 0 (0%) |
| 4 | 0 (0%) |
| IMDC risk score | |
| Good | 7 (18%) |
| Intermediate | 20 (56%) |
| Poor | 9 (26%) |
| Histology | |
| Clear cell | 38 (100%) |
| Sarcomatoid dedifferentiation | 4 (11%) |
| Nephrectomy status | |
| Status post nephrectomy | 32 (84%) |
| Primary in situ | 6 (16%) |
| High-risk sites of metastatic disease | |
| Bone metastasis | 22 (63%) |
| Brain metastasis | 6 (17%) |
| Liver metastasis | 9 (24%) |
| Line of systemic therapy | |
| Second line | 6 (16%) |
| Third line | 10 (26%) |
| Fourth line or later | 22 (58%) |
| Previous TKI | |
| Yes | 38 (100%) |
| No | 0 (0%) |
| Previous immune checkpoint inhibitor | |
| Yes | 24 (63%) |
| No | 14 (37%) |
Note. ECOG = Eastern Cooperative Oncology Group; IMDC = International Metastatic RCC Database Consortium; TKI = tyrosine kinase inhibitor.
RESULTS
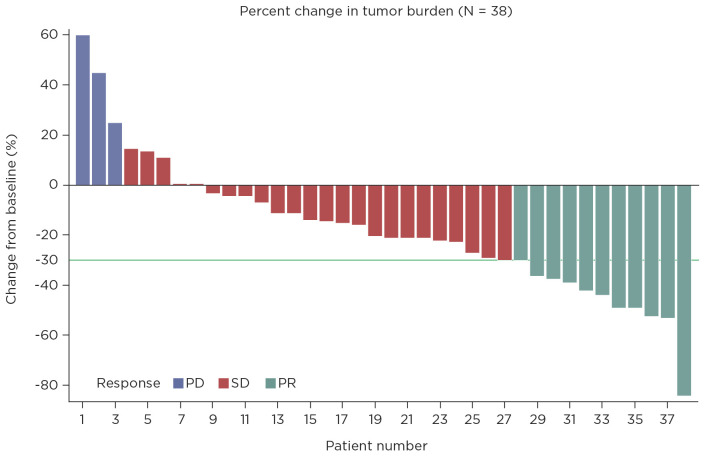
The median PFS for this cohort is 11.3 months (95% confidence interval [CI] = 7.9–not available) and median OS is 20.2 months (95% CI = 12.4–not available; Figures 1 and 2). The estimated median duration of treatment duration using the Kaplan-Meier method of treating treatment duration as time to event variable was 49.3 weeks (95% CI = 35–not available). The disease control rate was 92%; 20 patients had partial response, 10 had stable disease, and 8 had progressive disease (Figure 3). At cabozantinib initiation, 20 (53%) patients had unfavorable neutrophil-lymphocyte ratios (NLR) of ≥ 3, and 12 (34%) patients had a favorable shift of NLR to < 3 on cabozantinib (Table 2). On a logrank test, there was no significant association of NLR interpretation, IMDC risk score, and dose change with OS or PFS.
Figure 1.
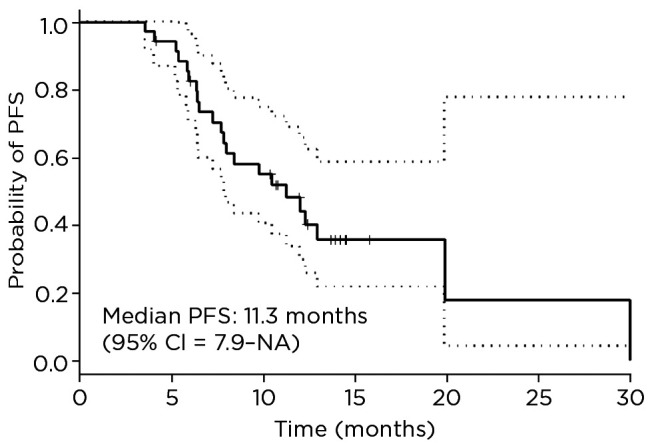
Progression-free survival of patients with metastatic clear cell renal cell carcinoma treated with cabozantinib at The University of Texas MD Anderson Cancer Center from 2015 to 2017. PFS = progression-free survival; CI = confidence interval; NA = not available.
Figure 2.
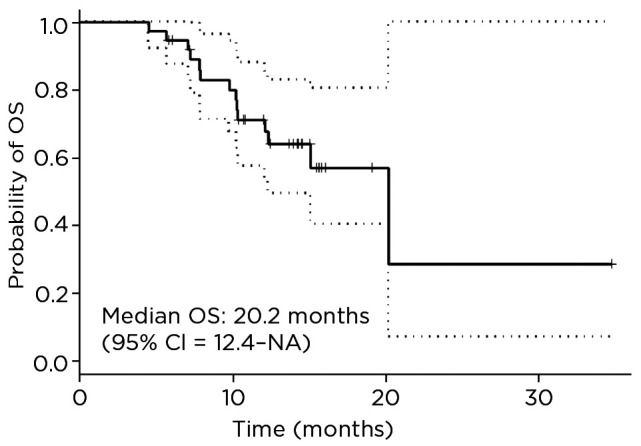
Overall survival of patients with metastatic clear cell renal cell carcinoma treated with cabozantinib at The University of Texas MD Anderson Cancer Center from 2015 to 2017. OS = overall survival; CI = confidence interval; NA = not available.
Figure 3.
Best overall response of patients with metastatic clear cell renal cell carcinoma treated with cabozantinib at The University of Texas MD Anderson Cancer Center from 2015 to 2017. PD = progressive disease; SD = stable disease; PR = partial response.
Table 2. Neutrophil-Lymphocyte Ratio Data.
| Variable | N (%) |
|---|---|
| Baseline NLR | |
| Median | 3.17 |
| Range | 0.8–49.09 |
| NLR on treatment | |
| Median | 2.05 |
| Range | 0.7–23.17 |
| NLR shift | |
| Favorable | 12 (34%) |
| No change | 23 (61%) |
Note. NLR = neutrophil-lymphocyte ratio.
Twenty-two patients (58%) required dose reduction and 24 (63%) required a treatment break due to cabozantinib-related toxicity (Table 3). The most common adverse events were fatigue (61%), hypothyroidism (34%), hand-foot syndrome (34%), and diarrhea (29%). The most common grade 3/4 toxicities were hypertension (13%) and fatigue (13%; Table 4).
Table 3. Cabozantinib Administration.
| Variable | N (%) |
|---|---|
| Cabozantinib starting dose | |
| 20 mg | 1 (3%) |
| 40 mg | 7 (18%) |
| 60 mg | 30 (79%) |
| Dose change while on cabozantinib | |
| Yes | 22 (58%) |
| No | 16 (42%) |
| Treatment break while on cabozantinib | |
| Yes | 24 (63%) |
| No | 14 (37%) |
Table 4. Adverse Events of Cabozantinib.
| Adverse event | Any grade, n (%) | Grade 3/4, n (%) |
|---|---|---|
| Hypertension | 9 (24%) | 5 (13%) |
| Diarrhea | 11 (29%) | 0 (0%) |
| Hand-foot syndrome | 13 (34%) | 1 (3%) |
| Transaminitis | 3 (8%) | 1 (3%) |
| Hypothyroidism | 13 (34%) | 0 (0%) |
| Fatigue | 23 (61%) | 5 (13%) |
| Weight loss | 5 (13%) | 0 (0%) |
| Mucositis | 7 (18%) | 1 (3%) |
| Anorexia | 7 (18%) | 0 (0%) |
DISCUSSION
With the introduction of next-generation VEGFR therapies such as cabozantinib, as well as immunotherapies and combinations of both, the landscape of therapies for metastatic RCC is rapidly changing. Cabozantinib is now a key player in both first- and second-line therapy for metastatic RCC; furthermore, trials combining cabozantinib with checkpoint blockade are ongoing. This study reports an impressive response rate in patients with clear cell histology despite having been heavily pretreated; however, in order for patients to obtain maximum benefit, toxicities must be identified and managed appropriately.
Adverse Event Management
The key principles of toxicity management include the appropriate use of dose reduction and treatment breaks. While the approved starting dose of cabozantinib is 60 mg by mouth daily, few patients can tolerate this dosing continuously. As previously mentioned, in this cohort, 58% of patients required dose reductions and 63% required treatment breaks to manage side effects. Diarrhea, fatigue, hypothyroidism, and hand-foot syndrome were the most frequently reported side effects. This is similar to the reported toxicity data from both the METEOR and CABOSUN trials (Choueiri et al., 2016, 2017).
Prior to making dose adjustments, many clinicians should attempt to manage side effects with medications and/or lifestyle changes. It is important to follow patients with regular clinic visits and laboratory assessments in order to achieve optimal tolerance of cabozantinib. For example, for patients with diarrhea, as-needed medications such as loperamide may be used, as well as the addition of probiotics (Schmidinger & Danesi, 2018). Fatigue can be an especially challenging side effect to manage, as it is often multifactorial; however, the most effective strategy to manage fatigue related to cancer therapies is physical exercise (Musanti, 2016). Patients with a thyroid stimulating hormone (TSH) > 10 mIU/L will require thyroid supplementation with levothyroxine along with continued monitoring of both TSH and free thyroxine (free T4; Schmidinger & Danesi, 2018). Lastly, hand-foot syndrome usually requires the use of topical creams and emollients, as well as pain medications in some cases (Schmidinger & Danesi, 2018). Topical creams or compounds that contain lidocaine, aloe vera, and collagen, such as Regenecare Wound Gel, have been especially efficacious for relieving hand-foot syndrome (Wong et al., 2010). Gerendash and Creel (2017) detail further management strategies related to cabozantinib-related toxicities that clinicians might find helpful.
When the above-mentioned side-effect management fails to achieve a tolerable toxicity profile, treatment breaks are then considered. For many patients, the first dose modification strategy attempting to mitigate side effects is taking weekend treatment breaks or taking the medication for 5 days per week. Another strategy employed in this trial was taking a longer break, ranging from 7 to 14 days, and then restarting cabozantinib at the prescribed dose. Often, if this does not help, a dose reduction is considered. Cabozantinib is currently available in 20-mg, 40-mg, and 60-mg tablets; therefore, most dose adjustments consist of reducing the dose from 60 mg to 40 mg or 40 mg to 20 mg. This retrospective study points to a clinical benefit despite many patients not receiving full-dose therapy, given there was no significant association with OS or PFS when the dose of cabozantinib was changed. It is certainly preferable for patients to be on a reduced dose of cabozantinib rather than having to change therapies due to toxicities, given the potential response to cabozantinib in the clear cell cohort observed in this study.
IMPLICATIONS FOR ADVANCED PRACTICE PROVIDERS
Advanced practice providers (APP) are well versed in side-effect identification, assessment, and management. Understanding the best management strategies to help patients best tolerate cabozantinib is important in order for patients to obtain therapeutic benefit. Advanced practice providers are well equipped to educate patients about anticipated side effects as well as when to contact their oncology providers to report uncontrolled side effects. Furthermore, APPs are well suited to make recommendations regarding treatment breaks and dose reductions as well as to prescribe any necessary medications for side-effect management, if warranted.
CONCLUSIONS
In this small retrospective study, a high disease control rate (92%) was observed despite a heavily pretreated population. For 58% of patients, cabozantinib was the fourth-line or greater systemic therapy used. There was no significant association between NLR interpretation or IMDC risk score with OS or PFS. Appropriate management strategies for cabozantinib-related side effects have been published previously, although with few suggestions as to the appropriate usage of treatment breaks and dose reductions (Schmidinger & Danesi, 2018). Furthermore, this study suggests that there is no association between dose reductions and PFS and OS. Given the impressive OS and PFS of cabozantinib despite dose reductions and treatment breaks, clinicians should not be afraid to employ such strategies. With careful monitoring, the appropriate use of treatment breaks and dose reductions, and side-effect management, patients can tolerate cabozantinib and ideally benefit from its clinical efficacy.
Footnotes
Ms. Lemke has no conflicts of interest to disclose. Dr. Shah has received consulting fees or honoraria from Eisai Medical Research Inc. and Roche Pharmaceuticals. Dr. Campbell has received consulting fees or honoraria from Eisai Medical Research Inc. and Pfizer Inc, and fees for participation in review activities from Genentech, Inc. Dr. Tannir has received consulting fees or honorarium from Exelixis for an advisory board meeting.
References
- American Cancer Society. (2019). Key statistics about kidney cancer. Retrieved from https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html
- Choueiri T. K., Escudier B., Powles T., Tannir N. M., Mainwaring P. N., Rini B. I.,…Motzer R. J. (2016). Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncology, 17(7), 917–927. 10.1016/s1470-2045(16)30107-3 [DOI] [PubMed] [Google Scholar]
- Choueiri T. K., Halabi S., Sanford B. L., Hahn O., Michaelson M. D., Walsh M. K.,…Morris M. J. (2017). Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: The Alliance A031203 CABOSUN Trial. Journal of Clinical Oncology, 35(6), 591–597. 10.1200/jco.2016.70.7398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choueiri T. K., & Motzer R. J. (2017). Systemic therapy for metastatic renal-cell carcinoma. New England Journal of Medicine, 376(4), 354–366. 10.1056/NEJMra1601333 [DOI] [PubMed] [Google Scholar]
- Gerendash B. S., & Creel P. A. (2017). Practical management of adverse events associated with cabozantinib treatment in patients with renal-cell carcinoma. OncoTargets and Therapy, 10, 5053–5064. 10.2147/OTT.S145295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer R. J., Tannir N. M., McDermott D. F., Aren Frontera O., Melichar B., Choueiri T. K.,…Escudier B. (2018). Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New England Journal of Medicine, 378(14), 1277–1290. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musanti R. (2016). How exercise can benefit patients with cancer. Clinical Journal of Oncology Nursing, 20(6 suppl), S2 10.1188/16.cjon.s2.2 [DOI] [PubMed] [Google Scholar]
- National Cancer Institute. (2017). Surveillance, Epidemiology, and End Results (SEER) program cancer stat facts: kidney and renal pelvis cancer. Retrieved from https://seer.cancer.gov/statfacts/html/kidrp.html
- National Comprehensive Cancer Network. (2018). NCCN Clinical Practice Guidelines in Oncology: Kidney cancer. V3.2018. Retrieved from https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf [DOI] [PubMed]
- Rini B. I., & Small E. J. (2005). Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. Journal of Clinical Oncology, 23(5), 1028–1043. 10.1200/jco.2005.01.186 [DOI] [PubMed] [Google Scholar]
- Schmidinger M., & Danesi R. (2018). Management of adverse events associated with cabozantinib therapy in renal cell carcinoma. Oncologist, 23(3), 306–315. 10.1634/theoncologist.2017-0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. F., Lindgren A., Mummaneni M., Byun T., Vasko C., Arenos R.,…Osann K. (2010). A prospective crossover pilot study to evaluate the use of a topical wound gel in patients with cutaneous toxicity caused by epidermal growth factor receptor inhibitors. Journal of Supportive Oncology, 8(5), 202–208. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21086877 [DOI] [PubMed] [Google Scholar]
- Zhou L., Liu X. D., Sun M., Zhang X., German P., Bai S.,…Jonasch E. (2016). Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene, 35(21), 2687–2697. 10.1038/onc.2015.343 [DOI] [PMC free article] [PubMed] [Google Scholar]