Abstract
The severe acute respiratory syndrome-coronavirus-2 (commonly known as SARS-CoV-2) is a novel coronavirus (designated as 2019-nCoV), which was isolated for the first time after the Chinese health authorities reported a cluster of pneumonia cases in Wuhan, China in December 2019. Optimal management of the Coronavirus Disease-2019 disease is evolving quickly and treatment guidelines, based on scientific evidence and experts’ opinions with clinical experience, are constantly being updated. On January 30, 2020, the World Health Organization declared the SARS-CoV-2 outbreak as a "Public Health Emergency of International Concern". The total lack of immune protection brought about a severe spread of the contagion all over the world. For this reason, diagnostic tools, patient management and therapeutic approaches have been tested along the way, in the desperate race to break free from the widespread infection and its fatal respiratory complications. Current medical knowledge and research on severe and critical patients’ management and experimental treatments are still evolving, but several protocols on minimizing risk of infection among the general population, patients and healthcare workers have been approved and diffused by International Health Authorities.
Keywords: Pandemics, Viruses, SARS-CoV-2/COVID-19, Sepsis, Acute respiratory distress syndrome, Acute respiratory distress syndrome, Translational medicine, Clinical microbiology, Clinical biochemistry, Antivirals, Emergency and critical care medicine
Core Tip: We describe the main epidemiological data and clinical features of coronavirus disease-2019 (COVID-19) human disease, analyzing principal signs and symptoms of its clinical manifestation and its current morbidity and mortality ratio. We also focus on the most important International Guidelines concerning both the recommended tests for COVID-19’s laboratory diagnosis and the acute patient’s management in the Emergency Room, paying attention to the appropriate personal protective equipment that medical staff must wear while in contact with infectious patients and to the current pharmacological approaches for hospitalized and symptomatic patients, such as use of antiviral drugs, immune enhancers, stem cells, and plasma therapy.
INTRODUCTION
The severe acute respiratory syndrome- (SARS-) coronavirus 2 (CoV-2), officially named as “coronavirus disease-2019 (COVID-19)” by the World Health Organization (WHO) on February 11, 2020, is actually the novel coronavirus responsible for one of the most severe worldwide pandemics in recent history[1]. On January 9, 2020, the Chinese Center for Disease Control and Prevention (CDC) reported and confirmed that a cluster of cases of acute pneumonia in people associated with the Huanan Seafood Wholesale Market in Wuhan, a city in the Hubei Province of China, were caused by a novel coronavirus, 2019-nCoV[1-3]. From that day, the epidemic spread throughout China, being followed by a rapidly increasing number of cases in other countries throughout the world, with a high morbidity and mortality ratio[4,5]. On 11 March, 2020, the Director-General of the WHO declared COVID-19 a global pandemic[6].
The coronavirus subfamily, Nidovirales order, includes four genera: alpha, beta, gamma, and delta coronaviruses. They are medium-sized, enveloped, positive-stranded RNA viruses which replicate using a nested set of mRNAs[7,8]. Their genetic material represents the largest known viral RNA genomes (between 27-32 kb). The host-derived membrane surrounds the genome, encased in a nucleocapsid, and contains glycoprotein spikes. Viral RNA replication by RNA polymerase occurs in the host cytoplasm. The coronaviruses genome encodes four or five structural proteins[8]. Coronaviruses are very common among birds and mammals, especially in bats, pigs and feline, which represent the major hosts[7,9].
The human coronaviruses (HCoVs) number seven. There are five non-SARS coronavirus serotypes (two beta coronaviruses — HCoV-OC43 and HCoV-HKU1 — and two alpha coronaviruses — HCoV-229E and HCoV-NL63)[8] and a novel coronavirus, the Middle East respiratory syndrome coronavirus (MERS-CoV), that emerged in 2012[9]. They are community-acquired viruses that continually circulate through the human population, causing asymptomatic infections, accounting for 5% to 10% of overall colds and upper respiratory tract infections during winter in adults and some proportion of lower respiratory illness in children[10,11]. In contrast, the last two beta coronaviruses – SARS-CoV and SARS-CoV-2 – jumped to the human population in 2002 and 2020 respectively, causing acute pneumonia, with a higher mortality rate. Respiratory coronaviruses probably spread in a fashion similar to that of the rhinoviruses, via direct contact with infected secretions or large aerosol droplets[12-15]. Protein-protein binding assays have confirmed that angiotensin-converting enzyme 2 (ACE2) is most likely to be the cell receptor through which the virus invades the host cell[16,17].
HOW DID COVID-19 START AND WHERE DID IT COME FROM? WAS IT REALLY WUHAN’S ANIMAL MARKET?
The scientific community is currently trying to identify the source of the infection, which is still uncertain. According to recent lines of evidence, in late 2019, someone at the Huanan Seafood Market in Wuhan was infected with SARS-CoV-2, but specific animal associations have not been confirmed. The viral infectious disease then spread from that first cluster in the capital of China’s Hubei province to a pandemic. Some argue that the involved animals would be bats and pangolins[18].
WHICH ARE THE ESTABLISHED RISK FACTORS FOR SEVERE COVID-19 DISEASE?
The spread of COVID-19 caused by the SARS-CoV-2 outbreak has been growing since its first identification in December 2019. On May 17, 2020, the WHO’s Coronavirus Disease Situation Report counted 4525497 confirmed cases globally since the beginning of the global pandemic and a total of 307395 deaths all over the world[19]. Actually, the case fatality rate of the ongoing COVID19 pandemic (the ratio between confirmed deaths and confirmed cases) is 6.78% for the world and 14.3% for Italy[20,21]. As a comparison for this global value with the case fatality rate of other coronavirus outbreaks, it was 10% for SARS-CoV and 34% for MERS-CoV[24], instead and approximately between 0.1%-0.2% for the seasonal flu[21-24].
COVID-19 is a new disease and there is limited information regarding risk factors for developing a severe case. What we know is that the COVID-19 pandemic has shown an opposite behaviour than that of other global infectious diseases. Indeed, for many other viral and bacterial diseases, such as the previous ‘Spanish flu’ pandemic in 1918 and malaria (which is still endemic in many areas of the world), the majority of deaths were young and children; for COVID-19 cases, the elderly are at the greatest risk of dying if infected with the virus. Yet, old age is not an isolated risk factor for developing a severe acute viral pneumonia by COVID-19. Based on currently available information, older adults, but also people of any age, who have serious underlying medical conditions might be at higher risk for severe illness. In fact, analysing the case fatality rate of each condition shows that those with an underlying health condition have a higher percentage than those without. More than 10% of people with a cardiovascular disease, more than 7% of people with diabetes, 6% with chronic respiratory disease or moderate to severe asthma, 6% with hypertension, and more than 5% with cancer who were diagnosed with COVID-19 have died[21].
Other important risk factors for developing severe complications of COVID-19 disease are related to immunocompromised status due to congenital and acquired immune-deficiencies (i.e., cancer treatment, bone marrow or organ transplantation, autoimmune deficiency syndrome, prolonged use of corticosteroids and immunosuppressive drugs), severe obesity (body mass index of 40 or higher), hypertension, liver disease, chronic kidney disease undergoing dialysis, and cerebrovascular diseases[21,25-27]. A possible reason why the elderly are most at risk of dying from COVID-19 might be the fact that they are also most likely to have underlying health conditions.
WHAT PREVENTIVE MEASURES SEEM TO HAVE BEEN THE MOST EFFECTIVE TO LIMIT THE COVID-19 CONTAGION?
Italy and other European countries emerged early on as the countries with the largest outbreaks of the novel SARS-CoV-2 outside Asia. Several prevention and containment measures have been applied worldwide to contain the COVID-19 disease. In Italy, the particular seriousness of COVID-19 disease regarding morbidity and mortality and the enormous overload of intensive care units (ICUs) brought about the Italian government’s establishment of a series of Decree Laws[28], from February to May 2020, to apply strict and extensive containment measures in all of the Italian territory. Most non-essential commercial activities were temporarily stopped. The decrees also restricted movements within the regional territory and beyond it, except for proven work reasons, absolute urgency, or for health reasons. But, first of all, to limit the spread of the contagion, careful personal hygiene measures were highly recommended, such as frequent hand-washing, interpersonal safety distancing of at least 1 m to avoid close contacts with potentially infected people, the mandatory use of masks in public closed spaces, and the constant sanitization of public spaces. Thanks to all these measures and practices, on May 4, 2020, the Italian government declared initiation of the so-called “Phase two”[28-30], a period of greater freedom but always with strong recommendations of respecting constant hygiene measures and being on alert for eventual rise in new cases.
Social distancing is fundamental to prevent the inter-human spread of COVID-19 though Flügge’s drops, produced as a result breathing, talking, sneezing, or coughing and able to contaminate surfaces. According to the latest release from the National Health Commission, known as the PRC, SARS-CoV-2 is believed to be transmitted mostly through respiratory droplets and close contacts. Prolonged exposure to high concentrations of aerosols may facilitate transmission. Spread is also possible through the conjunctiva. It will be very important during the summer of 2021 to better investigate the possible role of air conditioning systems in increasing the virus circulation. Finally, an observational study found that SARS-CoV-2 does not seem to be present in breast milk and its transmission may take place through respiratory droplets rather than the milk; for this reason, it is not recommended to interrupt breastfeeding[10,11].
SARS-CoV-2 persistence (and infectivity) on different surfaces (liquid, solid, or gaseous) is still debated. The 50% tissue culture infective dose (TCID50) is the measure of infectious virus titre. This endpoint dilution assay quantifies the amount of virus required to kill 50% of infected hosts or to produce a cytopathic effect in 50% of inoculated tissue culture cells. A recent experiment performed using aerosols (< 5 µm) containing SARS-CoV-2 (105.25 TCID50 per mL) showed a reduction from 103.5 to 102.7 TCID50 per L of air after 3 h, a reduction from 103.7 to 100.6 TCID50 per mL on plastic after 72 h, and the same reduction after 48 h on stainless steel[31]. These results show a persistence of SARS-CoV-2 for many hours on surfaces in experimental conditions, but further peer-reviewed investigation on this topic is needed because it represents an environmental and public health problem concerning hospitals (especially in COVID departments), schools, offices, and every day public places.
Furthermore, previous studies have found that air pollution is a risk factor for respiratory infection, by carrying microorganisms, but it also causes oxidative, pro-inflammatory and immunological damage to the lungs. Various recent studies have explored the relationship between ambient air pollutants and COVID-19 infection. Most of these have shown a relationship between long-term exposure to air pollution in cities and risk of infection[32-35]. Another study investigated in the United States whether long-term average exposure to fine particulate matter (PM2.5) is associated with an increased risk of COVID-19 death. It was found that an increase of only 1 μg/m3 in PM2.5 increases vulnerability to experiencing the most severe COVID-19 outcomes, with statistically significant evidence that this exposure is associated with a 15% increase in the COVID-19 mortality rate[36]. However, caution should be used in translating high values of conventional aerosol metrics, such as PM2.5 and PM10 concentrations, in a mortality predictive factor, because many biases may interfere in a real-life situation, relating to different temperature, humidity, and ultraviolet radiation.
We can interpret these data considering air pollution as an additional risk factor for COVID-19 disease, which might contribute to increasing the vulnerability and the clinical outcome, probably also through a previous increase in heart diseases, lung problems and cancer. This could also partially explain the prevalence of the infection in the most industrialized cities of the world, Northern Italy included, and the effect of national lockdown. Finally, it could provide implications for the control and prevention of this novel disease and underscores the importance of continuing to enforce existing air pollution regulations to protect human health both during and after the COVID-19 crisis.
WHICH ARE THE MOST TYPICAL CLINICAL SYMPTOMS AND SINGS OF COVID-19? CAN ANY PARAMETERS SUGGEST A SEVERE FORM OF COVID-19 INFECTION?
As the clinical spectrum of COVID-19 ranges widely from asymptomatic cases to severe pneumonia with a high risk of mortality, there is a need for more research to identify the earliest markers of disease severity. The incubation period for COVID-19 is currently estimated to be between 1 d and 14 d. Most infected people develop mild to moderate illness and recover without hospitalization. In the early phases of the disease, clinical manifestations are very unspecific, so differential diagnosis should include other infectious viral diseases that appear with the same symptoms, such as influenza and parainfluenza, the common cold caused by Rhinovirus, and those caused by human metapneumovirus, human respiratory syncytial virus and adenoviruses, but also with non-infectious (e.g., vasculitis, dermatomyositis) common respiratory disorders.
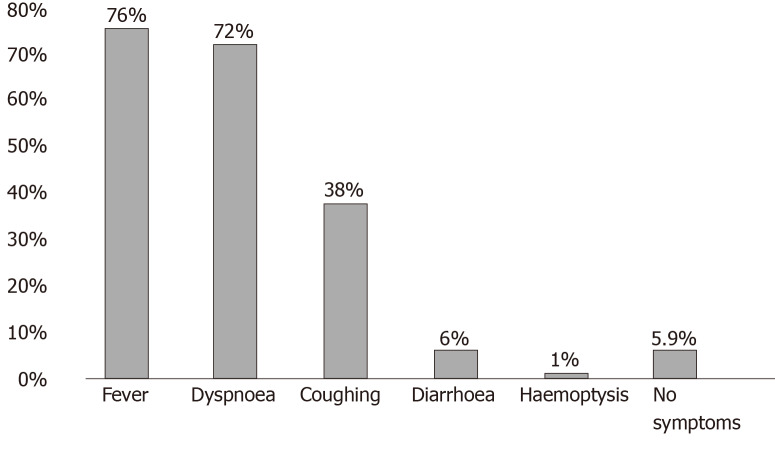
The most common symptoms of COVID-19 infection are fever (not very responsive to antipyretics), dry cough, dyspnoea and increased respiratory rate (in more fragile patients, the dyspnoea may appear at the onset of symptoms, while in younger subjects without other comorbidities it may appear later), myalgia, and intense fatigue[37-40]. Clinical studies have shown an incidence rate of diarrhoea ranging from 2% to 50%. It may precede or trail the respiratory symptoms[40]. Increasing evidence indicates possible faecal-oral transmission[41-43]. Other gastrointestinal symptoms are nausea and vomiting, abdominal pain, and loss of appetite[37-44] (Figure 1).
Figure 1.
Most commonly observed symptoms before hospitalization in a sample of 19996 patients who died of coronavirus disease-2019 in Italy. Source: Epicentro, Istituto Superiore di Sanità, Italy.
Less common symptoms include sore throat, headache, nasal congestion, hyposmia/anosmia, ageusia, diffuse aches and pains, and conjunctivitis[45]. The most common skin manifestations associated with COVID-19 infection include a maculo-papular or papulovesicular rash, urticarious lesions, painful acral red purple papules, livedo reticularis, and petechial lesions. The most common areas involved are the trunk, hands and feet, with little itching experienced. These symptoms usually present before the onset of respiratory symptoms and spontaneously disappear within 10 d in all patients. There is no demonstrated correlation, in the majority of the studies, between skin lesions and COVID-19 severity[46-48]. An Italian paper reported skin manifestations of COVID-19 in 3 young patients, 2 of whom were asymptomatic and potentially contagious. These lesions began as erythematous-violaceous patches in the acral sites and slowly evolved to purpuric and then to ulcero-necrotic lesions, followed by a complete “restitutio ad integrum” of tissues. Burning and itching were present with some of the lesions[49].
Data provided by the WHO Health Emergency Dashboard (May 21, 2020, 10.00 am CEST) indicated 4893186 confirmed cases worldwide since the beginning of the epidemic, and 103981 in the last 24 h. In total, 323256 cases were fatal, with 4467 in the last 24 h[50]. Looking at these data, the global situation still appears to be in a dramatic evolution. Trying to investigate the reasons why, in some patients, the COVID-19 infection rapidly evolves into a severe acute respiratory syndrome, has led to multiple organ dysfunction and even death being a focus of primary importance.
The largest cohort of patients with COVID-19 from China (more than 44,000) showed that illness severity can range from mild (81%) to severe and critical (14% and 5% respectively), as shown in Table 1[51]. Mild COVID-19 illness is characterized by a lower respiratory disease, evidenced by clinical assessment or imaging, and with blood O2 saturation level of > 93% on room air at sea level. These patients should be admitted to a healthcare facility for close observation. COVID-19 severe illness is defined by a blood O2 saturation level of ≤ 93% on room air at sea level, respiratory rate of > 30, arterial partial pressure of O2/fraction of inspiration O2 of ≤ 300 mmHg, or lung infiltrates of > 50%. These patients may experience rapid clinical deterioration into a critical disease state.
Table 1.
Main clinical manifestations according to the Chinese Center for Disease Control and Prevention
| Mild disease, 81% | Severe disease, 14% | Critical disease, 5% |
| Non-pneumonia; Mild pneumonia | Dyspnoea; Respiratory distress; Respiratory rate ≥ 30 per min; Oxygen saturation ≤ 93% at rest state; PaO2/FiO2 ≤ 300 mmHg, 1 mmHg = 0.133 kPa | Patients needing ICU: Respiratory failure needing mechanical; Ventilation; Septic shock; MOD or MOF |
FiO2: Fraction of inspiration O2; ICU: Intensive care unit; MOD: Multiple organ dysfunction; MOF: Multiple organ failure; PaO2: Arterial partial pressure of oxygen.
The clinical picture of critical patients with severe inflammatory-induced lung disease and with sepsis or septic shock needing intensive care support and mechanical ventilation is characterized by a wide range of signs and symptoms of life-threatening multiorgan dysfunction or failure, including dyspnoea, tachypnoea (respiratory rate of > 30/min), tachycardia, chest pain or tightness, hypoxemia, virus-induced distributive shock, cardiac dysfunction, elevations in multiple inflammatory cytokines, renal impairment with oliguria, altered mental status, functional alterations of organs expressed as laboratory data of hyperbilirubinemia, acidosis [serum lactate level > 2 mmol/L (18 mg/dL)], coagulopathy, and thrombocytopenia. Moreover, an exacerbation of underlying comorbidities is often possible[51-57]. Old-age patients with pre-existing comorbidities or dyspnoea should be hospitalized and closely monitored, especially at 1-2 wk after symptom onset. In fact, as already mentioned, in patients with other pre-existing diseases, COVID-19 may be fatal[21,25-27].
The sequential organ failure assessment (commonly known as SOFA) score is used for the evaluation of multiorgan damage and to predict ICU mortality risk based on lab results and clinical data[52,53], as well as for validation in a paediatric version[57]. A Kawasaki-like disease — a vasculitis for which diagnosis is based on the presence of persistent fever, polymorphic rash, lymphadenopathy, conjunctival injection, changes to the mucosa, swollen extremities and with coronary artery aneurysms as its main complication — has been described in children infected with COVID-19, with a monthly incidence much more higher than observed for Kawasaki disease across the previous 5 years. There was also a high proportion of shock in those children presenting with hypotension and requiring fluid resuscitation and some needing inotropic support. It is still uncertain, however, if this emerging phenomenon is a Kawasaki disease type, with SARS-CoV-2 as the triggering agent, or if it represents an emerging Kawasaki-like disease characterized by multisystem inflammation[58-60].
ARE DIAGNOSTIC IMAGING AND LABORATORY TESTS USEFUL FOR COVID-19 DIAGNOSIS?
Current studies are investigating the relationship between different variables and the risk of death of COVID-19 patients hospitalized for pneumonia. The pulmonary imaging techniques for diagnosis of COVID-19 Lung damage include an initial evaluation with chest X-ray, ultrasound, and, if indicated, computed tomography. Electrocardiogram should be performed if indicated, especially in patients with cardiovascular risk factors.
Laboratory testing includes a complete blood count with differential and a metabolic profile, including liver and renal function tests. A Chinese sex-, age- and comorbid illness-matched case-control study identified lymphopenia (CD3+CD8+ T-cells ≤ 75 cells· μL-1) and cardiac troponin I value of ≥ 0.05 ng· mL-1 as negative prognostic factors associated with an increase in risk of mortality from COVID-19 pneumonia[61].
Many studies have designed different increased laboratory results as early predictors of critical illness, such as leucocytosis with agranulocytosis, elevated lactate dehydrogenase, alanine aminotransferase, aspartate aminotransferase, bilirubin, creatine phosphokinase, myoglobin and cytokines [i.e., interleukin (IL)-2, IL-7, IL-10, granulocyte colony-stimulating factor, interferon gamma-induced protein 10, monocyte chemoattractant protein-1, macrophage inflammatory protein-1 alpha, and tumour necrosis factor-alpha]. Although, these findings remain to be validated by further studies. Measurements of inflammatory markers such as C-reactive protein, D-dimer, and ferritin, while not part of standard care, may have prognostic value[62-64].
WHAT HEALTHCARE FACILITIES CAN BE ADOPTED TO MINIMIZE RISK OF INFECTION AMONG PATIENTS AND HEALTHCARE WORKERS?
During infectious disease outbreaks, triage is particularly important to separate patients likely to be infected with the pathogen of concern. The CDC has released comprehensive guidelines for management of patients with COVID-19, including those who are critically ill[65]. It would be very important to make hotlines available that patients can call to notify the facility that they are seeking care and which can be used as telephone consultation for patients to determine the need to visit a healthcare facility; through this, patients could be informed, before arriving for triage, of preventive measures to take as they come to the facility (e.g., wearing a mask and having tissues to cover cough or sneeze). Moreover, healthcare facilities should consider telemedicine (e.g., cell phone videoconference or teleconference) to provide clinical support without direct contact with the patient[65]. Emergency departments should have a “respiratory waiting area” for patients coming in with respiratory symptoms (suspected COVID-19 patients), with clear signage at the entrance, physical barriers (e.g., glass or plastic screens) installed to limit close contact between registration desk personnel and potentially infectious patients. A facemask should be given to patients with respiratory symptoms as soon as possible, if they do not already have one. The number of accompanying family members in the waiting area should be limited.
Dedicated clinical staff (e.g., physicians or nurses) should be assigned for physical evaluation of patients presenting with respiratory symptoms at triage. These staff should be trained on triage procedures, COVID-19 case definition, and appropriate personal protective equipment (commonly referred to as PPE) use (e.g., mask, eye protection, gown, and gloves)[65]. All the hospital staff (healthcare workers, lab technician, cleaners) and visitors must protect themselves and others by correctly using the PPE and respecting standard precautions, which will include performing hand hygiene frequently (with an alcohol-based hand rub if your hands are not visibly dirty or with soap and water if hands are dirty), contact and droplet precautions, selection of PPE-based risk assessment (e.g., N95 respirators or powered, air-purifying respirators rather than a surgical mask, eye protection such as face shield or goggles, gowns, simple gloves or heavy-duty gloves, and boots or closed work-shoes), cleaning, disinfection and injection safety practices, and single-patient dedicated medical equipment (e.g., stethoscopes, blood pressure cuffs, and thermometers)[65-71].
The confirmed cases must be hospitalized, possibly in single, isolated rooms with negative air pressure as well as a dedicated bathroom and anteroom. If not possible, the confirmed cases must be, in any case, hospitalized in a single room with a dedicated bathroom and transferred as soon as possible to a safe structure. Confirmed COVID-19 patients may be hosted in the same room. If available, airborne infection isolation rooms should be used. COVID-19 patients are often very complex and require a multidisciplinary medical team which includes at least the following specialists: emergency doctor, pulmonologist, infectious disease specialist, critical care physician, and medical laboratory technician[72-74]. A first approach during triage is using the quick-SOFA (commonly referred to as the qSOFA) for a rapid identification of high-risk septic COVID-19 patients[51,52]. The American Disease CDC recommends some criteria priorities for testing patients with suspected COVID-19, as shown in Table 2.
Table 2.
Priorities for testing patients with suspected coronavirus disease-2019 infection
| Priority 1 | Ensure optimal care options for all hospitalized patients, lessen the risk of nosocomial infections, and maintain the integrity of the healthcare system: |
| Hospitalized patients | |
| Symptomatic healthcare workers | |
| Priority 2 | Ensure that those who are at highest risk of complication of infection are rapidly identified and appropriately triaged: |
| Patients in long-term care facilities with symptoms | |
| Patients 65 yr of age and older with symptoms | |
| Patients with underlying conditions with symptoms | |
| First responders with symptoms | |
| Priority 3 | As resources allow, test individuals in the surrounding community of rapidly increasing hospital cases to decrease community spread, and ensure health of essential workers: |
| Critical infrastructure workers with symptoms | |
| Individuals who do not meet any of the above categories with symptoms | |
| Healthcare workers and first responders | |
| Individuals with mild symptoms in communities experiencing high COVID-19 hospitalizations | |
| Non-priority | Individuals without symptoms |
Source: Center for Disease Control and Prevention. COVID-19: Coronavirus disease-2019.
For initial diagnostic testing for SARS-CoV-2, the CDC recommends collecting and testing an upper respiratory specimen (nasopharyngeal/oropharyngeal swab collected by a healthcare professional) for a rapid molecular in vitro diagnostic test, utilizing an isothermal nucleic acid amplification technology intended for the qualitative detection of nucleic acid from the SARS-CoV-2 viral RNA (real-time reverse transcriptase-polymerase chain reaction)[72,73]. Testing lower respiratory tract specimens is also an option. For patients who develop a productive cough, sputum should be collected and tested for SARS-CoV-2. The induction of sputum is not recommended. When under certain clinical circumstances (e.g., those receiving invasive mechanical ventilation), a lower respiratory tract aspirate or bronco-alveolar lavage sample should be collected and tested as a lower respiratory tract specimen[72,73]. Plasma cells take at least 5-10 d to develop antibodies against COVID-19. For this reason, serological tests are not sensitive enough to accurately diagnose a recent infection, even in symptomatic patients. Clinical recovery has been correlated with the detection of IgM and IgG antibodies, which signal the development of immunity. Actually, there are no data concerning the possibility of reinfection after recovery from COVID-19. Viral RNA shedding declines with resolution of symptoms and may continue for days to weeks. However, the detection of RNA during convalescence does not necessarily indicate the presence of viable infectious virus[71-73].
WHAT ARE THE NEW CHALLENGES IN COVID-19 TREATMENT?
Optimal management of COVID-19 is evolving quickly and treatment guidelines based on scientific evidence and experts’ opinions with clinical experience are updated frequently. Until now, there are no Food and Drug Administration (FDA)-approved drugs for COVID-19 and no vaccine is currently available (even if there are many experimental trials, such as the vaccine promoted by the United States’ biotech firm Moderna); hence, infected people primarily rely on symptomatic treatment and supportive care[74]. Meanwhile, an array of drugs approved for other indications as well as multiple investigational agents are being studied for the treatment of COVID-19 in several hundred clinical trials all over the world. Patients with severe infection are currently being treated with O2 therapy. Patients with viral pneumonia, hypoxemic respiratory failure/acute respiratory distress syndrome, sepsis and septic shock, cardiomyopathy and arrhythmia, and/or acute kidney injury often require non-invasive or mechanical ventilation and support in the ICU. Hemodynamic support is essential for managing septic shock. Hospitalization is also fundamental for the management of complications from prolonged hospitalization itself, including secondary bacterial infections, thromboembolism, gastrointestinal bleeding, and critical illness polyneuropathy/myopathy[74-77].
At present, the National Institutes of Health COVID-19 Treatment Guidelines do not recommend the use of any agents for pre-exposure prophylaxis and post-exposure prophylaxis against SARS-CoV-2 outside of the setting of a clinical trial, because no drug has actually been proven to be safe and effective for treating COVID-19[74-75]. Moreover, no specific treatment is also recommended for persons with suspected or confirmed asymptomatic or pre-symptomatic COVID-19 infection. To help reduce fever and diffuse aches related to COVID-19 infection, either acetaminophen or ibuprofen can be prudently used, without exceeding the recommended dose per day of 3000 mg[74,75]. Most of the recommendations for the management of severely and critically ill patients with COVID-19 are extrapolated from experience with other life-threatening infections and they do not deviate substantially from the management of other patients with severe diseases; although, special precautions in this infectious disease are required. These measures include high-flow nasal oxygen and non-invasive ventilation in non-severe forms of respiratory failure, while intubation and protective mechanical ventilation are required in severe forms. Prone position ventilation and extracorporeal membrane oxygenation have been used many times for very acute patients with refractory hypoxemia despite lung-protective ventilation[76]. Systemic corticosteroids and inappropriate administration of antibiotics are not recommended for the viral pneumonia’s treatment, although some centres recommend it but only in case of evidence of bacterial infection[52,53,69].
Although no antiviral treatments have been approved, several approaches have been proposed to limit viral reproduction, particularly drugs that have been used to treat malaria and autoimmune diseases and already used against past outbreaks, including those of SARS-CoV and MERS-CoV. These include antiviral drugs, such as lopinavir, ritonavir, nelfinavir, and remdesivir. The last one is an inhibitor of RNA polymerase with in vitro activity against multiple RNA viruses[77]. Alpha-interferon (e.g., 5 million units by aerosol inhalation twice per day), chloroquine (500 mg every 12 h), and hydroxychloroquine (200 mg every 12 h) are also used.
Chloroquine was introduced in clinical practice in 1946 to treat malaria, while hydroxychloroquine was introduced in 1955 and prescribed for the treatment of systemic lupus erythematosus[78]. The efficacy for systemic lupus erythematosus is based on the capability of this drug to inhibit Toll-like receptor signalling and to reduce cytokine production, especially that of IL-1 and IL-6. Starting from this consideration, hydroxychloroquine, and thereafter chloroquine, has been proposed as a helpful treatment for COVID-19 patients, in which some reports showed a direct antiviral effect in vitro due to an interference with ACE-2 receptors[79]. Chloroquine and hydroxychloroquine, which are not FDA approved for COVID-19, are available from the Strategic National Stockpile for hospitalized adults and adolescents (weighing ≥ 50 kg) under an Emergency Use Authorization[74,78,79].
There has been supposition (never proven) that azithromycin may help to reduce the overactive immune response to the SARS-CoV-2 infection that otherwise causes inflammatory damage. Unfortunately, the most recent human studies suggest no benefit and a strong statement was released, advising against the use of the combination of hydroxychloroquine and azithromycin, underlying the higher risk of death due to lethal heart arrhythmias with both hydroxychloroquine and azithromycin are used alone and especially when used in combination. In fact, it is well known that hydroxychloroquine and, moreover, chloroquine may have several side effects on the extrapyramidal, cardiovascular and digestive systems, which are more severe if associated with other medications (i.e. haemolysis with dapsone, severe hypoglycaemic effects with anti-diabetics, QT elongation, or torsades de pointes with ciprofloxacin and other antimicrobials, etc.)[80-82]. However, to date, this issue remains controversial[83].
Tocilizumab, a humanized IgG1 monoclonal antibody directed against the IL-6 receptor and commonly used in the treatment of rheumatoid arthritis, has already been used in a rhesus macaque model of MERS-CoV infection and is currently being used in experimentations against COVID-19. Two Chinese large randomized clinical trials, which enrolled over 700 patients and other studies which are also underway in European Countries and in the United States are likely to definitively answer the question of whether the drug is effective in treating COVID-19, and so it could be approved for use and produced in large amounts[84].
On 24 March, 2020, the FDA allowed convalescent plasma from recovered patients to be used in patients with serious or immediately life-threatening COVID-19 infections. These antibody-containing plasma, in many cases, showed the intended effect in fighting the illness, shortening the length or reducing the severity of the disease, but this treatment is still considered experimental and more randomized, controlled studies must be done to test its efficacy and safety. It has been estimated that herd immunity against COVID-19 (that is, an indirect protection given by recovered patients to those who are not immune to the disease) is around 50% to 66.66%[85].
Moreover, COVID-19 patients show a contemporary hypercoagulation and hypofibrinolytic state due to dysregulation of the coagulation and fibrinolytic systems, with elevated D-dimer and fibrinogen and deposition of fibrin in the air spaces and lung parenchyma caused by the activated tissue factor exposure on damaged alveolar endothelial cells and on the surface of leucocytes. The patients also show significantly elevated levels of plasminogen activator inhibitor 1 released from lung epithelium and endothelial cells. Prophylaxis treatment with low molecular weight heparin is considered important to limit COVID-19 patients’ coagulopathy, but, at the same time, it is fundamental to degrade pre-existing fibrin in the lung by promoting local fibrinolysis with tissue-type plasminogen activator as intravenous thrombolytic treatment. Its nebulizer form is currently in Phase II clinical trial and may provide a targeted approach in COVID-19 patients to degrade fibrin and improve oxygenation in critically ill patients[86].
New research fields are concerned with the use of immuno-enhancers (interferons, thymosin α-1, thymopentin, levamisole, cyclosporine A), vitamins (A, B, C, D, E), a-lipoic acids, minerals (selenium, zinc, iron), omega-6 polyunsaturated fatty acids, N-acetylcysteine and D-ribose-L-cysteine, probiotics, and the intravenous infusion of allogeneic expanded umbilical cord mesenchymal stem cells that show antiviral and antimicrobial properties, and which must be deepened. The Italian College of Anesthesia, Analgesia, Resuscitation and Intensive Care have reported guidelines to use these cells in COVID-19 patients, in the hope of decreasing the number of patients going to the ICU, and also getting them out of ICU relatively quickly[87,88]. Despite this, there are insufficient data to recommend either for or against the use of any antiviral or immunomodulatory therapy in patients with COVID-19 who have mild, moderate, severe, or critical illness[89]. Researchers are carrying out incessant efforts towards understanding these topics, including on translational regenerative approaches, such as mesenchymal stem cells[90,91].
CONCLUSION
The SARS-CoV-2 pandemic is a serious health problem and a challenge of global concern. During these months, we’ve had to learn, step-by-step, all about this novel coronavirus, ranging from its origins (which are still uncertain) to its mode of transmission, identifying people most at risk, and searching for old and new strategies which could help patients in fighting against this invisible enemy. Still, despite this, even more studies are needed to provide more effective preventive measures and treatment policies and to determine what is the proper social behaviour in public places and the rules of conduct for healthcare professionals’ management and resource planning; ultimately, the collective knowledge will lay a solid foundation for winning the battle against this epidemic.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interests.
Manuscript source: Invited manuscript
Peer-review started: May 15, 2020
First decision: June 4, 2020
Article in press: September 1, 2020
Specialty type: Infectious diseases
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ventura CA S-Editor: Liu M L-Editor: A P-Editor: Ma YJ
Contributor Information
Ioannis A Charitos, Department of Emergency and Urgency, National Poisoning Centre, Riuniti University Hospital of Foggia, Foggia 71122, Italy.
Andrea Ballini, Department of Biosciences, Biotechnologies and Biopharmaceutics, University Campus "E. Quagliariello", University of Bari “Aldo Moro”, Bari 70125, Italy. andrea.ballini@uniba.it; Department of Precision Medicine, University of Campania “Luigi Vanvitelli”, Naples 80138, Italy.
Lucrezia Bottalico, Interdepartmental Research Center for Pre-Latin, Latin and Oriental Rights and Culture Studies (CEDICLO), University of Bari, Bari 70121, Italy.
Stefania Cantore, Department of Interdisciplinary Medicine, University of Bari “Aldo Moro”, Bari 70124, Italy; Sorriso & Benessere - Ricerca e Clinica S.R.L, Bari 70129, Italy.
Pier Carmine Passarelli, Department of Head, Neck and Sense Organs, Division of Oral Surgery and Implantology, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, Rome 00168, Italy.
Francesco Inchingolo, Department of Interdisciplinary Medicine, Section of Dental Medicine, University of Bari “Aldo Moro”, Bari 70124, Italy.
Antonio D'Addona, Department of Head and Neck and Sensory Organs, Division of Oral Surgery and Implantology, Fondazione Policlinico Universitario A. Gemelli IRCCS — Università Cattolica del Sacro Cuore, Roma 00168, Italy.
Luigi Santacroce, Interdepartmental Research Center for Pre-Latin, Latin and Oriental Rights and Culture Studies (CEDICLO), University of Bari, Bari 70121, Italy; Ionian Department, Microbiology and Virology Laboratory, Policlinico University Hospital, University of Bari “Aldo Moro”, Bari 70124, Italy.
References
- 1.World Health Organization. Pneumonia of unknown cause — China. 2020 Jan 5 [Internet] Available from: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/ [Google Scholar]
- 2.World Health Organization. Novel coronavirus — China. 2020 Jan 12 [Internet] Available from: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/ [Google Scholar]
- 3.Santacroce L, Bottalico L, Charitos IA. The Impact of COVID-19 on Italy: A Lesson for the Future. Int J Occup Environ Med. 2020;11:151–152. doi: 10.34172/ijoem.2020.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Novel Coronavirus (2019-nCoV) Coronavirus disease 2019 (COVID-19) Situation Report–112. 2020 May 11 [Internet] Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200511-covid-19-sitrep-112.pdf?sfvrsn=813f2669_2. [Google Scholar]
- 6.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report –51. 2020 March 11 [Internet] Available from: https://apps.who.int/iris/bitstream/handle/10665/331475/nCoVsitrep11Mar2020-eng.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 7.International Committee on Taxonomy of Viruses. Naming the 2019 Coronavirus. 2020 March 15 [Internet] Available from: https://talk.ictvonline.org/information/w/news/1300/page. [Google Scholar]
- 8.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McIntosh K, Chao RK, Krause HE, Wasil R, Mocega HE, Mufson MA. Coronavirus infection in acute lower respiratory tract disease of infants. J Infect Dis. 1974;130:502–507. doi: 10.1093/infdis/130.5.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh K, Kapikian AZ, Turner HC, Hartley JW, Parrott RH, Chanock RM. Seroepidemiologic studies of coronavirus infection in adults and children. Am J Epidemiol. 1970;91:585–592. doi: 10.1093/oxfordjournals.aje.a121171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, Tamin A, Harcourt JL, Thornburg NJ, Gerber SI, Lloyd-Smith JO, de Wit E, Munster VJ. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Contini D, Costabile F. Does Air Pollution Influence COVID-19 Outbreaks? Atmosphere. 2020;11:377. [Google Scholar]
- 14.Wu X, Nethery RC, Sabath MB, Braun D, Dominici F. Exposure to air pollution and COVID-19 mortality in the United States: A nationwide cross-sectional study. medRxiv 2020.04.05.20054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setti L, Passarini F, De Gennaro G, Barbieri P, Pallavicini A, Ruscio M, Piscitelli P, Colao A, Miani A. Searching for SARS-COV-2 on Particulate Matter: A Possible Early Indicator of COVID-19 Epidemic Recurrence. Int J Environ Res Public Health. 2020;17:2986. doi: 10.3390/ijerph17092986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu J, Zhou B, Zhang L, Balaji KS, Wei C, Liu X, Chen H, Peng J, Fu J. Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19. Mol Biol Rep. 2020;47:4383–4392. doi: 10.1007/s11033-020-05478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Othman H, Bouslama Z, Brandenburg JT, da Rocha J, Hamdi Y, Ghedira K, Srairi-Abid N, Hazelhurst S. Interaction of the spike protein RBD from SARS-CoV-2 with ACE2: Similarity with SARS-CoV, hot-spot analysis and effect of the receptor polymorphism. Biochem Biophys Res Commun. 2020;527:702–708. doi: 10.1016/j.bbrc.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang T, Wu Q, Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr Biol. 2020;30:1578. doi: 10.1016/j.cub.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Coronavirus disease (COVID-19) situation report – 118 2020 May 17 [Internet] Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200517-covid-19-sitrep-118.pdf?sfvrsn=21c0dafe_10.
- 20.Santacroce L, Sardaro N, Topi S, Pettini F, Bottalico L, Cantore S, Cascella G, Del Prete R, Dipalma G, Inchingolo F. The pivotal role of oral microbiota in health and disease. J Biol Regul Homeost Agents. 2020;34:733–737. doi: 10.23812/20-127-L-45. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Estimating mortality from COVID-19. 2020 Aug 4 [Internet] Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1. [Google Scholar]
- 22.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A Novel Coronavirus Emerging in China - Key Questions for Impact Assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Estimated Influenza Illnesses, Medical visits, Hospitalizations, and Deaths in the United States-2018-2019 influenza season. 2020 Jan 8 [Internet] Available from: https://www.cdc.gov/flu/about/burden/2018-2019.html. [Google Scholar]
- 24.Rolfes MA, Foppa IM, Garg S, Flannery B, Brammer L, Singleton JA, Burns E, Jernigan D, Olsen SJ, Bresee J, Reed C. Annual estimates of the burden of seasonal influenza in the United States: A tool for strengthening influenza surveillance and preparedness. Influenza Other Respir Viruses. 2018;12:132–137. doi: 10.1111/irv.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epicentro - Istituto Superiore di Sanità. Characteristics of COVID-19 patients dying in Italy. 2020 July 22 [Internet] Available from: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-analysis-of-deaths. [Google Scholar]
- 26.Centers for Disease Control and Prevention. People Who Are at Higher Risk for Severe Illness. 2020 June 25 [Internet] Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-at-higher-risk.html. [Google Scholar]
- 27.Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with Coronavirus disease 2019 (COVID-19): A pooled analysis of published literature. Int J Stroke. 2020;15:385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 28.Italian government Presidency of the Council of Ministers. Coronavirus, le misure adottate dal Governo. 2020 July 31 [Internet] Available from: http://www.governo.it/it/coronavirus-misure-del-governo. [Google Scholar]
- 29.Italian Ministry of Health. FAQ - Covid-19, questions and answers. 2020 July 31 [Internet] Available from: http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioFaqNuovoCoronavirus.jsp?lingua=english&id=230#11. [Google Scholar]
- 30.World Health Organization. Advice on the use of masks in the community, during home care, and in health care settings in the context of COVID-19. Interim guidance, 19 March 2020. 2020 March 19 [Internet] Available from: https://apps.who.int/iris/bitstream/handle/10665/331493/WHO-2019-nCoV-IPC_Masks-2020.2-eng.pdf?sequence=14&isAllowed=y. [Google Scholar]
- 31.Meselson M. Droplets and Aerosols in the Transmission of SARS-CoV-2. N Engl J Med. 2020;382:2063. doi: 10.1056/NEJMc2009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y, Xie J, Huang F, Cao L. Association between short-term exposure to air pollution and COVID-19 infection: Evidence from China. Sci Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thakur M, Boudewijns EA, Babu GR, van Schayck OCP. Biomass use and COVID-19: A novel concern. Environ Res. 2020;186:109586. doi: 10.1016/j.envres.2020.109586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fattorini D, Regoli F. Role of the chronic air pollution levels in the Covid-19 outbreak risk in Italy. Environ Pollut. 2020;264:114732. doi: 10.1016/j.envpol.2020.114732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conticini E, Frediani B, Caro D. Can Atmospheric Pollution Be Considered a Co-Factor in Extremely High Level of SARS-CoV-2 Lethality in Northern Italy? Environ Pollut. 2020;261:114465. doi: 10.1016/j.envpol.2020.114465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domingo JL, Rovira J. Effects of air pollutants on the transmission and severity of respiratory viral infections. Environ Res. 2020;187:109650. doi: 10.1016/j.envres.2020.109650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020:e200994. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goh KJ, Choong MC, Cheong EH, Kalimuddin S, Duu Wen S, Phua GC, Chan KS, Haja Mohideen S. Rapid Progression to Acute Respiratory Distress Syndrome: Review of Current Understanding of Critical Illness from COVID-19 Infection. Ann Acad Med Singapore. 2020;49:108–118. [PubMed] [Google Scholar]
- 40.Lake MA. What we know so far: COVID-19 current clinical knowledge and research. Clin Med (Lond) 2020;20:124–127. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol. 2020;18:1663–1672. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li XY, Dai WJ, Wu SN, Yang XZ, Wang HG. The occurrence of diarrhea in COVID-19 patients. Clin Res Hepatol Gastroenterol. 2020;44:284–285. doi: 10.1016/j.clinre.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020;35:744–748. doi: 10.1111/jgh.15047. [DOI] [PubMed] [Google Scholar]
- 44.Cantore S, Ballini A. Coronavirus disease 2019 (COVID-19) Pandemic Burst and Its Relevant Consequences in Dental Practice. Open Dent J. 2020;14:111–112. [Google Scholar]
- 45.Passali GC, Bentivoglio AR. Comment to the article "Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the Coronavirus disease (COVID-19): a multicenter European study". Eur Arch Otorhinolaryngol. 2020;277:2391–2392. doi: 10.1007/s00405-020-06024-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marzano AV, Genovese G, Fabbrocini G, Pigatto P, Monfrecola G, Piraccini BM, Veraldi S, Rubegni P, Cusini M, Caputo V, Rongioletti F, Berti E, Calzavara-Pinton P. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: Multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83:280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, Ziv M, Leshem E, Dodiuk-Gad RP. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- 49.Guarneri C, Rullo EV, Pavone P, Berretta M, Ceccarelli M, Natale A, Nunnari G. Silent COVID-19: what your skin can reveal. Lancet Infect Dis. 2020:S1473–3099(20)30402-3. doi: 10.1016/S1473-3099(20)30402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Health Organization. Coronavirus disease (COVID-19) Situation Report– 122. 2020 May 21 [Internet] Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200521-covid-19-sitrep-122.pdf?sfvrsn=24f20e05_2. [Google Scholar]
- 51.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020:epub ahead of print. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 52.Wujtewicz M, Dylczyk-Sommer A, AszkieÅ‚owicz A, Zdanowski S, Piwowarczyk S, Owczuk R. COVID-19 - what should anaethesiologists and intensivists know about it? Anaesthesiol Intensive Ther. 2020;52:34–41. doi: 10.5114/ait.2020.93756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charitos IA, Topi S, Castellaneta F, D'Agostino D. Current Issues and Perspectives in Patients with Possible Sepsis at Emergency Departments. Antibiotics (Basel) 2019;8:56. doi: 10.3390/antibiotics8020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iba T, Nisio MD, Levy JH, Kitamura N, Thachil J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: a retrospective analysis of a nationwide survey. BMJ Open. 2017;7:e017046. doi: 10.1136/bmjopen-2017-017046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seymour CW, Kennedy JN, Wang S, Chang CH, Elliott CF, Xu Z, Berry S, Clermont G, Cooper G, Gomez H, Huang DT, Kellum JA, Mi Q, Opal SM, Talisa V, van der Poll T, Visweswaran S, Vodovotz Y, Weiss JC, Yealy DM, Yende S, Angus DC. Derivation, Validation, and Potential Treatment Implications of Novel Clinical Phenotypes for Sepsis. JAMA. 2019;321:2003–2017. doi: 10.1001/jama.2019.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D'Agostino D, Cappabianca G, Rotunno C, Castellaneta F, Quagliara T, Carrozzo A, Mastro F, Charitos IA, Beghi C. The Preoperative Inflammatory Status Affects the Clinical Outcome in Cardiac Surgery. Antibiotics (Basel) 2019;8:E176. doi: 10.3390/antibiotics8040176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matics TJ, Sanchez-Pinto LN. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr. 2017;171:e172352. doi: 10.1001/jamapediatrics.2017.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han H, Xie L, Liu R, Yang J, Liu F, Wu K, Chen L, Hou W, Feng Y, Zhu C. Analysis of heart injury laboratory parameters in 273 COVID-19 patients in one hospital in Wuhan, China. J Med Virol. 2020;92:819–823. doi: 10.1002/jmv.25809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Centers for Disease Control and Prevention. Approved respirator standards. 2006 Aug 21 [Internet] Available from: https://www.cdc.gov/niosh/npptl/standardsdev/cbrn/papr/default.html. [Google Scholar]
- 64.Liu R, Han H, Liu F, Lv Z, Wu K, Liu Y, Feng Y, Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Centers for Disease Control and Prevention. Standard Operating Procedure (SOP) for Triage of Suspected COVID-19 Patients in non-US Healthcare Settings: Early Identification and Prevention of Transmission during Triage. 2020 May 28 [Internet] Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/sop-triage-prevent-transmission.html. [Google Scholar]
- 66.World Health Organization. Water, sanitation, hygiene, and waste management for the COVID-19 virus: interim guidance. 2020, Apr. 2020 July 29 [Internet] Available from: https://www.who.int/publications-detail/water-sanitation-hygiene-and-waste-management-for-covid-19. [Google Scholar]
- 67.Centers for Disease Control and Prevention. Overview of Testing for SARS-CoV-2. 2020 July 2 [Internet] Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. [Google Scholar]
- 68.Zhdanov KV, Zakharenko SM, Kovalenko AN. [Delivery of healthcare to patients with infectious diseases during the pre-hospital stage] Voen Med Zh. 2013;334:39–43. [PubMed] [Google Scholar]
- 69.Rump A. [The prehospital use of antibiotics in military operations] Ann Fr Anesth Reanim. 2012;31:232–238. doi: 10.1016/j.annfar.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization. Rational use of personal protective equipment for Coronavirus disease 2019 (COVID-19). 2020 Feb. 27 [Internet] Available from: https://apps.who.int/iris/bitstream/handle/10665/331215/WHO-2019-nCov-IPCPPE_use-2020.1-eng.pdf. [Google Scholar]
- 71.Santacroce L. Letter in response to the article "Enhancing immunity in viral infections, with special emphasis on COVID-19: A review" (Jayawardena et al.) Diabetes Metab Syndr. 2020;14:927. doi: 10.1016/j.dsx.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19. 2020 May 22 [Internet] Available from: https://www.cdc.gov/coronavirus/2019-ncov/Lab/guidelines-clinical-specimens.html. [Google Scholar]
- 73.Pham VH, Gargiulo Isacco C, Nguyen KCD, Le SH, Tran DK, Nguyen QV, Pham HT, Aityan S, Pham ST, Cantore S, Inchingolo AM, Inchingolo AD, Dipalma G, Ballini A, Inchingolo F. Rapid and sensitive diagnostic procedure for multiple detection of pandemic Coronaviridae family members SARS-CoV-2, SARS-CoV, MERS-CoV and HCoV: a translational research and cooperation between the Phan Chau Trinh University in Vietnam and University of Bari "Aldo Moro" in Italy. Eur Rev Med Pharmacol Sci. 2020;24:7173–7191. doi: 10.26355/eurrev_202006_21713. [DOI] [PubMed] [Google Scholar]
- 74.National Institutes of Health. Management of Persons with COVID-19, in: COVID-19 Treatment Guidelines. 2020 June 11 [Internet] Available from: https://www.covid19treatmentguidelines.nih.gov/overview/management-of-covid-19/ [Google Scholar]
- 75.National Institutes of Health. Persons at Risk for Infection with SARS-CoV-2, in: COVID-19 Treatment Guidelines. 2020 July 17 [Internet] Available from: https://www.covid19treatmentguidelines.nih.gov/overview/prophylaxis/ [Google Scholar]
- 76.Zhan WQ, Li MD, Xu M, Lu YB. Successful treatment of COVID-19 using extracorporeal membrane oxygenation, a case report. Eur Rev Med Pharmacol Sci. 2020;24:3385–3389. doi: 10.26355/eurrev_202003_20705. [DOI] [PubMed] [Google Scholar]
- 77.Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 79.Cunningham AC, Goh HP, Koh D. Treatment of COVID-19: old tricks for new challenges. Crit Care. 2020;24:91. doi: 10.1186/s13054-020-2818-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Funck-Brentano C, Salem JE, Nguyen LS, Drici MD, Roden DM. Response to the editorial "COVID-19 in patients with cardiovascular diseases": Covid-19 treatment with hydroxychloroquine or chloroquine and azithromycin: A potential risk of Torsades de Pointes. Arch Cardiovasc Dis. 2020;113:367–368. doi: 10.1016/j.acvd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gérard A, Romani S, Fresse A, Viard D, Parassol N, Granvuillemin A, Chouchana L, Rocher F, Drici MD French Network of Pharmacovigilance Centers. "Off-label" use of hydroxychloroquine, azithromycin, lopinavir-ritonavir and chloroquine in COVID-19: A survey of cardiac adverse drug reactions by the French Network of Pharmacovigilance Centers. Therapie. 2020;May 7:S0040–5957(20)30091-3. doi: 10.1016/j.therap.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jankelson L, Karam G, Becker ML, Chinitz LA, Tsai MC. QT prolongation, torsades de pointes, and sudden death with short courses of chloroquine or hydroxychloroquine as used in COVID-19: A systematic review. Heart Rhythm. 2020;17:1472–1479. doi: 10.1016/j.hrthm.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mahase E. Covid-19: 146 researchers raise concerns over chloroquine study that halted WHO trial. BMJ. 2020;369:m2 197. doi: 10.1136/bmj.m2197. [DOI] [PubMed] [Google Scholar]
- 84.de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci USA. 2020;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Syal K. COVID-19: Herd immunity and convalescent plasma transfer therapy. J Med Virol. 2020:epub ahead of print. doi: 10.1002/jmv.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whyte CS, Morrow GB, Mitchell JL, Chowdary P, Mutch NJ. Fibrinolytic abnormalities in acute respiratory distress syndrome (ARDS) and versatility of thrombolytic drugs to treat COVID-19. J Thromb Haemost. 2020;18:1548–1555. doi: 10.1111/jth.14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res. 2018;52:751–762. doi: 10.1080/10715762.2018.1468564. [DOI] [PubMed] [Google Scholar]
- 88.Santacroce L, Charitos IA, Bottalico L. A successful history: probiotics and their potential as antimicrobials. Expert Rev Anti Infect Ther. 2019;17:635–645. doi: 10.1080/14787210.2019.1645597. [DOI] [PubMed] [Google Scholar]
- 89.Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ballini A, Scacco S, Coletti D, Pluchino S, Tatullo M. Mesenchymal Stem Cells as Promoters, Enhancers, and Playmakers of the Translational Regenerative Medicine. Stem Cells Int. 2017;2017:3292810. doi: 10.1155/2017/3292810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Atluri S, Manchikanti L, Hirsch JA. Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician. 2020;23:E71–E83. [PubMed] [Google Scholar]