Abstract
BACKGROUND
Group B Streptococcus (GBS) is a normal component of the gastrointestinal and genital microbiota in humans and can lead to important infections in newborns.
AIM
To compare GBS isolation and identification methods as well as to assess the antibiotic susceptibility and to identify resistance genes in GBS strains from pregnant women attended in healthcare services from the city of Vitória da Conquista, in Bahia State, Brazil.
METHODS
From January 2017 to February 2018, vaginorectal swabs were obtained from 186 participants and the samples were seeded onto chromogenic agar for GBS before and after inoculation in selective broth. Confirmatory identification using 3 CAMP and latex tests was performed in samples with GBS-suggestive colonies. Then, disk diffusion antibiograms were performed in GBS-positive samples, and the detection of the resistance genes ermB, ermTR, mefA, and linB in the clindamycin and/or erythromycin-resistant samples was carried out.
RESULTS
Thirty-two samples (17.2%) were GBS-positive. The culture in chromogenic agar after sample incubation in selective broth was the most sensitive method (96.9%) for GBS detection. All isolates were susceptible to penicillin, ampicillin, cefotaxime, and vancomycin. Clindamycin resistance was observed in 6 samples (18.8%), while 8 samples (25%) were erythromycin-resistant. All erythromycin and/or clindamycin-resistant GBS strains had negative D-tests. Two strains (25%) presented an M phenotype and 6 isolates (75%) presented a cMLSB phenotype. The ermB gene was identified in 4 samples (44.4%), the mefA gene was also found in 4 samples (44.4%), the ermTR gene was identified in 1 isolate (11.1%), and the linB gene was not found in any isolate.
CONCLUSION
This study evidenced that the screening for SGB can be performed by means of various methods, including chromogenic media, and that the chemoprophylaxis for pregnant women who cannot use penicillin must be susceptibility-guided.
Keywords: Streptococcus agalactiae, Pregnancy, Antimicrobial susceptibility
Core Tip: This study compares available methods for isolation and identification of Group B Streptococcus (GBS) in vaginorectal secretions from pregnant women and reveals the sensitivity profile of the isolated strains as well as evaluates the resistance genes related to the observed bacterial phenotypes. This study included 186 pregnant women and a 17.2% GBS prevalence was identified. Among isolates, 18.8% showed to be resistant to clindamycin while 25% was resistant to erythromycin. In the molecular analysis of the strains, the ermB, mefA, and ermTR were detected, and the linB was not identified.
INTRODUCTION
Streptococcus agalactiae (Group B Streptococcus GBS) is a bacterium that inhabits the gastrointestinal and genital tracts as a component of the normal human microbiota. Its clinical relevance is mainly due to infection in neonates. However, a growing number of GBS infections among non-pregnant adults has been reported[1,2]. The presence of GBS colonization in pregnant women during childbirth represents the main risk for the development of infections (pneumonia, meningitis, and/or sepsis) in the newborn through vertical transmission from the colonized mother[3,4].
The estimated prevalence of GBS colonization in pregnant women is 10%-35% in various countries, including Brazil[1-3]. In a recent systematic review, the average estimated prevalence was 20%, with the highest prevalence being reported in African countries. Nonetheless, there was a significant variation in the prevalence of maternal colonization that may be associated with factors that need to be further studied[5].
GBS vertical transmission occurs in approximately half of the newborns from colonized mothers, and, when antibiotic prophylaxis is not instituted, it is estimated that 1%-4% of neonates develop an early or a late GBS infection[6,7]. The prevention of GBS infection includes identifying the colonized pregnant women by means of vaginorectal swab culture between 35th and 37th gestational weeks[3,8,9].
The vaginal/rectal secretions are inoculated in an antibiotic-supplemented, enriched, selective, Todd Hewitt broth and, then, cultivated in a 5% sheep blood agar[3,10]. Other GBS isolation and identification methods include CAMP test, serogrouping, chromogenic media, and molecular tests[3]. Each of them presents different sensitivity and specificity levels for GBS detection as well as distinct costs.
Penicillin is the drug of choice for prevention and treatment of GBS infection, and no bacterial resistance to that drug has been reported in this scenario[11,12]. However, reduced penicillin susceptibility associated with penicillin-binding protein mutation was observed in some cases[13-15]. Moreover, using erythromycin and clindamycin is indicated for penicillin-allergic pregnant women and, if bacterial resistance to these drugs occurs or there is a lack of information, vancomycin is chosen[12]. In the last decades, a growing prevalence of erythromycin and clindamycin-resistant GBS strains has been observed (25%-32% and 13%-20%, respectively)[3,16-21]. Such resistance is associated with the acquirement and expression of the erm genes, resulting in a MLSB phenotype that is related to the occurrence of erythromycin and clindamycin resistance. Furthermore, mef and msrA genes confer erythromycin resistance and clindamycin susceptibility, characterizing an M phenotype (resistance to macrolides only). In its turn, linB gene, which is only linked to the clindamycin resistance, leads to an L phenotype (resistance to lincosamides only)[21,22]. Moreover, the transfer of these genes between bacteria occurs by means of plasmids or transposons[12,16,18].
The clinical and epidemiological impacts of the GBS neonatal infections are significant and there is a tendency to an antimicrobial resistance rising that has already been shown in other Brazilian studies[23-28]. Expanding the investigation of the antimicrobial resistance profile in more Brazilian cities can contribute for the better characterization of the prevalence and the susceptibility profile of the GBS as well as for the development of suitable guidelines in both national and regional scenarios.
Even though the local data do not directly influence the antibiotics choice since the international standards are taken into account for prevention and treatment of the GBS infection, such information may justify the implementation of GBS screening protocols in order to replace the risk-based chemoprophylaxis already adopted in some countries. That measure would reduce not only the local infant mortality, but also the costs related to the prolonged hospitalizations of GBS-infected newborns.
This study aimed to compare identification methods, to verify the susceptibility profile, and to determine the resistance genes of the GBS strains isolated from pregnant women in prenatal care in the basic health units from the county of Vitória da Conquista, in Bahia State, Brazil.
MATERIALS AND METHODS
Study design, period, region, and population
This cross-sectional study analyzed 186 vaginal and rectal secretion samples from pregnant women attended in nine Family Basic Health Unit of the county of Vitória da Conquista, in Bahia State, Brazil. The sampling was based on 5170 child-births occurred in the above-mentioned county in the year 2015 and on the GBS colonization prevalence (17%) described by the pilot study conducted by Oliveira et al[29], with a 5% margin of error and a 95% confidence level. The sample collection was performed from January 2017 to February 2018.
Inclusion and exclusion criteria
Pregnant women with a 32-to-40 gestational age who lives in the urban area of the county were eligible, but the participants were excluded if they had used antibiotics in the last 7 d before collection.
Samples collection
The biologic samples were collected by means of the use of single vaginorectal swabs without using speculum. The material was collected from the lower middle third region of the vagina and from perianal region, mandatorily. The samples were packaged in Stuart transport media and carried in specific boxes to the Clinical Analysis Laboratory of the Multidisciplinary institute of Health of the Federal University of Bahia within 8 h after the collection to perform the experiments.
Bacterial isolation and identification
The samples were seeded by depletion technique onto Streptococcus chromIDTMStreptoB chromogenic medium, inoculated in Todd Hewitt broth (BIOMERIÉUX), and placed at a 35°C-37°C bacteriological incubator for 24 h. After, the samples were subcultured in chromogenic agar and kept in the incubator under the same above-mentioned conditions for 24 h.
All pink or red colonies (characteristic for GBS identification) underwent legitimized identification tests by means of CAMP test using the kit composed by Todd Hewitt agar and Hemolisinabac (PROBAC) and serogrouping using the SLIDEX®Strepto Plus B kit (BIOMERIÉUX) for species confirmation.
All serogrouping-confirmed isolates (gold standard method for GBS identification) were forwarded for antibiogram and an aliquot of each of them was cryopreserved at -20ºC in a Brain Heart Infusion (BHI) broth with 15% glycerol.
Antimicrobial susceptibility testing
All serogrouping-confirmed specimens underwent the disk diffusion antimicrobial susceptibility test (Kirby e Bauer) as recommended by the Clinical and Laboratory Standards Institute[30]. The tested antibiotics were penicillin (10 units), ampicillin (10 µg), cefotaxime (30 µg), erythromycin (15 µg), clindamycin (2 µg), and vancomycin (30 µg). They were chosen according the Clinical and Laboratory Standards Institute[30] and considering the drugs availability in the public health network of the studied county.
Determination of erythromycin resistance phenotypes
A D-test was performed for each erythromycin and/or claritromycin-resistant sample. Comercial Erythromycin (15 µg) and Clindamycin (2 µg) discs were placed 12 mm apart on Mueller Hinton agar plates supplemented with 5% blood and kept at a 35-37ºC, 5% CO2, incubator for 24 h with subsequent observation of the bacterial growth inhibition halo. The clindamycin and erythromycin resistances were considered indicators of constitutive MLSB phenotype (cMLSB). The reduction of the bacterial growth inhibition halo around the clindamycin disc in the region close to the erythromycin disc defined the inductive MLSB phenotype (iMLSB). Isolated sensitivity to clindamycin indicated the M phenotype and the sensitivity to erythromycin alone defined the L phenotype[21,22].
Determination of the resistance genetic profile
The presence of resistance genes in erythromycin and/or clindamycin resistant strains according to the phenotypic test was assessed by multiplex Polymerase Chain Reaction (PCR) technique.
The evaluated resistance genes were ermB, ermTR, and mefA, for erythromycin, and linB for clindamycin, using specific primers (Table 1). The primers were synthesized and the reaction conditions were performed according described by Bolukaoto et al[21]. The bacterial DNA for the PCR performing was extracted by boiling method[31].
Table 1.
Comparison between the isolation and identification methods used in vaginorectal secretions from pregnant women (n = 186)
|
Method |
||||
| CRO1, n (%) | CRO2, n (%) | CAMP, n (%) | SERO, n (%) | |
| Positive | 26 (14.0) | 32 (17.2) | 32 (17.2) | 32 (17.2) |
| Negative | 160 (86.0) | 154 (82.8) | 154 (82.8) | 154 (82.8) |
CRO1: Biologic samples directly seeded in chromogenic media; CRO2: Biologic samples seeded in chromogenic media after growth in Todd Hewitt broth; CAMP: Christie, Atkins, and Munch-Petersen test; SERO: Serogrouping.
The PCR results were analyzed according the presence or absence of bands in 2% agarose gel in 1x TAE buffer solution, stained with ethidium bromide and visualized on UV transilluminator after electrophoretic run. One Streptococcus agalactiae reference strain was used as negative control.
Statistical analysis
The research database was made in Microsoft Office Excel 2007 spreadsheets and the descriptive statistical tests were done with the EPI-INFO 3.5.1 version statistical package. In order to perform frequency comparisons, chi-square tests were performed with a 5% significance level.
Aiming the evaluation of the microbial identification tests, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated, considering serogrouping as the gold standard[32].
Ethical considerations
The study was approved by the Ethical Committee of Research in Human Beings of the Multidisciplinary institute of Health - Campus Anísio Teixeira from the Federal University of Bahia (IMS-CAT/UFBA) under the protocol number 58104116. 8.000.5556.
RESULTS
From the 186 analyzed samples, 32 presented GBS strains according serogrouping, what represents a 17.2% colonization prevalence among the studied pregnant women.
Using the chromogenic medium (CRO1) without previously passing the sample through the Todd Hewitt medium, 26 samples were positive for GBS colonies and one of them was not confirmed by serogrouping or CAMP test. When a previous sample passage through the Todd Hewitt medium was performed (CRO2), 32 samples were GBS-positive and one of them was not confirmed by serogrouping.
Regarding the cross analysis between CRO1 and CRO2, one sample was GBS-positive only in CRO1, 24 were positive in both, and 7 were positive only in CRO2. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated by means of the analysis of the CRO1, CRO2 and serogrouping results (CRO1 X CRO2/CRO1 X serogrouping/CRO2 X serogrouping). The GBS detection through CRO2 was the method that presented the best performance, with sensitivity (96.9%), PPV (96.9%), and NPV (99.3%) very close to those observed in serogrouping.
All the samples that were identified as positive for SBG after serogrouping underwent the antibiogram test. The susceptibility profile showed that all the GBS strains were susceptible to penicillin, ampicillin, cefotaxime, and vancomycin. Six (18.8%) GBS-positive strains were resistant to clindamycin and eight (25.0%) were resistant to erythromycin.
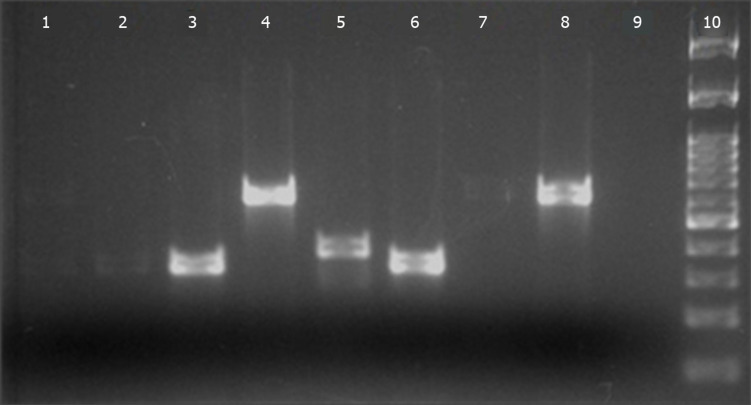
The erythromycin and/or clindamycin-resistant isolates (8) presented negative D-tests. Only 2 strains (25%) presented the M phenotype and 6 isolates (75%) presented the cMLSB phenotype. The Figure 1 shows the results of the 2% agarose gel electrophoresis performed from the GBS isolates that presented resistance to erythromycin and/or clindamycin in the phenotypic test, aiming genotypic evaluation by PCR.
Figure 1.
Electrophoresis in 2% agarose gel for erythromycin and clindamycin-resistant GBS isolates. Lines 1, 2, 3, and 6 (samples 32, 79, 98, and 174, respectively): mefA gene presence (348 pb); Line 5 (sample 136): ermTR gene presence (400 pb); Lines 1, 4, 7, and 8 (samples 32, 128, 177, and 209, respectively): ermB gene presence (640 pb); Line 9: Negative control; Line 10: Molecular weight marker (100 kb).
In 4 isolates (44.4%), the resistance was associated with ermB genes. The mefA gene was present in 4 isolates (44.4%) and the ermTR gene was detected in only one isolate (11.1%). In 7 isolates (87.5%), it was detected only one resistance gene (ermB, ermTR, or mefA). In one sample (12.5%), the presence of two resistance genes was verified (mefA + ermB). No strain carried the linB gene.
In 4 isolates (44.4%), the resistance was associated with ermB genes. Similarly, mefA gene was observed in 4 isolates (44.4%), while the ermTR gene was found in a single isolate (11.1%). The detection of a single resistance gene (ermB or ermTR or mefA) occurred in 7 isolates (87.5%). In a sample (12.5%), it was observed the presence of two resistance genes (mefA and ermB). None of the strains had linB gene.
DISCUSSION
The GBS colonization prevalence of 17.2% found in this study is similar with those described by Oliveira et al[29] and Borger et al[33]. That percentage is also befitting with the data from the Center for Disease Control and Prevention (CDC)[3] that point towards a colonization prevalence from 10% to 30%. Moreover, the prevalence described in this study corroborates to the data observed in Taiwan[4] (21.8%), Italy[16] (25.5%), Ethiopia[17] (19.0%), and Pakistan[34] (17.0%). A recent systematic review estimated a world maternal colonization by GBS of 18% with regional variations from 11% to 35%[35].
Unfortunately, GBS screening of the pregnant women during prenatal evaluation is not performed in the studied county. In that context, the significant GBS prevalence observed in this study may contribute to the development of invasive infections by GBS in newborns, since CDC estimates that it occurs in about 1%-2% of newborns from colonized women[3]. These data justify the adoption of a screening protocol for GBS by the health system from that county.
According CDC recommendations, the GBS isolation and identification of GBS biologic samples should be performed in selective enrichment broths such as Todd Hewitt broth, followed by microbial cultivation in 5% sheep blood agar, presumptive identification by CAMP test, and confirmatory identification by serogrouping[3]. Such recommendations are based on the assumption that the direct seeding of the vaginorectal sample onto any culture medium, without a previous passage in a selective enrichment broth, increases the chance of false-negative results. In addition, although the use of chromogenic media for GBS identification is mentioned by CDC, such an organization highlights that the available media were not able to identify non-hemolytic strains, which represent up to 4% of the GBS strains[3]. Furthermore, it was demonstrated that non-hemolytic strains are associated with severe infections in newborns[36,37]. However, the last CDC guidelines for the isolation and identification of GBS date back from 2010. Since then, chromogenic media that are able to detect hemolytic and non-hemolytic strains have emerged, including the ChromIDStrepto B (BIOMÉRIEUX) medium, which was used in this study.
Some studies had already demonstrated that the direct inoculation of vaginorectal samples onto chromogenic culture media is as sensitive as the method recommended by CDC[36-40]. In our study, it was observed an important sensitivity difference between the GBS isolation and identification using direct seeding in chromogenic medium (78.1%) when compared to when that procedure is preceded by sample inoculation in selective media for GBS (96.9%). Regarding specificity, there was no difference, being that 96.3% for each of these methods.
In this study, 32 samples were isolated/identified through CRO2 and, among them, only 1 was not confirmed by serogrouping. These data reveal that CRO2 presented a 96.9% sensitivity and a 99.3% specificity. Moreover, seven samples that were positive in serogrouping were not isolated/identified by CRO1, but only by CRO2. It shows that, besides a high sensitivity (96.9%), the high negative predictive value (99.3%) presented by CRO2 makes it stand out in this study as the best method for GBS colonization screening, since the minimization of false-negative results, which potentially lead to an increased risk of newborn infection by GBS, is one or the most important requirements for a screening protocol.
A previous research reported a 100% sensitivity for GBS detection through chromIDTMStrepto B (BIOMERIÉUX) media and a 79% sensitivity when blood agar was used, even after the enrichment in selective media[39]. In our study, when the isolation/identification of GBS colonies in chromogenic media after the passage in Todd Hewitt broth is compared with the serogrouping results, a 96.9% agreement is observed. Although the chromogenic agar media manufacturers and CDC recommend the confirmation of the detection of GBS-suggestive colonies in chromogenic media by means of biochemical or immunological tests, the findings from this study as well as results obtained by previous researches, such as the one carried out by Morita et al[39], suggest that the chromogenic media could be used without performing confirmatory tests. Furthermore, such method is considered an easy and fast diagnostic option that does not demand further technological sources, reducing test-related costs. Nowadays, besides the high detection rates, the chromogenic media provide other advantages such as the easy visualization of the colonies by means of colors, the detection of hemolytic and non-hemolytic strains, and the incubation in anaerobic environment[39].
In our investigation, the CAMP test showed 100% sensitivity in relation to serogrouping. However, that test should not be used as a confirmatory method for GBS identification, but only as a presumptive method, since the CAMP factor is not only found in GBS but also in other bacteria such as some streptococci of serogroups A, C, F, and G as well as Listeria monocytogenesstrains[41]. In addition, our study revealed that CAMP test should be performed in an extremely thorough way, using a standard Staphylococcus aureus strain that has not been frozen for a long time, and the blood agar plates should be obtained commercially in order to reach a high accuracy. The use of commercial kits also does not seem to be advantageous because they present a short shelf life and, after the opening of the kit label, the hemolysin strips dry out quickly.
Besides the comparative study of the diagnostic methods, we evaluated the susceptibility profile of the GBS strains that are circulating in the county where this study was carried out. Such investigation is crucial to institute proper antimicrobial prophylaxis and to reduce the incidence of neonatal group B streptococcal disease.
The erythromycin (25%) and clindamycin (18.8%) resistance rates found in this study were lower than in some previous Brazilian studies but higher than in others such as those carried out by Melo et al[12], Dutra et al[25] and Borger et al[33]. The resistance rates for erythromycin and clindamycin in these studies were, respectively, 8.1% and 5.9% in the first, 4.1% and 3.0% in the second, and 9.4% and 6.2% in the third. When compared to the erythromycin and clindamycin resistance prevalence found by studies carried out in other countries, the prevalence showed by our results is also higher. In South Africa, Bolukaoto et al[21] found that erythromycin and clarithromycin resistance rates were 21.1% and 17.2%, respectively, while Mengist et al[17], in Ethiopia, found resistance rates of 6.5% for erythromycin and of 3.2% for clindamycin. Moreover, Frohlicher et al[20] observed resistance prevalence of 14.5% for erythromycin and of 8.2% for clindamycin in Switzerland, and Pinheiro et al[18] found resistance rates of 15% and 9.6% for erythromycin and clindamycin, respectively. These data suggest that the use of erythromycin and clindamycin as an alternative for penicillin-allergic pregnant women with high anaphylaxis risk should be accompanied by susceptibility tests for the choice of the proper therapy for each case. However, it is important to highlight that the cases of penicillin allergy are not frequent.
The rates of phenotypic and genotypic resistances found in this study influence the clinical management of the patients regarding the choice of the therapeutic scheme. Our results confirm that GBS isolates from the study population present macrolides resistance genes and, considering that these genes can be transferred from one bacterium to another, the monitoring of the GBS antimicrobial resistance should be performed regularly in penicillin-allergic patients. The high prevalence of antimicrobial-resistant GBS strains limit the therapeutic options for the antimicrobial prophylaxis and, increasingly, the use of broad-spectrum antibiotics have been necessary, which leads to a selective pressure over other microorganisms, contributing to the antimicrobial resistance growth[3,12,16,18,19,42].
Although most studies show that the GBS susceptibility to penicillin is uniform, its reduction had already been reported by some authors[14,15,43]. In this study, we did not observe any GBS isolate that presented resistance or even a reduced sensitivity to penicillin.
The D-test results were negative in 100% of the isolated GBS strains that showed to be resistant to erythromycin. In other words, none of those strains presented an erythromycin-induced reduction of sensitivity to clindamycin. The resistance phenotypes observed in our study were cMLSB (75%) and M (25%). Similar studies carried out in Brazil by Dutra et al[25] and in Portugal by Pinheiro et al[18] also observed the iMLSB phenotype, besides cMLSB and M phenotypes. In its turn, the study by Bolukaoto et al[21] found four resistance phenotypes (cMLSB, iMLSB, M, and L).
The mefA gene was detected by means of molecular test in the two samples that presented the M phenotype, corroborating with the data from the available literature, which indicate that the mefA gene confer resistance only to macrolides[21]. Moreover, 5 samples presented the cMLSB phenotype had only the ermB gene or the ermTR gene, also being examples of concordance between the phenotypic and genotypic tests, since it is known that genes from erm family are associated with bacterial resistance to macrolides, lincosamides, and streptogramins B[21]. The mefA and ermB genes were detected in one isolate that presented a cMLSB phenotype, suggesting the participation of two different genes in the promotion of the antimicrobial resistance. The finding of a cMLSB phenotype sample whose genotypic test only detected the mefA gene, which confers resistance only to macrolides, show that the clindamycin resistance could be related to another gene that was not tested. The study by Dutra et al[25], which identified resistance genes of GBS strains in different Brazilian regions did not detect the mefA gene, contrasting with our results.
CONCLUSION
This is the first study that compared the identification methods and evaluated the antimicrobial susceptibility profile of GBS strains in Vitória da Conquista, providing valuable information to the development of public health policies, including the elaboration of screening and antibiotic prophylaxis protocols against that microorganism. Subsequently, it would be possible to minimize the GBS transmission to newborns during the deliveries, which may contribute not only to the decrease of the child mortality but also to the reduction of the costs with prolonged hospitalizations of infected newborns.
ARTICLE HIGHLIGHTS
Research background
Streptococcus agalactiae (Group B Streptococcus, SGB) is a bacterium that inhabits the gastrointestinal and genital tracts of men and women as a component of their normal microbiota. The colonization of pregnant women by GBS in childbirth represents the main risk factor for the development of infections (pneumonia, meningitis, and sepsis) in newborns through a vertical transmission that occurs in approximately a half of the newborns from colonized mothers and, when antibiotic prophylaxis is not performed, about 1%-4% of the neonates develops an early or a late GBS infection.
Research motivation
The great importance of Group B streptococcal disease for clinical practice and health systems demands the expansion of the knowledge on GBS colonization detection methods and eradication regimens in order to aid in the development of health policies against that infection.
Research objectives
To compare the identification methods, to verify the sensitivity profile, and to determine resistance genes in the GBS strains isolated from pregnant women in prenatal care in basic health units from the county of Vitória da Conquista, in Bahia State, Brazil.
Research methods
This is a quantitative transversal study that analyzed 186 samples of vaginal and rectal secretions from pregnant women attended in nine basic health units from the county of Vitória da Conquista, in Bahia State, Brazil. Pregnant women with gestational ages from 32 to 40 wk were eligible for this study. The biologic samples were collected with a single vaginorectal swab without speculum use, seeded by depletion onto Streptococcus chromIDTM Strepto B chromogenic media, inoculated in Todd Hewitt broth (BIOMERIÉUX), and placed at a bacteriological incubator under a 35ºC-37ºC temperature during 24 h. Subsequently, the Todd Hewitt broth samples were subcultured in chromogenic agar plates and kept in the incubator for other 24 h in the above-mentioned conditions.
All pink or red colonies (characteristics for GBS identification) underwent legitimate identification tests: CAMP test using the KIT composed by Todd Hewitt agar and Hemolisinabac (PROBAC DO BRASIL) and serogrouping using the SLIDEX®Strepto Plus B KIT (BIOMERIÉUX) for species confirmation.
All serogrouping-confirmed isolates (gold standard method for GBS identification) were forwarded for antibiogram performing by means of the disk-diffusion technique (Kirby and Bauer), following the Clinical and Laboratory Standards Institute recommendations, and an aliquot was preserved at -20ºC in a 15% glycerol brain-heart infusion broth for resistance genes investigation in erythromycin- and/or clindamycin-resistant strains through multiplex Polymerase Reaction Chain.
Research results
One hundred and eighty-six samples were analyzed by this study, and GBS strains were identified in 32 of them, representing a 17.2% prevalence among the included pregnant women. The GBS detection after subculturing in Todd Hewitt broth presented the higher sensitivity (96.9%). In six GBS isolates (18.8%), it was verified a clindamycin resistance, and eight isolates (25.0%) presented erythromycin resistance. The resistance was associated with ermB genes in 4 isolates. The mefA gene was detected in 4 strains and the ermTR gene was found in a single strain. In seven isolates, it was detected only one resistance gene (ermB or ermTR or mefA). One strain had two resistance genes (mefA+ermB).
Research conclusions
For the first time, we compared identification methods and analyzed the antimicrobial susceptibility profile of GBS strains in the above-mentioned county. The data from this study may be useful in the development of future screening and antibiotic prophylaxis protocols aiming the prevention of GBS neonatal infection.
Research perspectives
The information provided by this study is applicable for the elaboration of GBS colonization screening and eradication strategies. Therefore, this study potentially contributed to the reduction of children morbidity and mortality as well as to the decrease of health system costs with the hospitalization of neonates.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Ethical Committee of Research in Human Beings of the Multidisciplinary institute of Health-Campus Anísio Teixeira from the Federal University of Bahia (IMS-CAT/UFBA) under the protocol number 58104116.8.000.5556.
Informed consent statement: All study participants provided written consent prior to study enrollment.
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other coauthors contributed their efforts in this manuscript.
Manuscript source: Unsolicited manuscript
Peer-review started: April 24, 2020
First decision: April 29, 2020
Article in press: August 22, 2020
Specialty type: Infectious diseases
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kassir R S-Editor: Wang DM L-Editor: A P-Editor: Liu JH
Contributor Information
Fabrícia Almeida Fernandes Santana, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil.
Tais Viana Ledo de Oliveira, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil.
Marcelo Barreto de Souza Filho, Laboratório Oliveira Ltda, LABO, Vitória da Conquista 45020740, Bahia, Brazil.
Lucas Santana Coelho da Silva, Campus Soane Nazaré de Andrade, Universidade Estadual de Santa Cruz, Ilhéus 45662900, Bahia, Brazil.
Breno Bittencourt de Brito, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil.
Fabrício Freire de Melo, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil. freiremelo@yahoo.com.br.
Cláudio Lima Souza, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil.
Lucas Miranda Marques, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil; Campus Soane Nazaré de Andrade, Universidade Estadual de Santa Cruz, Ilhéus 45662900, Bahia, Brazil.
Márcio Vasconcelos Oliveira, Instituto Multidisciplinar em Saúde, Universidade Federal da Bahia, Vitória da Conquista 45029-094, Bahia, Brazil.
Data sharing statement
There is no additional data available.
References
- 1.Honig E, Mouton JW, van der Meijden WI. The epidemiology of vaginal colonisation with group B streptococci in a sexually transmitted disease clinic. Eur J Obstet Gynecol Reprod Biol. 2002;105:177–180. doi: 10.1016/s0301-2115(02)00162-8. [DOI] [PubMed] [Google Scholar]
- 2.Meehan M, Cafferkey M, Corcoran S, Foran A, Hapnes N, LeBlanc D, McGuinness C, Nusgen U, O'Sullivan N, Cunney R, Drew R. Real-time polymerase chain reaction and culture in the diagnosis of invasive group B streptococcal disease in infants: a retrospective study. Eur J Clin Microbiol Infect Dis. 2015;34:2413–2420. doi: 10.1007/s10096-015-2496-5. [DOI] [PubMed] [Google Scholar]
- 3.Verani JR, McGee L, Schrag SJ Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention (CDC) Prevention of perinatal group B streptococcal disease--revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 4.Lee WT, Lai MC. High prevalence of Streptococcus agalactiae from vaginas of women in Taiwan and its mechanisms of macrolide and quinolone resistance. J Microbiol Immunol Infect. 2015;48:510–516. doi: 10.1016/j.jmii.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Kwatra G, Cunnington MC, Merrall E, Adrian PV, Ip M, Klugman KP, Tam WH, Madhi SA. Prevalence of maternal colonisation with group B streptococcus: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1076–1084. doi: 10.1016/S1473-3099(16)30055-X. [DOI] [PubMed] [Google Scholar]
- 6.Reinheimer C, Kempf VA, Wittekindt BE, Allendorf A, Wichelhaus TA, Hogardt M, Schlößer RL, Fischer D. Group B streptococcus infections in neonates admitted to a German NICU: Emphasis on screening and adherence to pre-analytical recommendations. Early Hum Dev. 2016;103:37–41. doi: 10.1016/j.earlhumdev.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Le Doare K, Heath PT. An overview of global GBS epidemiology. Vaccine. 2013;31 Suppl 4:D7–12. doi: 10.1016/j.vaccine.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Committee on Infectious Diseases; Committee on Fetus and Newborn, Baker CJ, Byington CL, Polin RA. Policy statement—Recommendations for the prevention of perinatal group B streptococcal (GBS) disease. Pediatrics. 2011;128:611–616. doi: 10.1542/peds.2011-1466. [DOI] [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists Committee on Obstetric Practice. ACOG Committee Opinion No. 485: Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2011;117:1019–1027. doi: 10.1097/AOG.0b013e318219229b. [DOI] [PubMed] [Google Scholar]
- 10.El Beitune P, Duarte G, Maffei CM. Colonization by Streptococcus agalactiae during pregnancy: maternal and perinatal prognosis. Braz J Infect Dis. 2005;9:276–282. doi: 10.1590/s1413-86702005000400002. [DOI] [PubMed] [Google Scholar]
- 11.Money D, Allen VM, Infectious Diseases Committee. The prevention of early-onset neonatal group B streptococcal disease. J Obstet Gynaecol Can. 2013;35:939–948. doi: 10.1016/S1701-2163(15)30818-5. [DOI] [PubMed] [Google Scholar]
- 12.Melo SC, Santos NC, Oliveira M, Scodro RB, Cardoso RF, Pádua RA, Silva FT, Costa AB, Carvalho MD, Pelloso SM. ANTIMICROBIAL SUSCEPTIBILITY OF Streptococcus agalactiae ISOLATED FROM PREGNANT WOMEN. Rev Inst Med Trop Sao Paulo. 2016;58:83. doi: 10.1590/S1678-9946201658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banno H, Kimura K, Tanaka Y, Kitanaka H, Jin W, Wachino J, Yamada K, Shibayama K, Arakawa Y. Characterization of multidrug-resistant group B streptococci with reduced penicillin susceptibility forming small non-Beta-hemolytic colonies on sheep blood agar plates. J Clin Microbiol. 2014;52:2169–2171. doi: 10.1128/JCM.00226-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura K, Suzuki S, Wachino J, Kurokawa H, Yamane K, Shibata N, Nagano N, Kato H, Shibayama K, Arakawa Y. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008;52:2890–2897. doi: 10.1128/AAC.00185-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper K, Abbott F, Gould IM. Reduced penicillin susceptibility of group B Streptococcus: an assessment of emergence in Grampian, Scotland. Br J Biomed Sci. 2016;73:25–27. doi: 10.1080/09674845.2016.1144550. [DOI] [PubMed] [Google Scholar]
- 16.Matani C, Trezzi M, Matteini A, Catalani C, Messeri D, Catalani C. Streptococcus agalactiae: prevalence of antimicrobial resistance in vaginal and rectal swabs in Italian pregnant women. Infez Med. 2016;24:217–221. [PubMed] [Google Scholar]
- 17.Mengist A, Kannan H, Abdissa A. Prevalence and antimicrobial susceptibility pattern of anorectal and vaginal group B Streptococci isolates among pregnant women in Jimma, Ethiopia. BMC Res Notes. 2016;9:351. doi: 10.1186/s13104-016-2158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinheiro S, Radhouani H, Coelho C, Gonçalves A, Carvalho E, Carvalho JA, Ruiz-Larrea F, Torres C, Igrejas G, Poeta P. Prevalence and mechanisms of erythromycin resistance in Streptococcus agalactiae from healthy pregnant women. Microb Drug Resist. 2009;15:121–124. doi: 10.1089/mdr.2009.0895. [DOI] [PubMed] [Google Scholar]
- 19.Mousavi SM, Nasaj M, Hosseini SM, Arabestani MR. Survey of strain distribution and antibiotic resistance pattern of group B streptococci (Streptococcus agalactiae) isolated from clinical specimens. GMS Hyg Infect Control. 2016;11:Doc18. doi: 10.3205/dgkh000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fröhlicher S, Reichen-Fahrni G, Müller M, Surbek D, Droz S, Spellerberg B, Sendi P. Serotype distribution and antimicrobial susceptibility of group B streptococci in pregnant women: results from a Swiss tertiary centre. Swiss Med Wkly. 2014;144:w13935. doi: 10.4414/smw.2014.13935. [DOI] [PubMed] [Google Scholar]
- 21.Bolukaoto JY, Monyama CM, Chukwu MO, Lekala SM, Nchabeleng M, Maloba MR, Mavenyengwa RT, Lebelo SL, Monokoane ST, Tshepuwane C, Moyo SR. Antibiotic resistance of Streptococcus agalactiae isolated from pregnant women in Garankuwa, South Africa. BMC Res Notes. 2015;8:364. doi: 10.1186/s13104-015-1328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fassina JR, Pimenta-Rodrigues MV. Aspectos Laboratoriais da Identificação de Streptococcus agalactiae em Gestantes: uma mini revisão. Interbio. 2013;7(1):26–40. [Google Scholar]
- 23.Botelho ACN, Oliveira JG, Damasco AP, Santos KTB, Ferreira AFM, Rocha GT, Marinho PS, Bornia RBG, Pinto TCA, Américo MA, Fracalanzza SEL, Teixeira LM. Streptococcus agalactiae carriage among pregnant women living in Rio de Janeiro, Brazil, over a period of eight years. PLoS One. 2018;13:e0196925. doi: 10.1371/journal.pone.0196925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barros RR, de Souza AF, Luiz FB. Polyclonal spread of Streptococcus agalactiae resistant to clindamycin among pregnant women in Brazil. J Antimicrob Chemother. 2016;71:2054–2056. doi: 10.1093/jac/dkw085. [DOI] [PubMed] [Google Scholar]
- 25.Dutra VG, Alves VM, Olendzki AN, Dias CA, de Bastos AF, Santos GO, de Amorin EL, Sousa MÂ, Santos R, Ribeiro PC, Fontes CF, Andrey M, Magalhães K, Araujo AA, Paffadore LF, Marconi C, Murta EF, Fernandes PC Jr, Raddi MS, Marinho PS, Bornia RB, Palmeiro JK, Dalla-Costa LM, Pinto TC, Botelho AC, Teixeira LM, Fracalanzza SE. Streptococcus agalactiae in Brazil: serotype distribution, virulence determinants and antimicrobial susceptibility. BMC Infect Dis. 2014;14:323. doi: 10.1186/1471-2334-14-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kruk CR, Feuerschuette OH, Silveira SK, Cordazo M, Trapani Júnior A. Epidemiologic profile of Streptococcus agalactiae colonization in pregnant women attending prenatal care in a city of southern of Brazil. Braz J Infect Dis. 2013;17:722–723. doi: 10.1016/j.bjid.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souza VC, Kegele FC, Souza SR, Neves FP, de Paula GR, Barros RR. Antimicrobial susceptibility and genetic diversity of Streptococcus agalactiae recovered from newborns and pregnant women in Brazil. Scand J Infect Dis. 2013;45:780–785. doi: 10.3109/00365548.2013.810814. [DOI] [PubMed] [Google Scholar]
- 28.Palmeiro JK, Dalla-Costa LM, Fracalanzza SE, Botelho AC, da Silva Nogueira K, Scheffer MC, de Almeida Torres RS, de Carvalho NS, Cogo LL, Madeira HM. Phenotypic and genotypic characterization of group B streptococcal isolates in southern Brazil. J Clin Microbiol. 2010;48:4397–4403. doi: 10.1128/JCM.00419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira MV, Teles MF, Viana TA. Prevalência e fatores de risco associados à colonização por streptococcusagalactiae em gestantes atendidas no hospital municipal esaú matos em vitória da conquista–ba. C&D-Revista Eletrônica da Fainor. 2013; 6(1): 172–184. [Google Scholar]
- 30.Weinstein MP, Patel JB, Bobenchik AM Clinical and Laboratory Standards Institute. 2017. Performance standards for antimicrobial susceptibility testing. M100, 29th ed, Clinical and Laboratory Standards Institute, Pensylvania USA; pp. 148–149. [Google Scholar]
- 31.Fan HH, Kleven SH, Jackwood MW. Application of polymerase chain reaction with arbitrary primers to strain identification of Mycoplasma gallisepticum. Avian Dis. 1995;39:729–735. [PubMed] [Google Scholar]
- 32.Guerrero C, Martínez J, Menasalvas A, Blázquez R, Rodríguez T, Segovia M. Use of direct latex agglutination testing of selective broth in the detection of group B strepptococcal carriage in pregnant women. Eur J Clin Microbiol Infect Dis. 2004;23:61–62. doi: 10.1007/s10096-003-1052-x. [DOI] [PubMed] [Google Scholar]
- 32.Borger IL, d’sOliveira REC, Castro ACD de, Mondino SSB de. Streptococcus agalactiae em gestantes: prevalência de colonização e avaliação da suscetibilidade aos antimicrobianos. RevBrasGinecol e Obs. 2005;27(10):575–579. [Google Scholar]
- 34.Najmi N, Jehan I, Sikandar R, Zuberi NF. Maternal genital tract colonisation by group-B streptococcus: a hospital based study. J Pak Med Assoc. 2013;63:1103–1107. [PubMed] [Google Scholar]
- 35.Russell NJ, Seale AC, O'Driscoll M, O'Sullivan C, Bianchi-Jassir F, Gonzalez-Guarin J, Lawn JE, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Le Doare K, Madhi SA, Rubens CE, Schrag S, Sobanjo-Ter Meulen A, Vekemans J, Saha SK, Ip M GBS Maternal Colonization Investigator Group. Maternal Colonization With Group B Streptococcus and Serotype Distribution Worldwide: Systematic Review and Meta-analyses. Clin Infect Dis. 2017;65:S100–S111. doi: 10.1093/cid/cix658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salem N, Anderson JJ. Evaluation of four chromogenic media for the isolation of Group B Streptococcus from vaginal specimens in pregnant women. Pathology. 2015;47:580–582. doi: 10.1097/PAT.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 37.Tazi A, Réglier-Poupet H, Dautezac F, Raymond J, Poyart C. Comparative evaluation of Strepto B ID chromogenic medium and Granada media for the detection of Group B streptococcus from vaginal samples of pregnant women. J Microbiol Methods. 2008;73:263–265. doi: 10.1016/j.mimet.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 38.El Aila NA, Tency I, Claeys G, Saerens B, Cools P, Verstraelen H, Temmerman M, Verhelst R, Vaneechoutte M. Comparison of different sampling techniques and of different culture methods for detection of group B streptococcus carriage in pregnant women. BMC Infect Dis. 2010;10:285. doi: 10.1186/1471-2334-10-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morita T, Feng D, Kamio Y, Kanno I, Somaya T, Imai K, Inoue M, Fujiwara M, Miyauchi A. Evaluation of chromID strepto B as a screening media for Streptococcus agalactiae. BMC Infect Dis. 2014;14:46. doi: 10.1186/1471-2334-14-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry JD, Oliver M, Nicholson A, Wright J, Gould FK. Evaluation of a new chromogenic agar medium for isolation and identification of Group B streptococci. Lett Appl Microbiol. 2006;43:615–618. doi: 10.1111/j.1472-765X.2006.02023.x. [DOI] [PubMed] [Google Scholar]
- 41.Hardie JM, Whiley RA. Classification and overview of the genera Streptococcus and Enterococcus. J Appl Microbiol. 1997;83:1S–11S. doi: 10.1046/j.1365-2672.83.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 42.Numanović F, Smajlović J, Gegić M, Delibegović Z, Bektaš S, Halilović E, Nurkić J. Presence and resistance of Streptococcus agalactiae in vaginal specimens of pregnant and adult non-pregnant women and association with other aerobic bacteria. Med Glas (Zenica) 2017;14:98–105. doi: 10.17392/876-16. [DOI] [PubMed] [Google Scholar]
- 43.Dahesh S, Hensler ME, Van Sorge NM, Gertz RE, Jr, Schrag S, Nizet V, Beall BW. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob Agents Chemother. 2008;52:2915–2918. doi: 10.1128/AAC.00461-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no additional data available.