Abstract
BACKGROUND
Individuals’ interest in sports activities has been increasing, contributing to more stress fracture occurrences in uncommon locations on the skeleton. In this study, several cases of stress fractures in atypical locations are presented, and the possibility of combining diagnostic methods to make accurate and quick diagnoses is explored. Additionally, different causes of stress fractures, as well as various modalities of treatment, are highlighted. Other potential factors of stress fractures were identified by a literature review.
CASE SUMMARY
Six cases of stress fractures in the calcaneus, intermediate cuneiform bone, sacrum, tibia (bilateral), navicular bone and femoral neck are presented, with different types of diagnostic imaging and treatments. All of the cases were associated with an aspect of mobility because all of the patients were physically active in various sport disciplines.
CONCLUSION
The type of therapeutic procedure selected should depend on the specific clinical case, i.e., the patient’s condition and level of physical activity.
Keywords: Stress fractures, Case report, Calcaneus, Intermediate cuneiform bone, Sacral bone, Navicular bone
Core Tip: The cause of stress fracture is multifactorial and difficult to diagnose, particularly when it occurs in uncommon locations. This case report demonstrates the different possible locations and causes of stress fractures, as well as the various methods used to diagnose and treat these fractures. Here we show that the cause of stress fractures, except for well-known factors, may also lie in motor skill development. It also highlights that treatments should be individualized according to each patient’s clinical condition.
INTRODUCTION
Individuals’ interest in recreational and professional sport activities has been increasing, contributing to the occurrence of increasingly more stress fractures, as well as fractures at atypical locations on the skeleton, which are often difficult to diagnose. Patient-reported symptoms that result directly from the occurrence of fatigue-type fractures require a comprehensive diagnostic approach when they appear in less frequently encountered locations[1,2]. The locations in which stress fractures develop depend primarily on the type of physical activity performed by the individual, parts of the body involved in the activity and the stresses exerted on a particular anatomic structure[3-5]. Early diagnosis is crucial for the success of therapeutic management. Unfortunately, because stress fractures require a high index of clinical suspicion, they are frequently underdiagnosed and undertreated[6,7].
Stress fractures represent approximately 10% of all injuries in sports. Stress injuries most frequently occur when individuals, particularly females, perform a new activity or make changes in the type of training they perform, as well as when fatigue fractures are present in the medical history of a patient[8,9]. The additional intrinsic and extrinsic factors that increase the risk of fatigue fractures are a hard training surface, inappropriate footwear, and a low intake of vitamin D and calcium[10].
Diagnostic imaging methods used for fatigue bone fractures include a combination of radiography, magnetic resonance imaging (MRI), 3-phase skeletal scintigraphy and, in rare cases, computed tomography (CT) or color Doppler ultrasonography (periosteal imaging). A diagnostic method is selected according to the location and stage of development with regard to healing[11].
Typical fatigue fractures have a promising prognosis for return to sports. This type of fracture is also accompanied by a slight risk of complications. There is a risk of severe complications and the treatments for these injuries occurring in uncommon locations are difficult and require a long period of time. Fractures characterized by a high tensile load or low blood flow are called high-risk stress fractures. There is a risk of delayed healing, fracture progression or even nonunion[10,12].
The paper presents several cases of stress fractures in unusual locations: The calcaneus, intermediate cuneiform bone, sacrum, tibias (bilateral), navicular bone and femoral neck. All of the fractures were associated with an aspect of mobility because all of the patients had an increased level of physical activity in various sport disciplines. In this retrospective report, when considering individual clinical cases, the authors that in the case of fatigue fractures, in typical or atypical locations, the diagnostic process is often based on the stages of the more common overuse syndrome procedure, which mostly involves soft tissue injuries.
CASE PRESENTATION
From December 2014 to August 2018, six patients (four women and two men) with an average age of 25.3 years (age range 17-42 years) were diagnosed with stress fractures. The presented series of clinical cases occurred in four professional athletes, a novice athlete and an amateur athlete: A handball player, a tennis player, a swimmer who transitioned to the triathlon, a runner, one amateur who attended fitness activities and one amateur triathlete. In accordance with the suspected diagnosis determined on the basis of the physical examination, the patients underwent medical imaging: One patient underwent a CT scan, three underwent MRI scans, and two underwent both types of imaging examinations. Five patients had a stress fracture in a lower extremity, and four fatigue fractures occurred in atypical locations: The calcaneus, intermediate cuneiform bone, sacrum and femoral neck. In two cases, the stress fractures occurred bilaterally, which is also uncommon. According to Fullem[13], stress fractures were bilateral in 16.6% of the cases in his study group of 320 patients diagnosed with fatigue fractures. Table 1 presents an overview of all cases.
Table 1.
An overview of all clinical cases
| Case | Sex | Age | Activity1 | Location of stress fracture | Laboratory examination | Imaging examination | Follow-up (mo) | Control imaging examination |
| 1 | F | 42 | Zumba workout (R) | Calcaneus | None | MRI | 1.5 | MRI after 1.5 mo |
| 2 | M | 21 | Handball (P) | Intermediate cuneiform bone | Level of 25-hydroxyvitamin D3 | MRI, CT | 2.5 | CT after 1.5 mo CT after 2.5 mo |
| 3 | F | 17 | Tennis (P) | Sacral bone | None | MRI | - | - |
| 4 | F | 18 | Triathlon (P) | Bilateral tibia fracture | None | CT | 5 | CT after 5 mo |
| 5 | F | 18 | Running (P) | Navicular bone | None | X-ray, MRI | - | - |
| 6 | M | 36 | Triathlon (R) | Femoral neck | Level of 25-hydroxyvitamin D3 | X-ray, CT, MRI | 16.5 | MRI after 5 wk MRI after 11 wk CT after 13 wk MRI after 16.5 mo |
P: Professional; R: Recreational. MRI: Magnetic resonance imaging; CT: Computed tomography.
Chief complaints
Case 1: A 42-year-old woman [body mass index (BMI) 20.58 kg/m2] was referred to the clinic because of pain in her left ankle accompanied by significant swelling of the joint.
Case 2: A 21-year-old man (BMI 24.44 kg/m2), who was a professional handball player, was referred to the clinic because of pain in his left foot.
Case 3: A 17-year-old woman (BMI 22.39 kg/m2) who started playing tennis at 9 years of age was referred to the clinic because of low back pain in the sacral region.
Case 4: An 18-year-old woman (BMI 21.72 kg/m2) with a 10-year history of competitive swimming was referred to the clinic because of pain in both of her lower extremities. She suffered from pain on the border between the midpoint and the distal third point of the right tibia and the distal portion of the left tibia.
Case 5: An 18-year-old woman (BMI 19.23 kg/m2), who was a competitive runner, was referred to the clinic due to persistent pain in both calves.
Case 6: A 36-year-old man (BMI 24.69 kg/m2) was referred to the clinic because of pain in his right hip joint.
History of present illness
Case 1: The patient’s symptoms started two mo earlier, after participation in Zumba classes, and were exacerbated following more intense and prolonged exercises. Acute ankle pain occurred periodically.
Case 2: The pain lasted for four mo, and its onset was not associated with an injury. Before referral to the clinic, the patient underwent physical therapy, but he did not observe any improvement. Due to severe pain, the patient stopped the training one month before referral to the clinic.
Case 3: The pain lasted for six wk, and its onset was not associated with an injury.
Case 4: The onset of pain corresponded to when the patient switched from competitive swimming to training for a triathlon.
Case 5: The patient’s training routine included 8-10 km of running daily, but the distance was extended up to 20 km during the preparatory camps. The patient’s symptoms started approximately three mo before referral, and she did not have a previous injury.
Case 6: The patient’s symptoms started 10 days before the referral while running during a triathlon competition. The patient used crutches due to severe pain in the hip, which radiated to the knee. Discomfort and pain had occurred during preparation for the competition.
History of past illness
Cases 1-4, and Case 6: The patients’ medical history was unremarkable.
Case 5: The patient had a history of stress fractures in the left fibula and left metatarsal bone that occurred 3 years earlier. The fractures healed after supplementation with vitamin D.
Physical examination
Case 1: By palpation, the pain was determined to be localized on the medial side near the lateral malleolus along the tendon of the tibial muscle. The patient presented with substantial swelling of the left ankle. The ultrasound examination revealed edema of the tibial muscle tendon.
Case 2: During palpation, the patient reported pain in the tarsometatarsal joint and the area corresponding to the second metatarsal bone.
Case 3: During palpation, the patient reported pain in the left iliosacral joint. Moreover, she complained of pelvic pain during the push and squeeze test of the iliac bend and presented with a painful, limp gait.
Case 4: Upon palpation, the pain was determined to be localized in the distal thirds of both tibias. The pain was exacerbated during walking.
Case 5: Upon palpation, there was tenderness in the distal thirds of both fibulas but not in the peroneal muscles. The patient was unable to complete the deep squat test.
Case 6: The patient's gait was abnormal, with limping on the right leg. During the functional tests, the right hip showed limited flexion range of motion, no internal rotation range of motion and limited abduction range of motion, with pain in the terminal phase of gait.
Laboratory examinations
Case 2 and Case 6: A laboratory test was conducted for the concentration of 25-hydroxyvitamin D3.
Imaging examinations
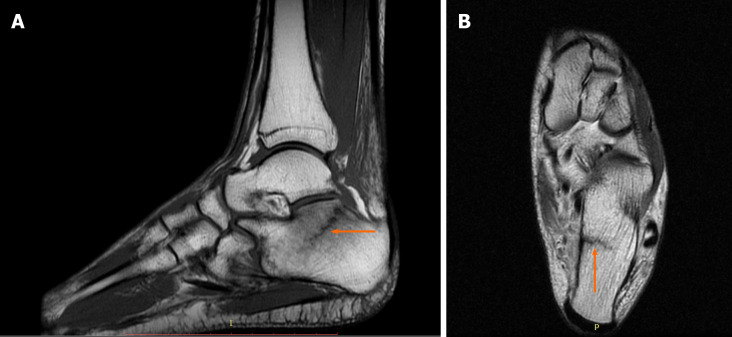
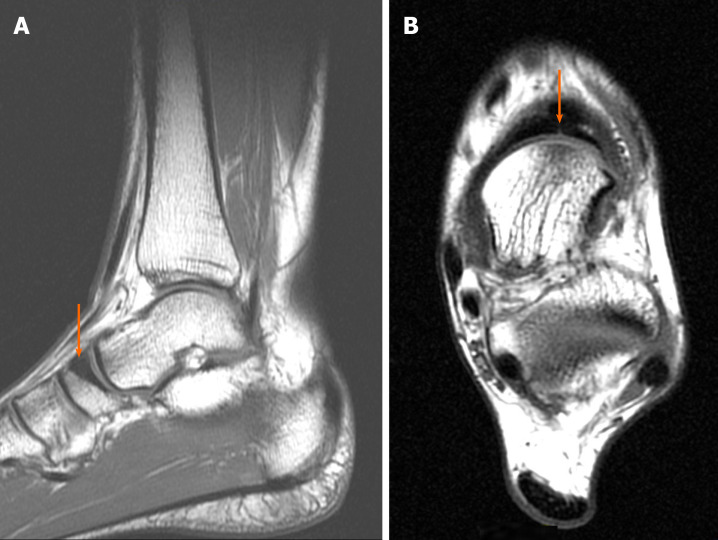
Case 1: The patient underwent an MRI scan because damage to the posterior tibialis tendon was suspected. The examination revealed a calcaneal fracture with bone marrow edema and swelling of the adjacent soft tissues (Figure 1).
Figure 1.
Diagnostic images from the magnetic resonance imaging examination. A: T1-weighted sagittal fast spin echo cross-section of the left foot; B: T2-weighted axial FSE cross-section, with the fracture site marked (orange arrow).
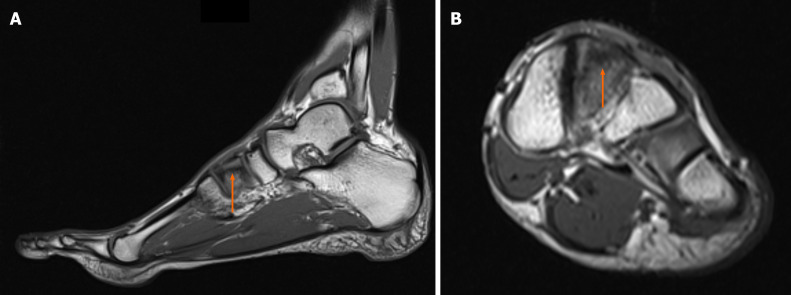
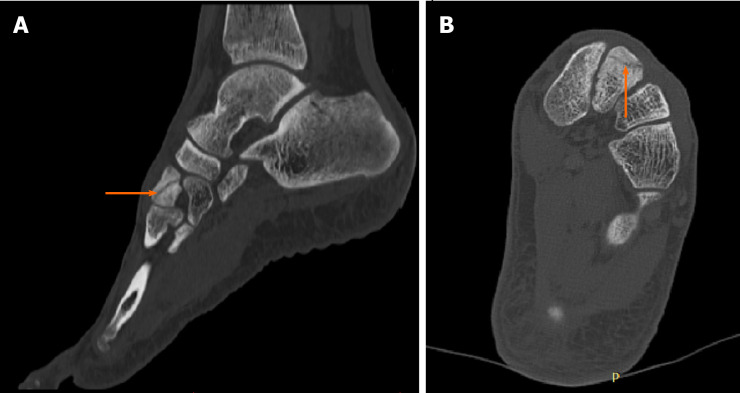
Case 2: The MRI results demonstrated edema around the intermediate cuneiform bone, which suggested the presence of a fracture (Figure 2). Indeed, a tiny cleft with sclerotic remodeling of the adjacent bone was observed on the CT scans, which were recommended to determine whether there was a fracture (Figure 3).
Figure 2.
Diagnostic images from the magnetic resonance imaging examination. A: T1-weighted sagittal cross-section of the left foot; B: T1-weighted coronal cross-section, with the fracture site marked (orange arrow).
Figure 3.
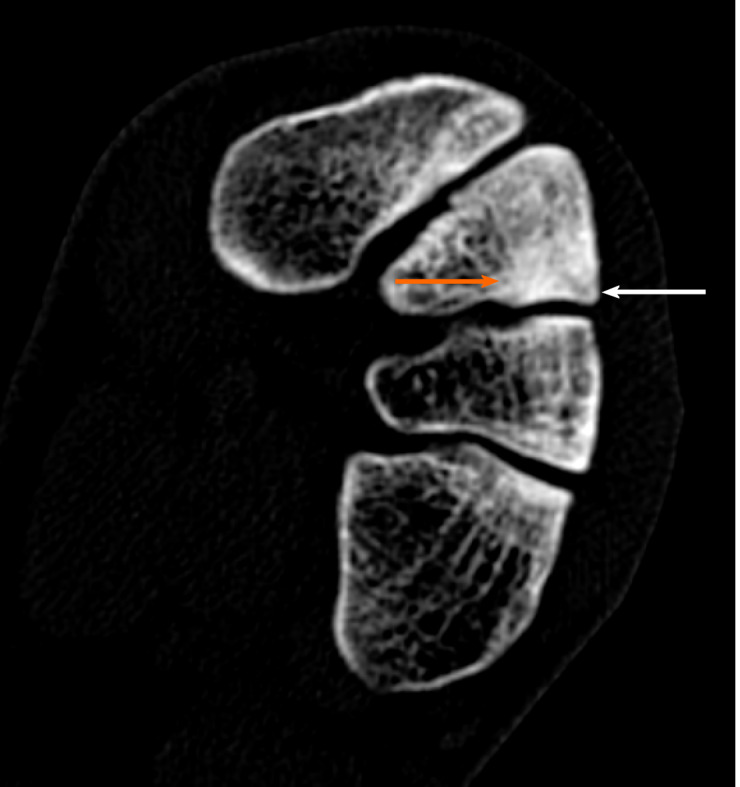
Diagnostic images from the computed tomography examination; sclerotic remodeling of bone tissue–orange arrow, very small cleft of the fracture–white arrow.
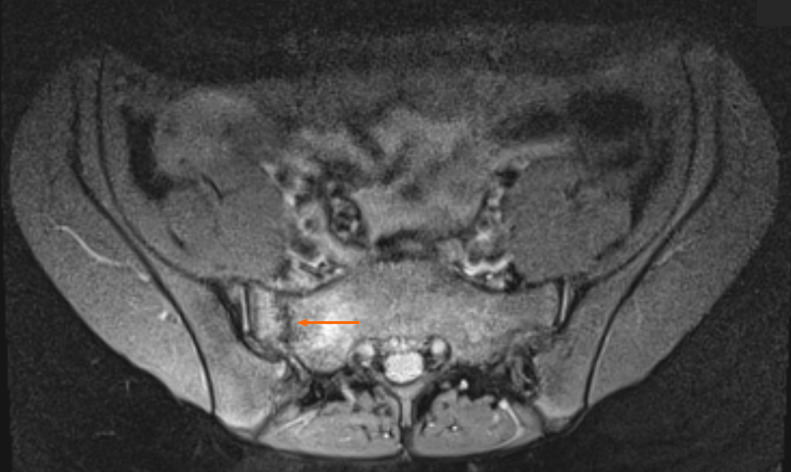
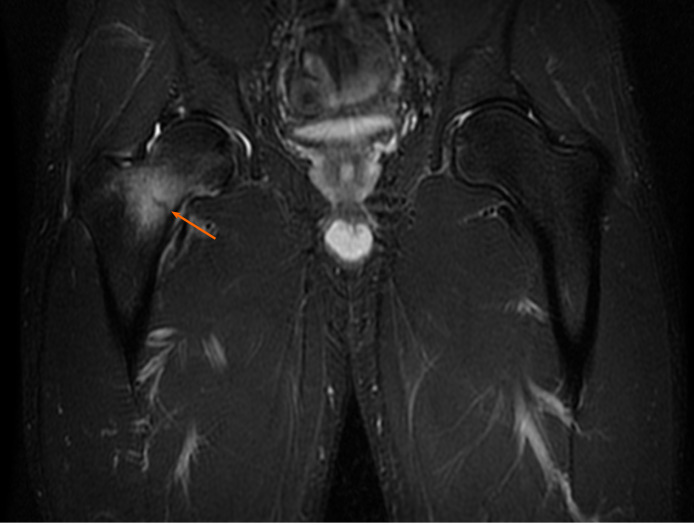
Case 3: MRI revealed a fracture in the sacrum, involving zone I, (ala, lateral to the sacral foramina) according to the Denis sacral injury classification system[14,15] (Figure 4).
Figure 4.
Diagnostic proton density weighted fat-suppressed (fs) axial images from the magnetic resonance imaging examination, with the fracture site marked (orange arrow).
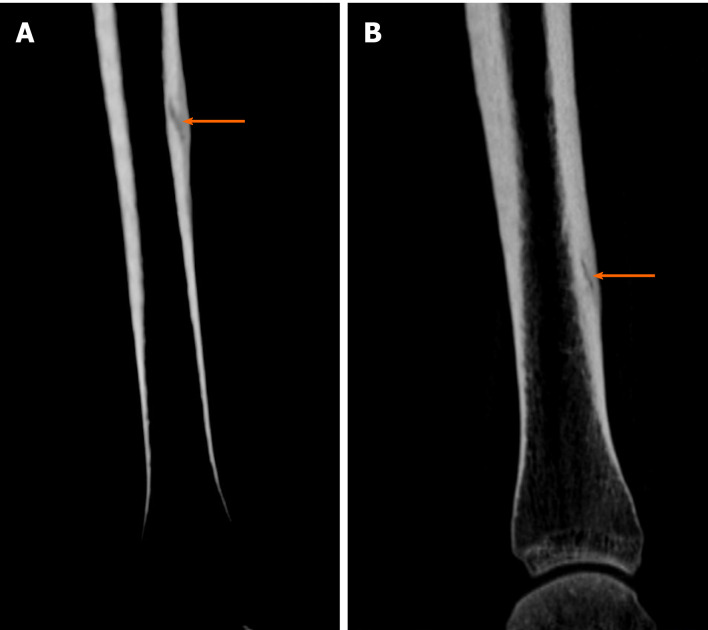
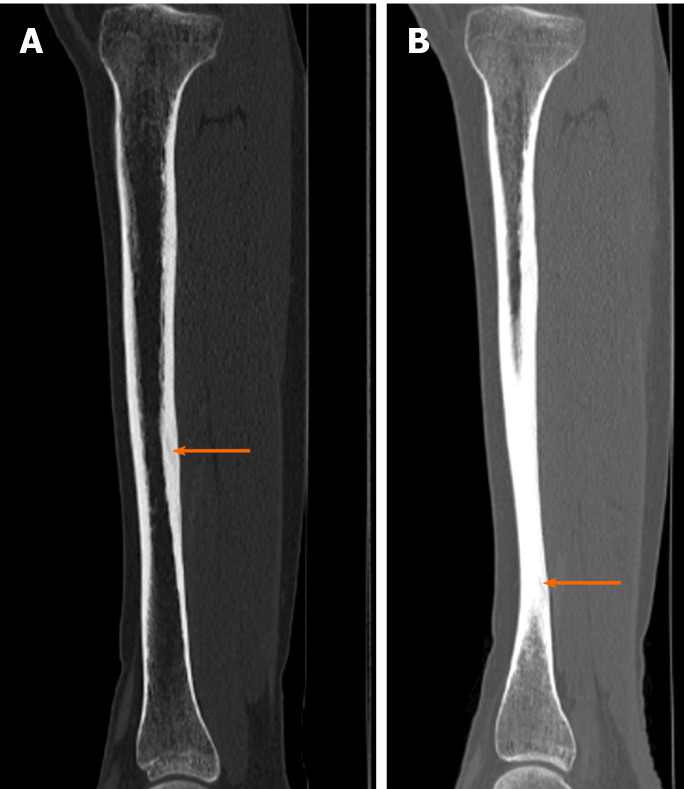
Case 4: Tibial fatigue fractures were found bilaterally on the CT scans (Figure 5).
Figure 5.
Diagnostic images from the computed tomography examination. A: Right tibia; B: Left tibia, with the fracture site marked (orange arrow).
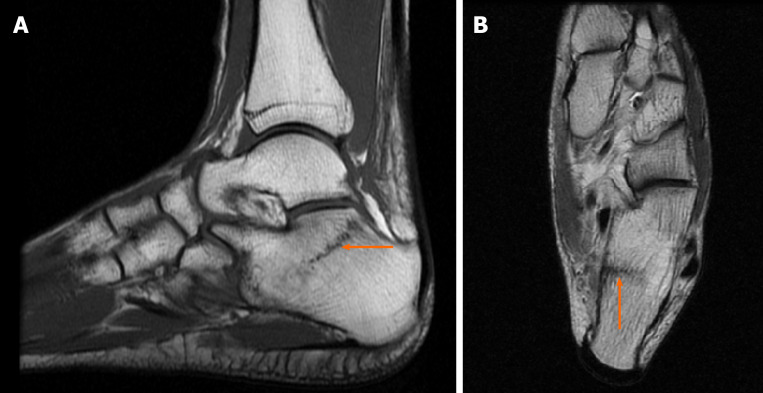
Case 5: No evidence of fractures was found on conventional radiograms. MRI demonstrated a bilateral fracture of the navicular bones (Figure 6 and Figure 7).
Figure 6.
Magnetic resonance imaging diagnostic images of the left ankle. A: Saggital T1-weighted image: bone sclerosis is indicated by the orange arrow; B: Axial T1-weighted image: the small crack is indicated by the orange arrow.
Figure 7.
Magnetic resonance imaging diagnostic images of the right ankle. A: Saggital T1-weighted image: bone sclerosis is indicated by the orange arrow; B: Coronal T1: bone sclerosis (orange arrow) and cleft of the fracture are indicated by the white arrow.
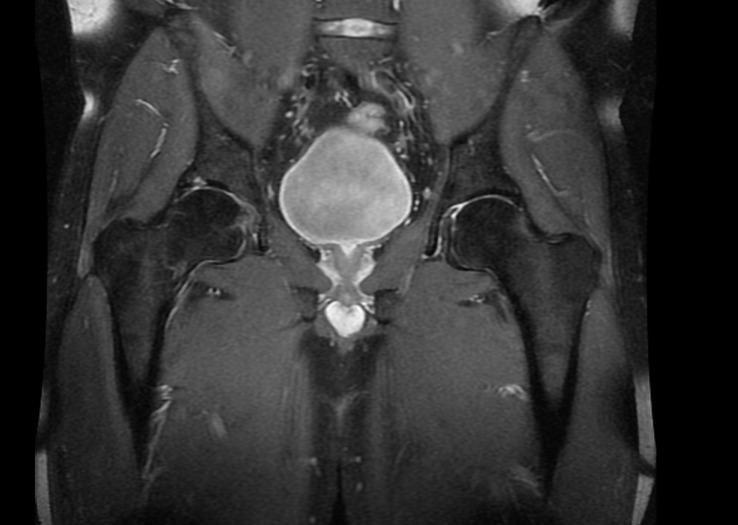
Case 6: No evidence of fractures was found on the conventional radiograms. The patient underwent CT and MRI scans because femoral head necrosis or a stress fracture was suspected. The CT scan did not reveal an obvious bone fracture. However, the MRI scan demonstrated edema of the femoral neck bone marrow with a visible hypointensive fracture cleft at the medial edge, without evidence of cortical layer rupture (Figure 8). The examination revealed swelling of the surrounding soft tissue, as well as slight effusion in the hip joint.
Figure 8.
Diagnostic images from the magnetic resonance imaging examination. Coronal short T1 inversion recovery sequence. Edema and the fracture cleft are indicated by the orange arrow.
FINAL DIAGNOSIS
Case 1: The final diagnosis of the presented case was a stage III chronic calcaneal stress fracture, according to the classification system proposed by Keading and Miller[16].
Case 2: The final diagnosis of the presented case was a stress fracture of the intermediate cuneiform bone in the left foot.
Case 3: The final diagnosis of the presented case was a sacral stress fracture.
Case 4: The final diagnosis of the presented case was an incomplete bilateral tibial stress fracture.
Case 5: The final diagnosis of the presented case was a bilateral navicular bone stress fracture.
Case 6: The final diagnosis of the presented case was an early phase of a fatigue fracture of the femoral neck.
TREATMENT
Case 1: The patient was instructed to walk with forearm crutches to avoid weight-bearing on the affected extremity, to rest with the limb elevated, and to apply cold compresses on the ankle.
Case 2: The patient was recommended to refrain from training and to use an ultrasound bone healing system once a day for 14 days. Furthermore, he was prescribed manual therapy and a single administration of platelet-rich plasma. The platelet-rich plasma, containing 4-5 times more platelets than whole blood, was obtained by centrifugation of the patient’s blood for 8 minutes and administered under ultrasonographic guidance, as recommended elsewhere, 2 wk after manual therapy was started[17].
Case 3: The patient was recommended to refrain from sports activities for 8 wk, after which a control MRI scan was scheduled.
Case 4: The patient was recommended to refrain from physical activity for eight wk, to rest frequently with the lower extremities elevated, to walk with forearm crutches, and to undergo pulsed signal therapy.
Case 5: The recommended treatment included the control of her vitamin D levels, the administration of platelet-rich plasma, and myofascial and manual therapy.
Case 6: The patient was instructed to walk with forearm crutches to avoid weight-bearing on the affected extremity, to rest with the limb elevated, to use an ultrasound bone healing system once a day for 6 wk, and to control his level of 25-hydroxyvitamin D3. Furthermore, he was advised to begin rehabilitation on day 5 after the visit.
OUTCOME AND FOLLOW-UP
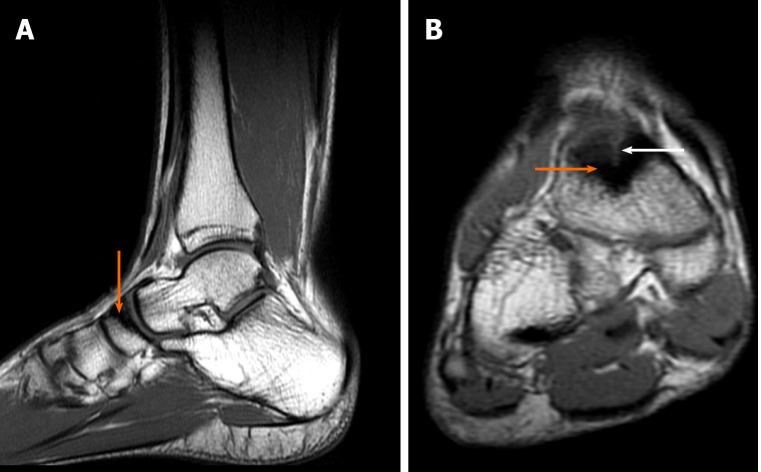
Case 1: After one and half mo, the patient returned for a follow-up visit and underwent a control MRI scan, which demonstrated a decrease in bone marrow edema, which likely occurred due to progressive bone consolidation (Figure 9). Since the last examination, the pain has gradually subsided. The patient did not report pain while walking or loading the extremity.
Figure 9.
Diagnostic images from the magnetic resonance imaging examination from the control visit. A: T1-weighted sagittal fast spin echo cross-section of the left foot; B: T2-weighted axial FSE cross-section, with the fracture site marked (orange arrow).
Case 2: One and half mo after the first visit, the patient no longer reported pain upon palpation or discomfort during walking. However, the CT scans acquired during the visit demonstrated widening of the fracture cleft, which involved the entire length of the intermediate cuneiform bone (Figure 10). The laboratory tests revealed a substantially decreased concentration of 25-hydroxyvitamin D3 (16.53 ng/mL; normal range 30-100 ng/mL). The patient was instructed to further refrain from physical activity, to control his levels of electrolytes and 25-hydroxyvitamin D3, to wear insoles lifting the medial longitudinal arch of the foot, and to continue treatment with the ultrasound bone healing system for another 14 days. During the subsequent control visit, one month later, consolidation of the bone within the medial margin of the fracture cleft was observed on the CT scans, but sclerotic edges were still observed within the lateral margin (Figure 11).
Figure 10.
Diagnostic images from the computed tomography examination from the first control visit. A: Sagittal multiplanar reconstruction; B: Axial multiplanar reconstruction image with the fracture site marked (orange arrow).
Figure 11.
Diagnostic images from the computed tomography examination from the second control visit. A: Sagittal multiplanar reconstruction; B: Axial multiplanar reconstruction image with the fracture site marked (orange arrow).
Case 3: The patient did not attend the scheduled control visit and did not undergo the MRI examination due to an improved well-being, and she returned to her preinjury level of training.
Case 4: Control CT scans obtained 5 mo after the initial referral demonstrated progressive ossification at the fracture sites, with virtually no evidence of clefts (Figure 12).
Figure 12.
Diagnostic images from the control computed tomography examination after 5 mo. A: right tibia; B: left tibia, with the fracture site marked (orange arrow).
Case 5: The patient did not attend the scheduled control visit.
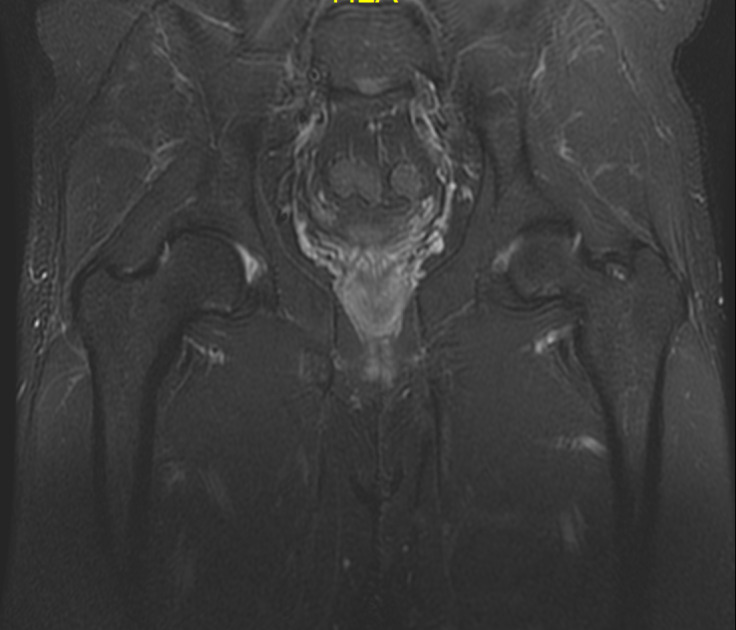
Case 6: After 5 wk, the patient underwent a control MRI scan, which demonstrated a decrease in bone marrow edema, edema of the surrounding soft tissue and effusion in the hip joint, which likely corresponded to progressive bone consolidation. Since the last examination, the pain has gradually subsided. The patient received a double dose of platelet-rich plasma and was advised to continue rehabilitation and to walk with forearm crutches for another 4 wk to avoid weight-bearing on the affected extremity. The laboratory tests revealed a low concentration of 25-hydroxyvitamin D3 (43.00 ng/mL; normal range 30-100 ng/mL), so the patient was instructed to take the vitamin D supplementation. At the subsequent control visit, one month later, the patient received a single dose of platelet-rich plasma. He was recommended to walk with one forearm crutch and to continue rehabilitation using an antigravity treadmill. The next MRI scan, performed approximately 6 wk after the first control MRI scan, demonstrated evolution of the repair processes at the site of the preexisting fatigue fracture (Figure 13). The structure of the bone and the degree of its calcification were assessed on the basis of the CT scans obtained 2 wk later. The CT results demonstrated consolidation of the bone, without a visible fracture gap and cortical layer rupture. The patient was recommended to increase the intensity of rehabilitation and start basic motor training to improve his fitness, based on the correction of movement patterns. He was also instructed to control his level of vitamin D with supplementation. Thirteen months after the last CT scan, the patient attended another control visit, during which another MRI scan of the right hip was performed. The MRI examination revealed proper healing of the fracture cleft, the absence of edema and the absence of a visible fracture cleft (Figure 14).
Figure 13.
Control magnetic resonance imaging scan of the right hip. Coronal proton density fat-suppressed sequence.
Figure 14.
Control magnetic resonance imaging scan of the right hip. Coronal short T1 inversion recovery sequence.
DISCUSSION
Fatigue fractures are the result of the application of unusual, multiple in compressive loads during weight-bearing activities, which correspond largely to a change in the mechanical integrity of bone structures, especially bone structures that have been weakened by other factors. The cases presented in this paper show the ambiguous characterization of stress fractures. Therefore, the treatment for this type of injury should be tailored as much as possible to each clinical case, from the diagnostic stage to the personalized therapy, with the daily activities of the patient taken into account[18]. For many elite and professional athletes, the conventional treatment of analgesics, immobilization, and extended rest from training is often unacceptable. Introducing modern strategies for treating stress fractures is superior to ensure a fast and safe return to sport[19]. The decision to supplement the therapeutic protocol with orthobiologic methods for stimulating and supporting bone healing is desired nowadays to expedite recovery and decrease the risk of nonunion. The widespread use of platelet-rich plasma in orthopedics has shown positive outcomes in bone healing as well[20,21]. Nevertheless, randomized studies with standardized protocols (relating preparation and administration phase) are still required to confirm efficacy of treatments in human stress fractures[19]. Another therapeutic strategy is the application of low-intensity pulsed ultrasound (LIPUS). A meta-analysis of the effect of LIPUS on the bone fracture healing indicated it is moderate to low in quality[22,23] . At this stage of knowledge[23,24], LIPUS would not be suggested for routine treatment of fatigue fractures but it might be a choice for non-union, or in professional athletes. As before, more high quality research is needed to further assess the effects of LIPUS on stress fracture healing, taking into account different dosages and sites[23].
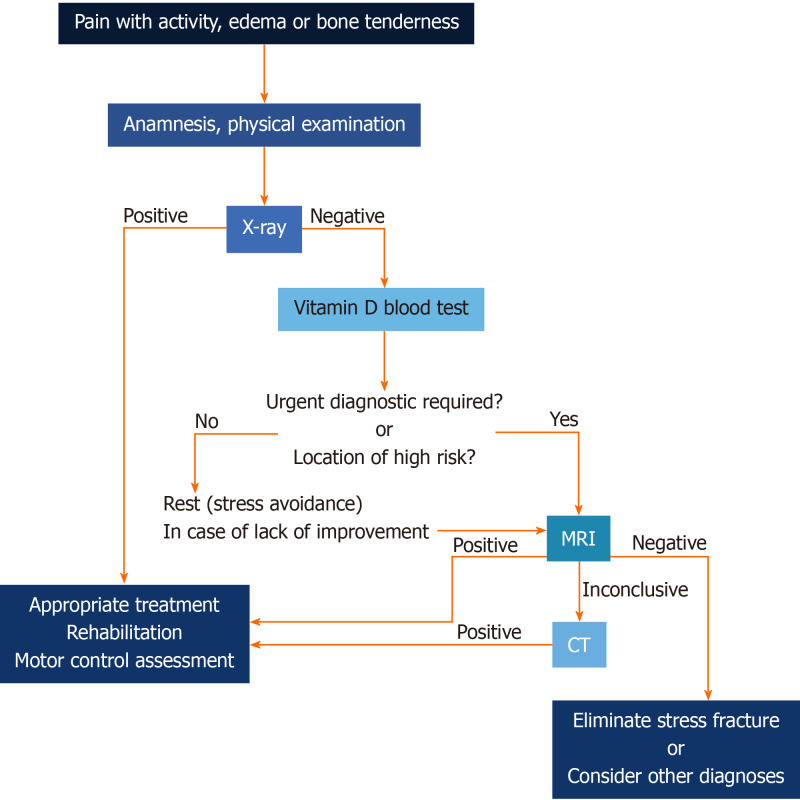
It is worth noting that in the majority of cases, a stress fracture is not suspected; therefore, the diagnostic process is based on the stages of the more common overuse syndrome procedure, which mostly involves soft tissue injuries. In the development and management of the type of maladies presented in the paper, both intrinsic and extrinsic factors play a crucial role. What is important to recognize is that some of these factors can be managed, and therefore, injuries may actually be preventable. Many algorithms were proposed for the stress fracture diagnostic and treatment stage[25-28]. Our algorithm (Figure 15) is based on principles derived from the literature[25,28,29], guided by epidemiological recommendation and our observations: Effects of vitamin D deficiency and disorders in basic movement patterns. The main aim of testing vitamin D level is to support bone remodeling[30], and prevent future fractures as it was stated by Lappe et al[31] that supplementation of calcium and vitamin D reduces incidence of stress fractures by as much as 20%. For the same purpose, the need of biomechanical movement patterns assessment should be emphasized, as studies[5,32,33] show that there is an association with the risk of lower extremities fracture. The assessment of basic movement patterns that are learned at an early stage of varies according to individual lifestyle, and the fact that they are changeable cannot be neglected[10]. Therefore motor control evaluation should be performed before and after treatment. Additionally, based on the presented cases, the physician-radiologist cooperation should be highlighted in the management algorithm, as a key to make accurate and quick diagnoses. Radiologists who know a patient’s medical history can assess whether the edema observed in the MR images is related to the symptoms and then suggest a CT scan, another form of medical imaging, is additionally performed to confirm or eliminate a stress fracture.
Figure 15.
Diagnostic algorithm. CT: Computed tomography.
Radiography, despite the low sensitivity and specificity, is still considered a first-line diagnostic method, due to the fact that it allows for other causes of pain to be differentiated[34]. The main advantage of MRI it has the best spatial resolution and specificity, therefore MRI can easily detect minor stress reactions (including bone contusions), especially on a short T1 inversion recovery (STIR) sequence or a fat-suppressed T2-weighted fast spin echo (FSE) sequence. Moreover, MRI examination is sensitive enough to detect further malignant entities which could necessitate a bone marrow replacement, and which, in consequence, would make the bone prone to insufficiency fracture. The biggest advantage of scintigraphy [the 3-phase skeletal scintigraphy with technetium-99m (99mTc) methylene diphosphonate (MDP)], in comparison to MRI, is that the entire skeleton can be screened. The increasing role of MRI, which is now the gold standard in fatigue fracture assessment, has replaced sensitive but nonspecific scintigraphy[10,32]. Computed tomography and ultrasound examination should be reserved for special cases. USG, together with vascular assessment, allows for the detection of bloodshot periosteal sites and sites for the initiation of tissue repair processes.
Considering the influence of vitamin D levels on fatigue fractures, it should be noted that at least half of the patients diagnosed with this type of malady are characterized as having a low level of this vitamin (levels below 40 ng/mL are considered insufficient based on the standards established by the vitamin D council[35]; levels below 30 ng/mL are considered insufficient based on the recommendations by the United States Endocrine Society[36]). Vitamin D concentration is closely related to the absorption of dietary calcium and phosphorus and individuals’ metabolism. In addition, individuals with vitamin D deficiency absorb approximately only 10%-15% of dietary calcium and 50%-60% of dietary phosphorus. A decrease in the calcium level can lead to a decrease in bone mineralization and structural integrity. Vitamin D could also have a positive, through moderate, effect on aerobic performance in athletes who do high-intensity training with limited exposure to sunlight[37].
Stress fractures occur in the vast majority of lower extremities, including the tibia (23.6%), navicular bone (17.6%), metatarsal and fibula (approximately 16%), and femur (6.6%), and they occur less frequently in the pelvic bone (1.6%) and spine (0.6%)[38]. Significantly less frequently occurring cases of stress fractures are those in unusual locations, such as the patella, sphenoid bone, femoral neck (tension side), calcaneus, and sacrum. Calcaneal fatigue fractures are uncommon and therefore can be easily misdiagnosed, which may result in malunion. The recommended therapeutic procedure for calcaneus stress fractures is primarily a reduction in activity and an extended period of absence from training sessions for athletes[33].
Despite the fact that fatigue fractures of the foot are relatively common, the cuneiform bones and navicular bones are not usually affected[39]. The fifth metatarsal, cuboid bone and three cuneiform bones form a structure called the transverse or ‘Roman arch’ and together form the Lisfranc joint, which enables articulation between the midfoot and forefoot. In view of the fact that the midfoot is a very difficult place to image, radiography is not a sufficient testing method for diagnosis. If a fatigue fracture is suspected with accompanying midfoot pain and swelling, more advanced imaging techniques, such as CT and MRI, should be considered to determine whether there is a stress fracture[39]. Regarding fatigue fractures of the cuneiform bones, two main forces should be taken into account. Due to the location of the cuneiform bones in the midfoot, bending and shear forces are applied across them.
The sacral bone is the keystone arch of the pelvis. As in the case of calcaneal stress fractures, a high level of discernment, suspicion and precision is required for the diagnosis of sacrum fatigue fractures. A comprehensive review of the current literature presented by Yoder et al[40] presents a set of pertinent risk factors associated with sacral stress fractures. For patients who exhibit risk factors and report the insidious onset of lower-back and buttock pain, the possibility of a sacral stress fracture should be considered. High-risk patients, such as female running athletes, are particularly noteworthy[41-43]. Unfortunately, these types of fractures are underreported, which is likely, in part, due to the initial physical examination findings, which are frequently cursory and often not localized to the sacral bone. Also, in the case of female athletes, some studies[44,45] indicated that regular use of the oral contraceptive pill (OCP) may protect against stress fracture occurrence since menstrual disturbances were assumed to directly relate to decreased bone mineral density[46]. On the other hand, based on the review of literature, there have been no randomized studies which show that the use of OCP reduces the stress fracture rate in female athletes, therefore the risk of stress fracture in such sport participants is not known[47]. In addition, this result is partially caused by a mismatch between the type of diagnostic imaging used and type required to diagnose the disease. Digital radiography seldom reveals a sacral stress fracture and therefore, in this case, should not be considered the primary diagnostic method. In CT images of the fracture area, the characteristic appearances are lucency or thickening of the cortical bone and focal linear sclerosis of the cancellous bone. MRI is most appropriate for diagnosing fatigue fractures. A distinctive feature of sacral stress fractures in MRI scans is an area of decreased signal intensity on the T1-weighted images in the superior portion of the sacral bone, while in T2-weighted images, a high signal intensity corresponding to the fracture line is visible, often surrounding a linear region of low signal intensity[40,48].
It has also been highlighted that stress injuries can occur with normal strain. Athletes experience abnormally high levels of physiologic strain in activities but also exhibit risk factors for bone failure, which puts some subpopulations of athletes at an increased risk of fractures[49]. Neither amateur nor professional athletes perform compensatory training. The main objective of compensatory training is synergistic movements and the compensation of existing disproportions between the use of and neglected parts of the body. In addition to a compensation effect, it also has an analgesic influence, as it can reduce or even eliminate the sensation of pain.
Due to the types of recreational sports activities currently performed, injuries and illnesses are less commonly reported in specific populations, such as professional athletes, than in nonprofessional sports participants. Injury mainly occur due to improper or even a total lack of preparation of the body for physical activity. A lack of motor preparation is often observed in amateurs who initiate intense workouts, often without proper knowledge or specialist support. People practicing sports more professionally, in a single sport discipline, may have certain motor skills related to motor development to become more specialized, often to the detriment of primary motor skill development[50,51].
Another possible factor that should be considered in the pathophysiology of stress fractures, particularly in athletes, is the influence of skeletal muscles. In the literature, it has been noted that muscles can protect the tibia during running by producing shear forces that counteract the joint reaction forces. Various authors[52,53] have hypothesized that reduced lower leg muscle strength increases the risk of stress fractures. Therefore, a biomechanical analysis with a large sample size should be conducted, particularly in professional athletes. Currently, the utilization of advanced multisensory recording systems, which allow motion data to be recorded (called motion capture systems), and even wireless surface electromyography or textile-based electromyography have been introduced for the clinical analysis, rehabilitation, evaluation and recording of the human balance system[54-57].
In addition to all these strengths, the study also has its weaknesses. Since the data were retrospectively collected, bone healing (based on diagnostic images) cannot be evaluated because not all patients attended the control visits. Additionally, not all patients had their vitamin D level tested; based on our retrospective case series report and the currently available information, we emphasized the need to verify patients’ vitamin D levels. The influences of treatment methods used in our study (such as the application of platelet-rich plasma or an ultrasound bone healing system) are unpredictable, since they did not have the same affects for all patients; treatments should be individualized according to each patient’s clinical condition, severity of injury and financial status.
CONCLUSION
Advanced diagnostic imaging techniques, including CT and MRI, are advantageous in identifying stress fractures. The type of therapeutic procedure selected should depend to a large extent on the specific clinical case, including the patient's condition and level of physical activity. A variety of diagnostic tools are available, and the modern tools enable faster diagnoses than the traditional tools, thereby increasing the accuracy of diagnoses and treatment effectiveness. The inclusion of vitamin D tests, in addition to standard medical imaging methods, at the diagnostic stage when a fatigue fracture is suspected can provide preliminary information about the possible causes of the symptoms and, more importantly, their nature, allowing the appropriate therapeutic treatment to be selected.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Sports Traumatology, Knee Surgery & Arthroscopy, 42947684.
Peer-review started: April 24, 2020
First decision: May 15, 2020
Article in press: August 20, 2020
Specialty type: Orthopedics
Country/Territory of origin: Poland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bäcker HC S-Editor: Wang DM L-Editor: A P-Editor: Li JH
Contributor Information
Krzysztof Ficek, Department of Science, Innovation and Development, Galen-Orthopaedics, Bierun 43-150, Poland; Department of Physiotherapy, The Jerzy Kukuczka Academy of Physical Education, Katowice 40-065, Poland. krzysztof.ficek@galen.pl.
Paulina Cyganik, Industry Cooperation Department, University of Silesia, Katowice 40-007, Poland.
Jolanta Rajca, Department of Science, Innovation and Development, Galen-Orthopaedics, Bierun 43-150, Poland.
Agnieszka Racut, Department of Science, Innovation and Development, Galen-Orthopaedics, Bierun 43-150, Poland.
Aleksandra Kiełtyka, Diagnostic Imaging Department, Helimed Diagnostic Imaging, Katowice 40-760, Poland.
Jerzy Grzywocz, Department of Spine Surgery, District Hospital of Orthopedics and Trauma Surgery, Piekary Śląskie 41-940, Poland.
Grzegorz Hajduk, Department of Science, Innovation and Development, Galen-Orthopaedics, Bierun 43-150, Poland.
References
- 1.Ekstrand J, Torstveit MK. Stress fractures in elite male football players. Scand J Med Sci Sports. 2012;22:341–346. doi: 10.1111/j.1600-0838.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- 2.Eggink KM, Raven EEJ, Bullens P. Fatigue fracture of the calcaneus: A case report. Clin Res Foot Ankle. 2014;2:2–5. [Google Scholar]
- 3.Astur DC, Zanatta F, Arliani GG, Moraes ER, Pochini Ade C, Ejnisman B. Stress fractures: definition, diagnosis and treatment. Rev Bras Ortop. 2016;51:3–10. doi: 10.1016/j.rboe.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romani WA, Gieck JH, Perrin DH, Saliba EN, Kahler DM. Mechanisms and management of stress fractures in physically active persons. J Athl Train. 2002;37:306–314. [PMC free article] [PubMed] [Google Scholar]
- 5.Miller TL, Kaeding CC. Stress Fractures in Athletes. Diagnosis and management. 1st ed. London: Springer International Publishing, 2015. [Google Scholar]
- 6.Serrano S, Figueiredo P, Páscoa Pinheiro J. Fatigue Fracture of the Calcaneus: From Early Diagnosis to Treatment: A Case Report of a Triathlon Athlete. Am J Phys Med Rehabil. 2016;95:e79–e83. doi: 10.1097/PHM.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 7.Deschamps Perdomo A, Tome-Bermejo F, Piñera AR, Alvarez L. Misdiagnosis of sacral stress fracture: an underestimated cause of low back pain in pregnancy? Am J Case Rep. 2015;16:60–64. doi: 10.12659/AJCR.892631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck TJ, Ruff CB, Shaffer RA, Betsinger K, Trone DW, Brodine SK. Stress fracture in military recruits: gender differences in muscle and bone susceptibility factors. Bone. 2000;27:437–444. doi: 10.1016/s8756-3282(00)00342-2. [DOI] [PubMed] [Google Scholar]
- 9.Milgrom C, Giladi M, Stein M, Kashtan H, Margulies JY, Chisin R, Steinberg R, Aharonson Z. Stress fractures in military recruits. A prospective study showing an unusually high incidence. J Bone Joint Surg Br. 1985;67:732–735. doi: 10.1302/0301-620X.67B5.4055871. [DOI] [PubMed] [Google Scholar]
- 10.McInnis KC, Ramey LN. High-Risk Stress Fractures: Diagnosis and Management. PM R. 2016;8:S113–S124. doi: 10.1016/j.pmrj.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Matcuk GR, Jr, Mahanty SR, Skalski MR, Patel DB, White EA, Gottsegen CJ. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emerg Radiol. 2016;23:365–375. doi: 10.1007/s10140-016-1390-5. [DOI] [PubMed] [Google Scholar]
- 12.Tomlinson RE, McKenzie JA, Schmieder AH, Wohl GR, Lanza GM, Silva MJ. Angiogenesis is required for stress fracture healing in rats. Bone. 2013;52:212–219. doi: 10.1016/j.bone.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fullem BW. Managing stress fractures in athletes. Pod Today. 2012;25:54–60. [Google Scholar]
- 14.Denis F, Davis S, Comfort T. Sacral fractures: an important problem. Retrospective analysis of 236 cases. Clin Orthop Relat Res. 1988;227:67–81. [PubMed] [Google Scholar]
- 15.Kiszka J, Gutkowski K. Złamania miednicy u dorosłych: Epidemiologia, klasyfikacja, diagnostyka i leczenie. Prz Med Uniw Rzesz. 2010;1:87–93. [Google Scholar]
- 16.Kaeding CC, Miller T. The comprehensive description of stress fractures: a new classification system. J Bone Joint Surg Am. 2013;95:1214–1220. doi: 10.2106/JBJS.L.00890. [DOI] [PubMed] [Google Scholar]
- 17.Lana JFSD, Santana MHA, Belangero WD, Luzo ACM. Platelet-rich plasma. Regenerative medicine: Sports medicine, orthopedic, and recovery of musculoskeletal injuries. Berlin, Heidelberg: Springer, 2014. [Google Scholar]
- 18.Knapik JJ, Reynolds K, Hoedebecke KL. Stress Fractures: Etiology, Epidemiology, Diagnosis, Treatment, and Prevention. J Spec Oper Med. Summer;17:120–130. doi: 10.55460/SPMB-1E6L. [DOI] [PubMed] [Google Scholar]
- 19.Miller TL, Kaeding CC, Rodeo SA. Emerging Options for Biologic Enhancement of Stress Fracture Healing in Athletes. J Am Acad Orthop Surg. 2020;28:1–9. doi: 10.5435/JAAOS-D-19-00112. [DOI] [PubMed] [Google Scholar]
- 20.Calcei JG, Rodeo SA. Orthobiologics for Bone Healing. Clin Sports Med. 2019;38:79–95. doi: 10.1016/j.csm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra R, Kumar V, Garg B, Singh R, Jain V, Coshic P, Chatterjee K. Role of autologous platelet-rich plasma in treatment of long-bone nonunions: a prospective study. Musculoskelet Surg. 2015;99:243–248. doi: 10.1007/s12306-015-0378-8. [DOI] [PubMed] [Google Scholar]
- 22.Busse JW, Kaur J, Mollon B, Bhandari M, Tornetta P 3rd, Schünemann HJ, Guyatt GH. Low intensity pulsed ultrasonography for fractures: systematic review of randomised controlled trials. BMJ. 2009;338:b351. doi: 10.1136/bmj.b351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rome K. Edinburgh: Churchill Livingstone Elsevier; 2015. Management of chronic conditions in the foot and lower leg. 1st ed; pp. 180–213. [Google Scholar]
- 24.Rue JP, Armstrong DW, Frassica FJ, et al. The effect of pulsed ultrasound in the treatment of tibial stress fractures. Orthopedics. 2004;27:1192–1195. doi: 10.3928/0147-7447-20041101-18. [DOI] [PubMed] [Google Scholar]
- 25.Nye NS, Covey CJ, Sheldon L, Webber B, Pawlak M, Boden B, Beutler A. Improving Diagnostic Accuracy and Efficiency of Suspected Bone Stress Injuries. Sports Health. 2016;8:278–283. doi: 10.1177/1941738116635558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts CL, Meyering CD, Zychowicz ME. Improving the management of tibia stress fractures: a collaborative, outpatient clinic-based quality improvement project. Orthop Nurs. 2014;33:75–83; quiz 84-5. doi: 10.1097/NOR.0000000000000032. [DOI] [PubMed] [Google Scholar]
- 27.Scott SJ, Feltwell DN, Knapik JJ, Barkley CB, Hauret KG, Bullock SH, Evans RK. A multiple intervention strategy for reducing femoral neck stress injuries and other serious overuse injuries in U.S. Army Basic Combat Training. Mil Med. 2012;177:1081–1089. doi: 10.7205/milmed-d-12-00085. [DOI] [PubMed] [Google Scholar]
- 28.Wright AA, Hegedus EJ, Lenchik L, Kuhn KJ, Santiago L, Smoliga JM. Diagnostic Accuracy of Various Imaging Modalities for Suspected Lower Extremity Stress Fractures: A Systematic Review With Evidence-Based Recommendations for Clinical Practice. Am J Sports Med. 2016;44:255–263. doi: 10.1177/0363546515574066. [DOI] [PubMed] [Google Scholar]
- 29.Patel DS, Roth M, Kapil N. Stress fractures: diagnosis, treatment, and prevention. Am Fam Physician. 2011;83:39–46. [PubMed] [Google Scholar]
- 30.Ogan D, Pritchett K. Vitamin D and the athlete: risks, recommendations, and benefits. Nutrients. 2013;5:1856–1868. doi: 10.3390/nu5061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. J Bone Miner Res. 2008;23:741–749. doi: 10.1359/jbmr.080102. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs JM, Cameron KL, Bojescul JA. Lower extremity stress fractures in the military. Clin Sports Med. 2014;33:591–613. doi: 10.1016/j.csm.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Shindle MK, Endo Y, Warren RF, Lane JM, Helfet DL, Schwartz EN, Ellis SJ. Stress fractures about the tibia, foot, and ankle. J Am Acad Orthop Surg. 2012;20:167–176. doi: 10.5435/JAAOS-20-03-167. [DOI] [PubMed] [Google Scholar]
- 34.Tins BJ, Garton M, Cassar-Pullicino VN, Tyrrell PN, Lalam R, Singh J. Stress fracture of the pelvis and lower limbs including atypical femoral fractures-a review. Insights Imaging. 2015;6:97–110. doi: 10.1007/s13244-014-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feldman D. Volume 1: Biochemistry, Physiology and Diagnostics. 4th ed. Academic Press; 2017. Vitamin D; pp. 925–937. [Google Scholar]
- 36.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 37.Jastrzębska M, Kaczmarczyk M, Michalczyk M, Radzimiński Ł, Stępień P, Jastrzębska J, Wakuluk D, Suárez AD, López Sánchez GF, Cięszczyk P, Godlewski P, Król P, Jastrzębski Z. Can Supplementation of Vitamin D Improve Aerobic Capacity in Well Trained Youth Soccer Players? J Hum Kinet. 2018;61:63–72. doi: 10.2478/hukin-2018-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger FH, de Jonge MC, Maas M. Stress fractures in the lower extremity. The importance of increasing awareness amongst radiologists. Eur J Radiol. 2007;62:16–26. doi: 10.1016/j.ejrad.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Williams AA, DesJardins CE, Wilckens JH. Stress Fracture of the Lateral Cuneiform Bone in a Lacrosse Player. JBJS Case Connect. 2013;3:e31–ee3. doi: 10.2106/JBJS.CC.L.00230. [DOI] [PubMed] [Google Scholar]
- 40.Yoder K, Bartsokas J, Averell K, McBride E, Long C, Cook C. Risk factors associated with sacral stress fractures: a systematic review. J Man Manip Ther. 2015;23:84–92. doi: 10.1179/2042618613Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duckham RL, Peirce N, Meyer C, Summers GD, Cameron N, Brooke-Wavell K. Risk factors for stress fracture in female endurance athletes: a cross-sectional study. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen YT, Tenforde AS, Fredericson M. Update on stress fractures in female athletes: epidemiology, treatment, and prevention. Curr Rev Musculoskelet Med. 2013;6:173–181. doi: 10.1007/s12178-013-9167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joy EA, Campbell D. Stress fractures in the female athlete. Curr Sports Med Rep. 2005;4:323–328. doi: 10.1097/01.csmr.0000306294.72578.a8. [DOI] [PubMed] [Google Scholar]
- 44.Barrow GW, Saha S. Menstrual irregularity and stress fractures in collegiate female distance runners. Am J Sports Med. 1988;16:209–216. doi: 10.1177/036354658801600302. [DOI] [PubMed] [Google Scholar]
- 45.Myburgh KH, Hutchins J, Fataar AB, Hough SF, Noakes TD. Low bone density is an etiologic factor for stress fractures in athletes. Ann Intern Med. 1990;113:754–759. doi: 10.7326/0003-4819-113-10-754. [DOI] [PubMed] [Google Scholar]
- 46.Porter DA, Schon L. 2008. Baxter’s the foot and ankle in sport. 2nd ed. Elsevier; pp. 45–72. [Google Scholar]
- 47.Burr D, Milgrom C. New York: CRC Press; 2001. Musculoskeletal fatigue and stress fractures. 1st ed; pp. 35–54. [Google Scholar]
- 48.Kilcoyne A, Kavanagh EC. Unusual presentation of sacral fatigue fractures. Spine J. 2014;14:1063–1064. doi: 10.1016/j.spinee.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 49.Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17:309–325. doi: 10.1097/RMR.0b013e3180421c8c. [DOI] [PubMed] [Google Scholar]
- 50.Brukner PD, Bennell KL. Stress fractures in runners. J Back Musculoskelet Rehabil. 1995;5:341–351. doi: 10.3233/BMR-1995-5409. [DOI] [PubMed] [Google Scholar]
- 51.Kupferer KR, Bush DM, Cornell JE, Lawrence VA, Alexander JL, Ramos RG, Curtis D. Femoral neck stress fracture in Air Force basic trainees. Mil Med. 2014;179:56–61. doi: 10.7205/MILMED-D-13-00154. [DOI] [PubMed] [Google Scholar]
- 52.Haris Phuah A, Schache AG, Crossley KM, Wrigley TV, Creaby MW. Sagittal plane bending moments acting on the lower leg during running. Gait Posture. 2010;31:218–222. doi: 10.1016/j.gaitpost.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Sasimontonkul S, Bay BK, Pavol MJ. Bone contact forces on the distal tibia during the stance phase of running. J Biomech. 2007;40:3503–3509. doi: 10.1016/j.jbiomech.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 54.McGinley JL, Baker R, Wolfe R, Morris ME. The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture. 2009;29:360–369. doi: 10.1016/j.gaitpost.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 55.Milner CE, Ferber R, Pollard CD, Hamill J, Davis IS. Biomechanical factors associated with tibial stress fracture in female runners. Med Sci Sports Exerc. 2006;38:323–328. doi: 10.1249/01.mss.0000183477.75808.92. [DOI] [PubMed] [Google Scholar]
- 56.Postolache O, Mukhopadhyay S, Jayasundera K, Swain A. Sensors for everyday life: Healthcare Settings. 1st ed. Lisbon: Springer International Publishing; 2017. pp. 185–198. [Google Scholar]
- 57.Sakata T, Kimura Y, Hida T. First rib stress fracture in a sidearm baseball pitcher: a case report. J Sports Sci Med. 2005;4:201–207. [PMC free article] [PubMed] [Google Scholar]