Abstract
Background
Cancer seems to have an independent adverse prognostic effect on COVID-19-related mortality, but uncertainty exists regarding its effect across different patient subgroups. We report a population-based analysis of patients hospitalised with COVID-19 with prior or current solid cancer versus those without cancer.
Methods
We analysed data of adult patients registered until 24 May 2020 in the Belgian nationwide database of Sciensano. The primary objective was in-hospital mortality within 30 days of COVID-19 diagnosis among patients with solid cancer versus patients without cancer. Severe event occurrence, a composite of intensive care unit admission, invasive ventilation and/or death, was a secondary objective. These endpoints were analysed across different patient subgroups. Multivariable logistic regression models were used to analyse the association between cancer and clinical characteristics (baseline analysis) and the effect of cancer on in-hospital mortality and on severe event occurrence, adjusting for clinical characteristics (in-hospital analysis).
Results
A total of 13 594 patients (of whom 1187 with solid cancer (8.7%)) were evaluable for the baseline analysis and 10 486 (892 with solid cancer (8.5%)) for the in-hospital analysis. Patients with cancer were older and presented with less symptoms/signs and lung imaging alterations. The 30-day in-hospital mortality was higher in patients with solid cancer compared with patients without cancer (31.7% vs 20.0%, respectively; adjusted OR (aOR) 1.34; 95% CI 1.13 to 1.58). The aOR was 3.84 (95% CI 1.94 to 7.59) among younger patients (<60 years) and 2.27 (95% CI 1.41 to 3.64) among patients without other comorbidities. Severe event occurrence was similar in both groups (36.7% vs 28.8%; aOR 1.10; 95% CI 0.95 to 1.29).
Conclusions
This population-based analysis demonstrates that solid cancer is an independent adverse prognostic factor for in-hospital mortality among patients with COVID-19. This adverse effect was more pronounced among younger patients and those without other comorbidities. Patients with solid cancer should be prioritised in vaccination campaigns and in tailored containment measurements.
Keywords: COVID-19, cancer, mortality, pandemic, health policy
Key questions.
What is already known about this subject?
-
•
Cancer seems to have an independent adverse prognostic effect on COVID-19-related mortality, but uncertainty exists regarding its effect across different patient subgroups. This knowledge is necessary, in order to provide tailored healthcare management to these patients.
What does this study add?
-
•
In this population-based nationwide cohort study, we observed that hospitalised patients with solid cancer and COVID-19 were older and presented with fewer symptoms/signs and lung imaging alterations compared with patients without cancer. Moreover, hospitalised patients with solid cancer had a 34% higher chance of dying within 30 days of COVID-19 diagnosis compared with patients without cancer. This adverse prognostic effect was more pronounced among patients <60 years and/or those without other comorbidities.
How might this impact on clinical practice?
-
•
We demonstrate that solid cancer is an independent adverse prognostic factor for 30-day in-hospital mortality among patients with COVID-19, especially among those with a favourable baseline prognosis. Patients with solid cancer should be prioritised in vaccination campaigns and in tailored containment measurements and clinicians should be aware of this adverse prognostic effect when discussing cancer treatment plans, in case a new surge of COVID-19 cases is seen in their region.
Alt-text: Unlabelled Box
Introduction
The betacoronavirus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), causing the disease COVID-19, was first detected in Wuhan, China, by the end of 2019 and has spread across the world.1 The first case in Belgium was identified on 15 February 2020 and as of 6 August 2020, 70 555 cases of COVID-19 and 9852 deaths have been reported in the country.2 The first reports from China raised concerns regarding a possible higher risk and worse outcome of COVID-19 among patients with cancer.3., 4. In a cohort of over 20 000 patients hospitalised with COVID-19 in the UK, 10% had a malignancy and their adjusted HR for hospital death was 1.13 compared with patients without cancer (95% CI 1.02 to 1.24).5 In another report including 928 patients with cancer and COVID-19, 30-day mortality was 13% and independent adverse prognostic factors were advanced age, male gender, being a former smoker, number of comorbidities, Eastern Cooperative Oncology Group performance status of ≥2 and having an active cancer.6
Notwithstanding these findings, more data are urgently needed to explore the vulnerability of patients with cancer during the COVID-19 pandemic and to provide tailored healthcare management to this population. We performed a population-based nationwide analysis among patients hospitalised with COVID-19 in Belgium, to investigate the baseline characteristics, clinical presentation and outcomes of patients with a solid cancer compared with patients without cancer, overall and among different patient subgroups.
Methods
Data source
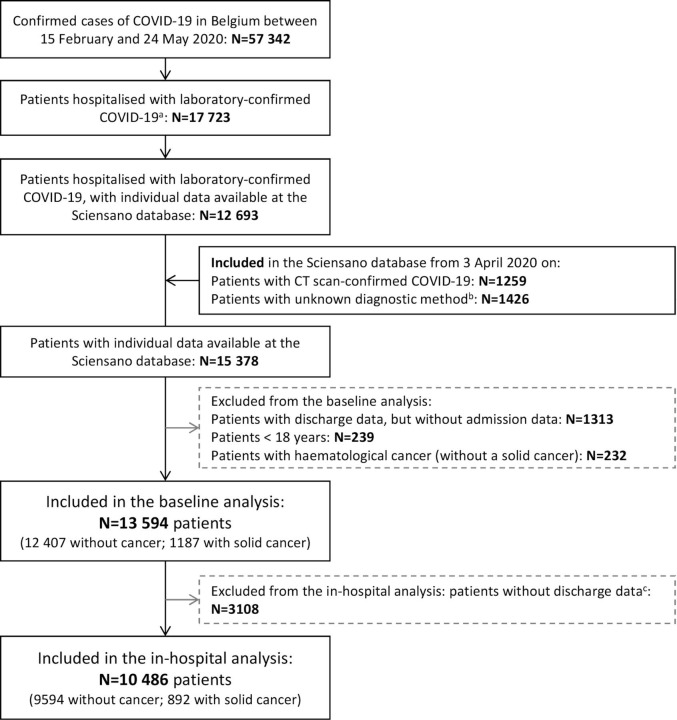
We analysed an anonymised subset of data from the nationwide population-based surveillance database of the Belgian public health institute Sciensano.7., 8. As part of its mission of national surveillance of infectious diseases, Sciensano collects/retrieves data from records of patients hospitalised with COVID-19, in particular patient demographics, comorbidities (including history of solid cancer), clinical presentation and outcomes. For each subject, data are captured at admission and at discharge/death through two different questionnaires. Although completion of these questionnaires is not mandatory, the database contains information on more than 70% of all patients hospitalised with COVID-19 in Belgium and is completed by more than 100 hospitals (figure 1 ). Data from Sciensano was shared with the Belgian Society of Medical Oncology on 26 May 2020 through a secured data transfer platform applying data encryption. The data set contained information collected until 24 May 2020.
Figure 1.
Flowchart of included patients up to 24 May 2020. aTotal number of new hospitalisations between 15 February 2020 and 24 May 2020 of patients with laboratory-confirmed COVID-19 at the moment of reporting and that were not referred from another hospital. bAmong these patients, 1313 did not have data on the admission questionnaire and, therefore, there was no information on the diagnostic method, as the method was only asked in the admission questionnaire; these patients were excluded from both baseline and in-hospital analysis. cUnavailability of discharge data may be due to different reasons: (I) the patient was still hospitalised at the time of data transfer; (II) the patient had already been discharged, but the discharge questionnaire had not yet been filled; and (III) the patient had already been discharged, the discharge questionnaire had been filled, but it could not be linked to the admission questionnaire (eg, due to errors on the insertion of the patient’s identifying information).
Patient population
Information on demographics, comorbidities, clinical presentation at hospital admission/COVID-19 diagnosis, method of COVID-19 diagnosis, administered treatments, clinical evolution and outcomes were included in this analysis. Adults (individuals ≥18 years) for whom prior or current solid cancer was reported were included in the cancer group and those without a history of solid or haematological malignancy were included in the non-cancer group. Diagnosis of COVID-19 was based on a molecular test (PCR or antigen test) and/or suggestive imaging alterations on chest CT scan combined with typical clinical presentation of COVID-19.
Analysis overview
Our primary objective was to compare in-hospital mortality within 30 days of COVID-19 diagnosis among patients with solid cancer versus those without cancer, adjusted for clinical characteristics. In addition, we compared baseline characteristics, clinical and imaging manifestations of COVID-19, clinical evolution and treatments administered during hospitalisation between the two groups. We also evaluated the occurrence of a severe event, a composite of intensive care unit (ICU) admission, invasive ventilation use and/or death. Baseline characteristics and presentation of COVID-19 were assessed in the entire population (baseline analysis), while clinical evolution, 30-day in-hospital mortality and severe event occurrence were only evaluated among patients with discharge data (in-hospital analysis). This study is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.9
Statistical analysis
Categorical variables were shown as frequencies with percentages and continuous variables as medians with IQR or means with SD. Baseline patient characteristics, clinical presentation, evolution and COVID-19-directed treatments during hospitalisation were compared between the two groups using χ2 or Fisher’s exact tests for categorical variables and Mann-Whitney U or two-sample t-tests for continuous variables. Multivariable logistic regression models were used to analyse the association of solid cancer with baseline characteristics and clinical/imaging manifestations of COVID-19, while correcting for age and gender (baseline analysis); and with clinical evolution and treatments administered during hospitalisation, adjusting for age, gender, comorbidities and the use of renin-angiotensin-aldosterone system inhibitors (RAASi) at the time of COVID-19 diagnosis (in-hospital analysis). Results are reported as ORs with 95% CI. A sensitivity analysis was performed excluding patients with symptom onset after admission (ie, who were hospitalised due to other reasons and were diagnosed with COVID-19 during hospitalisation).
A multivariable logistic regression analysis was performed to assess the effect of cancer on 30-day all-cause in-hospital mortality and severe event occurrence, adjusting for age, gender, comorbidities and RAASi use. These analyses were performed for patients with discharge data, overall and across patient subgroups. Baseline characteristics with a missingness ≥10% were not included in the multivariable model and a complete case analysis was performed. All tests were two-sided and results with a p value <0.05 were considered statistically significant. Given the exploratory nature of the secondary and subgroup analyses, no multiple testing correction was applied. Analyses were carried out in SAS V.3.8 statistical software (SAS Institute Inc, Cary, North Carolina, USA). Detailed information regarding the statistical analysis is provided in the online supplemental methods and online supplemental table 1.

Results
Baseline analysis
On 24 May 2020, 57 342 individuals had a laboratory-confirmed diagnosis of COVID-19 in Belgium, of whom 17 052 were or had been hospitalised. From 3 April 2020 on, CT scan-confirmed cases were also included in the Sciensano database, and by 24 May 2020, 15 378 hospitalised patients with laboratory-confirmed or CT-confirmed COVID-19 had data recorded in the database. We excluded 1313 patients without admission data, 239 subjects <18 years old and 232 patients with haematological malignancies (without a solid tumour). A total of 13 594 patients were evaluable for the baseline analysis (12 407 patients without and 1187 patients with cancer) and 10 486 for the in-hospital analysis (9594 patients without and 892 patients with cancer) (figure 1). Patients included in the in-hospital analysis were admitted earlier in time and were slightly younger than patients without available discharge data (mean 67.8 vs 70.1 years, respectively), but there were no significant differences regarding gender and most comorbidities (online supplemental figure 1 and online supplemental table 2). Diagnosis of COVID-19 was based on molecular testing in 12 446 patients (91.6%) and on imaging and clinical findings in 1038 patients (7.6%), while diagnostic modality was unknown for 110 patients (0.8%).
Patients with cancer were older and more frequently were men compared with patients without cancer. When adjusting for age and gender, patients with cancer had more chronic lung and liver diseases, were more often considered immunosuppressed, more likely to be current smokers and had more frequently received influenza vaccination compared with patients without cancer (table 1 ). Results were similar among the subset of patients with both admission and discharge data (online supplemental table 3).
Table 1.
Baseline characteristics and COVID-19 clinical presentation of patients included in the baseline analysis (n=13 594)
| Patients without cancer (n=12 407) | Patients with solid cancer (n=1187) | P value* | Adjusted OR (95% CI)† | |
|---|---|---|---|---|
| Age, in years | ||||
| Mean (SD) | 67.7 (17.1) | 74.5 (11.8) | <0.001 | -- |
| Median (IQR) | 70 (55 to 82) | 75 (67 to 83) | ||
| Age, in years - n (%) | ||||
| <50 | 1952 (15.7) | 34 (2.9) | <0.001 | 1 |
| 50–59 | 2002 (16.4) | 97 (8.2) | 2.83 (1.90 to 4.22) | |
| 60–69 | 2139 (17.2) | 240 (20.2) | 6.55 (4.53 to 9.48) | |
| 70–79 | 2463 (19.9) | 369 (31.1) | 8.78 (6.12 to 12.60) | |
| 80–89 | 2976 (19.9) | 343 (28.9) | 4.82 (4.75 to 9.79) | |
| ≥90 | 875 (7.1) | 104 (8.8) | 7.08 (4.74 to 10.56) | |
| Gender - n (%) | ||||
| Female | 5777 (46.9) | 528 (44.8) | 0.16 | 1 |
| Male | 6529 (53.1) | 55.2 (54.8) | 1.20 (1.06 to 1.63) | |
| Missing | 101 | 8 | ||
| Comorbidities (presence) - n (%) | ||||
| Cardiovascular disease | 4182 (33.7) | 497 (41.9) | <0.001 | 1.00 (0.88 to 1.14) |
| Hypertension | 4931 (39.7) | 511 (43.1) | 0.03 | 0.89 (0.79 to 1.01) |
| Diabetes | 2705 (21.8) | 269 (22.7) | 0.49 | 0.94 (0.81 to 1.09) |
| Chronic kidney disease | 1548 (12.5) | 188 (15.8) | 0.001 | 1.00 (0.84 to 1.18) |
| Chronic liver disease | 309 (2.5) | 52 (4.4) | <0.001 | 1.90 (1.41 to 2.58) |
| Chronic lung disease | 1777 (14.3) | 261 (22.0) | <0.001 | 1.55 (1.33 to 1.80) |
| Chronic neurological disease | 1099 (8.9) | 112 (9.4) | 0.49 | 0.93 (0.76 to 115) |
| Cognitive disorder | 1441 (11.7) | 119 (10.1) | 0.11 | 0.61 (0.50 to 0.75) |
| Missing | 62 | 5 | ||
| Immunosuppression, including HIV | 254 (2.1) | 64 (5.4) | <0.001 | 3.32 (2.49 to 4.42) |
| Haematological cancer | 0 | 30 (2.5) | <0.001 | NC |
| Pregnancy | 102 (0.8) | 0 | 0.002 | NC |
| Postpartum (<6 weeks) | 15 (0.1) | 0 | 0.64 | NC |
| Obesity | 822 (10.4) | 61 (7.1) | 0.002 | 0.87 (0.66 to 1.13) |
| Missing | 4533 | 326 | ||
| Other comorbidities | 1017 (14.1) | 89 (11.2) | 0.02 | 0.97 (0.77 to 1.22) |
| Missing | 5207 | 389 | ||
| No comorbidities‡ | 3019 (24.3) | 221 (18.6) | <0.001 | 0.93 (0.79 to 1.10) |
| Number of comorbidities‡- n (%) | ||||
| 0 | 3019 (24.3) | 221 (18.6) | <0.001 | 1 |
| 1 | 3518 (28.4) | 295 (24.9) | 0.87 (0.72 to 1.05) | |
| 2 | 2787 (22.5) | 294 (24.8) | 0.93 (0.77 to 1.13) | |
| ≥3 | 3083 (24.9) | 377 (31.8) | 1.01 (0.83 to 1.22) | |
| Current smoker - n (%) | ||||
| No | 5936 (47.8) | 569 (47.9) | <0.001 | 1 |
| Yes | 660 (5.3) | 100 (8.4) | 1.87 (1.48 to 2.36) | |
| Unknown | 5811 (46.8) | 518 (43.6) | 0.93 (0.82 to 1.06) | |
| Influenza vaccination (2019/2020 season) - n (%) | ||||
| No | 913 (7.4) | 43 (3.6) | <0.001 | 1 |
| Yes | 825 (6.7) | 94 (7.9) | 1.88 (1.28 to 2.42) | |
| Unknown | 10 669 (86.0) | 1050 (88.5) | 1.76 (1.28 to 2.75) | |
| RAASi use - n (%) | ||||
| No/unknown | 9479 (76.4) | 867 (73.0) | 0.01 | 1 |
| Yes | 2928 (23.6) | 320 (27.0) | 1.00 (0.87 to 1.15) | |
| Timing of symptoms onset - n (%) | ||||
| Before or day of hospitalisation | 11 680 (94.1) | 1030 (86.8) | <0.001 | 1 |
| During hospitalisation | 727 (5.9) | 157 (13.2) | 2.06 (1.71 to 2.48) | |
| Symptoms at presentation§- n (%) | ||||
| Systemic symptoms | 9120 (73.5) | 776 (65.4) | <0.001 | 0.75 (0.66 to 0.85) |
| Respiratory symptoms | 9037 (72.8) | 740 (62.3) | <0.001 | 0.70 (0.62 to 0.80) |
| Gastrointestinal symptoms | 2655 (21.4) | 206 (17.4) | 0.001 | 0.87 (0.74 to 1.02) |
| Neurological symptoms | 2146 (17.3) | 143 (12.1) | <0.001 | 0.68 (0.56 to 0.81) |
| Pain | 2740 (22.1) | 208 (17.5) | <0.001 | 0.95 (0.81 to 1.12) |
| No symptoms | 683 (5.5) | 100 (8.4) | <0.001 | 1.46 (1.17 to 1.83) |
| Signs at presentation¶- n (%) | ||||
| Respiratory signs | 10 064 (81.1) | 900 (75.8) | <0.001 | 0.76 (0.66 to 0.88) |
| Neurological signs | 104 (0.8) | 9 (0.8) | 0.77 | 0.83 (0.42 to 1.66) |
| Temperature ≥38°C (fever) | 3717 (30.0) | 285 (24.0) | <0.001 | 0.79 (0.68 to 0.90) |
| No signs | 1534 (12.4) | 197 (16.6) | <0.001 | 1.39 (1.18 to 1.64) |
NC, not calculable; RAASi, renin-angiotensin-aldosterone system inhibitors.
P value for the univariate comparison between the two groups (patients with solid cancer vs without cancer).
OR for age (as a categorical variable) was adjusted for gender; OR for gender was adjusted for age (as a continuous variable); all other ORs were adjusted for age (as a continuous variable) and gender.
Excluding the presence of cancer as a comorbidity.
Systemic symptoms cluster: presence of fever/chills reported by the patient and/or fatigue; respiratory symptoms cluster: presence of cough, sore throat, rhinorrhoea, anosmia and/or shortness of breath; gastrointestinal symptoms cluster: presence of diarrhoea and/or nausea/vomiting; neurological symptoms cluster: presence of headache and/or irritability/mental confusion.
Respiratory signs cluster: presence of pharyngeal exudate, dyspnoea/tachypnoea, abnormal pulmonary auscultation and/or abnormal lung imaging; neurological signs cluster: presence of coma and/or convulsions.
Patients with cancer were less likely to present with systemic, respiratory or neurological symptoms, temperature ≥38°C, respiratory signs or abnormalities consistent with COVID-19 on imaging. Overall, a higher proportion of patients with cancer were asymptomatic or had no signs at presentation compared with patients without cancer (8.4% vs 5.5%, and 16.6% vs 12.4%, respectively) (table 1 and online supplemental table 4). As patients with solid cancer had a higher likelihood of being diagnosed with COVID-19 while already hospitalised, we performed a sensitivity analysis excluding these patients, which showed similar findings (online supplemental table 5).
In-hospital analysis
Among the 10 486 patients with available discharge data, patients with cancer more frequently received corticosteroids during hospitalisation, but were less likely to receive COVID-19-directed treatment (table 2 ). Compared with patients without cancer, patients with solid cancer were less frequently admitted to an ICU (13.2% vs 8.6%, respectively; adjusted OR 0.70; 95% CI 0.55 to 0.90) and less likely to receive invasive ventilation (8.1% vs 4.9%, respectively; adjusted OR 0.67; 95% CI 0.49 to 0.93).
Table 2.
Patient evolution, treatment and outcomes during hospitalisation, among patients with discharge data (n=10 486)
| Patients without cancer (n=9594) | Patients with solid cancer (n=892) | P value* | Adjusted OR (95% CI)† | |
|---|---|---|---|---|
| Pneumonia at imaging exam‡- n (%) | ||||
| No | 1458 (15.2) | 161 (18.1) | 0.01 | 1 |
| Yes | 7679 (80.0) | 676 (75.8) | 0.83 (0.69 to 1.00) | |
| Unknown | 457 (4.8) | 55 (6.2) | 1.35 (0.95 to 1.89) | |
| ARDS - n (%) | ||||
| No | 7808 (81.4) | 726 (81.4) | 0.22 | 1 |
| Yes | 1277 (13.3) | 108 (12.1) | 0.87 (0.70 to 1.08) | |
| Unknown | 509 (5.3) | 58 (6.5) | 1.06 (0.79 to 1.43) | |
| Drugs received during hospitalisation - n (%) | ||||
| Hydroxychloroquine | 5523 (57.6) | 429 (48.1) | <0.001 | 0.75 (0.65 to 0.87) |
| Remdesivir | 7 (0.1) | 0 | 0.42 | NC |
| Lopinavir/ritonavir | 28 (0.3) | 2 (0.2) | 0.72 | 0.73 (0.17 to 3.17) |
| Tocilizumab | 29 (0.3) | 2 (0.2) | 0.68 | 1.22 (0.29 to 5.20) |
| Azithromycin | 809 (8.4) | 82 (9.2) | 0.45 | 1.26 (0.99 to 1.62) |
| Other antibiotics | 464 (4.8) | 53 (5.9) | 0.15 | 1.26 (0.93 to 1.71) |
| Other antivirals§ | 47 (0.5) | 2 (0.2) | 0.27 | 0.63 (0.15 to 2.60) |
| Corticosteroids | 597 (6.2) | 89 (10) | <0.001 | 1.31 (1.02 to 1.68) |
| Other immunomodulatory drugs¶ | 5 (0.1) | 1 (0.1) | 0.47 | 3.67 (0.41 to 32.62) |
| Anticoagulants | 47 (0.5) | 5 (0.6) | 0.77 | 0.91 (0.35 to 2.34) |
| Other drugs** | 92 (1.0) | 3 (0.3) | 0.06 | 0.35 (0.11 to 1.10) |
| No treatment reported | 3770 (39.3) | 406 (45.5) | 0.47 | 1.20 (1.04 to 1.39) |
| COVID-19-directed treatment††- n (%) | ||||
| No | 3816 (39.8) | 408 (45.7) | <0.001 | 1 |
| Yes | 5778 (60.2) | 484 (54.3) | 0.84 (0.73 to 0.98) | |
| Transfer to ICU - n (%) | ||||
| No | 8326 (86.8) | 815 (91.4) | <0.001 | 1 |
| Yes | 1268 (13.2) | 77 (8.6) | 0.70 (0.55 to 0.90) | |
| Use of invasive ventilation - n (%) | ||||
| No | 8620 (89.9) | 818 (91.7) | <0.001 | 1 |
| Yes | 772 (8.1) | 44 (4.9) | 0.67 (0.49 to 0.93) | |
| Unknown | 202 (2.1) | 30 (3.4) | 1.41 (0.94 to 2.13) | |
| Severe event occurrence within 30 days of COVID-19 diagnosis - n (%) | ||||
| No | 6827 (71.2) | 565 (63.3) | <0.001 | 1 |
| Yes | 2767 (28.8) | 327 (36.7) | 1.10 (0.95 to 1.29) | |
| Vital status within 30 days of COVID-19 diagnosis - n (%) | ||||
| Alive | 7672 (80.0) | 609 (68.3) | <0.001 | 1 |
| Deceased | 1922 (20.0) | 283 (31.7) | 1.34 (1.13 to 1.58) | |
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; NC, not calculable.
P value for the univariate comparison between the two groups (patients with solid cancer vs without cancer).
ORs adjusted for age (as a continuous variable), gender, all comorbidities (except ‘obesity’ and ‘other comorbidities’) and renin-angiotensin-aldosterone system inhibitors use.
Includes pneumonia observed at the CT-scan and/or at the thoracic X-ray.
Other antivirals include acyclovir, atazanavir, favipiravir and oseltamivir.
Other immunomodulatory drugs include anakinra (IL-1Ra), other anti-IL7 antibodies, siltuximab (anti-IL6) and immunoglobulin G.
Other drugs include vitamins (vitamin C, vitamin D, thiamine, among others), morphine, acetylcysteine, granulocyte colony-stimulating factor and anti-histaminics.
COVID-19-directed treatment includes hydroxychloroquine, remdesivir, lopinavir/ritonavir, tocilizumab, other antivirals, other immunomodulatory drugs, corticosteroids and azithromycin.
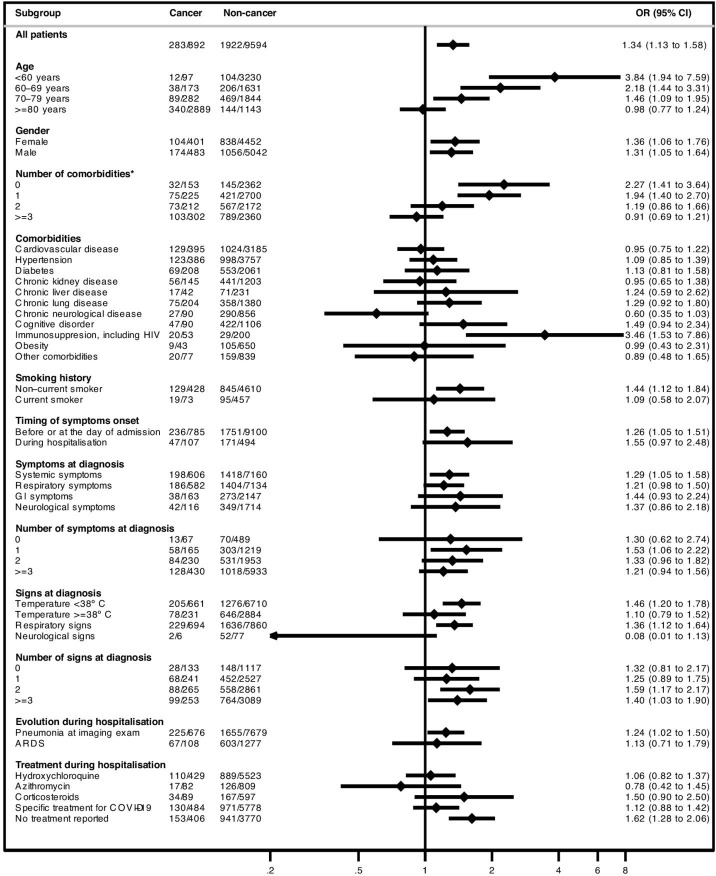
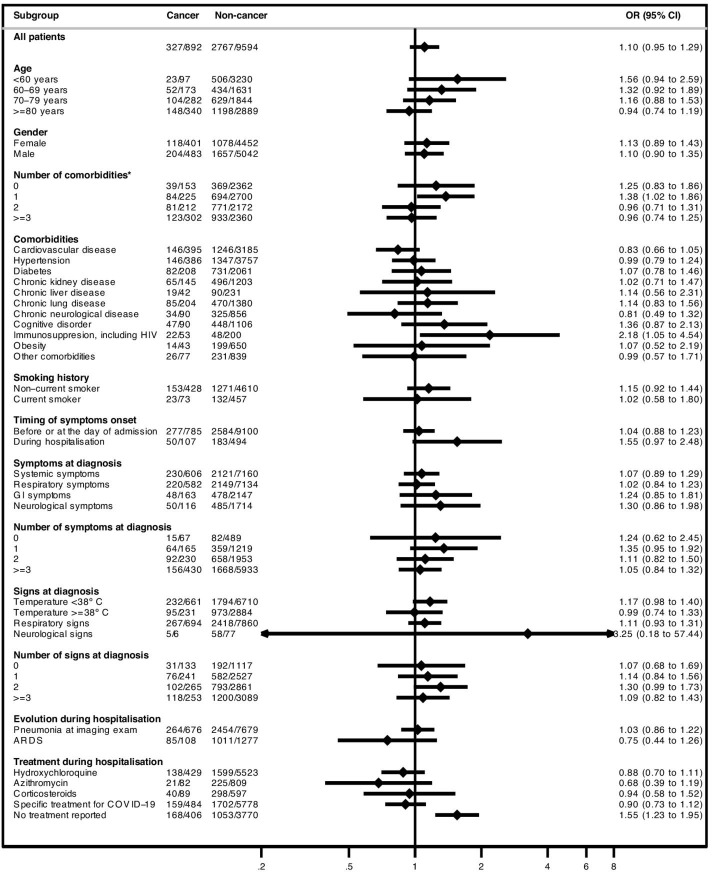
As of 24 May 2020, 2205 (21.0%) patients had died in the hospital within 30 days of COVID-19 diagnosis. After adjustment for age, gender, comorbidities and RAASi use, hospitalised patients with solid cancer had a higher likelihood of dying within 30 days of COVID-19 diagnosis compared with patients without cancer (31.7% vs 20.0%; adjusted OR 1.34; 95% CI 1.13 to 1.58). The effect of cancer on 30-day in-hospital mortality seemed to be more pronounced among younger patients (<60 years; 12.4% mortality in patients with cancer vs 3.2% in patients without cancer; adjusted OR 3.84; 95% CI 1.94 to 7.59) and patients without other comorbidities (20.9% vs 6.1%, respectively; adjusted OR 2.27; 95% CI 1.41 to 3.64) (figure 2 and online supplemental table 6). Regarding the composite endpoint of 30-day severe event occurrence, there were no significant differences between patients with or without cancer either in the overall population (36.7% vs 28.8%, respectively; adjusted OR 1.10; 95% CI 0.95 to 1.29) or across most subgroups (figure 3 and online supplemental table 7).
Figure 2.
Forest plot with subgroup analysis of 30-day in-hospital mortality according to the presence of solid cancer among patients included in the in-hospital analysis. ORs adjusted for age (as a continuous variable), gender, all comorbidities (except ‘obesity’ and ‘other comorbidities’) and renin-angiotensin-aldosterone system inhibitors use. Under the ‘cancer’ and ‘non-cancer’ columns is the description of the number of events (n) / number of patients in each subgroup (N) within the cancer and the non-cancer group, respectively. *Excluding the presence of cancer as a comorbidity. ARDS,acute respiratory distress syndrome; GI, gastrointestinal.
Figure 3.
Forest plot with subgroup analysis of 30-day severe event occurrence according to the presence of solid cancer among patients included in the in-hospital analysis ORs adjusted for age (as a continuous variable), gender, all comorbidities (except ‘obesity’ and ‘other comorbidities’) and renin-angiotensin-aldosterone system inhibitors use. Under the ‘cancer’ and ‘non-cancer’ columns is the description of the number of events (n) / number of patients in each subgroup (N) within the cancer and the non-cancer group, respectively. *Excluding the presence of cancer as a comorbidity. ARDS, acute respiratory distress syndrome; GI, gastrointestinal.
Discussion
We report a population-based nationwide analysis of the clinical course, treatment and outcomes of hospitalised patients with COVID-19 with prior or current solid cancer compared with patients without cancer. Patients with solid cancer comprised 8.7% of hospitalised patients with COVID-19 in Belgium and they were older, presented with fewer symptoms, signs and lung imaging alterations and were more likely to receive corticosteroids during hospitalisation compared with patients without cancer. In-hospital 30-day mortality among patients with solid cancer was 31.7% compared with 20.0% in the non-cancer group. After adjustment, patients with a solid malignancy had a 34% higher likelihood of in-hospital death within 30 days of COVID-19 diagnosis compared with patients without cancer. The effect of cancer on in-hospital mortality was more pronounced among patients <60 years of age and/or without other comorbidities. However, there were no differences regarding severe event occurrence, mainly due to the lower likelihood of ICU admission among patients with cancer.
This is a large population-based analysis including over 1000 patients with cancer, hospitalised for COVID-19 in both academic and non-academic hospitals in Belgium, for whom data was systematically registered into a public institutional database. Compared with registries with post hoc patient identification and data collection, this methodology is far less prone to recall and selection bias. Nonetheless, no details on cancer characteristics and anticancer treatments were available in the database. Yet, the probable inclusion of patients with prior cancer in the cancer group implies an underestimation of the adverse prognostic impact of current cancer on in-hospital mortality and, therefore, our study provides conservative estimates of its effect.
Since the Sciensano database only included hospitalised patients, we did not capture the full spectrum of COVID-19 manifestations and outcomes among patients with solid cancer. We assume that the likelihood of undergoing SARS-CoV-2 screening when paucisymptomatic or asymptomatic is higher in patients with cancer compared with the non-cancer group. However, even though the majority of these patients with a positive SARS-CoV-2 test were not admitted, differences in admission thresholds may have led to the higher proportion of admissions of less symptomatic patients in the cancer group. Moreover, patients with cancer may experience chronic symptoms that overlap with COVID-19 symptoms (eg, cough, fatigue) and thus do not report them. Nonetheless, despite being less symptomatic, patients with solid cancer still had a higher likelihood of in-hospital mortality compared with the non-cancer group and this effect was independent of the number/type of signs/symptoms at diagnosis (data not shown).
We were unable to include patients without discharge data in the in-hospital analysis as information regarding their current vital status is lacking. Nevertheless, we showed that despite being slightly older, there were no other major baseline differences compared with patients with discharge data. Additionally, we used the 30-day mortality outcome in order to limit the possible bias of excluding patients with long-duration hospitalisations. Given the rapid pace of new evidence, the Sciensano questionnaire was amended since its implementation in 14 March 2020. Some variables such as ‘obesity’ and ‘other comorbidities’ were introduced later, resulting in a significant proportion of missing data for these variables. For others, such as smoking status, a high number of unknown answers precluded its use in the multivariable model. Nonetheless, when further adjusting our model for smoking status and obesity in the group of patients with available data, the increased likelihood of 30-day in-hospital death among cancer patients was maintained (data not shown).
Our findings are consistent with other reports comparing COVID-19 characteristics and outcomes in patients with and without cancer. In the above mentioned cohort of over 20 000 patients hospitalised with COVID-19 in the UK, patients with cancer had an increase of 13% in the likelihood of dying.5 In a larger database comprising both hospitalised and non-hospitalised patients (the OpenSAFELY platform), patients with active cancer had higher mortality, with a HR that ranged from 1.72 (95% CI 1.50 to 1.96) for patients diagnosed with cancer <1 year ago to 1.15 (95% CI 1.05 to 1.27) for patients diagnosed 1 to 5 years ago and 0.96 (95% CI 0.91 to 1.03) among those diagnosed ≥5 years ago, compared with patients without cancer.10 However, none of these studies reported on the baseline characteristics, COVID-19 presentation or evolution of patients with cancer compared with those without cancer. Other reports also comprised both hospitalised and non-hospitalised patients, but only included patients with cancer. In a more recent report of the COVID-19 and Cancer Consortium, among 2186 cancer patients the 30-day mortality was 16%, but only half of patients had been admitted, explaining differences in mortality compared with cohorts limited to hospitalised patients.11 A mortality rate of 33% was reported among 428 patients with thoracic cancer, of whom 76% were hospitalised.12 Prior administration of chemotherapy was associated with increased risk of death, while immunotherapy and tyrosine kinase inhibitor use were not. In another report with 800 patients with cancer and symptomatic COVID-19 hospitalised in cancer centres in the UK, mortality was 28% and appeared to be mainly driven by age, gender and comorbidities.13 These poor prognostic factors were confirmed by the OnCovid registry, comprising 890 patients, mostly from Italy, Spain and the UK.14 In our report, we compared the clinical presentation, evolution and outcomes of COVID-19 between patients with and without solid cancer within a nationwide population-based cohort, without any a priori matching. In addition, we evaluated the prognostic effect of cancer among different patient subgroups, allowing a more accurate assessment of the impact of cancer on COVID-19-related outcomes.
Different factors may have contributed to the increased mortality observed in our study among cancer patients hospitalised with COVID-19. Older age, male gender and smoking are associated with an increased risk of cancer15., 16. and are also independent risk factors for adverse outcomes from COVID-19.5 Additionally, patients with cancer are more likely to have other comorbidities such as chronic lung or liver diseases, which were independent adverse prognostic factors for COVID-19 mortality (data not shown). Nonetheless, after adjustment for age, gender, comorbidities and RAASi use, patients with solid cancer still had an increased risk of in-hospital mortality compared with the non-cancer group. There are multiple justifications for this phenomenon. An immunocompromised status due to previous and/or current anticancer therapy, corticosteroids, or other immunosuppressive treatments can significantly impact the clinical course, treatment decisions and outcome of COVID-19. The cancer-related increase of thromboembolic risk could also contribute to COVID-19 severity.17 Since the Sciensano database only captured all-cause mortality, it is possible that, despite the short duration of hospitalisation, a small proportion of patients with cancer died because of their malignancy and not due to COVID-19. Finally, given the worse performance status and life-expectancy of some patients with cancer receiving palliative anticancer treatments, a substantial proportion of patients with cancer in Belgium may have had a non-resuscitation request. An imbalance in these treatment limitations would be expected between patients with and without cancer and this could contribute to the lower likelihood of ICU admission among patients with cancer, even after adjustment for age, gender and comorbidities.
The magnitude of the association of cancer with in-hospital mortality differed across patient subgroups. Surprisingly, this magnitude progressively decreased as the number of comorbidities per patient increased or as the age categories increased—from an OR of 3.84 among patients <60 years to an OR of 0.98 among those with ≥80 years. Older adults with cancer are expected to have a higher proportion of prior versus current cancer, more indolent cancers (eg, prostate cancer, hormone-receptor positive breast cancer) and are less likely to receive immunosuppressive anticancer treatments compared with younger patients. In addition, older patients with advanced cancer and severe COVID-19 may have a lower likelihood of hospitalisation, potentially leading to an underestimation of mortality among older patients with cancer. Moreover, given the baseline poor COVID-19-related prognosis among older patients or among those with several comorbidities, the presence of cancer might not significantly worsen their prognosis and might have a smaller impact on COVID-19-related treatment decisions. On the other hand, among patients with a more favourable COVID-19-related prognosis (young age and/or absence of other comorbidities), the presence of cancer might significantly increase the risk of death, given the above-mentioned reasons.
The COVID-19 outbreak provoked a dramatic disruption in cancer care around the globe, leading to delays in diagnosis, treatment and follow-up and to reductions in the intensity of cancer treatment.18., 19., 20. Additionally, several recommendations on how to manage cancer care during the COVID-19 outbreak have been published by oncological societies and by national groups, but given the absence of evidence on this subject, they are only expert-based.21., 22., 23. Therefore, our results have implications for clinicians and public health authorities and they may inform future recommendations about cancer care during the COVID-19 outbreak. We demonstrate that patients with solid cancer hospitalised with COVID-19 present with less symptoms, which might be due to lower thresholds for asymptomatic testing and hospitalisation, but nevertheless have higher in-hospital mortality compared with patients without cancer. We have also seen that among patients with favourable COVID-19-related prognosis (<60 years and/or without comorbidities), the presence of cancer led to a twofold to threefold increase in the likelihood of in-hospital death. Thus, clinicians should be aware of this potential adverse prognostic effect when discussing cancer treatment plans, in case a new surge of COVID-19 cases is seen in their region. Moreover, as lockdown strategies are being eased around the world and hope for an effective vaccine grows,24., 25. patients with solid cancer should be regarded as a vulnerable group for adverse outcomes of COVID-19 and thus be prioritised in vaccination campaigns and in tailored containment measurements.
Several questions remain unanswered regarding the effect of cancer type and anticancer treatments on the risk of severe events or death among cancer patients with COVID-19. Cancer-specific data is now being collected in a collaborative effort with participating oncologists from Belgian hospitals.
In conclusion, in this population-based nationwide analysis of hospitalised patients with COVID-19, we demonstrate that after adjustment for age, gender, comorbidities and RAASi use, patients with solid cancer have an increased likelihood of in-hospital death within 30 days of COVID-19 diagnosis compared with patients without cancer. This adverse prognostic effect seems to be more pronounced among patients <60 years and/or without other comorbidities.
Acknowledgements
The authors would like to acknowledge all hospitals and healthcare professionals who completed the information in the public database; to the Belgian Society of Medical Oncology Executive Board for their full support of this research; to Professor Nuno Lunet, for his comments; to Dr Gilberto Morgan, for proofreading this manuscript; and to Mrs Christie Freeman and Mr Hélio Silva, for collaborating in the video abstract.
Footnotes
EdA and MB are joint first authors.
TG and KP are joint senior authors.
Twitter: @E_de_Azambuja, @MarianaBrandao0, @kevinpunie
EdA and MB contributed equally.
TG and KP contributed equally.
Collaborators: List of participant members of the Belgian Collaborative Group on COVID-19 Hospital Surveillance: Kristof Bafort, MD (Mariaziekenhuis, Pelt); Leïla Belkhir, MD (Cliniques Universitaires Saint-Luc, Brussels); Nathalie Bossuyt, MD (Sciensano); Vincent Colombie, MD (Centre Hospitalier Epicura, Baudour); Nicolas Dauby, MD/PhD (Centre Hospitalier Universitaire Saint-Pierre, Brussels); Christine Daubresse, MD (Centre Hospitalier Chrétien, Liège); Paul De Munter, MD (Universitair Ziekenhuis Leuven, Leuven); Jessika Deblonde, PhD (Sciensano); Didier Delmarcelle, MD (Clinique St. Jean, Brussels); Mélanie Delvallee, MD (Centre Hospitalier de Wallonie Picarde, Tournai); Quentin Delefortrie, MD (Clinique Notre Dame de Grâce, Gosselies); Rémy Demeester, MD (Centre Hospitalier Universitaire de Charleroi, Charleroi); Thierry Dugernier, MD (Clinique Saint-Pierre, Ottignies); Xavier Holemans, MD (Grand Hôpital de Charleroi, Charleroi); Ingrid Louviaux, MD (Centre Hopsitalier Régional de la Citadelle, Liège); Pierre Yves Machurot, MD (Centre Hospitalier de l’Ardenne); Philippe Minette, MD (Centres Hospitaliers Jolimont); Saphia Mokrane, MD (Hôpitaux Iris Sud, Brussels); Catherine Nachtergal, MD (Cliniques de l’Europe, Brussels); Séverine Noirhomme, MD (Centre Hospitalier Régional de Namur); Denis Piérard, MD (Universitair Ziekenhuis Brussel, Brussels); Camelia Rossi, MD (Centre Hospitalier Universitaire Ambroise Paré, Mons); Carole Schirvel, MD (CHIREC, Brussels); Erica Sermijn, MD (A.S.Z. Ziekenhuis, Aalst); Frank Staelens, MD (OLV Ziekenhuis, Aalst); Filip Triest, MD (Algemeen Ziekenhuis Sint Lucas, Gent); Dominique Van Beckhoven, MD (Sciensano); Nina Van Goethem, MD (Sciensano); Jens Van Praet, MD (Algemeen Ziekenhuis Sint Jan, Brugge-Oostende); Anke Vanhoenacker, MD (Ziekenhuisnetwerk Antwerpen); Roeland Verstraete, MD (Algemeen Ziekenhuis. Monica); Elise Willems, MD (Algemeen Ziekenhuis Nikolaas, Sint-Niklaas); Chloé Wyndham-Thomas, MD (Sciensano).
Contributors: Conceptualisation: EdA, MB, TG, KP. Data curation: DVB, JD. Formal analysis: MB, AL. Investigation: All authors. Methodology: EdA, MB, AL, DVB, JD, TG, KP. Writing - original draft: EdA, MB, TG, KP. Writing - review and editing: All authors.
Funding: The Belgian Society of Medical Oncology (BSMO) was the legal sponsor of this project. No research funding for these analyses was received from third parties. Sciensano provided the database to BSMO free of charge. BSMO provided administrative support. Institut Jules Bordet and KU Leuven provided administrative support for protocol and manuscript writing.
Competing interests: EdA: honoraria and/or advisory board from Roche/GNE, Novartis, SeaGen and Zodiac; travel grants from Roche/GNE and GSK/Novartis; research grants to his institution from Roche/GNE, AstraZeneca, GSK/Novartis and Servier. MB: speaker honoraria and travel grants from Roche/GNE; research grants to her institution from Roche/GNE, AstraZeneca, GSK/Novartis and Servier. HW: his institution received consulting fees and honoraria from AstraZeneca, Biocartis, Lilly, Novartis, Pfizer, PUMA Biotechnology, Roche, Sirtex, Daiichi; his institution received unrestricted research grants from Roche and Novartis; he received travel support from Roche and Pfizer. AL: no conflicts of interest to disclose. SA: speaker fees from BMS, AstraZeneca, Roche, Sanofi and Novartis; travel grants from Pfizer, Roche; advisory board fee from Sanofi and Pierre Fabre. CF: advisory board fee from Lilly. JC: advisory board and lectures from Amgen, Servier, Bayer, Novartis, Pfizer, Celgen, Ipsen (paid to institution); travel grants from Roche, Pfizer, Amgen, Novartis. WL: honoraria and/or advisory board from BMS, MSD, Novartis, IPSEN and Bayer; travel support from Roche, MSD and IPSEN. JV: no conflicts of interest to disclose. AR: honoraria and/or advisory board from Sanofi, BMS, Novartis, Pierre Fabre, Roche; travel support from Roche, BMS, MSD. PV: honoraria and/or advisory board from Roche, Novartis, Pfizer, Lilly, MSD, Merus; travel grants from Roche, AstraZeneca, MSD and Pfizer; speaker fees from Pfizer and Roche (paid to institution). JCG: advisory board and lectures from Ipsen, BMS, Janssen, Bayer, AstraZeneca. WD: honoraria and/or advisory board from Amgen, Pierre Fabre. DVB: no conflicts of interest to disclose JD: no conflicts of interest to disclose. SR: consulting fees and honoraria from J&J, Roche, Pfizer, AstraZeneca, Novartis, Bayer, MSD, BMS, Ipsen; research grants from Roche, BSM and MSD; travel grants from Ipsen, Pfizer, Bayer. TG: no conflicts of interest to disclose. KP: honoraria for advisory/consultancy roles for AstraZeneca, Eli Lilly, Novartis, Pfizer, Pierre Fabre, Hoffmann/La Roche and Vifor Pharma (paid to institution); speaker fees for Eli Lilly, Mundi Pharma, Novartis, Pfizer and Hoffmann/La Roche (paid to institution); research funding from Sanofi (paid to institution); travel support from AstraZeneca, Novartis, Pfizer, PharmaMar and Hoffmann/La Roche.
Patient consent for publication: Not required.
Ethics approval: Concerning the overall hospital COVID-19 clinical surveillance, ethical and legal authorisations were granted to Sciensano, which is legally entitled for surveillance activities related to public health in Belgium. Collection of individual data for the hospital COVID-19 surveillance was authorised by the Belgian Data Protection Authority and the clinical surveillance was approved by the Ethics Committee of Ghent University Hospital (BC-07507). The present cancer substudy was performed on a subset of data shared with Belgian Society of Medical Oncology by Sciensano, after data anonymisation. The Ethics Committee of the Institut Jules Bordet approved this cancer substudy (reference CE3150).
Provenance and peer review: Not commissioned; internally peer reviewed.
Data availability statement:The datasets generated and analyzed during the current study are the property of Sciensano and are not publicly available. Nonetheless, data may be available from the corresponding author on reasonable request.
Supplementary data
References
- 1.WHO Director-General’s opening remarks at the media briefing on COVID-19. Available: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020 [Accessed 30 Mar 2020].
- 2.Belgium: who coronavirus disease (COVID-19) Dashboard. Available: https://covid19.who.int [Accessed 6 Aug 2020].
- 3.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. http://www.ncbi.nlm.nih.gov/pubmed/32066541 doi:10.1016/S1470-2045(20)30096-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10 doi: 10.1158/2159-8290.CD-20-0422. http://www.ncbi.nlm.nih.gov/pubmed/32345594 doi:10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Docherty A.B., Harrison E.M., Green C.A., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1985. http://www.ncbi.nlm.nih.gov/pubmed/32444460 doi:10.1136/bmj.m1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuderer N.M., Choueiri T.K., Shah D.P., et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395:1907–1918. doi: 10.1016/S0140-6736(20)31187-9. http://www.ncbi.nlm.nih.gov/pubmed/32473681 doi:10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sciensano. sciensano.be. Available: https://www.sciensano.be/en/home [Accessed 8 Jun 2020].
- 8.Goethem N.V., Vilain A., Wyndham-Thomas C., et al. Rapid establishment of a national surveillance of COVID-19 hospitalizations in Belgium. Research square. 2020 doi: 10.1186/s13690-020-00505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von E.E., Altman D.G., Egger M., et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. The Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 10.Williamson E.J., Walker A.J., Bhaskaran K., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. http://www.ncbi.nlm.nih.gov/pubmed/32640463 doi:10.1038/s41586-020-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivera D.R., Peters S., Panagiotou O.A., et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and cancer Consortium (CCC19) cohort study. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0941. http://www.ncbi.nlm.nih.gov/pubmed/32699031 doi:10.1158/2159-8290.CD-20-0941 doi [Epub ahead of print 22 Jul 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horn L., Whisenant J.G., Torri V., et al. Thoracic cancers international COVID-19 collaboration (TERAVOLT): impact of type of cancer therapy and COVID therapy on survival. JCO. 2020;38:LBA111. doi:10.1200/JCO.2020.38.18_suppl.LBA111 [Google Scholar]
- 13.Lee L.Y.W., Cazier J.-B., Angelis V., et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. The Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. doi:10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinato D.J., Zambelli A., Aguilar-Company J., et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov. 2020 doi: 10.1158/2159-8290.CD-20-0773. http://www.ncbi.nlm.nih.gov/pubmed/32737082 doi:10.1158/2159-8290.CD-20-0773 doi [Epub ahead of print 31 Jul 2020] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danaei G., Vander Hoorn S., Lopez A.D., et al. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. http://www.ncbi.nlm.nih.gov/pubmed/16298215 doi:10.1016/S0140-6736(05)67725-2 [DOI] [PubMed] [Google Scholar]
- 16.Wu S., Zhu W., Thompson P., et al. Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05467-z. http://www.ncbi.nlm.nih.gov/pubmed/30154431 doi:10.1038/s41467-018-05467-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. http://www.ncbi.nlm.nih.gov/pubmed/32437596 doi:10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poggio F., Tagliamento M., Di Maio M., et al. Assessing the impact of the COVID-19 outbreak on the attitudes and practice of Italian oncologists toward breast cancer care and related research activities. JCO Oncol Pract. 2020 doi: 10.1200/OP.20.00297. http://www.ncbi.nlm.nih.gov/pubmed/32574131 doi:10.1200/OP.20.00297 OP.20.00297. [DOI] [PubMed] [Google Scholar]
- 19.Wallis C.J.D., Novara G., Marandino L., et al. Risks from Deferring treatment for genitourinary cancers: a collaborative review to aid triage and management during the COVID-19 pandemic. Eur Urol. 2020;78:29–42. doi: 10.1016/j.eururo.2020.04.063. http://www.ncbi.nlm.nih.gov/pubmed/32414626 doi:10.1016/j.eururo.2020.04.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aeppli S., Eboulet E.I., Eisen T., et al. Impact of COVID-19 pandemic on treatment patterns in metastatic clear cell renal cell carcinoma. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000852. http://www.ncbi.nlm.nih.gov/pubmed/32669298 doi:10.1136/esmoopen-2020-000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Azambuja E., Trapani D., Loibl S., et al. ESMO management and treatment adapted recommendations in the COVID-19 era: breast cancer. ESMO Open. 2020;5:e000793. doi: 10.1136/esmoopen-2020-000793. http://www.ncbi.nlm.nih.gov/pubmed/32439716 doi:10.1136/esmoopen-2020-000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lambertini M., Toss A., Passaro A., et al. Cancer care during the spread of coronavirus disease 2019 (COVID-19) in Italy: young oncologists' perspective. ESMO Open. 2020;5 doi: 10.1136/esmoopen-2020-000759. http://www.ncbi.nlm.nih.gov/pubmed/32229501 doi:10.1136/esmoopen-2020-000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ueda M., Martins R., Hendrie P.C., et al. Managing cancer care during the COVID-19 pandemic: Agility and collaboration toward a common goal. J Natl Compr Canc Netw. 2020;18:366–369. doi: 10.6004/jnccn.2020.7560. doi:10.6004/jnccn.2020.7560 [DOI] [PubMed] [Google Scholar]
- 24.Folegatti P.M., Ewer K.J., Aley P.K., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. The Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. doi:10.1016/S0140-6736(20)31604-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson L.A., Anderson E.J., Rouphael N.G., et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med Overseas Ed. 2020 doi: 10.1056/NEJMoa2022483. doi:10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials