Abstract
Patient: Female, 35-year-old
Final Diagnosis: Fulminant liver failure
Symptoms: Coagulopathy • hepatic encephalopathy • jaundice
Medication:—
Clinical Procedure: Liver transplantation
Specialty: Gastroenterology and Hepatology
Objective:
Unusual clinical course
Background:
Drug-induced liver failure is a rare complication of pregnancy and occasionally requires liver transplantation. However, fulminant liver failure arising from in vitro fertilization (IVF) therapy involving progestogens (e.g. dydrogesterone) is extremely rare and has not been reported in pregnancy. Furthermore, dydrogesterone-mediated hepatic dysfunction has not previously necessitated liver transplantation and is usually conservatively managed. We report the first Australian case of a pregnant woman with delayed fulminant liver failure and in utero fetal death requiring a liver transplant from dydrogesterone use.
Case Report:
A 35-year-old multiparous (G5P2) woman presented with painless jaundice and transaminitis (alanine amino-transferase and aspartate aminotransferase of 2800 U/L and 2990 U/L respectively). She was pregnant at 14 weeks’ gestation after successful IVF in Thailand four months before involving dydrogesterone therapy. She was diagnosed with delayed, subfulminant liver failure arising from previous dydrogesterone use. Initially, she was not encephalopathic and conservative management strategies were instituted. Her hepatic dysfunction progressed and she deteriorated clinically with encephalopathy, necessitating an emergent liver transplantation. Fetal death was confirmed in utero four days before transplantation. A combined orthotopic liver transplant and hysterotomy with fetal evacuation were performed without complication.
Conclusions:
Fulminant liver failure in pregnancy due to idiosyncratic drug reactions are rare. Dydrogesterone may cause significant, albeit delayed, liver dysfunction in pregnancy necessitating the need for liver transplantation. Early recognition of progressive liver failure despite best supportive care efforts should prompt early considerations for liver transplantation. Delays in liver transplantation with prolonged hyperbilirubinemia and coagulopathy may exacerbate fetal death in utero.
MeSH Keywords: Dydrogesterone; Liver Failure, Acute; Liver Transplantation; Pregnancy
Background
Fulminant liver failure represents a rare complication of pregnancy and is more common during the third trimester [1]. Half of these cases are attributable to hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome, and acute fatty liver of pregnancy (AFLP) [1–3]. The remainder are caused by severe viral hepatitis, particularly hepatitis E in the developing world, as well as intrahepatic cholestasis of pregnancy (ICP), drug-induced liver injury, and Budd Chiari syndrome secondary to hepatic vein thrombosis [1–9]. A significant proportion of cases are often cryptogenic in their etiology [10–16]. Acute liver failure occurring during the first and second trimesters of pregnancy are associated with poor fetal outcomes, including developmental delays, neurological abnormalities, spontaneous abortions, and neonatal death [6–16]. However, only ~16% of pregnant women with fulminant liver failure will require liver transplantation [2].
Drug-induced liver injury in pregnancy seldom causes fulminant hepatic failure requiring liver transplantation [5,7–9]. The most frequently implicated agents include acetaminophen, isoniazid, valproate, phenytoin, and propylthiouracil [5]. The latter has caused fulminant liver failure in two pregnant patients requiring emergency liver transplantation [7,8]. Of these, only one case resulted in fetal preservation and delivery of an infant at 37 weeks’ gestation with significant neurological morbidity and developmental delay [8]. Fulminant liver failure arising from in vitro fertilization (IVF) therapies, particularly progestogens (eg dydrogesterone), are extremely rare. Multiple trials and reviews have evaluated the safety profile of dydrogesterone use in IVF and have reported minimal adverse side effects (ie nausea, vomiting, dizziness, constipation) [17]. No large cohort study or trial to date has shown any hepatic dysfunction arising from dydrogesterone [17]. Only two case reports have associated the use of dydrogesterone with the development of jaundice, acute hepatitis, and in one of these patients, co-morbid autoimmune hemolytic anemia [18,19]. However, neither patient achieved pregnancy nor required a liver transplant.
We report the first Australian case of a 35-year-old multiparous, pregnant woman requiring an emergency liver transplant secondary to dydrogesterone-induced fulminant hepatic failure with associated fetal death. We demonstrate that dydrogesterone-induced liver failure in pregnancy may be delayed. This case highlights the need for early liver transplantation in progressive drug-induced liver failure despite the best supportive care efforts. Furthermore, delays in transplantation that prolong maternal hyperbilirubinemia and coagulopathy may exacerbate fetal death.
Case Report
A 35-year-old multiparous (G5P2) Australian woman of Indian descent at 14 weeks’ gestation, previously fit and well, was admitted to our hepatology service for investigation and management of painless jaundice and elevated transaminases. The patient had reported the onset of scleral jaundice three days before her hospital admission with associated pruritus affecting the palms of her hands and soles of her feet. She denied any abdominal, stool, or urinary symptoms. Her past medical history included previous gestational diabetes, an elective termination of pregnancy four years prior, and a left salpingectomy one year ago after a previous ectopic pregnancy. She had undergone two previous spontaneous vaginal deliveries at term without complication. Her previous term pregnancies were unremarkable, with no prior history of liver dysfunction, eclampsia, HELLP, AFLP, or ICP. She had no history of any underlying liver or gallbladder disease. She did not smoke or drink alcohol. She did not have any tattoos and had no previous blood transfusions.
She had traveled to Thailand approximately four months before her hospital admission for IVF therapy. She had received a regular IVF regimen comprising three months’ duration of oral dydrogesterone (10 mg), oral estradiol (1 mg), and per vaginal progesterone. Embryo transfer and implantation was successful, and she returned to Australia for ongoing antenatal care. She reported only taking routine pregnancy supplements including Ferrograd-C, vitamin B complex, and cholecalciferol while pregnant. She did not use any herbal supplements, analgesic agents, antibiotics, or over-the-counter medications. On examination, she was hypotensive at 90/60 mmHg, but her other vitals were within normal limits. She was clinically jaundiced with evidence of marked scleral icterus. Peripheral stigmata of chronic liver disease were absent. She had no features of hepatic encephalopathy. Abdominal examination revealed a smooth liver edge that was nontender. Her abdomen was soft with no evidence of ascites.
Peripheral blood investigations on admission (day 0, Figure 1) noted abnormally elevated transaminases (ie alanine amino-transferase [ALT] 2800 U/L and aspartate aminotransferase [AST] 2990 U/L), and albumin of 36 g/L. Total and conjugated bilirubin levels were elevated at 215 μmol/L and 183 μmol/L respectively. Her international normalized ratio (INR) was mildly elevated at 1.3 with a normal platelet count of 310×109/L. Her liver enzyme profile was within normal limits before this pregnancy. Elevations of her total white cell count (11.98×109/L), neutrophils (8.39×109/L), ferritin (1260 μg/L), and lactate dehydrogenase (672 U/L) were noted. Her hemoglobin and eosinophil counts were normal at 144 g/L and 0.24×109/L respectively. Blood film analysis showed no features of hemolysis, including echinocytes and erythrocyte fragments. A hemolysis screen was unremarkable, including normal haptoglobin and reticulocyte counts with negative Coombe’s test. Uric acid levels were normal (0.18 mmol/L). Urinalysis and culture were un-remarkable, with normal urine albumin (<2.0 mg/L) and urine albumin/creatinine ratio (<0.1 mg/mmol). A urine drug screen was not performed. Additional serology, stool, and viral polymerase chain reaction testing for hepatitis viruses A through to E, Epstein-Barr virus, cytomegalovirus (CMV), human immunodeficiency virus, herpes simplex virus, adenovirus, human herpesvirus-6, Leptospira, and Listeria species were negative. Sputum and serological testing for tuberculosis were negative. An autoimmune screen including antinuclear antibody, anti-neutrophilic cytoplasmic antibody, anti-smooth muscle antibodies, anti-liver-kidney microsome antibody, mitochondrial antibodies, anti-soluble liver antigen antibodies, anti-liver cytosol-1 antibodies, and immunoglobulin levels were unre-markable. Alpha-1 anti-trypsin levels were normal. Vitamin D levels were replete at 72 nmol/L. Her estradiol level was 33 000 pmol/L. Follicle-stimulating hormone and luteinizing hormone levels were <0.1 and 0.2 U/L respectively. All other routine biochemistry was unremarkable. Routine Doppler ultrasonography of the liver and abdomen performed on admission revealed normal appearances with no focal hepatic lesions, thromboses, or biliary dilatation. A viable fetus was noted at this time. Features of HELLP, AFLP, ICP, and infective etiologies for her liver failure were excluded on the basis of the above investigations. Supportive care was initiated during her admission including an N-acetyl infusion, albumin, and vitamin K supplementation.
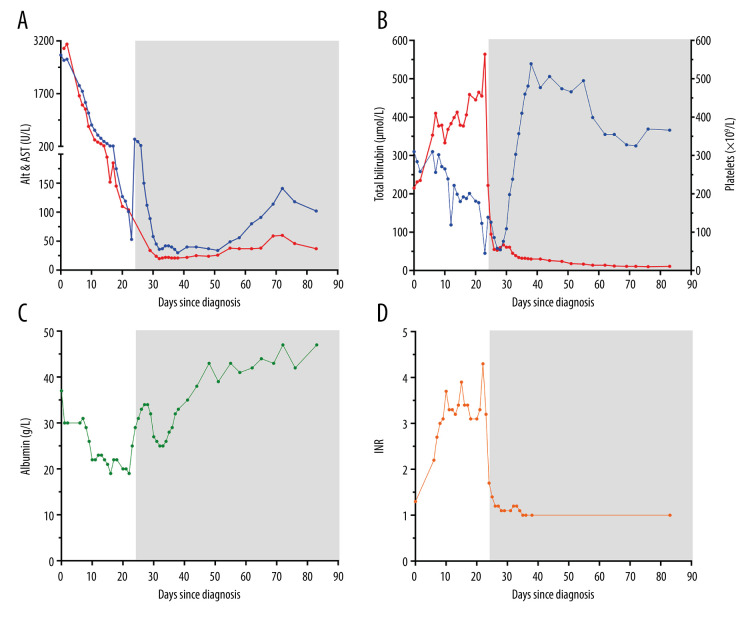
Figure 1.
Longitudinal biochemical data illustrating the patient’s progressive hepatic failure since her hospital admission (day 0). Transplantation occurred at day 24 with subsequent post-transplant biochemical data illustrated thereafter by gray shading. (A) Alanine aminotransferase (ALT, blue) and aspartate aminotransferase (AST, red) parameters showing initial significant transaminitis with progressive normalization post-liver transplantation. Hepatic failure was further illustrated by progressive (B) hyperbilirubinemia (red), thrombocytopenia (blue), (C) hypoalbuminemia, and (D) coagulopathy (international normalized ratio [INR]). Post-transplantation, all parameters normalized.
Her ALT and AST levels declined (Figure 1A) over the next week. However, ongoing biochemical deterioration was observed, including profound exacerbations of her hyperbilirubinemia, thrombocytopenia, hypoalbuminemia, and associated coagulopathy (Figure 1B–1D). At this time, she remained clinically asymptomatic and was not encephalopathic, excluding her persistent jaundice. Serum ceruloplasmin levels were within normal limits, but a 24-hour urine copper collection revealed a mildly elevated copper level (3.66 μmol/day, reference range: <0.5 μmol/day). An atypical form of Wilson’s disease unmasked by the current pregnancy was considered. She proceeded to have an ophthalmic review and Kayser-Fleischer rings were excluded. Concurrently, a transjugular biopsy of our patient’s liver was collected and showed features of submassive hepatic necrosis involving ~40% of the parenchyma without fibrosis. Focal steatosis with variable ballooning and cholestasis were noted. Predominant Kupffer cells and lymphocyte infiltrates were seen admixed among neutrophils and eosinophils. The liver biopsy dry copper weight was normal (0.5 mmol/kg, reference range: <0.6 mmol/kg) and Wilson’s disease was excluded. Possible differential diagnoses considered at this time included a drug-induced liver injury (ie dydrogesterone), toxic hepatic injury, or an undiagnosed fulminant viral hepatitis.
Despite our best supportive care efforts, our patient continued to deteriorate biochemically (Figure 1). She was activated for liver transplantation on day 10 of her admission and an urgent multidisciplinary liver transplantation workup as per our local hospital protocols and national policies was undertaken. Obstetric experts were consulted. A termination of the previable fetus before liver transplantation was not recommended. Although the fetus was at high risk of not surviving the current state of maternal liver failure, liver transplantation during pregnancy was considered a viable option that may sustain fetal survival. At day 21–22 after her initial admission, the patient clinically decompensated and became acutely encephalopathic. Her Glasgow coma scale decreased (E3V4M6) and she developed a new sinus tachycardia (130 beats per min) with oliguria. Her biochemistry at this time showed an ALT 119 U/L, AST 110 U/L, total bilirubin 465 μmol/L, albumin 19 g/L, platelet count 123×109/L, creatinine 142 μmol/L, and INR 4.3. She was admitted to the intensive care unit for ongoing management. A repeat Doppler ultrasound was performed and showed new ascites with increased echogenicity of the liver. An obstetric ultrasound was also performed, which confirmed fetal death in utero at 17+5 weeks’ gestation. Obstetric opinion recommended that the deceased fetus not be evacuated before the liver transplantation unless she developed severe per vaginal bleeding in her current coagulopathic state. Central venous access was obtained, and she was fluid resuscitated with crystalloid and albumin replacement. Intravenous terlipressin and, later, continuous venous-venous hemodiafiltration were instituted to support her hepatorenal syndrome. She was transfused with fresh frozen plasma, cryoprecipitate, vitamin K, and tranexamic acid to correct her underlying coagulopathy. Chest radiography noted progressive pulmonary edema and with the progressive deterioration in her conscious state, she was subsequently intubated and ventilated.
Her model for end-stage liver disease (MELD) score at this time was 40. A liver from a dead donor was obtained from a neighboring state within 48 hours. On day 24 after her initial hospital admission, the patient underwent a combined orthotopic liver transplantation with hysterotomy and evacuation of the deceased fetus without significant intraoperative complication. Histopathological analysis of the explanted liver (Figure 2) showed submassive hepatocellular necrosis with admixture of confluent necrosis (involving >50% of hepatic parenchyma) and regenerative nodules. There was no evidence of any underlying dysplastic, neoplastic, or infiltrative disorders (eg iron, copper). Features were in keeping with drug-induced fulminant hepatic failure, likely secondary to previous dydrogesterone use. Placental histology showed features of acute chorioamnionitis with early fibrin deposition and ischemic necrosis (Figure 2).
Figure 2.
Representative macroscopic and microscopic images of the patient’s liver and placenta. (A) Gross morphology of the liver with submassive hepatic necrosis demonstrating capsular wrinkling and flattening (*) with developing regenerative nodularity (arrow) in portions of the liver. (B) Histologic examination of the liver demonstrating submassive hepatic necrosis characterized by complete loss of hepatocytes and inflammation (right and upper portion of image), with early regenerative nodularity (lower left portion of image). (Original magnification ×40.) (C) Histologic examination of the placenta demonstrating evidence of chorioamnionitis characterized by subchorionic fibrin (*) with admixed neutrophils and neutrophilic infiltration of the chorionic plate (arrows). (Original magnification ×400.) (D) Histologic examination of the placenta demonstrating extensive accumulation of intervillous blood with perivillous fibrin deposition (*) and early ischemic change of villi (arrows). (Original magnification ×200.)
Our patient’s postoperative course was essentially unremarkable. She continued to improve clinically and biochemically, with resolution of her transaminitis, coagulopathy, and jaundice (Figure 1). Her post-transplant immunosuppression regimen consisted of tacrolimus 4 mg twice a day, azathioprine 75 mg daily, and weaning prednisolone. Valganciclovir 900 mg daily and Bactrim 800/160 mg thrice weekly were provided routinely for CMV and Pneumocystis jiroveci pneumonia prophylaxis respectively. Psychological support was offered through our local Perinatal Loss Support Service. Regular outpatient follow-up and biochemical monitoring were performed. Mild elevations in the patient’s transaminases were noted at ~68– 70 days after the patient’s initial admission to hospital. A repeat abdominal Doppler ultrasound was performed and no underlying liver pathology was identified. She continues to remain well and is planning a future pregnancy.
Discussion
Drug-induced liver failure in pregnancy is an uncommon event and represents a diagnosis of exclusion [5]. Offending agents typically include acetaminophen and occasionally propylthiouracil, and if fulminant or progressive, may necessitate a liver transplant [5,7–9]. Hepatic injury related to IVF drugs is rare. ICP secondary to IVF occurs in 0.01–2.7% of pregnancies [4,20,21]. Dydrogesterone-related hepatic failure has not been previously reported in pregnancy [17]. To the best of our knowledge, we have reported the first Australian case of a multiparous pregnant woman with dydrogesterone-induced fulminant hepatic failure requiring a liver transplant complicated by fetal death during the second trimester.
Dydrogesterone is an old progestogen that has been available since the 1970s [17]. It is still commonly used in several developing countries for routine IVF therapy with significant efficacy. In comparison with other IVF options, dydrogesterone has minimal maternal adverse side effects and holds a reputable safety profile among multiple clinical trials and reviews [17]. However, recent reports have associated its use with profound neurological, genital, and cardiological abnormalities in the developing fetus [22]. To date, liver dysfunction associated with dydrogesterone use has only been reported in two patients from Turkey and Malaysia with mild, self-resolving hepatitis [18,19]. In both cases, the onset of jaundice and hepatitis occurred within two weeks of commencing dydrogesterone. The Turkish patient presented with recurrent bouts of hepatitis after dydrogesterone use over a four-year period with comorbid autoimmune hemolytic anemia, suggesting an underlying autoimmune hepatitis etiology [19]. In contrast, there was a significant delay of four months before our patient developed rapidly progressive hepatic failure requiring liver transplantation. The mechanisms underpinning dydrogesterone-mediated liver injury are not understood. Mustafa and coworkers (2014) have proposed that it potentially mimics ICP by means of upregulating estradiol [18]. Peak estradiol levels in the third trimester, when ICP is particularly prevalent, may promote cholestasis by unknown signaling cascades [4,18]. Others have suggested a similar role for progesterone and its metabolites, which are similarly found to be upregulated in ICP [4]. It is plausible that concurrent estrogen administration with dydrogesterone during IVF therapy may have potentiated our patient’s hepatic failure through an ICP-mediated process. However, Germain et al. [23] have shown that no serological differences exist in estrogen levels between women with normal pregnancies and ICP, limiting the utility of its routine measurement in IVF pregnancies. Others have also shown that elevated bile acids in IVF pregnancies are more frequently associated with ICP, but its clinical sensitivity in predicting ICP remains uncertain [24]. Altintas et al. (2004) have proposed that dydrogesterone may facilitate hepatic injury through an idiosyncratic reaction that involves a complex, albeit unknown, interplay between genetic and autoimmune factors that influence the metabolism of itself and its downstream metabolites [19]. Moreover, its hepatotoxic effects are unpredictable and can last up to several months before becoming clinically evident [19]. Overall, the idiosyncrasy of dydrogesterone in mediating hepatocellular necrosis and progressive liver failure remains to be elucidated.
Greenberg and colleagues (2009) previously established an algorithm for managing acute liver failure in pregnancy [13]. Under these circumstances, maternal survival outweighs fetal welfare, irrespective of the stage of pregnancy. Should the fetus be previable (≤20–25 weeks’ gestation), careful consideration toward a termination of pregnancy versus ongoing maternal and fetal support should be given, particularly as the latter carries significantly higher morbidity and mortality. Reviews of previous case reports and cohort studies have shown that fetal death during pregnancy both pre- and post-liver transplantation is high (up to 23.8%), particularly during the second trimester [1–3,5–7,9–11,15]. Of those that survive, developmental delays arising from neurological sequalae are occasionally reported [8]. Development and maturation of the central nervous system occurs during week three of embryo-genesis until the 16–17th week of gestation [25]. The fetus is particularly sensitive to maternal coagulopathy and hyperbilirubinemia, with the former contributing to placental ischemia and fetal death [26,27]. Persistent placental ischemia and dys-function may inadvertently facilitate the placental transfer of excess bilirubin more readily to the fetus, exacerbating cerebral neurotoxicity through several molecular mechanisms [28]. It is likely that the onset of severe hyperbilirubinemia and coagulopathy in our patient caused significant impaired neurological development in the fetus at a time when its central nervous system was developmentally sensitive and did not support life. To our knowledge, no study to date has isolated the overall thresholds for hyperbilirubinemia and coagulopathy parameters that may predict fetal death. Further, the temporal relationship involving a previable fetus’ ability to withstand such serological abnormalities and its gestation period in relation to its survival have not been established. Nevertheless, recognition of progressive hepatocellular necrosis through persistent hyperbilirubinemia, coagulopathy, and pseudo-“improvements” in transaminases should prompt early progression toward liver transplantation during pregnancy, which may enable fetal survival and avoid significant neonatal morbidity.
Conclusions
Dydrogesterone-mediated hepatic injury and progression to fulminant hepatic failure is an extremely rare event during pregnancy. It represents an idiosyncratic reaction that may mimic ICP through excess estrogen- and progesterone-mediated effects [4,18,19]. Progressive liver failure despite conservative management strategies should prompt an early consideration for liver transplantation. Maternal well-being in these circumstances outweighs fetal survival, but the latter can be augmented by early liver transplantation and effective resolution of maternal coagulopathy and hyperbilirubinemia.
Acknowledgments
The authors express their gratitude to PathWest Laboratory Medicine staff for blood sampling, histological processing, and technical assistance; Prof. Luc Delriviere for his surgical liver transplantation expertise; and Dr. Jan Dickenson for her obstetric assistance and fetal medicine expertise. We also thank all clinical and allied health staff at Sir Charles Gairdner Hospital for their inpatient care, coordination efforts to facilitate a liver transplant, and ongoing outpatient care for this patient.
Footnotes
Conflicts of interest
None.
References:
- 1.Pandey CK, Karna ST, Pandey VK, Tandon M. Acute liver failure in pregnancy: Challenges and management. Indian J Anaesth. 2015;59:144–49. doi: 10.4103/0019-5049.153035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casey LC, Fontana RJ, Nelson DB, et al. Hepatology. 2020. Acute liver failure (ALF) in pregnancy: How much is pregnancy-related? [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson DB, Yost NP, Cunningham G. Acute fatty liver of pregnancy: Clinical outcomes and expected duration of recovery. Am J Obstet Gynecol. 2013;209:e1–7. doi: 10.1016/j.ajog.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Pusl T, Beuers U. Intrahepatic cholestasis of pregnancy. Orphanet J Rare Dis. 2007;2:26. doi: 10.1186/1750-1172-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo MW, Galanko JA, Shrestha R, et al. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- 6.Bhattia V, Singhal A, Panda SK, Acharya SK. A 20-year single-centre experience with acute liver failure during pregnancy: Is the prognosis really worse? Hepatology. 2008;48:1577–85. doi: 10.1002/hep.22493. [DOI] [PubMed] [Google Scholar]
- 7.Morris CV, Goldstein RM, Cofer JB, et al. An unusual presentation of fulminant hepatic failure secondary to propylthiouracil therapy. Clin Transpl. 1989:311. [PubMed] [Google Scholar]
- 8.Sequeria E, Wanyonyi S, Dodia R. Severe propylthiouracil-induced hepatotoxicity in pregnancy managed successfully by liver transplantation: A case report. J Med Case Rep. 2011;5:461. doi: 10.1186/1752-1947-5-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanogawa N, Kanda T, Ohtsuka M, et al. Acute liver failure occurring during the first trimester of pregnancy successfully treated with living donor liver transplantation. Case Rep Transpl. 2013;2013:309545. doi: 10.1155/2013/309545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kato T, Nery JR, Morcos JJ, et al. Successful living related liver transplantation in an adult with fulminant hepatic failure. Transplantation. 1997;64:415–17. doi: 10.1097/00007890-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 11.Eguchi S, Yanaga K, Fujita F, et al. Living-related right lobe liver transplantation for a patient with fulminant hepatic failure during the second trimester of pregnancy: Report of a case. Transplantation. 2002;73:1970–71. doi: 10.1097/00007890-200206270-00025. [DOI] [PubMed] [Google Scholar]
- 12.Jarufe N, Soza A, Pérez-Ayuso RM, et al. Successful liver transplantation and delivery in a women with fulminant hepatic failure occurring during the second trimester of pregnancy. Liver Int. 2006;26:494–97. doi: 10.1111/j.1478-3231.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg M, Daugherty TJ, Elihu A, et al. Acute liver failure at 26 weeks’ gestation in a patient with sickle cell disease. Liver Transpl. 2009;15:1236–41. doi: 10.1002/lt.21820. [DOI] [PubMed] [Google Scholar]
- 14.Morena EG, Garcia GI, Gomez SR, et al. Fulminant hepatic failure during pregnancy successfully treated by orthotopic liver transplantation. Transplantation. 1991;52:923–26. [PubMed] [Google Scholar]
- 15.Lo CM, Gertsch P, Fan ST. Living unrelated liver transplantation between spouses for fulminant hepatic failure. Br J Surg. 1995;82:1037. doi: 10.1002/bjs.1800820811. [DOI] [PubMed] [Google Scholar]
- 16.Maddukuri VC, Stephenson CD, Eskind L, et al. Liver transplantation for acute liver failure at 11-week gestation with successful maternal and fetal outcome. Case Rep Transpl. 2012;2012:484080. doi: 10.1155/2012/484080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carp H. A systematic review of dydrogesterone for the treatment of recurrent miscarriage. Gynecol Endocrinol. 2015;31:422–30. doi: 10.3109/09513590.2015.1006618. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa K, Abdul Hamid H, Abdul Karim A, et al. Severe jaundice post embryo transfer: A case report. World Congr Gynecol Endocrinol. 2014;36:229–31. [Google Scholar]
- 19.Altintaş E, Oğuz D, Kaçar S, et al. Dydrogestreone-induced hepatitis and autoimmune hemolytic anemia. Turk J Gastroenterol. 2004;15:49–52. [PubMed] [Google Scholar]
- 20.Reyes H, Simon FR. Intrahepatic cholestasis of pregnancy: An estrogen-related disease. Semin Liver Dis. 1993;13:289–301. doi: 10.1055/s-2007-1007357. [DOI] [PubMed] [Google Scholar]
- 21.Koivurova S, Hartikainen A, Karinen L, et al. The course of pregnancy and delivery and the use of maternal healthcare services after standard IVF in Northern Finland 1990–1995. Hum Reprod. 2002;17:2897–903. doi: 10.1093/humrep/17.11.2897. [DOI] [PubMed] [Google Scholar]
- 22.Koren G. Dydrogesterone exposure in the first trimester of pregnancy and fetal malformations. Motherisk Int J. 2020;1:11. [Google Scholar]
- 23.Germain AM, Carvajal JA, Glasinovic JC, et al. Intrahepatic cholestasis of pregnancy: An intriguing pregnancy-specific disorder. J Soc Gynecol Investig. 2002;9:10–14. doi: 10.1016/s1071-5576(01)00144-7. [DOI] [PubMed] [Google Scholar]
- 24.Bolukbas FF, Bolukbas G, Balaban HY, et al. Intrahepatic cholestasis of pregnancy: Spontaneous vs. in vitro fertilization. Euroasian J Hepatogastroenterol. 2017;7:126–29. doi: 10.5005/jp-journals-10018-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore KL, Persaud TVN, Torchia MG. The developing human: Clinically oriented embryology. 10th ed. Philadelphia (USA): Saunders; 2015. [Google Scholar]
- 26.Burke CJ, Tannenberg AE. Prenatal brain damage and placental infarction: An autopsy study. Dev Med Child Neurol. 1995;37:555–62. doi: 10.1111/j.1469-8749.1995.tb12042.x. [DOI] [PubMed] [Google Scholar]
- 27.Eloundou SN, Lee JY, Wu D, et al. Placental malperfusion in response to intrauterine inflammation and its connection to fetal sequelae. PLoS One. 2019;14:e0214951. doi: 10.1371/journal.pone.0214951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watchoko JF. Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromolec Med. 2006;8:513–29. doi: 10.1385/NMM:8:4:513. [DOI] [PubMed] [Google Scholar]