Abstract
Objective
To assess the levels of glycoprotein GPIV (CD36) expression on peripheral blood monocyte subsets, in a mouse model of glucose intolerance. Moreover, to determine the effect of; low-dose aspirin (LDA) alone, LDA combined with metformin, or clopidogrel alone, on the expression of CD36 on subsets of circulating monocytes.
Method
The study consisted of two experimental phases. In experiment one, the mice (n = 14) were randomised to receive a low-fat diet (LFD) or a high-fat diet (HFD) for eight weeks. Whereas the secondary phase of the experiment, comprised of twenty-four HFD-fed mice treated with LDA alone (3 mg/kg), or in combination with metformin (150 mg/kg), or clopidogrel alone (10 mg/kg) for six weeks. The surface expression of CD36 on monocytes was measured using flow cytometry.
Result
The levels of CD36 expression on monocytes were upregulated in the HFD-fed compared to LFD-fed group (p < 0.05). In addition, HFD group showed; no significant changes in body weight (p = 0.3848), however, blood glucose (p = 0.0002) and insulin (p = 0.0360) levels were markedly increased following HFD-feeding. Interestingly, all treatments reduced the expression of CD36 on monocytes, decreased fasting blood glucose levels (p = 0.0024) and increased circulating monocyte levels (p = 0.0217) when compared to the untreated HFD group. Moreover, treatment with LDA alone increased basophils levels (p = 0.0272), while when combined with metformin showed an improved effect in enhancing eosinophil levels (p = 0.0302).
Conclusion
HFD-feeding increased the expression of CD36 on monocyte subsets. LDA as a monotherapy or combined with metformin was as effective as clopidogrel monotherapy, in downregulating the expression of CD36 on monocyte subsets. These treatments may be of relevance in preventing cardiovascular complications associated with impaired glucose tolerance.
Keywords: Prediabetes, Impaired glucose tolerance, T2DM, Monocytes, Inflammation, CD36, Antiplatelet drug
Highlights
-
•
High fat-diet feeding increases levels of peripheral blood MФ and M1 monocytes expressing glycoprotein IV (CD36).
-
•
Cyclooxygenase-1 inhibition decreases the expression of CD36.
-
•
Impaired glucose tolerance is associated with increased levels of M1 monocytes that express elevated levels CD36.
1. Introduction
Chronic inflammation and the persistent activation of peripheral monocytes and neutrophils are associated with the pathogenesis of type 2 diabetes mellitus (T2DM). Activated monocytes can further initiate atherosclerosis and increase the risk of complications in people living with T2DM [[1], [2], [3]]. For example, the monocyte chemoattractant protein 1 (MCP-1) plays a pivotal role in the recruitment of monocytes in response to atherogenic stimuli such as hyperglycaemia and dyslipidaemia [4]. The efficacy of low-dose aspirin (LDA) in combination with clopidogrel in the secondary prevention of cardiovascular disease (CVD) has been reported [5,6]. In addition, the pleiotropic properties of metformin, a widely used first-therapy drug in patients with T2DM, include both anti-inflammatory and anti-thrombotic properties [7]. Metformin has also been shown to suppress the production of tumour necrosis factor-alpha (TNF)-α and reactive oxygen species (ROS), while also downregulating the expression of the membrane glycoprotein IV (CD36) [8]. Importantly, CD36 is a class B scavenger receptor that plays a crucial role in innate immune responses [9,10], including the modulation of lipid metabolism and inflammation [9,11].
The ligation of CD36 by oxidised low-density lipoprotein (oxLDL) initiates the formation of foam cells with the subsequent formation of an atherosclerotic plaque [12]. In such a case, CD36 plays an essential role in the development of atherosclerosis [1]. Notably, in hyperglycaemic states, activated monocytes can upregulate the expression of CD36 [9], which has a high affinity for oxLDL and apoptotic cell surfaces [13,14], as well as advanced glycation end products (AGEs) [15]. Furthermore, the levels of the soluble form of CD36 (sCD36) are increased in patients living with T2DM, and can also accelerate the risk of CVD in these patients [16,17]. Currently, the use of low-dose aspirin (LDA) in the primary prevention of cardiovascular events in patients living with T2DM remains controversial [18]. Interestingly, LDA has been shown to partially downregulate sCD36 levels, consistent with reduction of platelet activation in patients with T2DM [9]. Furthermore, a recently published preclinical study showed beneficial effects of LDA in suppressing vascular inflammation and maintaining atherosclerotic plaques stability in LDL receptor–deficient mice [19].
Although CD36 is highly expressed on monocytes and upregulated in atherosclerosis and T2DM, it remains unclear whether the levels of circulating monocyte subsets differentially express CD36 in a state of impaired glucose tolerance. To date, the differential expression of CD36 expression on circulating monocyte subsets in a state of impaired glucose metabolism remains unclear. The enumeration and quantification of monocyte subsets in a state of impaired glucose tolerance may allow for risk stratification of individuals at increased risk of atherosclerosis. In this study, short term high-fat diet (HFD) was used to induce glucose intolerance in C57BL/6 male mice, which is an important model to study complications linked with early development of T2DM, as demonstrated elsewhere [20,21]. Therefore, the current study reports on the expression of CD36 on monocyte subsets in HFD fed mice with glucose intolerance. Furthermore, we assessed whether treatment with; low-dose aspirin (LDA) alone; LDA in combination with metformin or clopidogrel alone could modulate the expression of CD36 on monocyte subsets in glucose tolerance.
2. Materials and methods
2.1. Animal care and feeding
In this study, six-week-old male C57BL/6 mice (n = 36) were purchased from the Biomedical Research Unit (BRU) at the University of KwaZulu-Natal, South Africa. The UKZN Animal Research Ethics Committee (AREC) approved the animal experiments and study procedures, complying with existing UKZN animal handling standards which are in accordance to the principle and guidelines of Canadian Council on Animal Care (protocol number: AREC/086/016) and reported as per Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines (Supplementary doc 1). The animals were housed in a temperature-regulated room (22 ± 2 °C) in a 12-h light and 12-h dark cycle (light was switched on from 06:00 p.m.-06:00 a.m.). After a 1-week acclimatisation period, the mice were assigned into two diet groups matched for macronutrient type and source. These mice were enclosed in clean cages (n = 6/cage). In addition, they had unlimited access to water, food pellets and fresh air throughout the experiments.
2.1.1. The diet composition of a high and low-fat diet
Low-fat diet (LFD; D12450J) contained 20% kcal protein, 70% kcal carbohydrate and 10 kcal% of fat. While the high-fat diet (HFD; D12492J) contained 20% kcal protein, 20% kcal carbohydrates and 60 kcal% of fat.
2.2. Study design and experimental protocols
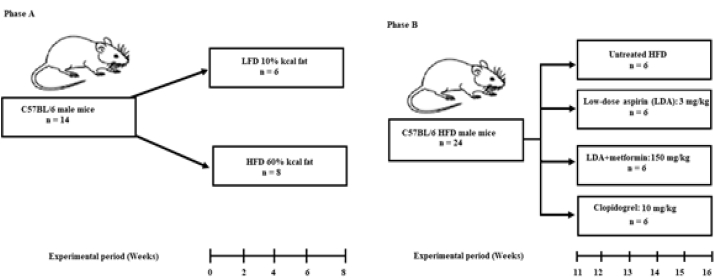
The experiments comprised of two phases (Fig. 1). The first phase of the experiment included mice (n = 14) with six kept on a low-fat diet (LFD), and eight mice kept on HFD for eight weeks (Fig. 1A). The second phase of the experiment comprised of mice (n = 24) kept on an HFD diet for eight weeks (Fig. 1B). In the second phase of the study, the animals were randomly assigned into four groups (n = 6/group) comprised of an untreated HFD group and three treatment groups, following short-term six-week treatment.
Fig. 1.
Study design. The figure illustrates the two experimental phases. The first phase was used to establish the pathological state (A), where fourteen animals were randomly allocated into a high-fat diet (HFD) (n = 8) or low-fat diet (LFD) (n = 6). The mice were kept on the respective diets for eight weeks. The oral glucose tolerance test (OGTT) was then performed to determine glucose tolerance. In the second phase (B), twenty-four mice fed on HFD for eight weeks showing signs of impaired glucose tolerance and were then randomised into four groups (n = 6/group); (i) untreated HFD group; (ii) Low-dose aspirin group (LDA); (iii) Low-dose aspirin in combination with metformin (LDA + Met); and (iv) Clopidogrel group. The treatment was administered daily, through oral gavage for six weeks.
Experimental phase 1: In the first phase of the experiment, we measured peripheral blood monocyte subtypes and basal CD36 expression on classical and pro-inflammatory monocytes following a short-term HFD-feeding. In this phase of the study, fourteen six-week-old male C57BL/6 mice were assigned into two experimental diet groups, the LFD (n = 6) and HFD (n = 8). The animals were kept on the respective diets for eight weeks (Fig. 1A). We then measured the haematological parameters using the AcT 5 diff haemo-analyser (Beckman Coulter, Brea, CA, USA). Plasma insulin and glucose levels were measured at experimental week 8, while body weights were measured weekly. The oral glucose tolerance test (OGTT) was also performed at experimental week 8, following previously described methods [22]. All glucose measurements were performed using the OneTouch® Select® handheld glucometer (LifeScan Inc., Milpitas, CA, USA). While fasting serum insulin levels were determined following, using an enzyme-linked immunosorbent assay (ELISA) kit (Thermo Fisher, Massachusetts, U.S.A).
Experimental phase 2: To determine whether the anti-inflammatory and antithrombotic drugs, modulate the expression of CD36 on monocyte subsets in conditions of impaired glucose tolerance. A total of 24 HFD-fed mice were randomly assigned into four groups (n = 6/group); (i) untreated HFD; (ii) LDA alone (3 mg/kg); (iii) LDA in combination with metformin (150 mg/kg); (iv) clopidogrel alone (10 mg/kg) (Fig. 1B). All drugs were administered daily, for six weeks via oral gavage. Blood was drawn after six weeks of treatment, and the analysis of glycoprotein IV (CD36) was performed using flow cytometry (Fig. 1 and S). The animals were then euthanised using halothane.
2.2.1. Biochemical and haematological analysis
Blood (200 μl) was drawn from the lateral tail vein into serum separator (SST) and ethylenediaminetetraacetic acid (EDTA) microtainer tubes (BD Bioscience, USA), as previously reported [23]. Haematological parameters were measured using the AcT 5 diff haemo-analyser (Beckman Coulter, Brea, California, United States).
2.2.2. Monocytes isolation
For monocyte isolation, whole blood samples were incubated with microbeads and then magnetic cell sorting (MACS) buffer at 4 °C for 15 min. MACS consisted of phosphate-buffered saline (PBS) (Gibco BRL, Frederick, MD, USA) with bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA) and 2 mM of EDTA (VWR international, Leuven, Belgium). MACS buffer was used at 4 °C for all washing stages with centrifugation at 600 g for 5 min. Ice-cold LD and MS MACS columns were kept at 4 °C, and air bubbles were prevented during incubation and magnetic separation. Isolation and characterisation of mouse monocytes were performed, as explained previously [24].
2.2.3. Immunophenotyping of monocyte subsets
All data acquisition was carried out using the BD FACS CANTO II flow cytometer (Becton Dickson, NJ, USA). In order to control for non-specific binding a fluorescence minus one (FMO) control was used to control for non-specific binding. Antibody titrations were also performed to determine the optimal antibody concentrations used. The aantibody cocktail included CD11c-PE/Cy7 and CD36-PE (BD Biosciences, San Diego, CA) (Table 1S). CD11c-PE/Cy7 antibody was used to identify monocytes, and the CD36 antibody was used to measure the expression of glycoprotein IV (CD36) on monocyte subsets. Briefly, 50 μL (50 μl) of isolated monocytes sample were stained with titrated volumes (1:100) of the antibody cocktail. For each sample, at least a 1000 events were acquired at a medium flow rate. Monocyte subsets were identified by their distinctive forward and side scatter properties and further classified as classic (CD11c+) or pro-inflammatory (CD11c++) monocytes.
Table 1.
Baseline metabolic and haematological characteristics of low-fat diet (LFD) and high-fat diet (HFD)-fed mice.
| Parameters | LFD (n = 6) | HFD (n = 8) | p-value |
|---|---|---|---|
| Weight (g) | 24.93 ± 1.91 | 25.81 ± 1.98 | 0.3771 |
| Insulin (μU/L) | 4.63 ± 0.19 | 6.02 ± 1.43 | 0.0465 |
| 2 HOUR POSTPRANDIAL GLUCOSE | |||
| Glucose (mmol/L∗120 min) | 3.1 (3.0–3.85) | 6.75 (6.45–7.05) | 0.0002 |
| AUC (mmol/L∗ 120 min) | 557.40 ± 201.80 | 1032.00 ± 194.10 | 0.0151 |
| WBC (103/μL) | 5.98 ± 1.50 | 8.08 ± 2.99 | 0.1155 |
| Monocytes (103/μL) | 0.08 ± 0.04 | 0.09 ± 0.06 | 0.8774 |
| Neutrophils (103/μL) | 0.42 ± 0.19 | 0.72 ± 0.29 | 0.0848 |
| Lymphocytes (103/μL) | 4.54 ± 0.76 | 7.16 ± 2.73 | 0.1094 |
| Basophils (103/μL) | 0.01 (0.01–0.01) | 0.01 (0.00–0.01) | 0.3215 |
| Eosinophils (103/μL) | 0.01 (0.00–0.01) | 0.01 (0.01–0.01) | 0.8852 |
| RBC (106/μL) | 6.56 ± 1.01 | 5.93 ± 1.41 | 0.3502 |
| Hgb (g/dL) | 25.43 ± 3.33 | 19.04 ± 4.13 | 0.0571 |
| Hct (%) | 28.68 ± 4.25 | 22.01 ± 5.56 | 0.0746 |
| MCV (fl) | 42.00 ± 0.89 | 43.13 ± 0.83 | 0.0016 |
| MCH (pg) | 48.00 ± 1.31 | 38.44 ± 10.60 | 0.1141 |
| RDW (%) | 10.53 ± 0.63 | 10.38 ± 0.42 | 0.6086 |
| Platelets (103/μL) | 671.0 (625.5–842.8) | 739.0 (570.3–782.5) | 1.0000 |
Significance (p < 0.05) shown in boldface. Data reported as mean ± SD or median (IQR).
Abbreviations: LFD: low-fat diet; HFD: high-fat diet; AUC: area under the curve; WBC: white blood cell; RBC; red blood cell; Hgb: haemoglobin; Hct: haematocrit; MCV: mean corpuscular volume; MCH: mean corpuscular haemoglobin; RDW: red cell distribution width.
2.3. Statistical analysis
Statistical analysis was performed using GraphPad Prism 8 version 8.2.1 (441) Software, (GraphPad Software Inc, San Diego, CA, USA). For normality testing, Kolmogorov-Smirnov (KS) normality test was performed. Differences between LFD and HFD groups in normal distribution was assessed using a two-tailed unpaired Student’s t-test, while the Mann-Whitney test was used for nonparametric data. In instances of normally distributed data, a one-way ANOVA was used with Tukey’s as a post hoc test while for nonparametric data, the Kruskal-Wallis test was used followed by a Dunn’s post-hoc test. A p-value of < 0.05 was considered statistically significant. The effect size was also calculated using Cohen’s D method.
3. Results
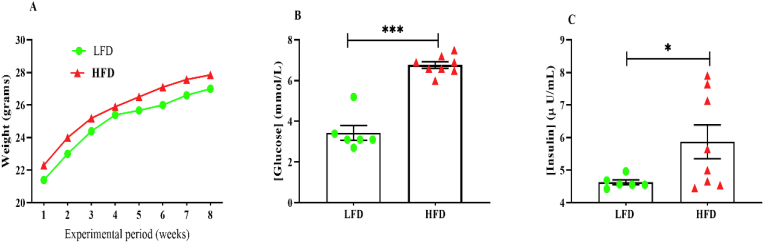
Initially, we determined whether the HFD induced a state of impaired glucose tolerance following short-term 8-week HFD-feeding period. Blood glucose and insulin levels were determined after animals were kept on their respective diets for eight weeks. As anticipated, HFD-feeding resulted in impaired glucose tolerance with a higher postprandial blood glucose level (6.75 (6.45–7.05) when compared to the LFD-feed group [3.1 (3.0–3.85)], p = 0.0002 (Fig. 2B and C). Furthermore, the elevated levels of postprandial blood glucose levels were associated with a 1.9-fold increase in the area underneath the curve (AUC) compared to the LFD mice (p = 0.0068) (Table 1). In a similar manner, insulin levels (μU/L) were increased in HFD (6.02 ± 1.43), p = 0.0360) compared to LFD-fed mice (4.63 ± 0.19) (Fig. 2B, Table 1). However, the animal weights across both diet groups were comparable (p = 0.3848) following the short-term diet feeding of 8 weeks (Table 1). There were no adverse events observed in any of the experimental phases of this study.
Fig. 2.
Metabolic changes following eight weeks of high-fat diet (HFD) and low-fat diet (LFD) feeding. Figure A-Illustrates the changes in body weight from experimental week 1–8. Figure B and C demonstrates the postprandial glucose and insulin levels following eight weeks of HFD-feeding compared to LFD. Data are reported as mean ± SEM, ∗ p < 0.05, ∗∗∗ p < 0.0001 vs. LFD group.
3.1. Changes in haematological indices following short-term HFD compared to LFD
The haemoglobin concentration (g/dL) were comparable between the HFD group (19.04 ± 4.13) when compared to the LFD (25.43 ± 3.33), p = 0.0571 (Table 1). The haematocrit levels were also comparable between the HFD (22.01 ± 5.56) compared to the LFD group (28.68 ± 4.52), p = 0.0746. While the mean corpuscular volume (MCV) was slightly increased in HFD group (43.13 ± 0.83) compared to LFD group (42.00 ± 0.89), p = 0.0016 (Table 1). Lastly, the levels of circulating monocytes, basophils and eosinophils were comparable (p > 0.05) between the two groups. Whereas, neutrophils and lymphocytes were increased in the HFD compared to the LFD Group, p < 0.05 (Table 1).
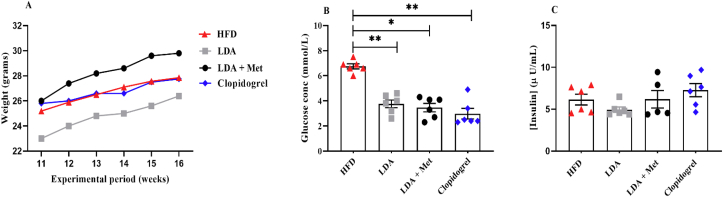
3.2. Short-term treatment with LDA alone, or in combination with metformin improves glucose tolerance in HFD-fed mice
To assess the changes in the body weights, glucose and insulin levels following short-term treatment. A one-way analysis of variance (ANOVA) was performed. There were significant changes in the body weights following the short-term treatment with the various drugs (F (3, 20) = 9.474, p = 0.0004). Tukey’s post hoc analysis, showed a significant reduction in the body weights of mice treated with LDA monotherapy (24.80 ± 1.19), when compared to those on HFD only (25.81 ± 1.19), or when LDA was used in combination with metformin (28.27 ± 1.42), p < 0.05 (Table 2). In addition, the treatments also altered the blood glucose levels (H = 18.38, p = 0.0024). The Dunn’s post-hoc analysis showed a marked reduction in the blood glucose levels in; LDA + metformin [3.7 (2.60–4.15)], and clopidogrel alone [2.45 (2.38–3.78)], treated groups compared to the untreated HFD group [6.75 (6.45–7.05)], p = 0.0024. However, all treatments did not affect insulin levels (F (3, 21) = 1.992, p = 0.1460) (Table 2, Fig. 3).
Table 2.
Haematological, and metabolic changes following the short-term treatment with low-dose aspirin (LDA); LDA + Met or clopidogrel.
| Parameters | HFD | LDA | LDA + Met | Clopidogrel | p-value |
|---|---|---|---|---|---|
| Weight (g) | 25.81 ± 1.98 | 24.80 ± 1.19a | 28.27 ± 1.42b | 26.71 ± 0.78c | 0.0004 |
| Glucose (mg/dL) | 6.75 (6.45–7.05) | 3.90 (3.05–4.45)a | 3.7 (2.6–4.15)d | 2.45 (2.38–3.78)e | 0.0024 |
| Insulin (mmol/L) | 6.02 ± 1.43 | 4.95 ± 0.79 | 6.19 ± 2.33 | 7.28 ± 1.94 | 0.1460 |
| WBC (103/μL) | 8.08 ± 2.99 | 8.43 ± 0.42 | 7.87 ± 1.70 | 7.17 ± 3.76 | 0.8564 |
| Monocytes (103/μL) | 0.09 ± 0.06 | 0.26 ± 0.11a | 0.26 ± 0.10d | 0.23 ± 0.17 | 0.0217 |
| Neutrophils (103/μL) | 0.63 (0.98–0.51) | 0.69 (0.79–0.60) | 0.77 (1.3–0.59) | 0.50 (0.68–0.40) | 0.1959 |
| Lymphocytes (103/μL) | 7.16 ± 2.73 | 7.45 ± 0.29 | 6.64 ± 1.34 | 6.35 ± 3.45 | 0.8414 |
| Basophils (103/μL) | 0.01 (0.01–0.0) | 0.02 (0.02–0.02)a | 0.01 (0.02–0.01) | 0.01 (0.02–0.01) | 0.0272 |
| Eosinophils (103/μL) | 0.01 (0.01–0.00) | 0.01 (0.02–0.00) | 0.02 (0.09–0.01)f | 0.00 (0.0–0.00) | 0.0302 |
| RBC (106/μL) | 6.55 ± 1.77 | 6.99 ± 1.13 | 7.21 ± 0.89 | 6.23 ± 1.71 | 0.2644 |
| Hgb (g/dL) | 19.04 ± 4.13 | 28.97 ± 4.97a | 30.57 ± 3.90d | 21.73 ± 8.00 | 0.0015 |
| Hct (%) | 22.01 ± 5.56 | 28.97 ± 4.97 | 30.57 ± 3.90d | 25.55 ± 7.35 | 0.0420 |
| MCV (fL) | 43.13 ± 0.83 | 41.50 ± 0.55a | 42.50 ± 0.82 | 41.00 ± 1.41e | 0.0019 |
| MCH (pg) | 38.44 ± 10.60 | 44.75 ± 1.49 | 31.80 ± 17.25 | 42.87 ± 1.60 | 0.5504 |
| RDW (%) | 10.38 ± 0.42 | 10.72 ± 0.74 | 10.95 ± 1.14 | 11.20 ± 1.35 | 0.4278 |
| Platelets (103/μl) | 739 (782.5–570.3) | 839 (966.5–670.5) | 820.5 (940.8–756.3) | 756 (874–552.3) | 0.4337 |
Significant values are shown in boldface (p > 0.05).
Abbreviations: WBC: white blood cell; RBC: red blood cell; Hgb: haemoglobin; MCV: mean corpuscular volume; MCH: mean cell haemoglobin; RDW: red blood cell distribution width.
HFD vs LDA.
LDA vs LDA + Metformin.
LDA vs clopidogrel.
HFD vs LDA + Metformin.
HFD vs clopidogrel.
Clopidogrel vs LDA + metformin.
Fig. 3.
Changes in metabolic parameters following six-week treatment with low-dose aspirin (LDA) alone; LDA in combination with metformin (LDA + Met) or clopidogrel alone in high-fat diet (HFD)-fed mice. Figure A, B, and C show the body weights, blood glucose and insulin levels in untreated HFD-fed mice versus treated group following a 6-week short term treatment. Data are reported as mean ± SEM, (n = 6/group), ∗ p < 0.05, ∗∗ p < 0.005.
3.3. The effect of short-term treatment on haematological parameters in HFD-fed mice
The changes in the haematological indices following treatment with the anti-inflammatory and anti-thrombotic drugs were assessed. Treatment had a no effect on levels of circulating white blood cells (WBC) (F (3, 22) = 0.2557, p = 0.8564) and red blood cells (RBC) (F (3, 22) = 1.417, p = 0.2644). Whereas all the treatment altered the haemoglobin (F (3, 18) = 7.803, p = 0.0015); haematocrit (F (3, 22) = 3.230, p = 0.0420) and mean corpuscular volume (MCV) (F (3, 22) = 6.905, p = 0.0019), while other RBC indices remained unchanged (p > 0.05) (Table 2). Notably, the haemoglobin levels were increased following treatment with LDA alone (28.97 ± 4.97, p = 0.0090), or when combined with metformin (30.57 ± 3.90, p = 0.0025), in comparison to the untreated HFD group (19.04 ± 4.13) (Table 2). Moreover, a significant decrease in MCV was observed in both LDA alone (41.50 ± 0.55) and clopidogrel (41.00 ± 1.41) treatments when compared to the untreated HFD group (43.13 ± 0.83), p < 0.05 (Table 2). LDA + Met significantly increased the haematocrit levels when compared to the untreated HFD group (p = 0.041), (Table 2).
There were significant interactions between various treatments and levels of circulating monocytes (F (3, 22) = 3.937, p = 0.0217). While no interactions were observed between the treatment and the levels of circulating lymphocytes (F (3,22) = 0.2769, p = 0.8414) (Table 2). Unexpectedly, monocytes significantly increased in mice treated with LDA alone (0.26 ± 0.11) and in LDA + Met (0.26 ± 0.10), when compared to the untreated HFD group (0.09 ± 0.06) (Table 2). Treatment also altered the levels of circulating basophils and eosinophils. Notably, treatment with LDA alone was effective at increasing the levels of circulating basophils when compared to the untreated HFD group (p = 0.0179). Whereas LDA + Met increased the levels of circulating eosinophils when compared to clopidogrel treated mice (p = 0.0177) (Table 2).
3.4. Pro-inflammatory monocytes (M1) in HFD-fed mice express elevated levels of CD36
Upregulation in the expression of CD36 is associated with an increased risk of thrombosis. However, the cellular origins of CD36 in metabolic disease remain unclear. We measured the levels of CD36 circulating classical (MФ) and pro-inflammatory (M1) monocytes following short-term HFD feeding. The levels of circulating monocytes subsets were elevated in HFD compared to LFD (p < 0.05). As expected, the levels of CD36 expression on MФ and M1 monocytes were also elevated in the HFD group when compared to the LFD-fed group (p < 0.001) (Table 3).
Table 3.
Monocyte subsets and the expression of CD36 in the high-fat diet (HFD)-fed compared to low-fat diet (LFD)-fed mice.
| Parameter | LFD (n = 6) | HFD (n = 8) | p-value |
|---|---|---|---|
| Monocyte subsets | |||
| MФ | 72.29 ± 26.06 | 79.70 ± 12.95 | 0.0005 |
| M1 | 6.75 ± 5.54 | 10.25 ± 8.50 | 0.0001 |
| MФ/M1 | 15.78 ± 8.35 | 20.33 ± 18.96 | 0.5525 |
| Monocyte-CD36 expression | |||
| MФ | 39.72 ± 14.50 | 47.44 ± 15.15 | 0.0002 |
| M1 | 97.79 ± 2.5 | 99.56 ± 0.75 | < 0.0001 |
| MФ/M1 | 0.39 ± 0.14 | 0.49 ± 0.16 | 0.3053 |
Significant values are shown in boldface.
MФ - classical monocyte subset; M1-pro-inflammatory monocyte subset.
Furthermore, we investigated the effects of treatment on the mean differences of monocyte subsets (MФ and M1). Interestingly, in comparison to the untreated HFD group (52.60 [95% CI: 35.57–69.62], p = 0.0005); treatment with; LDA alone (43.70 [95%CI: 23.87–63.53], p = 0.0024); LDA + Met (44.89 [95%CI: 26.86–62.91], p = 0.0014) or clopidogrel alone (51.42 [95%CI: 33.18–69.65], p = 0.0008) significantly reduced the mean difference of circulating monocyte subsets in the LDA only group (43.70 [95%CI: 23.87–63.53], p = 0.0024); or when combined with (Table 4).
Table 4.
The effect of treatment on the expression of CD36 on monocyte subsets in the HFD-fed mice.
| Groups | MФ | M1 | Mean difference (95% CI) | p-value | |
|---|---|---|---|---|---|
| HFD | 43.29 ± 14.08 | 95.89 ± 3.66 | 52.60 (35.57–69.62) | 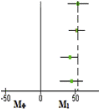 |
0.0005 |
| LDA | 54.39 ± 19.80 | 98.09 ± 2.11 | 43.70 (23.87–63.53) | 0.0024 | |
| LDA + Met | 52.13 ± 18.35 | 97.02 ± 2.62 | 44.89 (26.86–62.91) | 0.0014 | |
| Clopidogrel | 46.59 ± 16.52 | 98.01 ± 1.63 | 51.42 (33.18–69.65) | 0.0008 | |
Significant values are shown in boldface (p < 0.05). HFD, high-fat-diet; LDA, low-dose aspirin; Met, metformin; MФ-classical monocyte subset; M1-pro-inflammatory monocyte subset.
3.4.1. The effect of treatment on the expression of CD36 on monocytes subsets of HFD-fed mice
To determine which of the treatments induced a more significant effect on the levels of circulating monocytes expressing CD36. An effect size estimation was performed using Cohen’s d method [25]. There was a larger reduction in the levels of M1 monocytes expressing CD36 following short-term treatment with LDA alone (Cohen’s d (ds) = 0.71) and clopidogrel monotherapy (ds = 0.66), compared to the dual therapy of LDA and metformin (ds = 0.3) which showed smaller reduction in circulating M1 monocytes expressing CD36 (Table 5).
Table 5.
Mean difference in the expression of CD36 on MФ and M1 subsets post treatment in high fat diet-fed mice.
| Groups | MФ Mean Difference [95%CI] |
M1 Mean difference [95%CI] |
p-value |
|---|---|---|---|
| LDA | 11.10 (-8.34–30.54) | 2.20 (-1.18–5.58) | < 0.05 |
| LDA + Met | 8.84 (-9.67–27.35) | 1.13 (-2.47–4.73) | < 0.05 |
| Clopidogrel | 3.30 (-14.07–20.67) | 2.12 (-1.09–5.33) | < 0.01 |
Significant values are shown in boldface (p < 0.05). HFD, high-fat-diet; LDA, low-dose aspirin; Met, metformin; MФ - classical monocyte subset; M1-pro-inflammatory monocyte subset.
4. Discussion
In this study, we evaluated the expression of glycoprotein IV (CD36) on monocyte subsets in a mouse model of high-fat diet (HFD)-induced impaired glucose tolerance. Furthermore, we assessed whether LDA alone; LDA + Met or clopidogrel alone, could modulate the expression of CD36 on monocyte subsets. Our results showed elevated pro-inflammatory monocytes (M1) in a mouse model of glucose intolerance and these levels of pro-inflammatory (M1) monocytes were associated with a high expression of glycoprotein IV (CD36). Moreover, all treatments were able to reduce the levels of CD36 expression on M1 monocytes when compared to classical monocytes (MФ) in HFD-fed mice. In this study, it was also evident that the short-term exposure to HFD could induce glucose intolerance and hyperinsulinemia, which were independent of increased body weight. This has been described in previous studies, where three major phases of HFD have been characterised, which include an early, intermediate and late phase [26,27]. Notably, the early phase occurs within three days of HFD and lasting until 12 weeks of HFD. The early phase is characterised by insulin insensitivity and a fold increase in the glucose tolerance area underneath the curve (AUC) without any marked changes in body weights [26]. Our findings point to the changes that occur in insulin sensitivity and impaired glucose metabolism during the early phase of HFD-feeding.
Interestingly, LDA as a monotherapy or in combination with metformin improved glycaemic index and this was independent of insulin. As, indicated by persistently elevated insulin levels even during treatment with the anti-inflammatory (LDA alone, LDA + Met) and antithrombotic drugs (clopidogrel) monotherapy. In addition, short-term LDA treatment led to a reduction in animal body weights, whereas a gradual increase in body weights of animals on LDA in combination with metformin and clopidogrel alone was observed. Consistently, although short-term LDA treatment is known to lower blood glucose levels [28], it provides no long-term therapeutic benefit in the prevention of clinical T2DM [29]. The administration of antithrombotic and anti-inflammatory drugs in high-fat diet C57BL/6 model, has been shown to ameliorate states of obesity and diabetes [[30], [31], [32]]. Moreover, impaired glucose tolerance was associated with elevated neutrophil and lymphocyte counts. This may further support the well-established association between insulin insensitivity and increased risk of thrombosis. As elevated neutrophils [33,34] and lymphocytes have been associated with chronic inflammation and thrombosis [33]. The increased levels of circulating neutrophils and lymphocytes persisted even during treatment with LDA alone; LDA + Met or clopidogrel alone. Notably, the monocyte, basophil, and eosinophil levels were also increased following treatment with the various anti-inflammatory and antithrombotic drugs in HFD-fed mice. Although the RBC was normal, changes in the haemoglobin, haematocrit and mean corpuscular volume were observed. Interleukin-6, an anti-erythropoietic agent, alters the sensitivity of erythropoietin progenitors resulting in the destruction of immature red blood cells and a decreased concentration of haemoglobin [[35], [36], [37]].
Furthermore, due to their importance in regulating immune response [38], it was essential to explore the involvement and regulation of peripheral blood MФ and M1 monocytes in conditions of impaired glucose tolerance. In the first phase of the study, we showed increased levels of peripheral blood MФ and M1 monocytes following short-term HFD. Elevated levels of M1 monocytes have been reported in patients living with T2DM [39]. Concomitantly, increased levels of circulating monocytes were associated with increased CD36 expression following HFD feeding. It is hypothesised that CD36 is upregulated during the early phases of atherosclerosis; this involves the differentiation of monocytes into macrophages which subsequently engulf oxLDL and promote the formation of foam cells [40,41]. Notably, the levels of sCD36 are associated with an increased risk of insulin resistance and an increased risk of T2DM [16]. Contradictory findings regarding the role of CD36 in T2DM exist, with a clinical study reporting on similar levels of sCD36 in patients with diabetes and healthy controls [42]. The cellular origins of sCD36 in T2DM remain unclear [43]. However, erythrocyte-derived micro-particles have been described as a significant contributor to the levels of sCD36 in patients with T2DM [43].
In the second phase of our study, the short-term treatment with either cyclooxygenase-1 (COX-1) dependent (LDA) or independent (clopidogrel) antithrombotic drugs reduced the expression of CD36 on circulating M1 and MФ monocytes. Interestingly, it has been previously demonstrated that LDA can promote CD36 expression on macrophage via peroxisome proliferator-activated-receptor (PPAR) γ independent pathways [44]. Moreover, it is known that aspirin can be metabolised into its active form, salicylic acid, and in the process, block the adhesion of monocytes to low-density-lipoprotein activated endothelial cells through mechanisms involving the inhibition of nuclear factor kappa-light-chain-enhancer of activated β cells (NF-κβ) activity [45]. In T2DM, advanced glycation end products are also known to activate the NF-κβ pathway, which promotes the polarisation of macrophages into the M1 phenotype [46]. Importantly, CD36 orchestrates the patrolling of M1 monocytes on the endothelium during the initial stages of atherogenesis [47]. In addition, HFD feeding enhances the ratio of pro-inflammatory monocytes involved in CD36-dependent surveillance of peripheral blood vessels [47]. Elevated CD36 levels are associated with macrophage trapping, impaired insulin signalling, and thrombosis [48]. Altogether, our findings may suggest that an increased polarisation of monocytes accompanies the initial phases of impaired glucose tolerance into M1 monocytes that express elevated levels of CD36. Although this did not establish mechanistic insights into the propensity of M1 derived microparticles expressing CD36, our findings may suggest that M1 monocytes may be a source of sCD36 in patients with insulin resistance.
A limitation of the current study includes the lack of lipid profiling and lipid-binding activity of monocyte subsets. However, previous studies have associated the upregulation of CD36 with increased oxLDL uptake and clearance of apoptotic cells [47,49]. In conclusion, the current study reports on differential expression of CD36 on classical and pro-inflammatory monocytes in a state of impaired glucose tolerance. Moreover, pro-inflammatory monocytes, express elevated levels of CD36, which may suggest that basal CD36 expression on classical and pro-inflammatory monocytes may enhance the formation of foam cells. Hence, the enumeration and quantification of monocyte subsets in prediabetes may allow for risk stratification of individuals at increased risk of atherosclerosis. In this study, LDA and clopidogrel monotherapies showed a more significant reduction in the levels of CD36 expression on circulating M1, as indicated by an effect size of > 0.6. While LDA in combination with metformin showed a smaller effect in reducing the levels of CD36 expression on circulating pro-inflammatory monocytes. The discordance between the efficacy of LDA compared to LDA combined with metformin could be due to the reported effect of metformin of increasing free fatty acid (FFA) uptake and reduced mitochondria oxidation [50] and thus modulating the expression of CD36, which is also an FFA transporter.
Funding statement
BBN is partially funded by the National Research Foundation of South Africa (Grant Number: 107519) and research reported in this publication was supported by the South African Medical Research Council (SAMRC) under a Self-Initiated Research (Grant Number: 9894). BBN is also a University of KwaZulu-Natal (UKZN) Developing Research Innovation, Localisation and Leadership in South Africa (DRILL) fellow. DRILL is an NIH D43 grant (D43TW010131) awarded to UKZN in 2015 to support a research training and induction programme for early-career academics. PV Dludla was partially supported as a Post-Doctoral Fellow by funding from the SAMRC through its division of Research Capacity Development under the Intra-Mural Postdoctoral Fellowship Programme from funding received from the South African Treasury. The content hereof is the sole responsibility of the authors and do not necessarily represent the official views of the SAMRC or the funders.
Authors contribution
Kabelo Mokgalaboni: Data curation, writing- Original draft preparation, investigation. Phiwayinkosi V. Dludla: Conceptualization, Writing-reviewing and Editing, Supervision. Zibusiso Mkandla: Data curation, validation, Writing-Reviewing and Editing. Tinashe Mutize: Data Curation, validation, Writing-Reviewing and editing. Tawanda Maurice Nyambuya: Data curation, validation, Writing-Reviewing and Editing. Vuyolwethu Mxinwa: Validation, Writing-Reviewing and Editing, investigation. Bongani B. Nkambule: Conceptualization, Methodology, Supervision, Writing-reviewing and Editing, Supervision
Declaration of competing interest
The authors have no conflict of interests regarding the work presented in this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.metop.2020.100047.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Flynn M.C., Pernes G., Lee M.K.S., Nagareddy P.R., Murphy A.J. Monocytes, macrophages, and metabolic disease in atherosclerosis. Front Pharmacol. 2019;10:1–13. doi: 10.3389/fphar.2019.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rendra E., Riabov V., Mossel D.M., Sevastyanova T., Harmsen M.C., Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224:242–253. doi: 10.1016/j.imbio.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Mokgalaboni K., Dludla P.V., Nyambuya T.M., Yakobi S.H., Mxinwa V., Nkambule B.B. Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus : a systematic review and meta-analysis of pre-clinical and clinical studies. JRSM Cardiovasc Dis. 2020 doi: 10.1177/2048004019900748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piemonti L., Calori G., Lattuada G., Mercalli A., Ragogna F., Garancini M.P. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32:2105–2110. doi: 10.2337/dc09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito Y., Okada S., Ogawa H., Soejima H., Sakuma M., Nakayama M. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus. Circulation. 2017;135:659–670. doi: 10.1161/CIRCULATIONAHA.116.025760. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt D.L., Fox K.A.A., Hacke W., Berger P.B., Black H.R., Boden W.E. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 7.Kelly B., Tannahill G.M., Murphy M.P., O’Neill L.A.J. Metformin inhibits the production of reactive oxygen species from NADH: ubiquinone oxidoreductase to limit induction of interleukin-1β (IL-1β) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. J Biol Chem. 2015;290:20348–20359. doi: 10.1074/jbc.M115.662114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyun B., Shin S., Lee A., Lee S., Song Y., Ha N.-J. Metformin down-regulates TNF-α secretion via suppression of scavenger receptors in macrophages. Immune Netw. 2013;13:123. doi: 10.4110/in.2013.13.4.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liani R., Halvorsen B., Sestili S., Handberg A., Santilli F., Vazzana N. Plasma levels of soluble CD36, platelet activation, inflammation, and oxidative stress are increased in type 2 diabetic patients. Free Radic Biol Med. 2012;52:1318–1324. doi: 10.1016/j.freeradbiomed.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Young M.P., Febbraio M., Silverstein R.L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy D.J., Kuchibhotla S., Westfall K.M., Silverstein R.L., Morton R.E., Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. 2011;89:604–613. doi: 10.1093/cvr/cvq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Podrez E.A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 13.Glatz J.F.C., Luiken J.J.F.P. Dynamic role of the transmembrane glycoprotein CD36 (SR-B2) in cellular fatty acid uptake and utilization. J Lipid Res. 2018;59:1084–1093. doi: 10.1194/jlr.R082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicholson A.C., Han J., Febbraio M., Silversterin R.L., Hajjar D.P. Role of CD36, the macrophage class B scavenger receptor, in atherosclerosis. Ann N Y Acad Sci. 2006;947:224–228. doi: 10.1111/j.1749-6632.2001.tb03944.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu W., Li W., Silverstein R.L. Advanced glycation end products induce a prothrombotic phenotype in mice via interaction with platelet CD36. Blood. 2012;119:6136–6144. doi: 10.1182/blood-2011-10-387506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handberg A., Norberg M., Stenlund H., Hallmans G., Attermann J., Eriksson J.W. Soluble CD36 (sCD36) clusters with markers of insulin resistance, and high sCD36 is associated with increased type 2 diabetes risk. J Clin Endocrinol Metab. 2010;95:1939–1946. doi: 10.1210/jc.2009-2002. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Carmona M.D., Plaza-Seron M.C., Vargas-Candela A., Tinahones F.J., Gomez-Huelgas R., Bernal-Lopez M.R. CD36 overexpression: a possible etiopathogenic mechanism of atherosclerosis in patients with prediabetes and diabetes. Diabetol Metab Syndrome. 2017;9:1–10. doi: 10.1186/s13098-017-0253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ekström N., Cederholm J., Zethelius B., Eliasson B., Fhärm E., Rolandsson O. Aspirin treatment and risk of first incident cardiovascular diseases in patients with type 2 diabetes: an observational study from the Swedish National Diabetes Register. BMJ Open. 2013;3:1–9. doi: 10.1136/bmjopen-2013-002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cyrus T., Sung S., Zhao L., Funk C.D. Effect of low-dose aspirin on vascular inflammation. 2020. [DOI] [PubMed]
- 20.Asha G.V., Reddy R.G., Mahesh M., Vajreswari A., Jeyakumar S.M. Male mice are susceptible to high fat diet-induced hyperglycaemia and display increased circulatory retinol binding protein 4 (RBP4) levels and its expression in visceral adipose depots. Arch Physiol Biochem. 2016;122:19–26. doi: 10.3109/13813455.2015.1126609. [DOI] [PubMed] [Google Scholar]
- 21.Morselli Eugenia, Criollo Alfredo, Rodriguez-Navas Carlos, Djc Chronic high fat diet consumption impairs metabolic health of male mice. Inflamm Cell Signal. 2015;1:1–11. doi: 10.14800/ics.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrikopoulos S., Blair A.R., Deluca N., Fam B.C., Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab. 2008;295:1323–1332. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 23.Mkandla Z., Mutize T., Dludla P.V., Nkambule B.B. Impaired glucose tolerance is associated with enhanced platelet-monocyte aggregation in short-term high-fat diet-fed mice. Nutrients. 2019;11:1–10. doi: 10.3390/nu11112695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Goncalves R., Mosser D.M. The isolation and characterization of murine macrophages. Curr Protoc Im. 2008:1–14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. Routledge; 2013. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 26.Williams L.M., Campbell F.M., Drew J.E., Koch C., Hoggard N., Rees W.D. The development of diet-induced obesity and glucose intolerance in C57BL/6 mice on a high-fat diet consists of distinct phases. PloS One. 2014;9 doi: 10.1371/journal.pone.0106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin S., Thomas T.C., Storlien L.H., Huang X.F. Development of high fat diet-induced obesity and leptin resistance in C57B1/6J mice. Int J Obes. 2000;24:639–646. doi: 10.1038/sj.ijo.0801209. [DOI] [PubMed] [Google Scholar]
- 28.Loss DB, Coe LM, Denison JD, Mccabe LR. Cellular physiology and biochemistr y biochemistry low dose aspirin therapy decreases blood glucose levels but does not prevent type I 2011;48824. [DOI] [PMC free article] [PubMed]
- 29.Pradhan A.D., Cook N.R., Manson J.E., Ridker P.M., Buring J.E. A randomized trial of low-dose Aspirin in the prevention of clinical type 2 Diabetes in women. Diabetes Care. 2009;32:3–8. doi: 10.2337/dc08-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coe L.M., Denison J.D., McCabe L.R. Low dose aspirin therapy decreases blood glucose levels but does not prevent type i diabetes-induced bone loss. Cell Physiol Biochem. 2011;28:923–932. doi: 10.1159/000335806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horakova O., Kroupova P., Bardova K., Buresova J., Janovska P., Kopecky J. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci Rep. 2019;9:1–11. doi: 10.1038/s41598-019-42531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui Y., Hirasawa Y., Sugiura T., Toyoshi T., Kyuki K., Ito M. Metformin reduces body weight gain and improves glucose intolerance in high-fat diet-fed C57BL/6J Mice. Biol Pharm Bull. 2010;33:963–970. doi: 10.1248/bpb.33.963. [DOI] [PubMed] [Google Scholar]
- 33.Haumer M., Amighi J., Exner M., Mlekusch W., Sabeti S., Schlager O. Association of neutrophils and future cardiovascular events in patients with peripheral artery disease. J Vasc Surg. 2005;41:610–617. doi: 10.1016/j.jvs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Downing L.J., Strieter R.M., Kadell A.M., Wilke C.A., Brown S.L., Wrobleski S.K. Neutrophils are the initial cell type identified in deep venous thrombosis induced vein wall inflammation. Am Soc Artif Intern Organs J. 1996;42:M677–M682. doi: 10.1097/00002480-199609000-00073. [DOI] [PubMed] [Google Scholar]
- 35.Fava S., Azzopardi J., Ellard S., Hattersley A.T. ACE gene polymorphism as a prognostic indicator in patients with type 2 diabetes and established renal disease. Diabetes Care. 2001;24:2115–2120. doi: 10.2337/diacare.24.12.2115. [DOI] [PubMed] [Google Scholar]
- 36.Barbieri Jéssica, Fontela Paula Caitano, Winkelmann Eliane Roseli, Zimmermann Carine Eloise Prestes, Sandri Yana Picinin, Mallet Emanelle Kerber Viera, Mnf Anemia in patients with diabetes mellitus. Med Pregl. 2015;1–7 doi: 10.2298/MPNS0706225D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akchurin O., Patino E., Dalal V., Meza K., Bhatia D., Brovender S. Interleukin-6 contributes to the development of anemia in juvenile CKD. Kidney Int Rep. 2019;4:470–483. doi: 10.1016/j.ekir.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varga G., Foell D. Anti-inflammatory monocytes—interplay of innate and adaptive immunity. Mol Cell Pediatr. 2018;5:8–11. doi: 10.1186/s40348-018-0083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang M., Gan H., Shen Q., Tang W., Du X., Chen D. Proinflammatory CD14 +CD16 + monocytes are associated with microinflammation in patients with type 2 diabetes mellitus and diabetic nephropathy uremia. Inflammation. 2012;35:388–396. doi: 10.1007/s10753-011-9374-9. [DOI] [PubMed] [Google Scholar]
- 40.Kimball A.S., Joshi A., Carson W.F., Boniakowski A.E., Schaller M., Allen R. The histone methyltransferase mll1 directs macrophage-mediated inflammation in wound healing and is altered in a murine model of obesity and type 2 diabetes. Diabetes. 2017;66:2459–2471. doi: 10.2337/db17-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunjathoor V.V., Febbraio M., Podrez E.A., Moore K.J., Andersson L., Koehn S. Scavenger receptors class AI/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 42.Castelblanco E., Sanjurjo L., Falguera M., Hernández M., Fernandez-Real J.-M., Sarrias M.-R. Circulating soluble CD36 is similar in type 1 and type 2 diabetes mellitus versus non-diabetic subjects. J Clin Med. 2019;8:710. doi: 10.3390/jcm8050710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alkhatatbeh M.J., Enjeti A.K., Acharya S., Thorne R.F., Lincz L.F. The origin of circulating CD36 in type 2 diabetes. Nutr Diabetes. 2013;3 doi: 10.1038/nutd.2013.1. e59-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viñals M., Bermúdez I., Llaverias G., Alegret M., Sanchez R.M., Vázquez-Carrera M. Aspirin increases CD36, SR-BI, and ABCA1 expression in human THP-1 macrophages. Cardiovasc Res. 2005;66:141–149. doi: 10.1016/j.cardiores.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 45.Eisele G., Schwedhelm E., Schieffer B., Tsikas D., Böger R.H. Acetylsalicylic acid inhibits monocyte adhesion to endothelial cells by an antioxidative mechanism. J Cardiovasc Pharmacol. 2004;43:514–521. doi: 10.1097/00005344-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Jin X., Yao T., Zhou Z., Zhu J., Zhang S., Hu W. Advanced glycation end products enhance macrophages polarization into M1 phenotype through activating RAGE/NF-κβ pathway. BioMed Res Int. 2015;2015 doi: 10.1155/2015/732450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marcovecchio P.M., Thomas G.D., Mikulski Z., Ehinger E., Mueller K.A.L., Blatchley A. Scavenger receptor CD36 directs nonclassical monocyte patrolling along the endothelium during early atherogenesis. Arterioscler Thromb Vasc Biol. 2017;37:2043–2052. doi: 10.1161/ATVBAHA.117.309123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang C.P., Han S., Okamoto H., Carnemolla R., Tabas I., Accili D. Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest. 2004;113:764–773. doi: 10.1172/JCI19528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greenberg M.E., Sun M., Zhang R., Febbraio M., Silverstein R., Hazen S.L. vol. 203. 2006. (Oxidized phosphatidylserine – CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musutova M., Elkalaf M., Klubickova N., Koc M., Povysil S., Rambousek J. The effect of hypoxia and metformin on fatty acid uptake, storage, and oxidation in L6 differentiated myotubes. Front Endocrinol (Lausanne) 2018;9:1–11. doi: 10.3389/fendo.2018.00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.