Abstract
Objective
To investigate the association between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension (PAH).
Methods
The relevant literature was retrieved from the PubMed® database and the data were extracted. STATA® version 12.0 software was used to calculate pooled odds ratios (ORs) and 95% confidence intervals (CI).
Results
Eight case–control studies qualified for inclusion in the meta-analysis. These studies included 1215 cases and 936 control subjects. There was no significant association between the SLC6A4 gene L/S polymorphism and PAH risk in the total population (LL versus SS: OR 1.83, 95% CI 0.95, 3.51; LS versus SS: OR 1.37, 95% CI 0.93, 2.02; dominant model: OR 1.38, 95% CI 0.97, 1.97; recessive model: OR 1.54, 95% CI 0.84, 2.83). Subgroup analysis based on study quality scores and Hardy–Weinberg equilibrium also showed no significant association.
Conclusion
The findings of this meta-analysis suggest that the SLC6A4 gene L/S polymorphism is unlikely to be related to PAH risk. Well-designed studies with more participants will be required to validate these results.
Keywords: 5-hydroxytryptamine transporter, pulmonary arterial hypertension, meta-analysis
Introduction
Pulmonary arterial hypertension (PAH), a relatively rare but lethal disease, is characterized by obliterative pulmonary vascular remodelling, which causes progressively enhanced pulmonary vascular resistance that leads to right heart failure. Although clear progress has been made in the modern treatment of this disease, the mortality for heritable and idiopathic PAH remains at approximately 10% annually.1,2 To some extent, the high mortality rate is in part due to the low efficacy of the approved therapy on pulmonary vascular pathology, including endothelial cell proliferation, inflammation and hyperplasia of fibroblasts and vascular smooth muscle cells.3 In addition, the substantially varied therapeutic responses among patients emphasize the insufficient understanding of the causes of PAH.
The critical effects of serotonin (5-hydroxytryptamine [5-HT]) has been recently noted in pulmonary vascular remodelling.4 Patients receiving appetite suppressants that block the 5-HT transporter (5-HTT) have been shown to have an elevated PAH risk.4 5-HTT enables the reuptake of excess 5-HT from the synaptic cleft, which is vitally important in regulating 5-HT synaptic function.5 However, there is controversy over the mechanism underlying the effects of 5-HT on pulmonary vasculature.6 The solute carrier family 6 member 4 (SLC6A4) gene is localized on chromosome 17q11.2-17q12 and it encodes 5-HTT.4 Polymorphisms of this gene can cause changes in 5-HT concentrations, including two polymorphisms (variable number tandem repeat [VNTR] and 5-HTTLPR), named the SLC6A4 gene L/S polymorphism.7 Cells with the ‘L/L’ 5-HTTLPR genotype have been reported to uptake more serotonin than those with the ‘S/L’ or ‘S/S’ genotypes.4 That is to say, the S allele indicates lower uptake activity.
The pathogenesis of PAH is multifactorial and complex, with a largely unclear mechanism.8 The relationship between the SLC6A4 gene L/S polymorphism and PAH risk has been reported by various studies, however, with controversial outcomes.8–10 Case–control studies with relatively limited sample sizes might not be the best option for comprehensively elucidating the complex relationship due to their inadequate statistical power. Undertaking a meta-analysis is helpful for analysing complex data from multiple case–control studies. Herein, this current meta-analysis aimed to investigate the relationship between the SLC6A4 gene L/S polymorphism and PAH risk by collecting all currently relevant and accessible articles.
Materials and methods
Publication search strategy
The electronic database PubMed® was searched from January 2000 to January 2020 to identify relevant studies using a combination of keywords and subject terms as follows: “serotonin transporter” or “5-HTT”, “polymorphism” and “pulmonary arterial hypertension”. The reference lists of included studies were also manually examined to identify other potentially eligible studies. For literature with overlapping data, studies with the largest number of cases were selected. This current meta-analysis was undertaken in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist.
Inclusion and exclusion criteria
The eligibility inclusion criteria were as follows: (i) case–control studies evaluating the correlation between the SLC6A4 gene L/S polymorphism and PAH risk; (ii) patients were clinically diagnosed with PAH; (iii) populations with accessible odds ratio (OR) with 95% confidence interval (CI) or adequate data to calculate OR with 95% CI. Studies were eliminated if they had no control or usable information.
Data extraction
All possible articles were independently reviewed by two investigators (F. Z. & M. Y.), followed by data extraction. Any discrepancies were resolved by discussion with another investigator (Y. H.). The following data were retrieved from each paper: country, number of cases and controls, publication year, genotype frequencies in cases and controls, first author name and Hardy–Weinberg equilibrium (HWE) evidence in control subjects.
Quality score assessment
The quality of the included studies was evaluated independently by two investigators (Y. C. & S. X.) according to a set of predetermined criteria (Table 1) modified from previous research.11 Any disagreements were resolved by discussion among the two investigators to reach consensus.11 Scores ranged from 0 (lowest) to 10 (highest) and studies with scores ≥ 6 were classified as high-quality studies and studies with scores < 6 were classified as low-quality studies.
Table 1.
Criteria used to assess the quality of the case–control studies included in a meta-analysis to investigate the relationship between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension (PAH).
| Criteria | Score |
|---|---|
| Source of cases | |
| Selected from population disease registry or multiple centre sites | 2 |
| Selected from hospital | 1 |
| Not described | 0 |
| Source of controls | |
| Population-based | 3 |
| Blood donors or volunteers | 2 |
| Hospital-based | 1 |
| Not described | 0 |
| Ascertainment of PAH | |
| Standard method confirmation | 2 |
| Not described | 0 |
| Genotyping examination | |
| Genotyping done under blinded conditions | 1 |
| Unblinded or not mentioned | 0 |
| Hardy–Weinberg equilibrium in controls | |
| Hardy–Weinberg equilibrium | 3 |
| Hardy–Weinberg disequilibrium | 0 |
| Association assessment | |
| Assessed association between genotypes and PAH with appropriate statistics and examining confounders and effect modifiers | 1 |
| Inappropriate statistics used | 0 |
Statistical analyses
The association between the SLC6A4 gene L/S polymorphism and PAH susceptibility was determined by ORs and corresponding 95% CIs using a homozygote comparison (LL versus SS), a heterozygote comparison (LS versus SS), a dominant model (LL+LS versus SS) and a recessive model (LL versus SS+LS) between groups. The I2 test was used to assess the potential heterogeneity among the articles. An I2 of > 50% suggested the presence of heterogeneity among the studies, so a random-effects model was employed; otherwise, a fixed-effects model was adopted. The stability of the results was determined by a one-way sensitivity analysis. Every individual study in the meta-analysis was sequentially omitted to identify the effects of this specific study on the pooled OR. The diversity among different studies were examined by subgroup analyses stratified by HWE. Moreover, the Begg’s analysis was used to determine the underlying publication bias (P < 0.05 indicated statistical significance). Statistical analyses were undertaken with STATA® version 12.0 software (STATA Corp., College Station, TX USA). The power of each study was computed as the probability of detecting an association between the SLC6A4 gene L/S polymorphism and PAH using a significance level of 0.05, assuming an OR of 1.5 (small effect size). Power analysis was performed using the statistical program PS: Power and Sample Size Calculation.12
Results
A flow diagram showing the study selection process is presented in Figure 1. Eight case–control studies were finally enrolled according to the inclusion criteria, involving 1215 cases and 936 controls.6,8–10,13–16 The genotype frequencies were consistent with the HWE in all studies except three.9,15,16All publications were written in English. The general features and the allele and genotype distributions were summarized in Table 2.6,8–10,13–16 The results of the quality score assessment ranged from 4 to 8. The statistical powers of these eight studies ranged from 12.6% to 48%. None of the studies had a statistical power that exceeded 80%.
Figure 1.
Flow diagram of eligible studies showing the number of citations identified, retrieved and included in the meta-analysis to investigate the relationship between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension.
Table 2.
Study selection and subject characteristics of studies included in a meta-analysis to investigate the relationship between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension.6,8–10,13–16
| Author | Year | Country | Cases(n) | Controls(n) | Genotypes of cases | Genotypes of controls | P-value for HWE | Quality scores |
|---|---|---|---|---|---|---|---|---|
| SS/SL/LL | SS/SL/LL | |||||||
| Eddahibi et al.8 | 2003 | France | 103 | 98 | 17/54/32 | 20/50/28 | P = 0.79 | 8 |
| Machado et al.9 | 2006 | England | 528 | 353 | 114/244/170 | 88/157/108 | P = 0.04 | 5 |
| Willers et al.10 | 2006 | USA | 223 | 125 | 46/99/78 | 19/63/43 | P = 0.60 | 8 |
| Cao et al.13 | 2009 | China | 140 | 140 | 71/51/18 | 86/45/9 | P = 0.35 | 8 |
| Ulrich et al.6 | 2010 | Switzerland | 27 | 22 | 5/16/6 | 8/12/2 | P = 0.40 | 8 |
| Baloira et al.14 | 2011 | Spain | 49 | 50 | 13/26/10 | 12/23/15 | P = 0.59 | 8 |
| Shivani et al.15 | 2011 | India | 65 | 100 | 16/28/21 | 57/19/24 | P < 0.01 | 4 |
| Ulasli et al.16 | 2013 | Turkey | 80 | 48 | 24/19/37 | 17/11/20 | P < 0.01 | 5 |
HWE, Hardy–Weinberg equilibrium.
The major outcomes in this study are summarized in Table 3. The SLC6A4 gene L/S polymorphism was not significantly associated with PAH under any genetic models (Figure 2; LL versus SS: OR 1.83, 95% CI 0.95, 3.51; LS versus SS: OR 1.37, 95% CI 0.93, 2.02; dominant model: OR 1.38, 95% CI 0.97, 1.97; recessive model: OR 1.54, 95% CI 0.84, 2.83). In the subgroup analysis where studies were stratified according to their quality scores, there was no significant association observed with high-quality studies. In the subgroup analysis where studies were stratified according to HWE, the heterogeneity was removed for stratification analysis after removing articles deviating from HWE.
Table 3.
Summary odds ratio (OR) and 95% confidence interval (CI) for total and subgroup meta-analysis of the relationship between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension.6,8–10,13–16
| LL versus SS | LS versus SS | Dominant model | Recessive model | ||
|---|---|---|---|---|---|
| Variables | n a | OR (95% CI) Model | OR (95% CI) Model | OR (95% CI) Model | OR (95% CI) Model |
| Total | 8 | 1.83 (0.95, 3.51) R | 1.37 (0.93, 2.02) R | 1.38 (0.97, 1.97) R | 1.54 (0.84, 2.83) R |
| HWE | |||||
| Yes | 5 | 1.18 (0.80, 1.74) F | 1.10 (0.80, 1.51) F | 1.16 (0.87, 1.56) F | 1.14 (0.84, 1.55) F |
| No | 3 | 2.92 (1.31, 6.48) R | 1.93 (0.75, 4.94) R | 1.79 (0.85, 3.78) R | 2.10 (0.83, 5.34) R |
| Study quality | |||||
| High quality | 5 | 1.18 (0.80, 1.74) F | 1.10 (0.80, 1.51) F | 1.16 (0.87, 1.56) F | 1.14 (0.84, 1.55) F |
| Low quality | 3 | 2.92 (1.31, 6.48) R | 1.93 (0.75, 4.94) R | 1.79 (0.85, 3.78) R | 2.10 (0.83, 5.34) R |
aNumber of comparisons.
R, random-effects model; HWE, Hardy–Weinberg equilibrium; F, fixed-effects model.
Figure 2.
Forest plot of a meta-analysis to investigate the relationship between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension. Data are pooled odds ratios (OR) with 95% confidence intervals (CI) determined using a random-effects model. Error bars indicate the 95% CIs.6,8–10,13–16
The stability of the present outcomes was assessed by a sensitivity analysis by sequentially removing a single study each time. The results demonstrated that no individual study significantly influenced the pooled ORs (Figure 3).
Figure 3.
Sensitivity analysis of the relationship between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension.
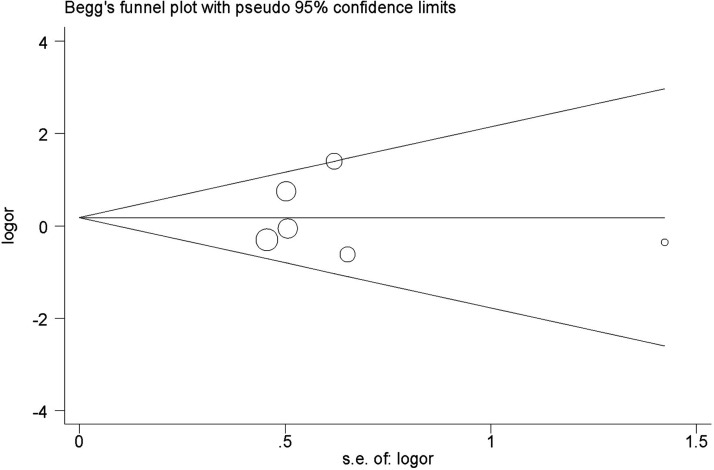
A funnel plot and Begg’s test was adopted to determine publication bias, which showed that there was no evidence of publication bias in this current study (Figure 4).
Figure 4.
Begg’s funnel plot of studies included in a meta-analysis to investigate the relationship between the solute carrier family 6 member 4 (SLC6A4) gene L/S polymorphism and pulmonary arterial hypertension to test for publication bias. Area of each circle represents the contribution of the study to the pooled odds ratio (OR).
Discussion
Pulmonary arterial hypertension, a severe and lethal disease, is characterized by progressively elevated pulmonary vascular resistance but normal left heart pressure. Despite the unclear pathogenesis, research has demonstrated that specific cellular pathways are involved in the development of PAH lesions.2 In 2000, bone morphogenetic protein receptor type 2 was reported to be responsible for the pathogenesis of hereditary PAH, which was considered as one of the most significant findings in this field of research.17 In addition, studies have investigated the potential genes associated with vascular regulation in recent years.8–10 5-HT can stimulate the proliferation of smooth muscle cells within the pulmonary vasculature.4 This current meta-analysis evaluated the relationship between the SLC6A4 gene L/S polymorphism and the risk of PAH.
To assess the role of the SLC6A4 gene L/S polymorphism in the susceptibility to PAH, eight case–control studies were included in this meta-analysis, involving 1215 cases and 936 healthy control subjects. There was no significant association between this variant and PAH susceptibility among the total population. The actual effects of any single gene or polymorphism in the 5-HTT system is likely to be less than expected. However, the non-significant relationship between the SLC6A4 gene L/S polymorphism and PAH does not necessarily eliminate the possibility that other variants or combinations of alleles at multiple loci within the same genes might be related to PAH. Therefore, it is necessary to systematically screen for functional variants within the SLC6A4 gene and other related genes, followed by functional assays to validate the causal variants and their epistatic interactions in PAH pathogenesis.18 In addition, significant between-study heterogeneity was displayed among all comparison models in the current meta-analysis. However, after removing articles deviating from HWE, the heterogeneity was removed for stratification analysis, suggesting that studies deviating from HWE were a significant source of the heterogeneity.19
This current meta-analysis had several limitations. First, only articles published in English-language journals were included, with unpublished or non-English-language articles being excluded although they might have met the inclusion criteria. Secondly, the OR value was acquired without correction, but OR would normally be corrected by ethnicity, age and other exposure factors possibly related to PAH risk in order to produce accurate outcomes. Thirdly, inter-gene and gene–environment interactions might also influence the accuracy of these current outcomes. A lack of the original data restricted further evaluation of the potential inter-gene and gene–environment interactions.
In conclusion, this current meta-analysis demonstrated that the SLC6A4 gene L/S polymorphism did not appear to be related to PAH risk. Based on the limitations described earlier, high-quality studies are required to validate these findings.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Chunping Ni https://orcid.org/0000-0002-1450-7027
References
- 1.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013; 62: D51–D59. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 3.Stacher E, Graham BB, Hunt JM, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulrich S, Szamalek-Hoegel J, Hersberger M, et al. Sequence variants in BMPR2 and genes involved in the serotonin and nitric oxide pathways in idiopathic pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: relation to clinical parameters and comparison with left heart disease. Respiration 2010; 79: 279–287. [DOI] [PubMed] [Google Scholar]
- 5.Fink KB, Gothert M. 5-HT receptor regulation of neurotransmitter release. Pharmacol Rev 2007; 59: 360–417. [DOI] [PubMed] [Google Scholar]
- 6.Ulrich S, Hersberger M, Fischler M, et al. Genetic polymorphisms of the serotonin transporter, but not the 2a receptor or nitric oxide synthetase, are associated with pulmonary hypertension in chronic obstructive pulmonary disease. Respiration 2010; 79: 288–295. [DOI] [PubMed] [Google Scholar]
- 7.Lam D, Ancelin ML, Ritchie K, et al. Genotype-dependent associations between serotonin transporter gene (SLC6A4) DNA methylation and late-life depression. BMC Psychiatry 2018; 18: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddahibi S, Chaouat A, Morrell N, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation 2003; 108: 1839–1844. [DOI] [PubMed] [Google Scholar]
- 9.Machado RD, Koehler R, Glissmeyer E, et al. Genetic association of the serotonin transporter in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 173: 793–797. [DOI] [PubMed] [Google Scholar]
- 10.Willers ED, Newman JH, Loyd JE, et al. Serotonin transporter polymorphisms in familial and idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 173: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camargo MC, Mera R, Correa P, et al. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms and gastric cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2006; 15: 1674–1687 [DOI] [PubMed] [Google Scholar]
- 12.Liu N, Wang Y. Association between angiotensinogen T174M polymorphism and the risk of diabetic nephropathy: A meta-analysis. J Renin Angiotensin Aldosterone Syst 2019; 20: 1470320318823927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao H, Gu H, Qiu W, et al. Association study of serotonin transporter gene polymorphisms and ventricular septal defects related possible pulmonary arterial hypertension in Chinese population. Clin Exp Hypertens 2009; 31: 605–614. [DOI] [PubMed] [Google Scholar]
- 14.Baloira A, Núñez M, Cifrian J, et al. Polymorphisms in the serotonin transporter protein (SERT) gene in patients with pulmonary arterial hypertension. Arch Bronconeumol 2012; 48: 77–80. [DOI] [PubMed] [Google Scholar]
- 15.Shivani V, Sujana K, Sastry BKS, et al. 5HTT Promoter Polymorphism in Idiopathic Pulmonary Arterial Hypertension. Int J Hum Genet 2011; 11: 111–115. [Google Scholar]
- 16.Ulasli SS, Eyuboglu FO, Verdi H, et al. Associations between endothelial nitric oxide synthase A/B, angiotensin converting enzyme I/D and serotonin transporter L/S gene polymorphisms with pulmonary hypertension in COPD patients. Mol Biol Rep 2013; 40: 5625–5633. [DOI] [PubMed] [Google Scholar]
- 17.Lane KB, Machado RD, Pauciulo MW, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. International PPH Consortium. Nat Genet 2000; 26: 81–84. [DOI] [PubMed] [Google Scholar]
- 18.Rhodes CJ, Batai K, Bleda M, et al. Genetic determinants of risk in pulmonary arterial hypertension: international genome-wide association studies and meta-analysis. Lancet Respir Med 2019; 7: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao Y, Dong Z, Zhu J, et al. Association between ACE A240T polymorphism and cancer risk: a meta-analysis. J Int Med Res 2019; 47: 5917–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]